Abstract
Growing perennial grasses is often cited as one of the possible and most affordable solutions for mitigating climate change. This practice is also recommended for sustainable soil management in agriculture. Our experiment involved timothy grass (Phleum pratense L.), red clover (Trifolium pratense L.), and their mixture; tall oat grass (Arrhenatherum elatius L.), alfalfa (Medicago sativa L.), and their mixture, with the aim of diversifying the annual rotation; and periodical, twice-per-season cultivated plots in the same area (the bare soil fallow). Soil samples were collected in late October after plant vegetation’s first, second, and third growth years from three field replicates at the soil layers 0–0.1 m, 0.1–0.2 m, and 0.2–0.3 m and plant roots—at the beginning of November in the second cultivation year. After three years, the SOC content increased in all the study areas occupied by plants, regardless of their species composition, while it decreased in fallow plots. Grass roots were characterized by the highest C/N ratio (38.2 and 45.5). The roots of the red clover–timothy grass mixture also reached a C/N ratio greater than 30. Based on our research, choosing a combination of at least two plants, such as legumes and grasses, is possibly more effective for enriching the soil with carbon compounds in a short period.
1. Introduction
As global food and forage production needs grow, agricultural strategies have concentrated on maximizing crop yields. This goal was commonly achieved with systems dominated by a single or few annual crop species [1]. However, this choice of crop rotation can have a negative impact on the sustainability of agricultural ecosystems, resulting in soil erosion, the loss of organic carbon and other nutrients, and the build-up of diseases and pests [2]. These problems become even more apparent in the background of climate change and force scientists, farmers, and politicians to search for solutions and consensus to mitigate the inevitable consequences. Also, the European Union aims to achieve by 2050 becoming a climate-neutral economy with net-zero greenhouse gas emissions, according to the European Green Deal and the duty to act on climate change under the Paris Agreement [3,4,5,6]. All these steps require, and in the future will need, even more knowledge, cooperation, planning, and, of course, financial costs, both in solving the current and future problems of sustainable farming [7,8].
Growing perennial grasses is often cited as a possible and the most affordable solution to these problems. Although perennial grasses are usually highlighted as beneficial for soil ecosystems in the long term (at least 10 years), the advantages can be recognized after a shorter period [9,10,11]. In annual rotations with excessive soil tillage, which leads to soil degradation, perennial grasses can be used to regenerate soil health and restore agroecosystem functions before the next period of annual grain crops as a part of the rotation or as cover crops [12,13]. The roots of perennial grasses are rich in organic matter and can improve agricultural soil quality through C sequestration, water, and nutrient cycling, as well as supporting biodiversity [14]. However, this process is slow, and the results are not always visible, so choosing plant species and their mixtures that best fulfill the needs and climatic conditions is essential [15,16,17]. It is also required to predict for what purpose the grasses’ biomass will be used: whether for feed, energy production, fibers, protein products, etc. [18].
Various perennial legumes and grasses are available for agricultural and other objectives. Although the legumes alfalfa (Medicago sativa L.) and red clover (Trifolium pratense L.) and grasses timothy grass (Phleum pratense L.) and tall oat grass (Arrhenatherum elatius L.) belong to different plant families, their needs for environmental conditions differ little (Table 1). Moreover, they grow in the natural Lithuanian environment and are not only cultivated in agriculture.
Legume–grass mixtures contain one or more legumes, such as red clover, alfalfa, etc., adjusted for various aims, such as forage, belowground biomass for nitrogen or carbon addition to the soil, erosion prevention, or other. They are widely used in considerable regions of Europe [2,19].

Table 1.
Biological characteristics of tested perennial grass species.
Table 1.
Biological characteristics of tested perennial grass species.
| Species | Growth Form | Storage Organ | Type of Clonal Growth Organ | Ellenberg-Type Ecological Indicator Values | |||
|---|---|---|---|---|---|---|---|
| Light 1 | Temperature 2 | Moisture 3 | Nutrient 4 | ||||
| Arrhenatherum elatius | Perennial clonal herb | Shoot tuber, tuft | hypogeogenous rhizome | 7 | 5x | 5x | 7 |
| Phleum pratense | epigeogenous rhizome | 7 | 5 | 5 | 7 | ||
| Medicago sativa | Polycarpic perennial non-clonal herb | Pleiocorm, primary storage root | - | 8 | 6 | 4 | 5x |
| Trifolium pratense | - | 7 | 5x | 5 | 6x | ||
1 7—half-light plant, mostly occurring at full light but also in the shade up to about 30% of diffuse radiation incident in an open area; 8—light plant, only exceptionally occurring at less than 40% of diffuse radiation incident in an open area. 2 5x—moderate heat indicator, occurring from lowland to montane belt, mainly in submontane-temperate areas (generalist); 5—moderate heat indicator, occurring from lowland to montane belt, mainly in submontane-temperate areas; 6—transition between values 5 and 7; 7—heat indicator, occurring in relatively warm lowlands. 3 4—a transition between values 3 and 5; 5—indicator of fresh soils, focus on soils of average moisture, missing on wet and on soils that frequently dry out; 5x—indicator of fresh soils, focus on soils of average moisture, missing on wet and on soils that frequently dry out (generalist). 4 5x—occurring at moderately nutrient-rich sites and less frequently at poor and rich sites (generalist); 6x—transition between values 5 and 7 (generalist); 7—occurring at nutrient-rich sites more often than at average sites and only exceptionally at poor sites. Note. Presented data are sourced from the Pladias Database of the Czech Flora and Vegetation [20].
Soil organic carbon (SOC) is essential for soil quality and health. Increasing SOC in agricultural systems can simultaneously reduce net CO2 emission, improve soil fertility, and increase productivity and resistance to climate change [21]. The highest amounts of SOC usually accumulate in the top layer of soil. The most sensitive and active SOC fraction is water-extractable organic carbon (WEOC), which makes up a few percent of SOC and can show the anthropogenic impact on the soil. Forests and grasslands hold higher concentrations of labile WEOC fractions than arable soils [22]. These carbon compounds have relatively simple structures, contain carbohydrates from plant roots, their exudates, microorganisms, and humus substances, and are easily mineralized. WEOC is also a bioavailable carbon that plants and particularly microorganisms absorb quickly and use as an energy source [22]. All these features make WEOC suitable to use as an integrated soil quality indicator. WEOC fractions in deeper layers are likely to be less sensitive to degradation. Thus, there is potential for SOC preservation in the future [22,23].
The roots of grasses are not evenly spread out in the soil. They are primarily concentrated in the upper 0–10 cm layer of soil, and their distribution throughout the entire soil profile depends on various factors, such as soil properties and plant species or mixtures. These differences affect how roots impact soil and carbon accumulation potential. The amount and composition of the organic matter from the roots carried into deeper soil layers play a crucial role in these effects [24,25,26]. The C/N ratio of biomass is a common indicator that can be used to predict root decomposition in soil. A higher C/N ratio implies lower soil mineral N, resulting in a slower decomposition of root organic matter. Consequently, the components of the organic matter remain in the soil for a more extended period [27,28]. In this way, the physical and chemical properties of the soil and the structure of the rhizosphere bacterial community are improved [29].
