A Quantitative Index for Evaluating Maize Leaf Wilting and Its Sustainable Application in Drought Resistance Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials
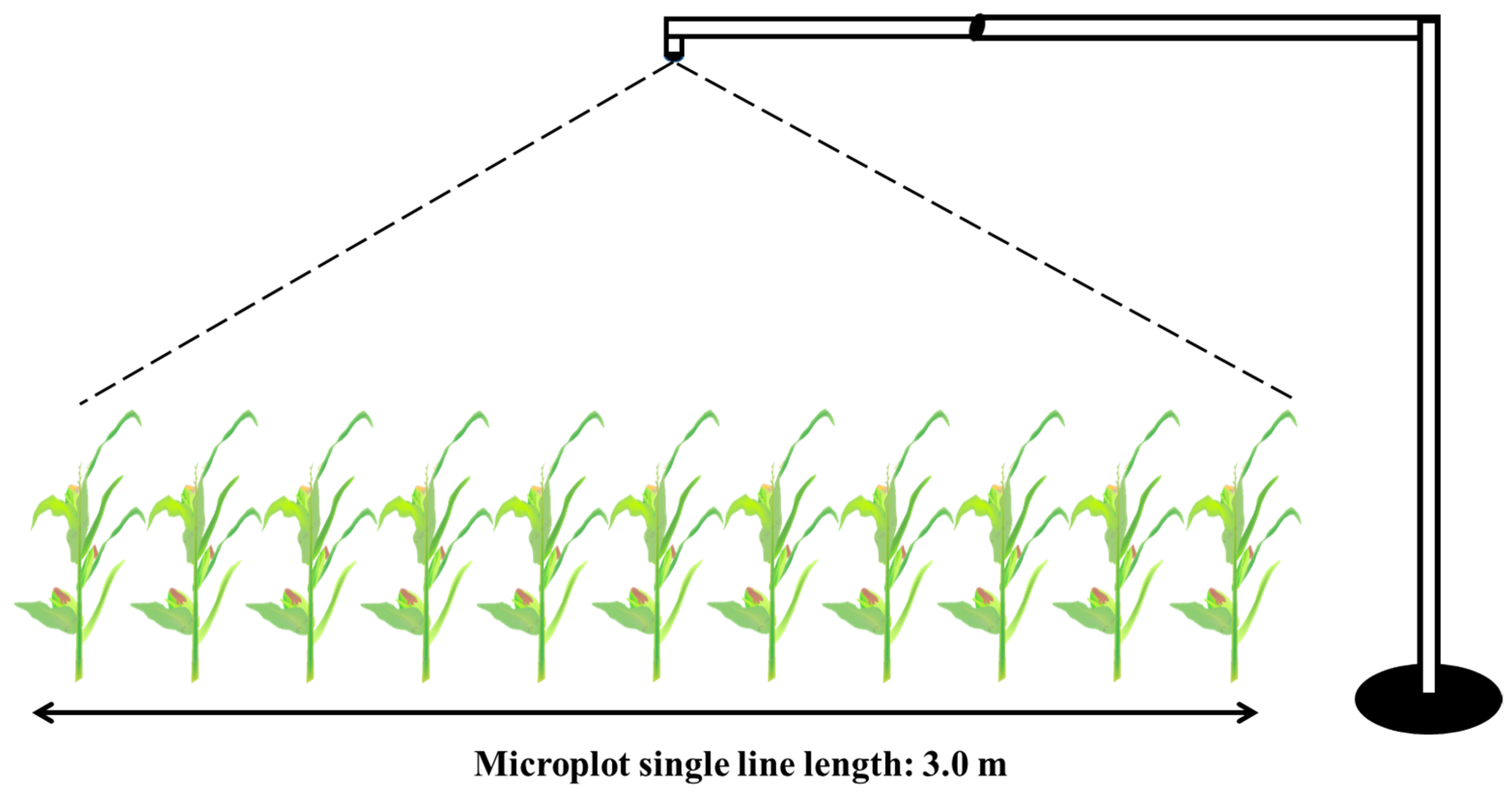
2.2. Design and Construction of the Image Acquisition Device
2.3. Image Processing
2.3.1. Principle of Extracting Green Parts
2.3.2. Visible Green Area Ratio Calculation
2.3.3. Effect of Light Intensity on Green Area Recognition
2.4. Leaf Wilting Ratio
2.5. Data Processing
3. Results
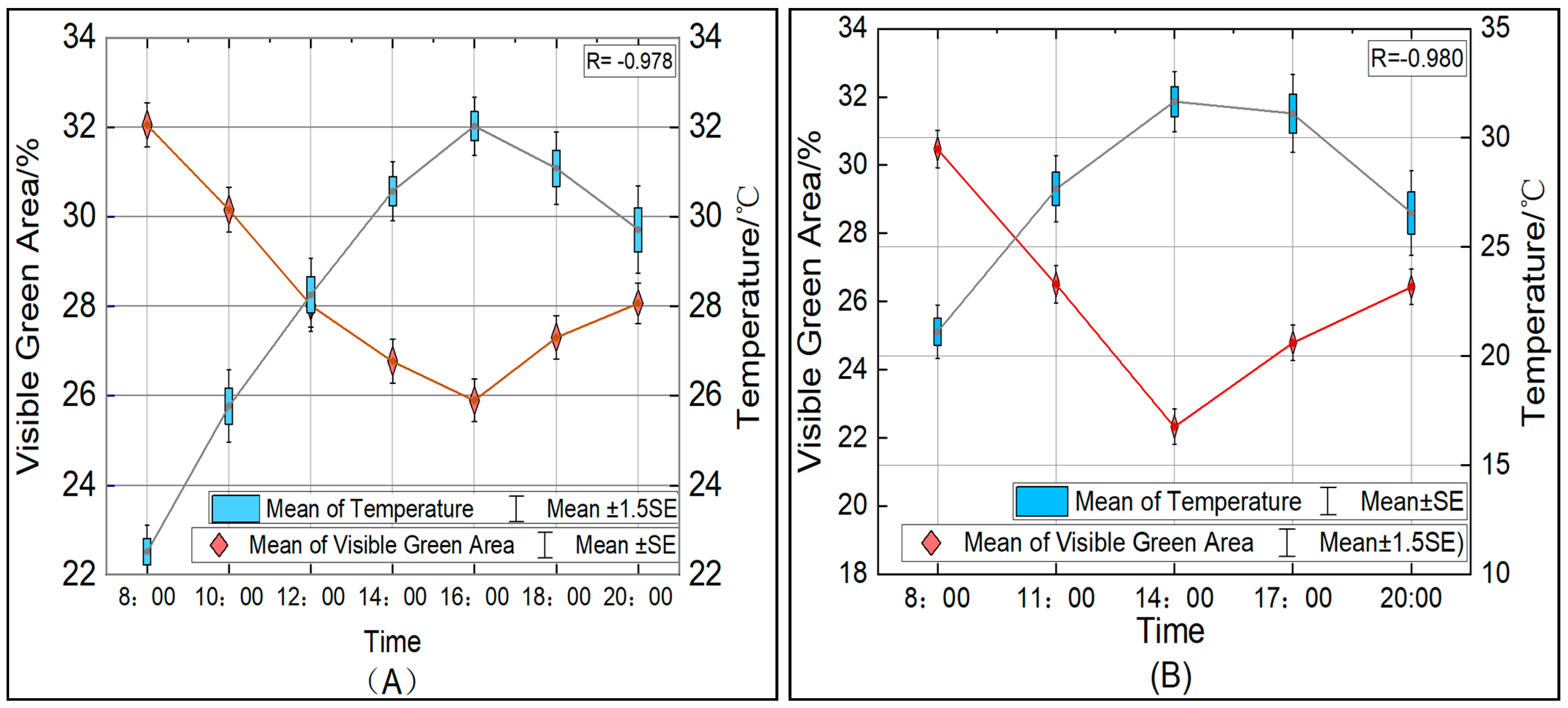
3.1. Daily Changes in Visible Green Leaf Area
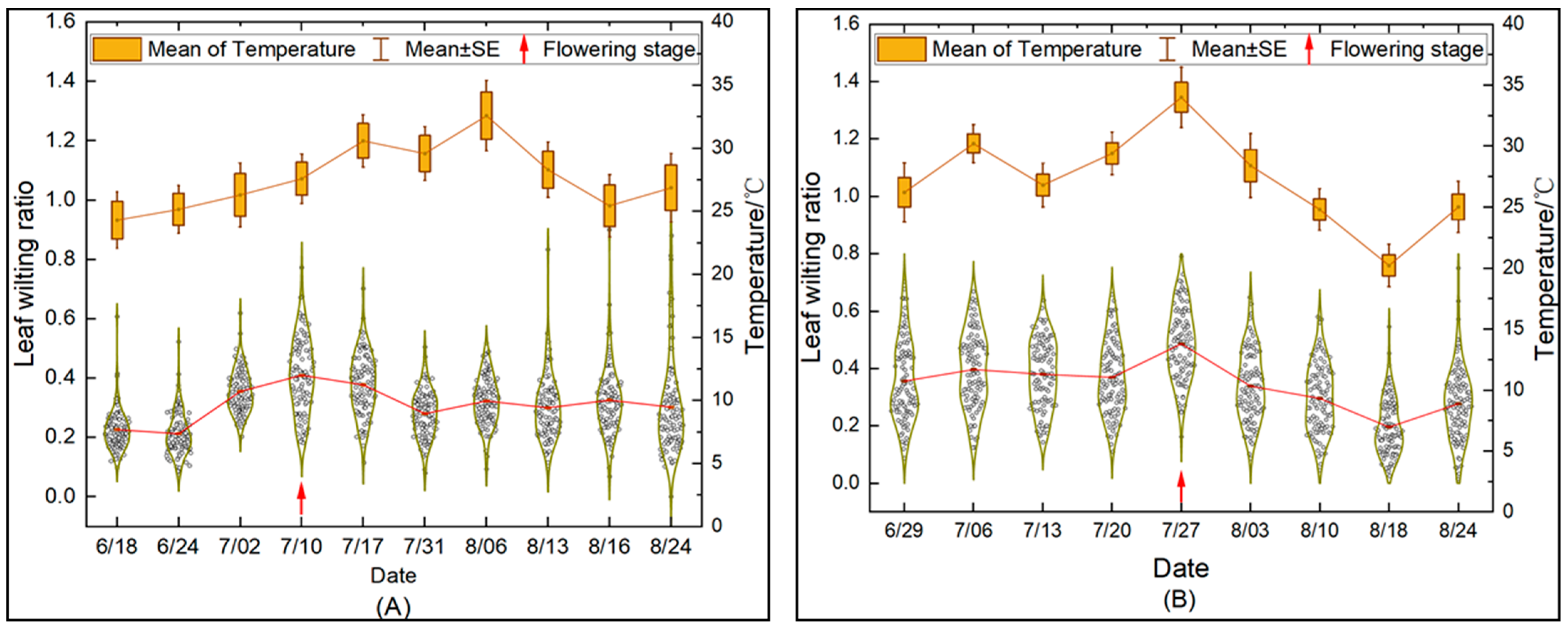
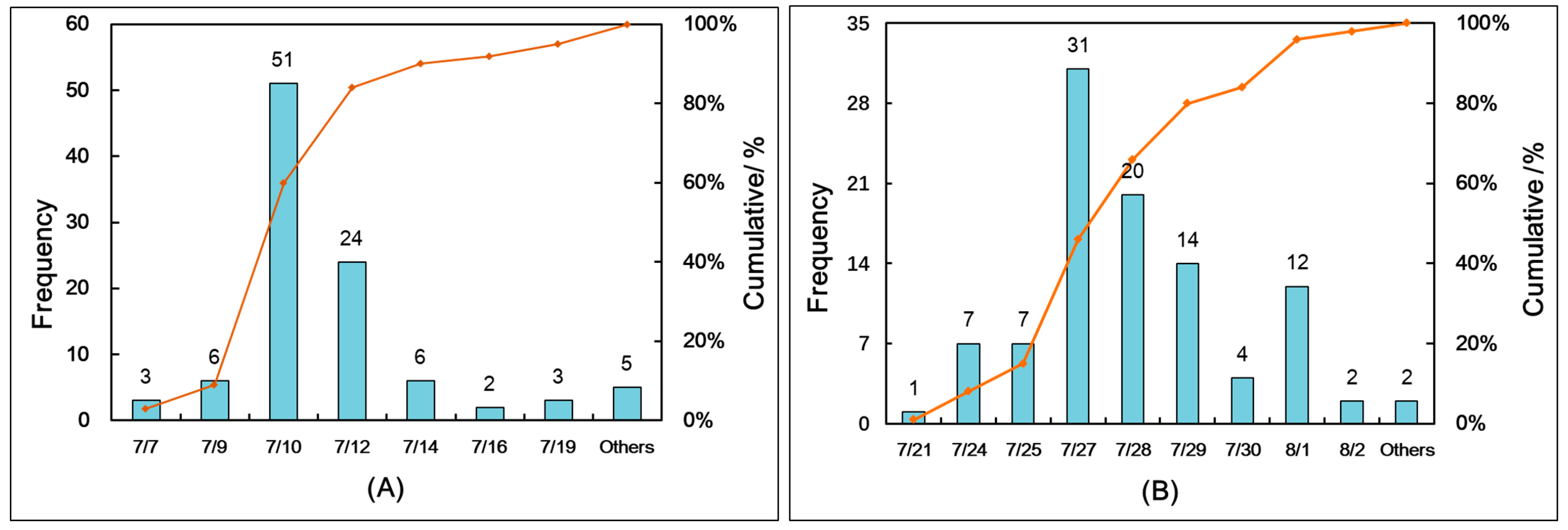
3.2. Changes in the Leaf Wilting Trend during the Entire Growth Period
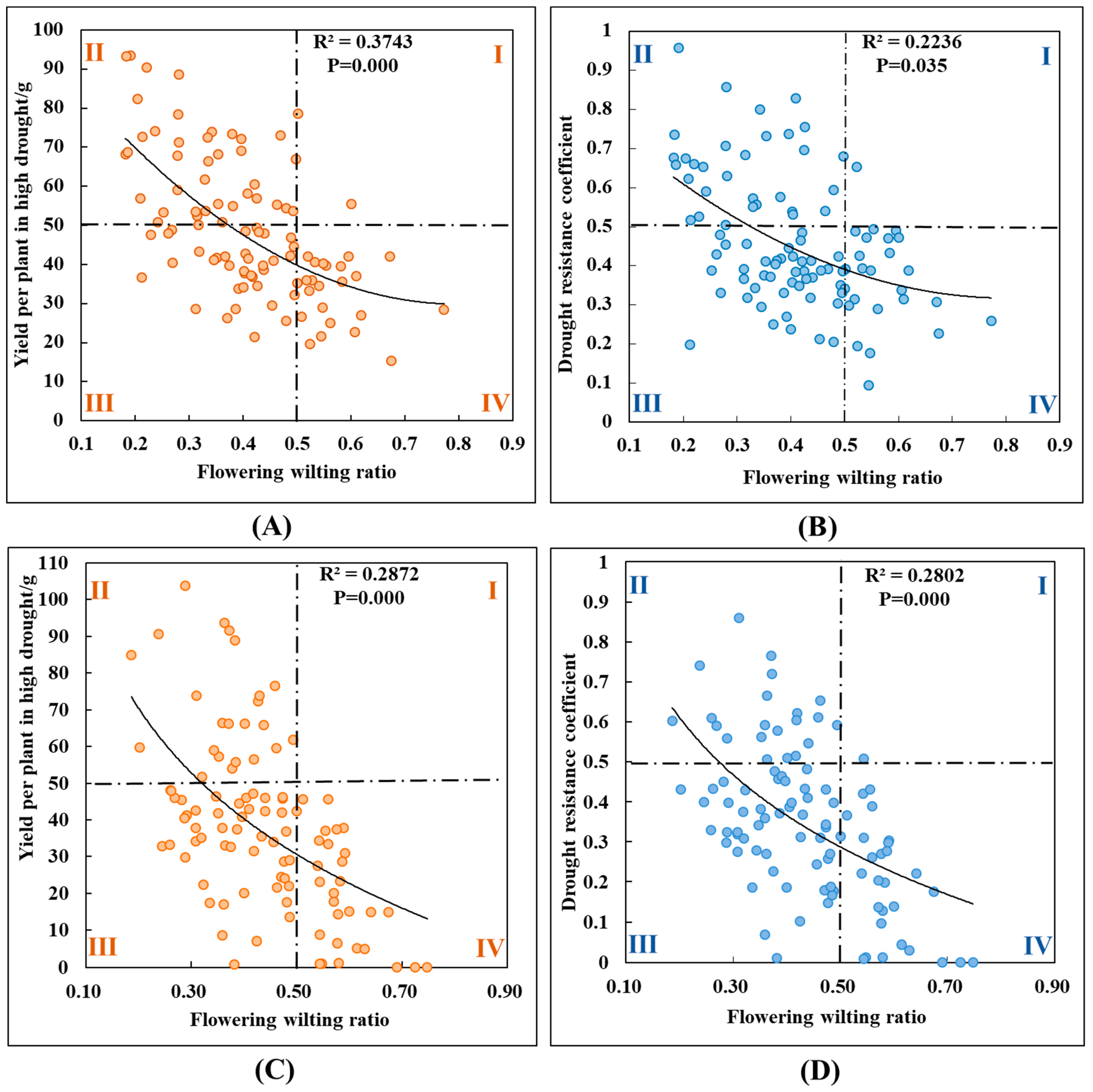
3.3. Leaf Wilting Amount, Yield, and Drought Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, R.; Memon, J.; Kumar, S.; Patel, D.A.; Sakure, A.A.; Patel, M.B.; Das, A.; Karjagi, C.G.; Patel, S.; Patel, U.; et al. Genetic Diversity and Population Structure of Maize (Zea mays L.) Inbred Lines in Association with Phenotypic and Grain Qualitative Traits Using SSR Genotyping. Plants 2024, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Farhangfar, S.; Bannayan, M.; Khazaei, H.R.; Baygi, M.M. Vulnerability assessment of wheat and maize production affected by drought and climate change. Int. J. Disaster Risk Reduct. 2015, 13, 37–51. [Google Scholar] [CrossRef]
