Recovery and Characterization of Calcium-Rich Mineral Powders Obtained from Fish and Shrimp Waste: A Smart Valorization of Waste to Treasure
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Sample
2.2. Proximate Composition Analysis
2.3. Determination of Amino Acid Composition
2.4. Determination of Protein Solubility
2.5. Water Holding Capacity (WHC)
2.6. Oil Holding Capacity (OHC)
2.7. Determination of Heavy Metals Content
2.8. Color Analysis
2.9. Field Emission Scanning Electron Microscopy and Energy-Dispersive X-ray Analysis
2.10. FTIR Spectroscopy
2.11. ABTS Radical Scavenging Activity
2.12. Statistical Analyses
3. Results and Discussion
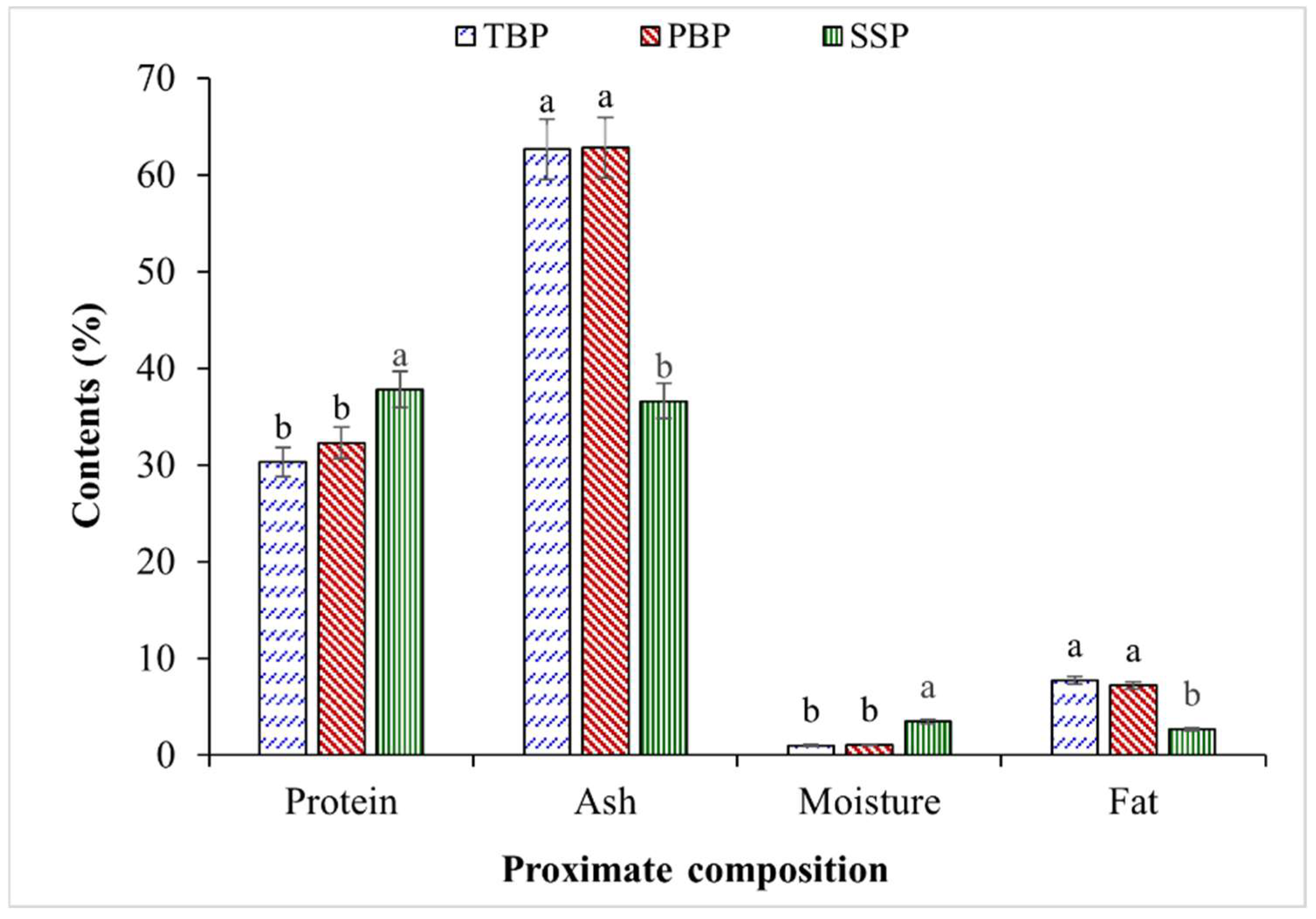
3.1. Proximate Composition of Fish Bone and Shrimp Shell Powders
3.2. The Composition and Contents of Total Amino Acids
3.3. Protein Solubility
3.4. Water and Oil Holding Capacity
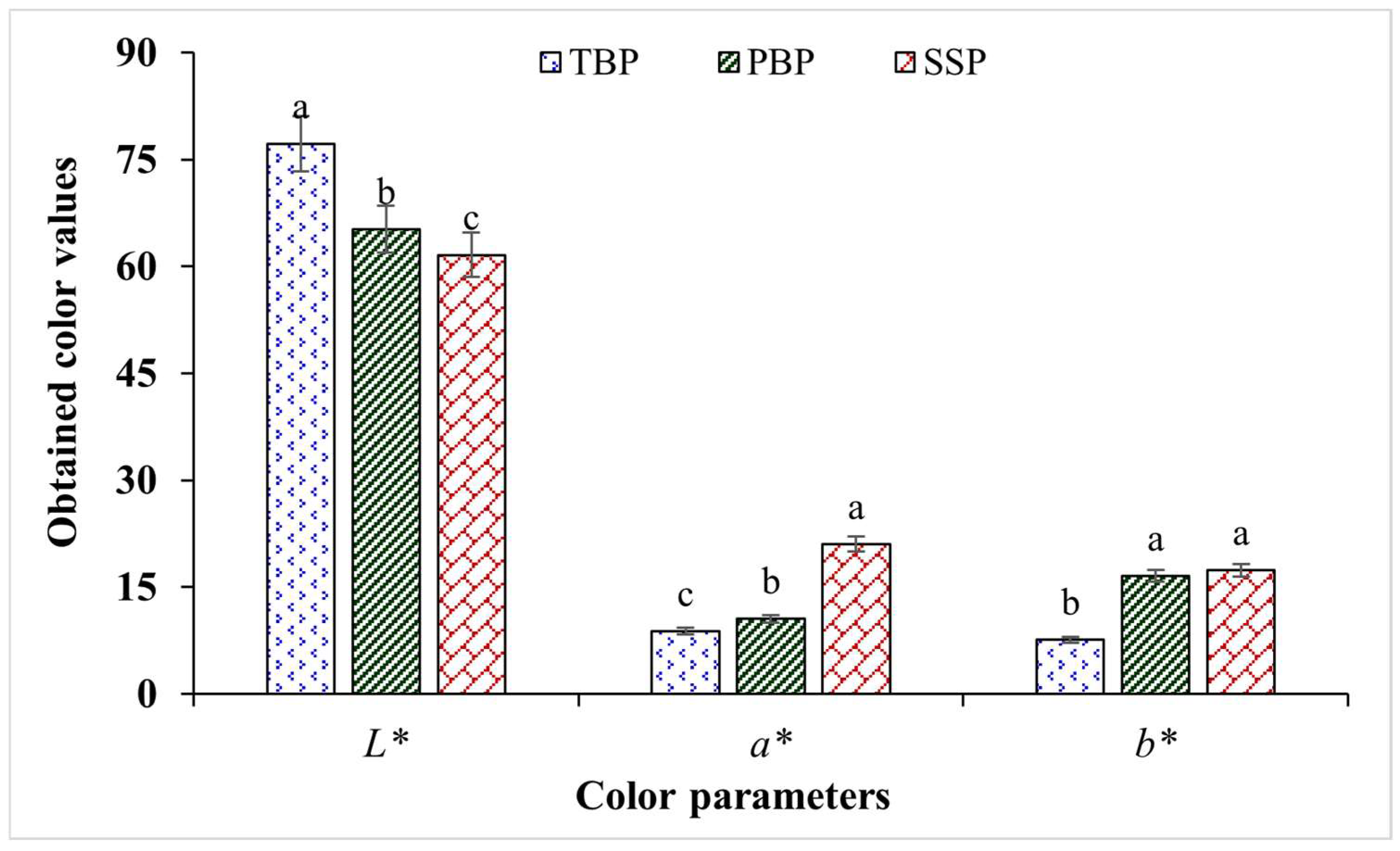
3.5. Color Analysis of Fish Bones and Shrimp Shell Powder
3.6. FE-SEM Analysis of Fish Bones and Shrimp Shell Powders
3.7. EDX Spectra of Fish Bones and Shrimp Shell Powders
3.8. FT-IR Analysis of Fish Bones and Shrimp Shell Powder
3.9. Heavy Metals Contents Analysis of FBP and SSP
3.10. ABTS Radical Scavenging Activity of Fish Bones and Shrimp Shell Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; pp. 1–223. [Google Scholar]
- Karayannakidis, P.D.; Zotos, A. Fish processing by-products as a potential source of gelatin: A review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Bin, M.I.; Dara, A.; Sontang, M.; Zuha, R.; Marlini, A.N. Fish bone waste utilization program for hydroxyapatite product: A case study of knowledge transfer from a university to coastal communities. J. Environ. Res. Dev. 2013, 7, 1274–1281. [Google Scholar]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Kamilah, H.; Huda, N.; Ariffin, F. In vitro calcium availability in bakery products fortified with tuna bone powder as a natural calcium source. Int. J. Food Sci. Nutr. 2016, 67, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef]
- Zhang, K.; Li, B.; Chen, Q.; Zhang, Z.; Zhao, X.; Hou, H. Functional calcium binding peptides from pacific cod (Gadus macrocephalus) bone: Calcium bioavailability enhancing activity and anti-osteoporosis effects in the ovariectomy-induced osteoporosis rat model. Nutrients 2018, 10, 1325. [Google Scholar] [CrossRef]