Given the relevance of this issue described above in the Introduction Section, this study aimed to determine how some indicators predicting carbon accumulation in soils previously used for annual or biennial crop rotations change after introducing perennial grasses and their mixtures. The most crucial question was whether, within a period of 3 years, differences could emerge that could be used to select perennial plant species with the most significant positive effect on soil carbon accumulation through crop diversification, which is important to sustainable agriculture.
2. Materials and Methods
2.1. Study Area and Sampling
This research was carried out in the central part of the Middle Lithuanian Lowland at the Lithuanian Research Centre for Agriculture and Forestry (LAMMC) experimental base in Akademija, Kedainiai district (Figure 1). Its location is at 55°39′65.9″ N 23°86′76.1″ E [30], with the soil type being Cambisol according to the classification by WRB (2022) [31].
Figure 1.
The experimental site in Akademija, Kedainiai district, Lithuania.
Based on Europe’s environmental stratification, the site is situated in the Nemoral zone, with a short growing season of 195 days [32]. According to the standard climate norm from 1991 to 2020, the mean annual precipitation is 695 mm, and the average temperature is 7.4 °C (Figure 2) [33].
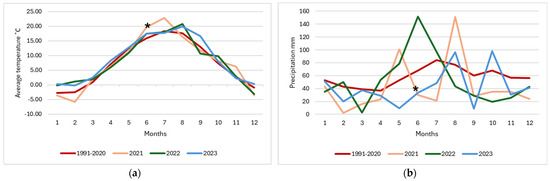
Figure 2.
Weather conditions from 1991 to 2020 and the years of the experiment 2021, 2022, and 2023. The average temperature of the months (a) and total monthly precipitation (b). The sowing time of the seeds is marked with an asterisk.
The experimental field before the experiment was used for the short-term cultivation of grasses for one or two years, managed by conventional fall plowing (0.2 m) after their vegetation. The field was fertilized with the complex fertilizer at a N 24 kg, P 72 kg, K 136 kg ha−1 rate before the experiment, and no additional fertilizer was used during the later stages of the study. The experiment involved the following plants: timothy grass, red clover, and their mixture; tall oat grass, alfalfa, and their mixture; and the bare soil fallow. Although some shortcomings in bare soil fallow agricultural management practices have been identified, such fallow is used as an ecological tool for weed control or to prepare fields for other crops after perennial vegetation [34].
Plant seeds were sown mechanically (seed rate 10–30 kg ha−1, depending on the plant species) in 9 m2 plots in three field replicates in separate blocks in June 2021. The plant mixtures consisted of legumes 40% and grasses 60%. During the season, the aboveground biomass was cut twice and removed from the field, as would be conducted in producing forage or preparing to use biomass for other purposes.
Soil samples were collected in late October after plant vegetation’s first, second, and third growth years from three field replicate plots using a steel auger at the soil layers 0–0.1 m, 0.1–0.2 m, and 0.2–0.3 m. Soil samples were analyzed for SOC content during the first and third years of plant growth and for WEOC dynamics over the entire three-year period. Plant roots were collected from the same areas and depths at the beginning of November after the end of active plant vegetation in the second cultivation year, using a metal frame of 1000 cm3. The soil and roots were separated using a wet sieving system with three different sieves, of which the densest was 0.25 mm, removing both stones and aboveground biomass residues and collecting roots for further analyses.
Before the experiment was set up, the soil properties were determined. The soil at a 0–30 cm depth contained 72.3% silt, 26.3% sand, and 1.4% clay and was classified as a sandy loam [35]. The soil pH was near neutral (pHKCl 6.9), and it contained 0.23 g kg−1 plant-available phosphorus (P2O5) and 0.37 g kg−1 plant-available potassium (K2O). The concentration of Kjeldahl nitrogen was 1.52 g kg−1.
2.2. Chemical Analyses of Soil and Roots
Chemical analyses of the soil and roots were carried out in the Chemical Research Laboratory of the Institute of Agriculture of LAMMC. The soil samples before chemical analyses were crushed, sieved through a 2 mm sieve, and mixed. For SOC and WEOC analysis, the samples were further ground and passed through a 0.25 mm sieve. SOC content was determined by the Nikitin-modified Tyurin dichromate oxidation method using wet combustion at 160 °C for 30 min. SOC measurement was performed by a spectrophotometer at a wavelength of 590 nm using glucose as a standard [36,37]. The roots’ organic carbon (Corg.) content was measured using the same dichromate oxidation method after drying and grinding the samples.
The WEOC analysis procedure was performed according to the methodology guided by SKALAR, using C8H5KO4 as a standard. The sieved soil was poured with distilled water at a ratio of 1:5, and the extract was prepared by shaking, centrifugation for 15 min at 4500 rpm, and filtration. Then, the automated measurement procedure was performed based on the IR detection method following UV-catalyzed persulfate oxidation under nitrogen. WEOC oxidized to carbon dioxide during this process and was measured using an ion analyzer (SKALAR, The Netherlands) [38,39].
The amount of Nt in the soil and roots was measured using the Kjeldahl method, which involved a spectrophotometric measurement at 655 nm. The reaction between nitrogen salicylate and hypochlorite ions in an alkaline solution, along with the presence of sodium nitroferricyanide, formed a blue-colored compound [40,41].
To prepare the roots for scanning their length, they were cut into 2 cm long pieces and then soaked in a solution of 20% ethyl alcohol and 0.05% neutral red dye for staining. Afterwards, the roots were washed several times with running water to remove any remaining dye solution, then spread in an acrylic tray with distilled water, and scanned. Each root sample was divided into several scans depending on the size to minimize the overlap of the roots, which can disturb the clear view of the roots. The root length analysis was conducted using the software WinRhizo [42,43].
Root biomass (as dry matter) was determined by weight after drying at +60 °C in an air flow oven to a constant weight. Soil bulk density was determined using core samples for the widely applied thermogravimetric method [44]. In this method, soil cores are dried in an oven at 105 °C and weighed to determine the soil mass without moisture. The bulk density is calculated by dividing the dried soil’s mass by the core sample’s volume [45].
2.3. Statistical Analysis
The experiment’s soil and root parameter results were calculated from three field replicates and presented as the mean and standard error (SE). These statistical analyses were performed using the SAS Enterprise software, version 7.1 (SAS Institute Inc., Cary, NC, USA). A one-way analysis of variance (ANOVA) was performed to assess significant differences among all treatment means. Fisher’s test was used to calculate p-values, and values of p < 0.05 were considered statistically significant. No between-block effects were found (p = 0.1–0.9) in all analyzed data sets. Pearson’s correlation was used to examine the relationship between root biomass, length, the root C/N of different perennial plants and their mixtures, SOC, and WEOC at a confidence level of 0.95.