- Pathan, S.M.; Lee, J.-D.; Sleper, D.A.; Fritschi, F.B.; Sharp, R.E.; Carter, T.E.; Nelson, R.L.; King, C.A.; Schapaugh, W.T.; Ellersieck, M.R.; et al. Two soybean plant introductions display slow leaf wilting and reduced yield loss under drought. J. Agron. Crop Sci. 2014, 200, 231–236. [Google Scholar] [CrossRef]
- Duncan, W.J.C.S. Leaf angles, leaf area, and canopy photosynthesis 1. Crop Sci. 1971, 11, 482–485. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.-F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Zhao, S.; Zeng, W.; Li, Z.; Peng, Y. Mannose regulates water balance, leaf senescence, and genes related to stress tolerance in white clover under osmotic stress. Biol. Plant. 2020, 64, 406–416. [Google Scholar] [CrossRef]
- Subashri, M.; Robin, S.; Vinod, K.K.; Rajeswari, S.; Mohanasundaram, K.; Raveendran, T.S. Trait identification and QTL validation for reproductive stage drought resistance in rice using selective genotyping of near flowering RILs. Euphytica 2009, 166, 291–305. [Google Scholar] [CrossRef]
- Briggs, L.J.; Shantz, H.L. A wax seal method for determining the lower limit of available soil moisture. Bot. Gaz. 1911, 51, 210–219. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.-M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Devi, E.L.; Kumar, S.; Singh, T.B.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Adaptation strategies and defence mechanisms of plants during environmental stress. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 359–413. [Google Scholar]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Saglam, A.; Kadioglu, A.; Demiralay, M.; Terzi, R. Leaf rolling reduces photosynthetic loss in maize under severe drought. Acta Bot. Croat. 2014, 73, 315–332. [Google Scholar] [CrossRef]