- Malde, M.K.; Graff, I.E.; Siljander-Rasi, H.; Venalainen, E.; Julshamn, K.; Pedersen, J.I.; Valaja, J. Fish bone a highly available calcium source for growing pigs. J. Anim. Physiol. Anim. Nutr. 2010, 94, 66–76. [Google Scholar] [CrossRef]
- Sefrienda, A.R.; Kumayanjati, B.; Setyono, D.E.D.; Herdian, H.; Novianty, H. Effects of physical treatments on size particle and nutritional properties of bone powder from Pangasius sp. IOP Conf. Ser. Earth Environ. Sci. 2022, 1119, 012037. [Google Scholar]
- Xavier, K.A.M.; Prabhu, U.A.; Nair, K.G.R.; Mathew, P.T. Utilization of fish bone as calcium supplement. In Seafood Safety; Society of Fisheries Technologists: Cochin, India, 2003; pp. 58–61. [Google Scholar]
- Gopal, T.K.S.; Ravishankar, C.N.; Bindu, J.; Ashok Kumar, K. Processing and Product Development from Tuna; Society of Fisheries Technologists: Cochin, India, 2008; pp. 104–127. [Google Scholar]
- Benjakul, S.; Karnjanapratum, S. Characteristics and nutritional value of whole wheat cracker fortified with tuna bone bio-calcium powder. Food Chem. 2018, 259, 181–187. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Carotenoids in different body components of Indian Shrimps. J. Sci. Food Agric. 2005, 85, 167–172. [Google Scholar] [CrossRef]
- Trung, T.S.; Phuong, P.T.D. Bioactive compounds from by-products of shrimp processing industry in Vietnam. J. Food Drug Anal. 2012, 20, 64. [Google Scholar] [CrossRef]
- Hemung, B.O.; Yongsawatdigul, J.; Chin, K.B.; Limphirat, W.; Siritapetawee, J. Silver carp bone powder as natural calcium for fish sausage. J. Aquat. Food Prod. Technol. 2018, 27, 305–315. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W.; Xiong, S. Physicochemical properties of nano fish bone prepared by wet media milling. LWT-Food Sci. Technol. 2015, 64, 367–373. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Hammad, H.H.M.; Liu, J.; Shahbaz, H.M.; Regenstein, J.M. Improved effect of autoclave processing on size reduction, chemical structure, nutritional, mechanical and in vitro digestibility properties of fish bone powder. Adv. Powder Technol. 2020, 31, 2513–2520. [Google Scholar] [CrossRef]
- Busca, K.; Wu, S.; Miao, S.; Govindan, A.; Strain, C.R.; O‘Donnell, S.T.; Whooley, J.; Gite, S.; Ross, R.P.; Stanton, C. An in vitro study to assess bioaccessibility and bioavailability of calcium from blue whiting (Micromesistius poutassou) fish bone powder. Ir. J. Agric. Food Res. 2022, 61, 229–240. [Google Scholar] [CrossRef]