3. Results and Discussion
3.1. Soil Organic Carbon
In our study, estimating SOC content in different swards at the end of the year of their installation, not many significant differences were found between treatments or soil layers (Figure 3a). Statistically significant differences (p = 0.03) were determined only in the middle of the investigated depths of 0.1–0.2 m. The plots under alfalfa and tall oat grass in this layer had the highest amount of SOC, with 17.7 and 17.2 g kg−1 content, respectively. These treatments’ lush plants also most densely covered the soil. The grass grew more slowly and less densely in other plots, especially timothy grass, because their germination period was very long in 2021. Similar views were observed in the red clover–timothy grass mixture plots. This was likely influenced by a long period of drought (Figure 2). According to the Lithuanian Hydrometeorological Service under the Ministry of Environment data, only about 20 mm of precipitation fell on the study site during the month of the experiment installation. At the start of the growing, delicate and shallow roots make timothy grass especially vulnerable to unfavorable weather conditions [46]. Most of the growing season in June and July was characterized by low rainfall, with heavy rain in August. These conditions made it challenging to grow aboveground and belowground biomass in swards.
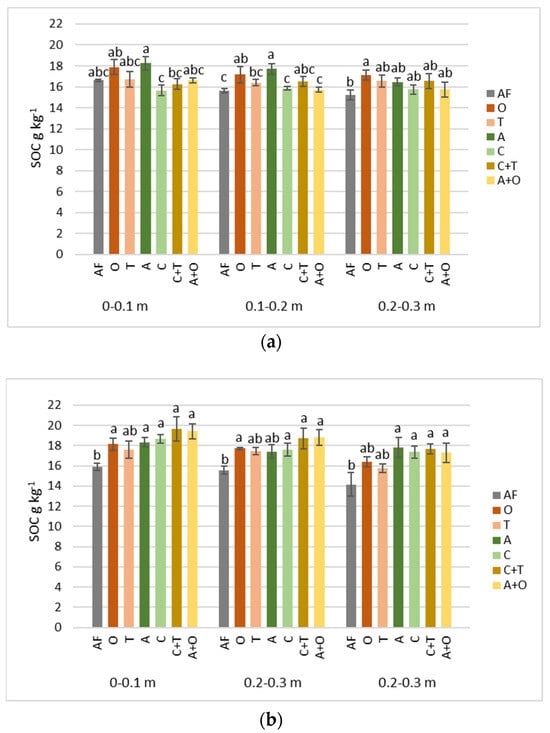
Figure 3.
SOC content as influenced by different perennial plants and their mixtures in 0–0.1, 0.1–0.2, and 0.2–0.3 m soil depth after the first (a) and third (b) years of vegetation. AF—arable field, O—tall oat grass, T—timothy grass, A—alfalfa, C—red clover, C + T—red clover + timothy grass, A + O—alfalfa + tall oat grass. Note. Significant differences in SOC (g kg−1) among various perennial plant species and their mixtures are shown at p ≤ 0.05 using letters; error bars are shown as the standard error (SE) of the mean.
Three years after the experiment was installed, more differences in SOC content were observed between the studied treatments in all soil layers (p = 0.04–0.05) (Figure 3b). However, based on these data, a statistically significant difference is perceptible only between the plots occupied by plants and fallow in the upper and deeper soil layers, regardless of the species. In all these cases, the amount of SOC tends to increase, while the opposite situation is observed in the case of fallow (Table 2). The most prominent change was found under mixtures’ swards at almost all depths (1.1–3.4 g kg−1). Only at a depth of 0.2–0.3 m under legumes was the SOC change greater than under mixtures (1.4–1.6 g kg−1), while grasses at the same depth showed a decrease (−0.7–0.8 g kg−1).

Table 2.
Change in SOC content in the swards under different perennial plants and their mixtures at depths of 0–0.1 m, 0.1–0.2 m, 0.2–0.3 m, and 0–0.3 m, after 2 years of cultivation (2023–2021).
Although evaluating only SOC data, we could not distinguish plants that are more favorable for soil carbon storage, except for the slight advantage of mixtures; plants’ benefit over fallow, which is used to solve specific problems in agricultural soils, is present. It is possible that these results would be more significant and show greater differences between species in a longer experiment, as seen in other similar studies [47]. However, there are limitations, such as the short lifespan of red clover, which showed a noticeable decline in plant growth after three years [46], which we also observed in our experimental plots as the thinning of the grass at the end of the third year of this study.
SOC accumulation is most active in the upper 0–0.30 m soil layer and can be reversed if sustainable farming practices are not implemented. At the same time, a C balance in the topsoil is expected to be achieved within 20 years of targeted land management [48,49]. Also, a realistic understanding and assessment of future SOC sequestration potential in grassland soils require practical studies under actual climate conditions [50]. Establishing perennial plants has led to an initial increase in SOC, marking the beginning of a complex and multifaceted process that must be sustained and enhanced to benefit from it fully.
3.2. Water-Extractable Organic Carbon
During a three-year vegetation period, the water-extractable organic carbon (WEOC) content (g kg−1) was also determined in the 0–0.1, 0.1–0.2, and 0.2–0.3 m soil depths under the same treatments (Figure 4).
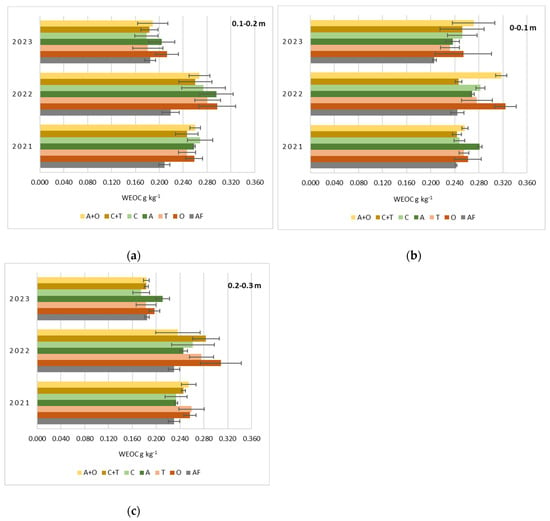
Figure 4.
Water-extractable organic carbon (WEOC) content (g kg−1) as influenced by different perennial plants and their mixtures at 0–0.1 (a), 0.1–0.2 (b), and 0.2–0.3 (c) m soil depth during the three-year vegetation period. AF—arable field, O—tall oat grass, T—timothy grass, A—alfalfa, C—red clover, C + T—red clover + timothy grass, A + O—alfalfa + tall oat grass.
Changes in land use, agronomical models, and vegetation affect soil properties, which impact WEOC [22]. However, we did not find statistically significant differences between the treatments in any observation years (p = 0.09–0.55). Our research time was relatively short, and WEOC is a labile component of carbon pool compounds, making it highly vulnerable to environmental changes. Based on previous studies, the amount of WEOC in grass-occupied soil increases over the cultivation period, especially when it reaches 6 years or more [51,52].