- Baret, F.; Madec, S.; Irfan, K.; Lopez, J.; Comar, A.; Hemmerlé, M.; Dutartre, D.; Praud, S.; Tixier, M.H. Leaf-rolling in maize crops: From leaf scoring to canopy-level measurements for phenotyping. J. Exp. Bot. 2018, 69, 2705–2716. [Google Scholar] [CrossRef]
- O’Toole, J.; Cruz, R.; Singh, T. Leaf rolling and transpiration. Plant Sci. Lett. 1979, 16, 111–114. [Google Scholar] [CrossRef]
- Matthews, R.; Azam-Ali, S.; Peacock, J. Response of four sorghum lines to mid-season drought. II. Leaf characteristics. Field Crop. Res. 1990, 25, 297–308. [Google Scholar] [CrossRef]
- Sirault, X.R.R.; Condon, A.G.; Wood, J.T.; Farquhar, G.D.; Rebetzke, G.J. “Rolled-upness”: Phenotyping leaf rolling in cereals using computer vision and functional data analysis approaches. Plant Methods 2015, 11, 52. [Google Scholar] [CrossRef]
- Rascio, A.; De Santis, G.; Sorrentino, G. A low-cost method for phenotyping wilting and recovery of wheat leaves under heat stress using semi-automated image analysis. Plants 2020, 9, 718. [Google Scholar] [CrossRef]
- Camargo, A.; Smith, J. Image pattern classification for the identification of disease causing agents in plants. Comput. Electron. Agric. 2009, 66, 121–125. [Google Scholar] [CrossRef]
- Wakamori, K.; Mineno, H. Optical flow-based analysis of the relationships between leaf wilting and stem diameter variations in tomato plants. Plant Phenomics 2019, 2019, 9136298. [Google Scholar] [CrossRef]