- Savlak, N.; Çağındı, Ö.; Erk, G.; Öktem, B.; Köse, E. Treatment method affects color, chemical, and mineral composition of seabream (Sparus aurata) fish bone powder from by-products of fish fillet. J. Aquat. Food Prod. Technol. 2020, 29, 592–602. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, TX, USA, 2005. [Google Scholar]
- Islam, M.A.; Mohibbullah, M.; Suraiya, S.; Sarower-E-Mahfuj, M.; Ahmed, S.; Haq, M. Nutritional characterization of freshwater mud eel (Monopterus cuchia) muscle cooked by different thermal processes. Food Sci. Nutr. 2020, 8, 6247–6258. [Google Scholar] [CrossRef]
- Wu, G.C.; Min, Z.; Wang, Y.Q.; Mothibe, K.J.; Chen, W.X. Production of silver carp bone powder using superfine grinding technology: Suitable production parameters and its properties. J. Food Eng. 2012, 109, 730–735. [Google Scholar] [CrossRef]
- Aziah, A.N.; Komathi, C. Physicochemical and functional properties of peeled and unpeeled pumpkin flour. J. Food Sci. 2009, 74, S328–S333. [Google Scholar] [CrossRef]
- Kumaravel, S.; Alagusundaram, K. Determination of mineral content in Indian spices by ICP-OES. Orient. J. Chem. 2014, 30, 631–636. [Google Scholar] [CrossRef]
- Suraiya, S.; Mohona, M.A.S.; Fatema, M.; Haq, M.; Rahman, M.A.; Mondal, S. Edible paper sheets from Alternanthera philoxeroides and Hypophthalmichthys molitrix: Smart biomass valorization. Biomass 2024, 4, 414–428. [Google Scholar] [CrossRef]
- Yin, T.; Du, H.; Zhang, J.; Xiong, S. Preparation and characterization of ultrafine fish bone powder. J. Aquat. Food Prod. Technol. 2016, 25, 1045–1055. [Google Scholar] [CrossRef]
- Ali, S.; Ho, T.C.; Razack, S.A.; Haq, M.; Roy, V.C.; Park, J.-S.; Kang, H.W.; Chun, B.-S. Oligochitosan recovered from shrimp shells through subcritical water hydrolysis: Molecular size reduction and biological activities. J. Supercrit. Fluids 2023, 196, 105868. [Google Scholar] [CrossRef]
- Nawaz, A.; Xiong, Z.; Xiong, H.; Chen, L.; Wang, P.; Ahmad, I.; Hu, C.; Irshad, S.; Ali, S.W. The effects of fish meat and fish bone addition on nutritional value, texture and microstructure of optimised fried snacks. Int. J. Food Sci. Technol. 2018, 54, 1045–1053. [Google Scholar] [CrossRef]