However, our graphs (Figure 4a–c) show an increase in WEOC in 2022, which drops significantly in 2023. This variation in soil WEOC was unexpected, as the literature predicts that WEOC and SOC increase when the land is converted from arable to grassland [23]. The formation process of WEOC in the soil depends not only on changes in soil management practices but also on the activity of microorganisms in the decomposition of organic matter, the intensity of root exudation, etc. [53,54]. These processes require suitable environmental conditions, primarily a favorable temperature and moisture ratio [54,55,56].
Our experimental site experienced above average rainfall in the entire summer during the second year of plant growth (about 290 mm), which was relatively evenly distributed throughout the period. The air temperature was also higher than the average temperature of the decade, reaching 18.7 °C (Figure 2). These conditions may have led to more intensive processes of recycling organic residues after previous plant rotations in the soil. This may have also enhanced plant mass growth, root development, and exudation. In 2022, the plots where tall oat grass grew had the highest WEOC content in all studied soil layers: 0.324–0.297–0.308 g kg−1, respectively. An increase in WEOC was observed in most of the different grass plots and at nearly all studied soil depths (Figure 4a–c).
After the third year of grass growth, WEOC decreased in all study plots, and in many cases, it was even lower than the results obtained after the first year of plant vegetation. Weather conditions during this period were also completely different: very dry beginning and middle of summer (June–July precipitation was 80 mm) and abundant precipitation at the end of August (96 mm); the average temperature was 17.7 °C (Figure 2).
According to a study by Homyak et al., such conditions are promising for forming WEOC [57]. The highest values of the analyzed indicator were determined during an extended period of drought. The data we collected might have been affected by the sampling time in the autumn after heavy rains had already occurred. This trend is also observed in the presented study, which investigates the long-term changes in WEOC levels in soil under various moisture regimes.
It is also likely that the consumption of WEOC exceeded the production capacity because these labile organic carbon compounds are characterized not only as potentially significant for carbon sequestration but also as being used by microorganisms as an energy source and involved in transporting nutrients to plants [23,58,59]. WEOC was probably utilized in grasslands to meet these needs as a readily available source of nutrients. The leaching of WEOC into deeper soil layers or adjacent areas is also likely, especially after a long drought during the summer followed by heavy rain in August, which was heavy rain lasting only 3 days [60]. These weather conditions are not typical of the local climatic zone but recur increasingly, which points to the need to pay attention to and prepare to adapt to climate change (Figure 2).
Regarding the significance of the plant species and their mixtures in our experiment on the amount of WEOC in the soil, the third-year data show even more minor differences between the study treatments. Still, we could single out alfalfa, tall oat grass, and a mixture of these grasses as the leaders. Although the root measurements were made only in the second year of plant growth (Figure 5 and Figure 6), it is likely that the roots’ mass and length of these plants also tended to increase further to the greatest extent. The roots of these plants possess biological properties that enable them to penetrate the deeper layers of the soil more effectively while also providing a greater surface area for exudation [61].
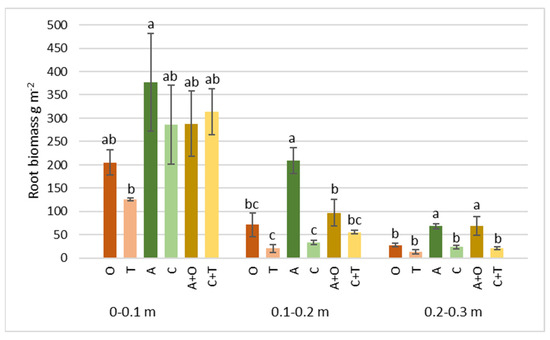
Figure 5.
Roots’ biomass of different perennial plants and their mixtures at 0–0.1, 0.1–0.2, and 0.2–0.3 m soil depth at the end of the second growth year. O—tall oat grass, T—timothy grass, A—alfalfa, C—red clover, C + T—red clover + timothy grass, A + O—alfalfa + tall oat grass. Note. Significant differences in root biomass (g m−2) among various perennial plant species and their mixtures are shown at p ≤ 0.05 using letters; error bars are shown as the standard error (SE) of the mean.
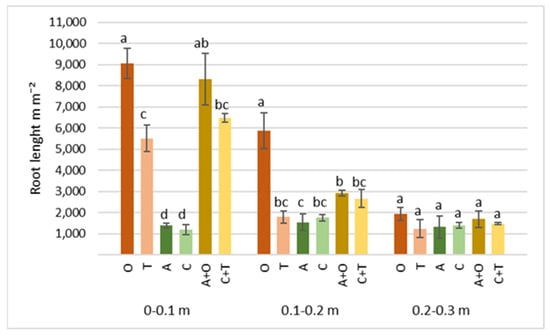
Figure 6.
Roots’ length of different perennial plants and their mixtures at 0–0.1, 0.1–0.2, and 0.2–0.3 m soil depth at the end of the second growth year. O—tall oat grass, T—timothy grass, A—alfalfa, C—red clover, C + T—red clover + timothy grass, A + O—alfalfa + tall oat grass. Note. Significant differences in root length (m m−2) among various perennial plant species and their mixtures are shown at p ≤ 0.05 using letters; error bars are shown as the standard error (SE) of the mean.
3.3. Roots Biomass and Length
Of all the studied treatments, the highest root biomass was determined in the upper 0–0.1 m soil layer, regardless of the plant species or the composition of the mixture. It represented 57.6–83.3% of the total mass in the 0–0.3 m soil layer (Figure 5). These data correspond to previous studies conducted in the same climatic zone, which indicate that the tremendous mass of roots is concentrated in the upper soil layer, where the most intensive nutrient turnover, water supply, etc., occur [62,63,64]. It appears that the combination of these conditions in the upper layer of soil was beneficial for the root development of all plants, with no statistically significant differences (p = 0.25) found among the treatments under study. The average root biomass content at this depth ranged from 127 g m−2 in timothy grass to 377 g m−2 in alfalfa swards.
Still, statistically significant differences between the study treatments were found in other soil layers (0.1–0.2; 0.2–0.3 m), representing p-values of 0.0002 and 0.0018, respectively. Although the mass of roots found at these depths is lower than in the upper layer, regardless of the plant species or their mixture, the importance of organic matter transferred here for the soil’s carbon sequestration potential is higher. According to articles published by other researchers, it provides long-term storage due to its excellent subsoil stability, lower temperature, and lower disturbance without intensive soil management, which results in the slower decomposition of organic matter [65,66].
The highest amount of root biomass at both discussed depths was found in alfalfa (209 and 68.7 g m−2) and alfalfa–tall oat grass mixture plots (97.3 and 69.2 g m−2) (Figure 5). Our findings support the conclusions of previous studies, which found that in legume swards with higher aboveground biomass, the root biomass is also greater and surpasses the root biomass of grasses. These differences are likely caused by larger root diameters at all soil depths, other biological characteristics of legumes (such as mycorrhizae), and soil characteristics [61,67,68].