- Chen, Y.; Ribera, J.; Delp, E.J. Estimating plant centers using a deep binary classifier. In Proceedings of the 2018 IEEE Southwest Symposium on Image Analysis and Interpretation (SSIAI), Las Vegas, NV, USA, 8–10 April 2018. [Google Scholar]
- Han, L.; Yang, G.; Dai, H.; Yang, H.; Xu, B.; Feng, H.; Li, Z.; Yang, X. Fuzzy clustering of maize plant-height patterns using time series of UAV remote-sensing images and variety traits. Front. Plant Sci. 2019, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, H.S.; Odindi, J.; Sibanda, M.; Mutanga, O.; Clulow, A.; Chimonyo, V.G.P.; Mabhaudhi, T. A comparative estimation of maize leaf water content using machine learning techniques and unmanned aerial vehicle (UAV)-based proximal and remotely sensed data. Remote. Sens. 2021, 13, 4091. [Google Scholar] [CrossRef]
- Yang, C.; Baireddy, S.; Méline, V.; Cai, E.; Caldwell, D.; Iyer-Pascuzzi, A.S.; Delp, E.J. Image-based plant wilting estimation. Plant Methods 2023, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Li, Q.Y.; Zhang, F.R. Comparison of soil genetic classification and soil taxonomy for plain soil in Xinjiang. CABI Digital Library 2011, 379, 312–317. [Google Scholar]
- Sytar, O.; Zivcak, M.; Olsovska, K.; Brestic, M. Perspectives in high-throughput phenotyping of qualitative traits at the whole-plant level. In Eco-Friendly Agro-Biological Techniques for Enhancing Crop Productivity; Springer: Berlin/Heidelberg, Germany, 2018; pp. 213–243. [Google Scholar]