- Wulandari, P.; Kusumasari, S. Effect of extraction methods on the nutritional characteristics of milkfish (Chanos chanos Forsskal) bone powder. IOP Conf. Series Earth Environ. Sci. 2019, 383, 012035. [Google Scholar] [CrossRef]
- Bubel, F.; Dobrzański, Z.; Jan Bykowski, P.; Chojnacka, K.; Opaliński, S.; Trziszka, T. Production of calcium preparations by technology of saltwater fish by product processing. Open Chem. 2015, 13, 1333–1340. [Google Scholar] [CrossRef]
- Hemung, B.O. Properties of tilapia bone powder and its calcium bioavailability based on transglutaminase assay. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 306. [Google Scholar]
- Tha, A.; Raju, C.; Lakshmisha, I.; Kumar, P.A.; Sarojini, A.; Endra, G.; Pal, J. Nutritional composition of fish bone powder extracted from three different fish filleting waste boiling with water and an alkaline media. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2942–2948. [Google Scholar] [CrossRef]
- Abbey, L.; Glover-Amengor, M.; Atikpo, M.O.; Atter, A.; Toppe, J. Nutrient content of fish powder from low value fish and fish byproducts. Food Sci. Nutr. 2017, 5, 374–379. [Google Scholar] [CrossRef]
- Phanat, K.; Soottawat, B.; Wonnop, V.; Takashi, N.; Munehiko, T. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar]
- Qin, X.; Shen, Q.; Guo, Y.; Li, X.; Liu, J.; Ye, M.; Wang, H.; Jia, W.; Zhang, C. Physicochemical properties, digestibility and anti-osteoporosis effect of yak bone powder with different particle sizes. Food Res. Int. 2021, 145, 110401. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Li, Y.; Li, P.; Qiu, X.; Guo, X.; Chen, J.; Wang, W. Improving grindability and bioaccessibility of chicken bone by in situ steam explosion. Int. J. Food Sci. Technol. 2023, 58, 3201–3208. [Google Scholar] [CrossRef]
- Kong, X.; Qiu, X.; Li, P.; Li, Y.; Zhang, Y.; Guo, X.; Kong, F. Enhancement of nutrient bioaccessibility and functional property of chicken bone powder through steam explosion. J. Agric. Food Res. 2024, 15, 100941. [Google Scholar] [CrossRef]
- Huey, Y.W.; Zulkipli, A.S.; Tajarudin, H.A.; Salleh, R.M. Physicochemical properties of pre-treated cuttlebone powder and its potential as an alternative calcium source. J. Food Process. Preserv. 2021, 45, e15831. [Google Scholar] [CrossRef]