The presence of roots significantly contributes to forming SOC through various mechanisms. These include root decomposition, which introduces root nutrients into the soil and increases SOC and total soil nitrogen [69]; root exudates as mediators of SOC input and stabilization supporting long-term SOC sequestration; and creating favorable conditions for the prospering of microbial communities in the soil ecosystem [70]. Therefore, research on various plant root systems and their mixtures is necessary to understand the interspecific differences better and the possibilities of application in agricultural and environmental practice.
In our study, we also measured the length of the roots of the investigated plants and their distribution at 0–0.1, 0.1–0.2, and 0.2–0.3 m soil depths (Figure 6). The highest average root length in all soil depths was found in tall oat grass swards, ranging from 1952 to 9063 m m−2. The research conducted thus far has revealed that the tall oat grass fibrous root system plays a significant role in its ecological adaptation and competitiveness. This is evident in the vertical distribution of root mass in both mono-crops and communities with other plants. This characteristic is particularly heightened under unfavorable environmental conditions such as drought [71,72]. Although the total root length in the mixture with alfalfa at a soil depth of 0–0.3 m was lower, it was still significantly higher than under the mono-component alfalfa sward. A similar positive trend can also be seen when measuring the root length of the red clover and timothy mixture.
However, at the soil depth of 0.2–0.3 m, statistically significant differences between the research treatments were not found, while in other layers, these differences were strongly represented (p < 0.001; p = 0.002). These results also support the claims that most perennial grass roots are concentrated in the upper soil layer [62]. In the top 0–0.1 m layer, plots under red clover and alfalfa had the most petite root length, 1205 and 1386 m m−2, respectively. These values of the analyzed indicator were influenced by the biological features of the taproot of leguminous plants, which are characterized by a large main root with a smaller number of branches in the upper part [67].
Plant root length is often noted as positively affecting the entire soil ecosystem. Some research has shown that grass species with extraordinary root lengths contribute more to resistance to soil erosion by improving aggregate stability through root nets and secretions acting as soil binders [68,71,73]. These species can also improve the soil’s physical characteristics, such as reducing compaction, enhancing water-holding capacity, and increasing microbial activity, which can increase carbon storage potential [74].
3.4. Root Carbon-to-Nitrogen Ratio
For long-time carbon sequestration in the soil, organic matter quality plays a significant role. The C/N ratio of roots and other residues is one of the most crucial indicators of this feature. SOC immobilization could be predicted when residues have a C/N ratio > 30, while residues with a C/N ratio < 20 often lead to SOC mineralization and loss due to CO2 emissions, leaching, etc. [75].
Our study determined the C/N ratio in live root samples. According to other studies, this ratio can be considered the residual roots’ C/N ratio since there is no significant difference between these two ratios [76].
Generally, at a depth of 0–0.3 m, the highest average C/N ratio, 45.5, was in the roots under timothy grass and a lower but still high 38.2 under tall oat grass swards (Table 3). The organic matter with higher C/N values, as mentioned above, is favorable for soil carbon storage. This value was lower for the roots of leguminous plants and mixtures with them and ranged from 22.1 to 31.3. Significant differences (p = 0.0009) in root carbon content were also seen. The highest carbon content was found in the roots of grasses: timothy grass—466 g kg−1 and tall oat grass—454 g kg−1. The lowest mean C values were found in the roots of the alfalfa and tall oat grass mixture—445 g kg−1 and alfalfa—447 g kg−1.

Table 3.
Carbon and nitrogen content, as well as the C/N ratio in the roots of different perennial plants and their mixtures at depths of 0–0.1 m, 0.1–0.2 m, 0.2–0.3 m, and 0–0.3 m, after 2 years of cultivation.
Despite this, the C/N ratios of the deepest studied soil layer, 0.2–0.3 m, which is presumably more significant for long-term carbon accumulation in the soil [66], were just 20.5–31.8. Only the timothy grass roots showed a statistically significant difference (p = 0.004). However, the mass and length of these roots were the smallest of all the investigated treatments (Figure 5 and Figure 6). Considering all the indicators, it is unlikely that there will be a notable accumulation of carbon in the deeper soil layers under timothy grass swards. Further detailed studies should confirm or decline this conclusion because it is known that as roots age, their C/N ratio tends to increase [77].
3.5. Correlation of SOC and WEOC
Carbon accumulation in the soil is influenced by numerous factors that are not always easy to determine. Based on the research data we received, we calculated correlations to determine which of the studied indicators had a higher influence on SOC accumulation (Table 4). Significant correlations were found between root biomass and SOC at 0.2–0.3 m depth and WEOC at 0.1–0.2 m depth. No correlation was found between other indicators. The C/N case tended to have a negative correlation, which was also insignificant and may indicate a higher carbon accumulation in the roots than in the soil.

Table 4.
Correlation coefficients between root biomass, length, root C/N of different perennial plants and their mixtures and SOC, WEOC.
Root studies require thorough work, because of replicates showing varying root amounts affecting chemical and physical indicators. Based on the data in this study, it is clear that there were only minor changes in SOC and WEOC during the first three years of perennial plant growth. However, these data provide valuable insights for future research on integrating these plants into land use practices to promote sustainable agriculture.
4. Conclusions
After analyzing the research data, we have to look back at the question presented at the beginning of the experiment: do differences emerge within 3 years of cultivation that could be used to select perennial plant species with the most significant positive effect on soil carbon accumulation through crop diversification?
After three years, the SOC content increased in all the study areas occupied by plants. The highest increase was found in the soil under mixtures consisting of legume and grass. The amount of WEOC in soil varied annually and was likely influenced more by climatic conditions than plant species, but in almost all cases, it was higher under the swards than in fallow plots. The correlation analysis between all the investigated indicators showed that the root biomass had the greatest influence on SOC accumulation.
Recently, there has been increasing talk of incorporating perennials into intensive farming rotations, and according to our research, selecting a combination of at least two plants, such as legumes and grasses, and with potentially higher root biomass is possibly a more effective option for enriching the soil with carbon compounds in a short period. Still, perennial plants might be cultivated in the field for more than three years to contribute significantly to the organic matter in the soil and to maintain it, so research on different plant combinations that would support better results in various climate conditions should also continue.
We believe the data we obtained are valuable for understanding the speed of processes in the soil after changes in management practices, even if the three-year period is only the first step towards increasing SOC and one of the ways to support the sustainability of agricultural land.
Author Contributions
Conceptualization, A.S. (Aida Skersiene), A.S. (Alvyra Slepetiene) and V.S.; methodology, A.S. (Aida Skersiene), A.S. (Alvyra Slepetiene) and V.S.; software, A.S. (Aida Skersiene); validation, A.S. (Aida Skersiene), A.S. (Alvyra Slepetiene), V.S. and E.N.; formal analysis, A.S. (Aida Skersiene); investigation, A.S. (Aida Skersiene), A.S. (Alvyra Slepetiene), V.S. and E.N.; resources, A.S. (Aida Skersiene), V.S. and E.N.; data curation, A.S. (Aida Skersiene) and A.S. (Alvyra Slepetiene); writing—original draft preparation, A.S. (Aida Skersiene); writing—review and editing, A.S. (Aida Skersiene), A.S. (Alvyra Slepetiene), V.S. and E.N.; visualization, A.S. (Aida Skersiene); supervision, A.S. (Alvyra Slepetiene). All authors have read and agreed to the published version of the manuscript.