- Chopin, J.; Laga, H.; Miklavcic, S.J. A hybrid approach for improving image segmentation: Application to phenotyping of wheat leaves. PLoS ONE 2016, 11, e0168496. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Park, S.; Kim, C. Simulation of morphology changes in drying leaves. Comput. Graph. Forum 2013, 32, 204–215. [Google Scholar] [CrossRef]
- Raju, S.K.K.; Adkins, M.; Enersen, A.; de Carvalho, D.S.; Studer, A.J.; Ganapathysubramanian, B.; Schnable, P.S.; Schnable, J.C. Leaf Angle eXtractor: A high-throughput image processing framework for leaf angle measurements in maize and sorghum. Appl. Plant Sci. 2020, 8, e11385. [Google Scholar] [CrossRef] [PubMed]
- Valliyodan, B.; Ye, H.; Song, L.; Murphy, M.; Shannon, J.G.; Nguyen, H.T. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J. Exp. Bot. 2017, 68, 1835–1849. [Google Scholar] [CrossRef]
- Kadioglu, A.; Terzi, R.; Saruhan, N.; Saglam, A. Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Sci. 2012, 182, 42–48. [Google Scholar] [CrossRef]
- Ye, H.; Song, L.; Schapaugh, W.T.; Ali, L.; Sinclair, T.R.; Riar, M.K.; Mutava, R.N.; Li, Y.; Vuong, T.; Valliyodan, B.; et al. The importance of slow canopy wilting in drought tolerance in soybean. J. Exp. Bot. 2019, 71, 642–652. [Google Scholar] [CrossRef]
- Veerala, P.; Chand, P.; Das, T.R.; Gangwar, L.K.; Kumar, R. Leaf rolling and senescence scores as selection criteria to identify drought-tolerant genotypes in basmati rice (Oryza sativa L.). Indian J. Genet. Plant Breed. 2024, 84, 124–126. [Google Scholar]