- Cho, Y.J.; Haq, M.; Park, J.S.; Lee, H.J.; Chun, B.S. Physicochemical and biofunctional properties of shrimp (Penaeus japonicus) hydrolysates obtained from hot-compressed water treatment. J. Supercrit. Fluids 2019, 147, 322–328. [Google Scholar] [CrossRef]
- Kusumawati, P.; Triwitono, P.; Anggrahini, S.; Pranoto, Y. Nano-calcium powder properties from six commercial fish bone waste in Indonesia. Squalen Bull. 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Shimp, L. Heat resistance of allograft tissue. Cell Tissue Bank. 2008, 9, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yin, T.; Xiong, S.; Li, Y.; Ikram, U.; Liu, R. Thermal treatments affect breakage kinetics and calcium release of fish bone particles during high-energy wet ball milling. J. Food Eng. 2016, 183, 74–80. [Google Scholar] [CrossRef]
- Nemati, M.; Huda, N.; Ariffin, F. Development of calcium supplement from fish bone wastes of yellowfin tuna (Thunnus albacares) and characterization of nutritional quality. Int. Food Res. J. 2017, 24, 2419–2426. [Google Scholar]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Ali, M.S.; Roy, V.C.; Park, J.S.; Haque, A.R.; Mok, J.H.; Zhang, W.; Chun, B.S. Protein and polysaccharide recovery from shrimp wastes by natural deep eutectic solvent mediated subcritical water hydrolysis for biodegradable film. Mar. Biotechnol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zainol, I.; Adenan, N.H.; Rahim, N.A.; Aiza Jaafar, C.N. Extraction of natural hydroxyapatite from tilapia fish scales using alkaline treatment. Mater. Today Proc. 2019, 16, 1942–1948. [Google Scholar] [CrossRef]
- Nam, P.V.; Hoa, N.V.; Trung, T.S. Properties of hydroxyapatites prepared from different fish bones: A comparative study. Ceram. Int. 2019, 45, 20141–20147. [Google Scholar] [CrossRef]
- Nauen, C.E. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish Circ. 1983, 764, 102. [Google Scholar]
- Boronat, Ò.; Sintes, P.; Celis, F.; Díez, M.; Ortiz, J.; Aguiló-Aguayo, I.; Martín-Gómez, H. Development of added-value culinary ingredients from fish waste: Fish bones and fish scales. Int. J. Gastron. Food Sci. 2023, 31, 100657. [Google Scholar] [CrossRef]







| Name of Amino Acid | TBP | PBP | SSP |
|---|---|---|---|
| Essential amino acids (EAAs) | |||
| Histidine | 0.46 ± 0.01 a | 0.46 ± 0.01 a | n.d. |
| Threonine | 1.38 ± 0.11 b | 1.76 ± 0.02 a | 1.98 ± 0.14 a |