Funding
The first author acknowledges the financial assistance provided by the Research Council of Lithuania Project Nr. 09.3.3-ESFA-V-711-01-0001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Part of this research was supported by the long-term research program “Biopotential and quality of plants for multifunctional use” implemented by the Lithuanian Research Centre for Agriculture and Forestry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Asbjornsen, H.; Hernandez-Santana, V.; Liebman, M.; Bayala, J.; Chen, J.; Helmers, M.; Ong, C.K.; Schulte, L.A. Targeting Perennial Vegetation in Agricultural Landscapes for Enhancing Ecosystem Services. Renew. Agr. Food Syst. 2000, 29, 101–125. [Google Scholar] [CrossRef]
- Weißhuhn, P.; Reckling, M.; Stachow, U.; Wiggering, H. Sustainability Supporting Agricultural Ecosystem Services through the Integration of Perennial Polycultures into Crop Rotations. Sustainability 2017, 9, 2267. [Google Scholar] [CrossRef]
- NASA. Carbon Dioxide Vital Signs—Climate Change: Vital Signs of the Planet. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 31 May 2024).
- UNFCCC. Report of the Conference of the Parties on Its Twenty-First Session, Held in Paris from 30 November to 13 December 2015. Part One: Proceedings. Available online: https://unfccc.int/documents/9096 (accessed on 31 May 2024).
- European Commission. The European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 31 May 2024).
- Mattila, T.J.; Hagelberg, E.; Söderlund, S.; Joona, J. How Farmers Approach Soil Carbon Sequestration? Lessons Learned from 105 Carbon-Farming Plans. Soil Tillage Res. 2022, 215, 105204. [Google Scholar] [CrossRef]
- Moerkerken, A.; Blasch, J.; Van Beukering, P.; Van Well, E. A New Approach to Explain Farmers’ Adoption of Climate Change Mitigation Measures. Clim. Change 2020, 159, 141–161. [Google Scholar] [CrossRef]
- Lantz, U.; Baldos, C.; Fuglie, K.O.; Hertel, T.W.; Baldos, L.C. The Research Cost of Adapting Agriculture to Climate Change: A Global Analysis to 2050. Agr. Econ. 2019, 51, 207–220. [Google Scholar] [CrossRef]
- Elias, D.M.O.; Mason, K.E.; Howell, K.; Mitschunas, N.; Hulmes, L.; Hulmes, S.; Lebron, I.; Pywell, R.F.; Mcnamara, N.P. The Potential to Increase Grassland Soil C Stocks by Extending Reseeding Intervals Is Dependent on Soil Texture and Depth. J. Environ. Manag. 2023, 334, 117465. [Google Scholar] [CrossRef] [PubMed]
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.B.; Silveira, M.L. Grassland Management Affects Delivery of Regulating and Supporting Ecosystem Services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Iepema, G.; Hoekstra, N.J.; de Goede, R.; Bloem, J.; Brussaard, L.; van Eekeren, N. Extending Grassland Age for Climate Change Mitigation and Adaptation on Clay Soils. Eur. J. Soil Sci. 2022, 73, e13134. [Google Scholar] [CrossRef]
- Nunes, M.R.; van Es, H.M.; Schindelbeck, R.; Ristow, A.J.; Ryan, M. No-till and Cropping System Diversification Improve Soil Health and Crop Yield. Geoderma 2018, 328, 30–43. [Google Scholar] [CrossRef]
- Acharya, B.S.; Rasmussen, J.; Eriksen, J. Grassland Carbon Sequestration and Emissions Following Cultivation in a Mixed Crop Rotation. Agric. Ecosyst. Environ. 2012, 153, 33–39. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Sawchik, J.; Taboada, M.A. Agronomic and Environmental Impacts of Pasture-Crop Rotations in Temperate North and South America. Ecosyst. Environ. 2014, 190, 18–26. [Google Scholar] [CrossRef]
- Ryan, M.R.; Crews, T.E.; Culman, S.W.; Dehaan, L.R.; Hayes, R.C.; Jungers, J.M.; Bakker, M.G. Managing for Multifunctionality in Perennial Grain Crops. Bioscience 2018, 68, 294–304. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Short Rotation Coppices, Grasses and Other Herbaceous Crops: Productivity and Yield Energy Value versus 26 Genotypes. Biomass Bioenerg. 2018, 119, 109–120. [Google Scholar] [CrossRef]
- Hu, T.; Chabbi, A. Grassland Management and Integration during Crop Rotation Impact Soil Carbon Changes and Grass-Crop Production. Agric. Ecosyst. Environ. 2022, 324, 107703. [Google Scholar] [CrossRef]
- Englund, O.; Mola-Yudego, B.; Börjesson, P.; Cederberg, C.; Dimitriou, I.; Scarlat, N.; Berndes, G. Large-Scale Deployment of Grass in Crop Rotations as a Multifunctional Climate Mitigation Strategy. GCB Bioenergy 2023, 15, 166–184. [Google Scholar] [CrossRef]
- Dhakal, D.; Anowarul Islam, M. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability 2018, 10, 2718. [Google Scholar] [CrossRef]
- PLADIAS. Database of the Czech Flora and Vegetation. Available online: https://pladias.cz/en/ (accessed on 31 May 2024).
- Villarino, S.H.; Studdert, G.A.; Laterra, P.; Cendoya, M.G. Agricultural Impact on Soil Organic Carbon Content: Testing the IPCC Carbon Accounting Method for Evaluations at County Scale. Agric. Ecosyst. Environ. 2014, 185, 118–132. [Google Scholar] [CrossRef]
- Ćirić, V.; Belić, M.; Nešić, L.; Šeremešić, S.; Pejić, B.; Bezdan, A.; Manojlović, M. Sensitivity of Water Extractable Soil Organic Carbon Fractions to Land Use in Three Soil Types. Arch. Agron. Soil Sci. 2016, 62, 1654–1664. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved Organic Matter. Biogeochemistry, Dynamics, and Environmental Significance in Soils. Adv. Agron. 2011, 110, 1–75. [Google Scholar] [CrossRef]
- Deru, J.; Van Eekeren, N.; De Boer, H. Rooting Density of Three Grass Species and Eight Lolium perenne Cultivars. In Grassland—A European Resource? Proceedings of the 24th General Meeting of the European Grassland Federation, Lublin, Poland, 3–7 June 2012; Goliński, P., Warda, M., Stypiński, P., Eds.; Organizing Committee of the 24th General Meeting of the European Grassland Federation and Polish Grassland Society: Poznań, Poland, 2012; pp. 604–606. [Google Scholar]
- Houde, S.; Thivierge, M.-N.; Fort, F.; Bélanger, G.; Chantigny, M.H.; Angers, D.A.; Vanasse, A. Root Growth and Turnover in Perennial Forages as Affected by Management Systems and Soil Depth. Plant Soil 2020, 451, 371–387. [Google Scholar] [CrossRef]
- Dupont, S.T.; Beniston, J.; Glover, J.D.; Hodson, A.; Culman, S.W.; Lal, R.; Ferris, H. Root Traits and Soil Properties in Harvested Perennial Grassland, Annual Wheat, and Never-Tilled Annual Wheat. Plant Soil 2014, 381, 405–420. [Google Scholar] [CrossRef]
- Lama, S.; Velescu, A.; Leimer, S.; Weigelt, A.; Chen, H.; Eisenhauer, N.; Scheu, S.; Oelmann, Y.; Wilcke, W. Plant Diversity Influenced Gross Nitrogen Mineralization, Microbial Ammonium Consumption and Gross Inorganic N Immobilization in a Grassland Experiment. Oecologia 2020, 193, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Silver, W.L.; Miya, R.K. Global Patterns in Root Decomposition: Comparisons of Climate and Litter Quality Effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, T.; Li, Y.; Chen, N.; Yin, Q.; Li, M.; Liu, H.; Liu, G. Organic Materials with High C/N Ratio: More Beneficial to Soil Improvement and Soil Health. Biotechnol. Lett. 2022, 44, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Geoportal.lt. Available online: https://www.geoportal.lt/geoportal/autoriu-teises (accessed on 31 May 2024).