- Wirojsirasak, W.; Songsri, P.; Jongrungklang, N.; Tangphatsornruang, S.; Klomsa-Ard, P.; Ukoskit, K. Determination of Morpho-Physiological Traits for Assessing Drought Tolerance in Sugarcane. Plants 2024, 13, 1072. [Google Scholar] [CrossRef] [PubMed]




| Line Number | Drought Resistance Coefficient | Line Number | Drought Resistance Coefficient |
|---|---|---|---|
| XJ006 | 0.56 | XJ 081 | 0.41 |
| XJ 019 | 0.50 | XJ 095 | 0.49 |
| XJ 022 | 0.65 | Z58 | 0.67 |
| XJ 038 | 0.39 | PH4CV | 0.47 |
| XJ 078 | 0.57 | PH6WC | 0.43 |
| Year | Precipitation | Normal Irrigation (100%) | Moderate Drought (50%) | Severe Drought (0%) |
|---|---|---|---|---|
| 2020 | 25.1 | 350 | 175 | 50 |
| 2021 | 51.8 | 350 | 175 | 50 |
| 2022 | 26.1 | 350 | 175 | 50 |
| Years | Item | Data | |||||||||
| 2020 | Data | 6/18 | 6/24 | 7/2 | 7/10 | 7/17 | 7/31 | 8/6 | 8/13 | 8/16 | 8/24 |
| Temperature/°C | 24.3 | 25.1 | 26.3 | 27.6 | 30.6 | 29.6 | 32.6 | 28.3 | 25.4 | 26.9 | |
| 2021 | Data | 6/29 | 7/6 | 7/13 | 7/20 | 7/27 | 8/3 | 8/10 | 8/18 | 8/24 | |
| Temperature/°C | 26.2 | 33.8 | 26.8 | 29.4 | 34 | 28.4 | 24.8 | 20.2 | 25 | ||
| Item | Variance Source | DF | SS | MS | F-Value | p |
|---|---|---|---|---|---|---|
| Wilting ratio | Irrigation | 2 | 4.444 | 2.222 | 90.170 | 0.000 |
| Varieties × Normal irrigation (100%) | 9 | 0.191 | 0.021 | 1.187 | 0.304 | |
| Varieties × Mild drought (50%) | 9 | 0.849 | 0.094 | 5.552 | 0.000 | |
| Varieties × Severe drought (0%) | 9 | 1.596 | 0.177 | 5.823 | 0.000 |
| 2020 | 2021 | ||||
|---|---|---|---|---|---|