| Arginine | 1.85 ± 0.12 c | 2.23 ± 0.14 b | 2.83 ± 0.11 a |
| Valine | 1.13 ± 0.05 b | 1.34 ± 0.12 a | 1.59 ± 0.12 a |
| Phenylalanine | 1.13 ± 0.08 | 1.31 ± 0.13 b | 1.70 ± 0.06 a |
| Isoleucine | 1.17 ± 0.10 b | 1.17 ± 0.11 b | 1.41 ± 0.04 a |
| Leucine | 1.87 ± 0.13 a | 1.48 ± 0.08 b | 1.98 ± 0.14 a |
| Lysine | 1.93 ± 0.12 b | 2.02 ± 0.06 b | 3.62 ± 0.17 a |
| Histidine | n.d. | n.d. | 0.36 ± 0.02 a |
| ƩEAAs | 10.92 | 11.77 | 15.47 |
| Non-essential amino acids (NEAAs) | |||
| Aspartic acid | 1.78 ± 0.04 b | 1.54 ± 0.14 a | 2.21 ± 0.05 b |
| Glutamic acid | 1.22 ± 0.12 b | 2.88 ± 0.11 c | 4.94 ± 0.14 a |
| Serine | 1.54 ± 0.11 c | 1.02 ± 0.17 b | 2.43 ± 0.11 a |
| Glycine | 4.78 ± 0.08 a | 3.72 ± 0.15 b | 3.26 ± 0.10 c |
| Proline | 3.17 ± 0.07 a | 3.12 ± 0.10 a | n.d. |
| Alanine | 1.36 ± 0.14 b | 1.43 ± 0.11 b | 1.77 ± 0.14 a |
| Taurine | 1.22 ± 0.12 a | 0.29 ± 0.05 c | 0.54 ± 0.10 b |
| Tyrosine | n.d. | 1.47 ± 0.10 a | 0.39 ± 0.03 b |
| ƩNEAAs | 15.07 | 15.47 | 15.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antu, M.A.R.; Ali, M.S.; Ferdous, M.J.; Ahmed, M.T.; Ali, M.R.; Suraiya, S.; Pangestuti, R.; Haq, M. Recovery and Characterization of Calcium-Rich Mineral Powders Obtained from Fish and Shrimp Waste: A Smart Valorization of Waste to Treasure. Sustainability 2024, 16, 6045. https://doi.org/10.3390/su16146045
Antu MAR, Ali MS, Ferdous MJ, Ahmed MT, Ali MR, Suraiya S, Pangestuti R, Haq M. Recovery and Characterization of Calcium-Rich Mineral Powders Obtained from Fish and Shrimp Waste: A Smart Valorization of Waste to Treasure. Sustainability. 2024; 16(14):6045. https://doi.org/10.3390/su16146045
Chicago/Turabian StyleAntu, Mst. Aspriya Rahman, Md Sadek Ali, Mst Jannatul Ferdous, Md. Tanvir Ahmed, Md. Rasal Ali, Sharmin Suraiya, Ratih Pangestuti, and Monjurul Haq. 2024. "Recovery and Characterization of Calcium-Rich Mineral Powders Obtained from Fish and Shrimp Waste: A Smart Valorization of Waste to Treasure" Sustainability 16, no. 14: 6045. https://doi.org/10.3390/su16146045
APA StyleAntu, M. A. R., Ali, M. S., Ferdous, M. J., Ahmed, M. T., Ali, M. R., Suraiya, S., Pangestuti, R., & Haq, M. (2024). Recovery and Characterization of Calcium-Rich Mineral Powders Obtained from Fish and Shrimp Waste: A Smart Valorization of Waste to Treasure. Sustainability, 16(14), 6045. https://doi.org/10.3390/su16146045