- Schad, P. World Reference Base for Soil Resources—Its Fourth Edition and Its History. J. Plant Nutr. Soil Sci. 2023, 186, 151–163. [Google Scholar] [CrossRef]
- Metzger, M.J.; Shkaruba, A.D.; Jongman, R.H.G.; Bunce, R.G.H. Descriptions of the European Environmental Zones and Strata; Alterra-Rapport; Wageningen University and Research Centre: Wageningen, The Netherlands, 2012; p. 2281. [Google Scholar]
- Meteo.lt. Available online: https://www.meteo.lt/klimatas/lietuvos-klimatas/klimato-indeksai/ (accessed on 31 May 2024).
- Nielsen, D.C.; Calderón, F.J. Fallow Effects on Soil. In Soil Management: Building a Stable Base for Agriculture; Hatfield, J.L., Thomas, J.S., Eds.; U.S. Department of Agriculture, Agricultural Research Service: Lincoln, NE, USA, 2015; pp. 287–300. [Google Scholar] [CrossRef]
- Groenendyk, D.G.; Ferré, T.P.A.; Thorp, K.R.; Rice, A.K. Hydrologic-Process-Based Soil Texture Classifications for Improved Visualization of Landscape Function. PLoS ONE 2015, 10, e0131299. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/3cb77bc5-0801-4ae8-bcc4-983c96013928/content (accessed on 31 May 2024).
- Slepetiene, A.; Slepetys, J.; Liaudanskiene, I. Standard and Modified Methods for Soil Organic Carbon Determination in Agricultural Soils. Agron. Res. 2008, 6, 543–554. [Google Scholar]
- Volungevičius, J.; Amalevičiūtė, K.; Liaudanskienė, I.; Šlepetienė, A.; Šlepetys, J. Chemical Properties of Pachiterric Histosol as Influenced by Different Land Use. Zemdirb. Agric. 2015, 102, 123–132. [Google Scholar] [CrossRef]
- Gershey, R.M.; Mackinnon, M.D.; Williams, P.J.B.; Moore, R.M. Comparison of Three Oxidation Methods Used for the Analysis of the Dissolved Organic Carbon in Seawater. Mar. Chem. 1979, 7, 289–306. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The Potential of Digestate as a Biofertilizer in Eroded Soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Kelly, C.; Haddix, M.L.; Byrne, P.F.; Francesca Cotrufo, M.; Schipanski, M.E.; Kallenbach, C.M.; Wallenstein, M.D.; Fonte, S.J. Long-Term Compost Amendment Modulates Wheat Genotype Differences in Belowground Carbon Allocation, Microbial Rhizosphere Recruitment and Nitrogen Acquisition. Soil. Biol. Biochem. 2022, 172, 108768. [Google Scholar] [CrossRef]
- Bouma, T.J.; Nielsen, K.L.; Koutstaal, B. Sample Preparation and Scanning Protocol for Computerised Analysis of Root Length and Diameter. Plant Soil 2000, 218, 185–196. [Google Scholar] [CrossRef]
- Gardner, C.M.K.; Robinson, D.A.; Blyth, K.; Cooper, J.D. Soil water content. In Soil and Environmental Analysis: Physical Methods, 2nd ed.; Smith, K., Mullins, C., Eds.; Marcell Dekker, Inc.: New York, NY, USA, 2000; pp. 1–64. [Google Scholar]
- Lobsey, C.R.; Viscarra, R.R.A. Sensing of Soil Bulk Density for More Accurate Carbon Accounting. Eur. J. Soil Sci. 2016, 67, 504–513. [Google Scholar] [CrossRef]
- Alberta Agriculture and Rural Development Information Management Services. Alberta Forage Manual, 2nd ed.; Kaulbars, C., Ed.; Alberta Agriculture and Rural Development Information Management Services: Alberta, AB, Canada, 2009. Available online: https://www1.agric.gov.ab.ca/$Department/deptdocs.nsf/all/agdex16/$FILE/120_20-1_2009.pdf (accessed on 31 May 2024).
- Chen, X.; Chen, H.Y.H.; Chen, C.; Ma, Z.; Searle, E.B.; Yu, Z.; Huang, Z. Effects of Plant Diversity on Soil Carbon in Diverse Ecosystems: A Global Meta-Analysis. Biol. Rev. 2020, 95, 167–183. [Google Scholar] [CrossRef]
- Batjes, N.H.; Niels Batjes, C.H. Technologically Achievable Soil Organic Carbon Sequestration in World Croplands and Grasslands. Land. Degrad. Dev. 2018, 30, 25–32. [Google Scholar] [CrossRef]
- IPCC. Agriculture, forestry and other land use. In IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4 (accessed on 31 May 2024).