| Wilting Ratio | Yield | Dc | Wilting Ratio | Yield | Dc |
| Wr (6/18) | −0.171 | −0.025 | Wr (6/29) | −0.397 ** | −0.395 ** |
| Wr (6/24) | −0.325 ** | −0.208 | Wr (7/06) | −0.387 ** | −0.428 ** |
| Wr (7/02) | −0.093 | −0.116 | Wr (7/13) | −0.447 ** | −0.492 ** |
| Wr (7/10) | −0.593 ** | −0.343 ** | Wr (7/20) | −0.394 ** | −0.459 ** |
| Wr (7/17) | −0.305 ** | −0.243 ** | Wr (7/27) | −0.559 ** | −0.560 ** |
| Wr (7/31) | −0.279 ** | −0.118 | Wr (8/03) | −0.394 ** | −0.419 ** |
| Wr (8/06) | −0.136 | −0.047 | Wr (8/10) | −0.454 ** | −0.476 ** |
| Wr (8/13) | −0.449 ** | −0.260 ** | Wr (8/18) | −0.527 ** | −0.405 ** |
| Wr (8/16) | −0.552 ** | −0.278 ** | Wr (8/24) | −0.471 ** | −0.402 ** |
| Wr (8/24) | −0.387 ** | −0.125 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Tang, H.; Xie, X.; Sun, B.; Liu, C. A Quantitative Index for Evaluating Maize Leaf Wilting and Its Sustainable Application in Drought Resistance Screening. Sustainability 2024, 16, 6129. https://doi.org/10.3390/su16146129
Zhang L, Tang H, Xie X, Sun B, Liu C. A Quantitative Index for Evaluating Maize Leaf Wilting and Its Sustainable Application in Drought Resistance Screening. Sustainability. 2024; 16(14):6129. https://doi.org/10.3390/su16146129
Chicago/Turabian StyleZhang, Lei, Huaijun Tang, Xiaoqing Xie, Baocheng Sun, and Cheng Liu. 2024. "A Quantitative Index for Evaluating Maize Leaf Wilting and Its Sustainable Application in Drought Resistance Screening" Sustainability 16, no. 14: 6129. https://doi.org/10.3390/su16146129