- Poeplau, C. Grassland Soil Organic Carbon Stocks along Management Intensity and Warming Gradients. Grass Forage Sci. 2021, 76, 186–195. [Google Scholar] [CrossRef]
- Chantigny, M.H. Dissolved and Water-Extractable Organic Matter in Soils: A Review on the Influence of Land Use and Management Practices. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile Organic Matter as an Indicator of Organic Matter Quality in Arable and Pastoral Soils in New Zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Xu, N.; Wilson, H.F.; Saiers, J.E.; Entz, M. Effects of Crop Rotation and Management System on Water-Extractable Organic Matter Concentration, Structure, and Bioavailability in a Chernozemic Agricultural Soil. J. Environ. Qual. 2013, 42, 179–190. [Google Scholar] [CrossRef]
- Chowaniak, M.; Głąb, T.; Klima, K.; Niemiec, M.; Zaleski, T.; Zuzek, D. Effect of Tillage and Crop Management on Runoff, Soil Erosion and Organic Carbon Loss. Soil Use Manag. 2020, 36, 581–593. [Google Scholar] [CrossRef]
- Chen, Q.; Niu, B.; Hu, Y.; Luo, T.; Zhang, G. Warming and Increased Precipitation Indirectly Affect the Composition and Turnover of Labile-Fraction Soil Organic Matter by Directly Affecting Vegetation and Microorganisms. Sci. Total Environ. 2020, 714, 136787. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.A.; Trumbore, S.E.; Davidson, E.A.; Vicca, S.; Janssens, I. Sensitivity of Decomposition Rates of Soil Organic Matter with Respect to Simultaneous Changes in Temperature and Moisture. J. Adv. Model. Earth Syst. 2015, 7, 335–356. [Google Scholar] [CrossRef]
- Homyak, P.M.; Blankinship, J.C.; Slessarev, E.W.; Schaeffer, S.M.; Manzoni, S.; Schimel, J.P. Effects of Altered Dry Season Length and Plant Inputs on Soluble Soil Carbon. Ecology 2018, 99, 2348–2362. [Google Scholar] [CrossRef]
- Guigue, J.; Lévêque, J.; Mathieu, O.; Schmitt-Kopplin, P.; Lucio, M.; Arrouays, D.; Jolivet, C.; Dequiedt, S.; Chemidlin Prévost-Bouré, N.; Ranjard, L. Water-Extractable Organic Matter Linked to Soil Physico-Chemistry Andmicrobiology at the Regional Scale. Soil Biol. Biochem. 2015, 84, 158–167. [Google Scholar] [CrossRef]
- Ma, W.; Li, Z.; Ding, K.; Zhou, Q. Dynamics of Water Extractable Organic Carbon at a Subtropical Catchment Using Fluorescence Excitation-Emission Matrix Spectroscopy Coupled with Parallel Factor Analysis. Eur. J. Soil Sci. 2021, 72, 871–885. [Google Scholar] [CrossRef]
- Surey, R.; Kaiser, K.; Schimpf, C.M.; Mueller, C.W.; Böttcher, J.; Mikutta, R. Contribution of Particulate and Mineral-Associated Organic Matter to Potential Denitrification of Agricultural Soils. Front. Environ. Sci. 2021, 9, 640534. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, F.; Jia, P.; Zhang, J.; Hou, F.; Wu, G. Leguminous Species Sequester More Carbon than Gramineous Species in Cultivated Grasslands of a Semi-Arid Area. Solid. Earth 2017, 8, 83–91. [Google Scholar] [CrossRef]
- Iepema, G.; Deru, J.G.C.; Bloem, J.; Hoekstra, N.; De Goede, R.; Brussaard, L.; Van Eekeren, N. Productivity and Topsoil Quality of Young and Old Permanent Grassland: An On-Farm Comparison. Sustainability 2020, 12, 2600. [Google Scholar] [CrossRef]
- Kuťáková, E.; Mészárošová, L.; Baldrian, P.; Münzbergová, Z.; Herben, T. Plant–Soil Feedbacks in a Diverse Grassland: Soil Remembers, but Not Too Much. J. Ecol. 2023, 111, 1203–1217. [Google Scholar] [CrossRef]
- Skuodienė, R.; Kinderienė, I.; Tomchuk, D.; Šlepetys, J.; Karčauskienė, D. Root Development of Temporary and Permanent Grasslands and Their Anti-Erosion Significance on a Hilly Terrain. Zemdirb. Agric. 2020, 107, 209–216. [Google Scholar] [CrossRef]
- Tian, D.; Xiang, Y.; Seabloom, E.; Wang, J.; Jia, X.; Li, T.; Li, Z.; Yang, J.; Guo, H.; Niu, S. Soil Carbon Sequestration Benefits of Active versus Natural Restoration Vary with Initial Carbon Content and Soil Layer. Commun. Earth Environ. 2023, 4, 83. [Google Scholar] [CrossRef]
- Lal, R.C. Digging Deeper: A Holistic Perspective of Factors Affecting Soil Organic Carbon Sequestration in Agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Roumet, C.; Lafont, F.; Sari, M.; Warembourg, F.; Garnier, E. Root Traits and Taxonomic Affiliation of Nine Herbaceous Species Grown in Glasshouse Conditions. Plant Soil 2008, 312, 69–83. [Google Scholar] [CrossRef]
- Razafintsalama, H.; Sauvadet, M.; Trap, J.; Autfray, P.; Ripoche, A.; Becquer, T. Legume Nitrogen Fixation and Symbioses in Low-Inputs Rainfed Rice Rotations. Sustainability 2021, 13, 12349. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Wang, J.; Zhu, X.; Qin, C.; Zeng, Y.; Zhen, W.; Fang, Y.; Shangguan, Z. Interactions of Soil Nutrients and Microbial Communities during Root Decomposition of Gramineous and Leguminous Forages. Land Degrad. Dev. 2023, 34, 3250–3261. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Moncada, V.Y.M.; Américo, L.F.; Duchini, P.G.; Guzatti, G.C.; Schmitt, D.; Sbrissia, A.F. Root Mass Vertical Distribution of Perennial Cool-Season Grasses Grown in Pure or Mixed Swards. Ciência Rural 2022, 52, e20210242. [Google Scholar] [CrossRef]
- Xiang, L.S.; Miao, L.F.; Yang, F. Neighbors, Drought, and Nitrogen Application Affect the Root Morphological Plasticity of Dalbergia odorifera. Front. Plant Sci. 2021, 12, 650616. [Google Scholar] [CrossRef]
- Mosebi, P.E.; Truter, W.F.; Madakadze, I.C. Smuts Finger Grass (Digitaria eriantha Cv Irene) Root Growth Assessment and Some Physicochemical Characteristics on Coal Mined Land Compacted Soil. J. Appl. Sci. Environ. Manag. 2018, 22, 1293–1296. [Google Scholar] [CrossRef]
- Land, M.; Haddaway, N.R.; Hedlund, K.; Jørgensen, H.B.; Kätterer, T.; Isberg, P.E. How Do Selected Crop Rotations Affect Soil Organic Carbon in Boreo-Temperate Systems? A Systematic Review Protocol. Environ. Evid. 2017, 6, 9. [Google Scholar] [CrossRef]
- Christopher, S.F.; Lal, R. Nitrogen Management Affects Carbon Sequestration in North American Cropland Soils. CRC Crit. Rev. Plant Sci. 2007, 26, 45–64. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, S.; Ciais, P.; Manzoni, S.; Fang, J.; Yu, G.; Tang, X.; Zhou, P.; Wang, W.; Yan, J.; et al. Climate and Litter C/N Ratio Constrain Soil Organic Carbon Accumulation. Natl. Sci. Rev. 2019, 6, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Hishi, T. Heterogeneity of Individual Roots within the Fine Root Architecture: Causal Links between Physiological and Ecosystem Functions. J. For. Res. 2007, 12, 126–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






