Real-Time Elemental Analysis Using a Handheld XRF Spectrometer in Scanning Mode in the Field of Cultural Heritage
Abstract
1. Introduction
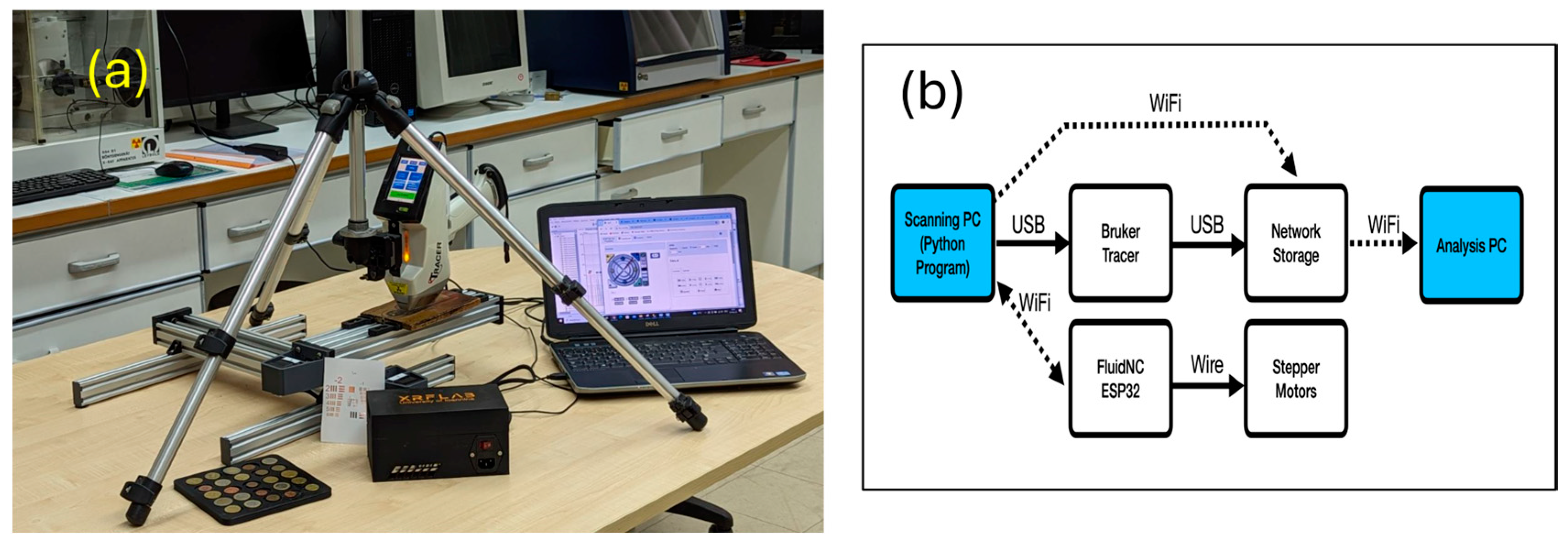
2. The X-ray Fluorescence Scanner
2.1. The Scanner
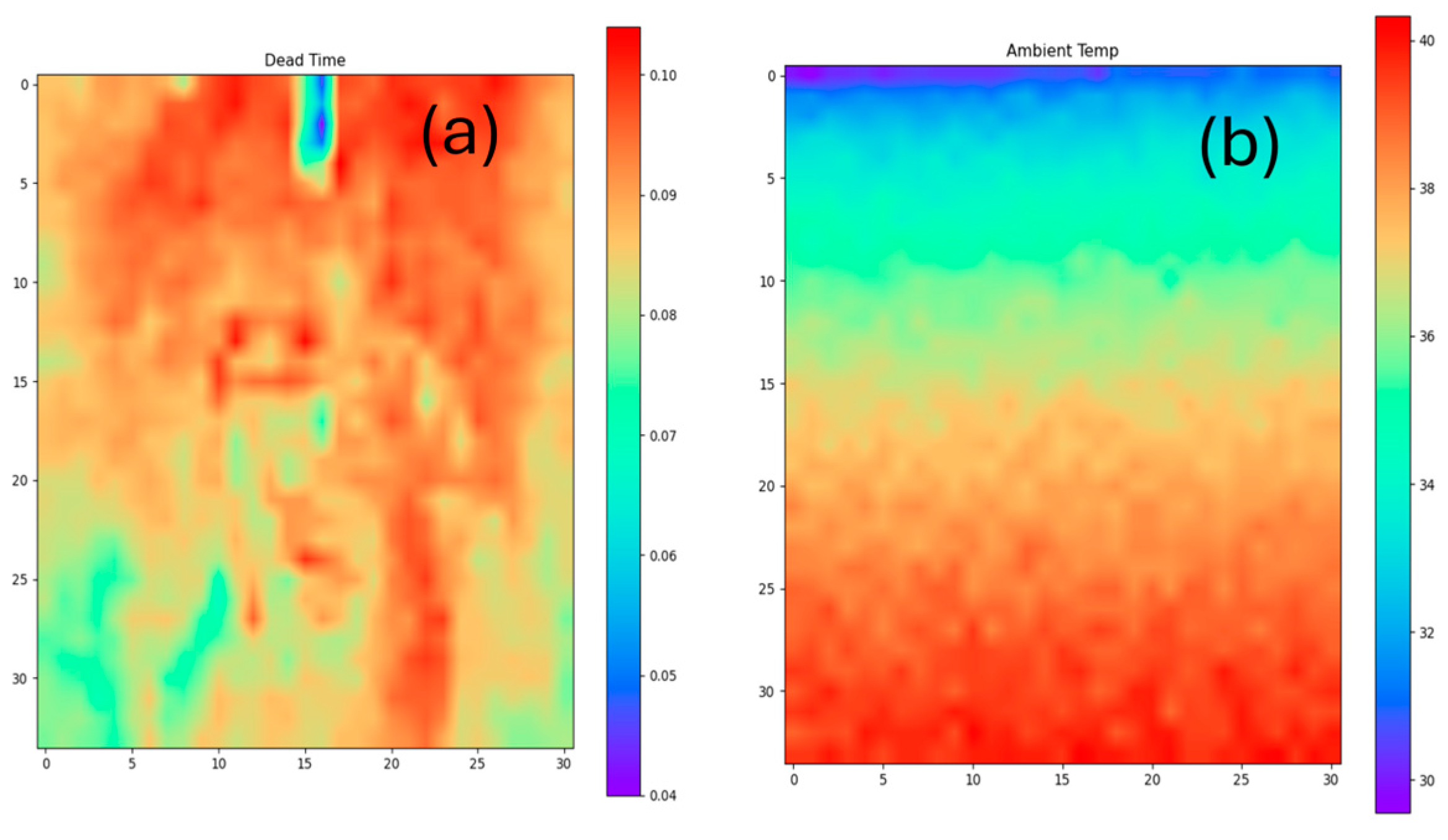
2.2. Data Handling—Data Analysis
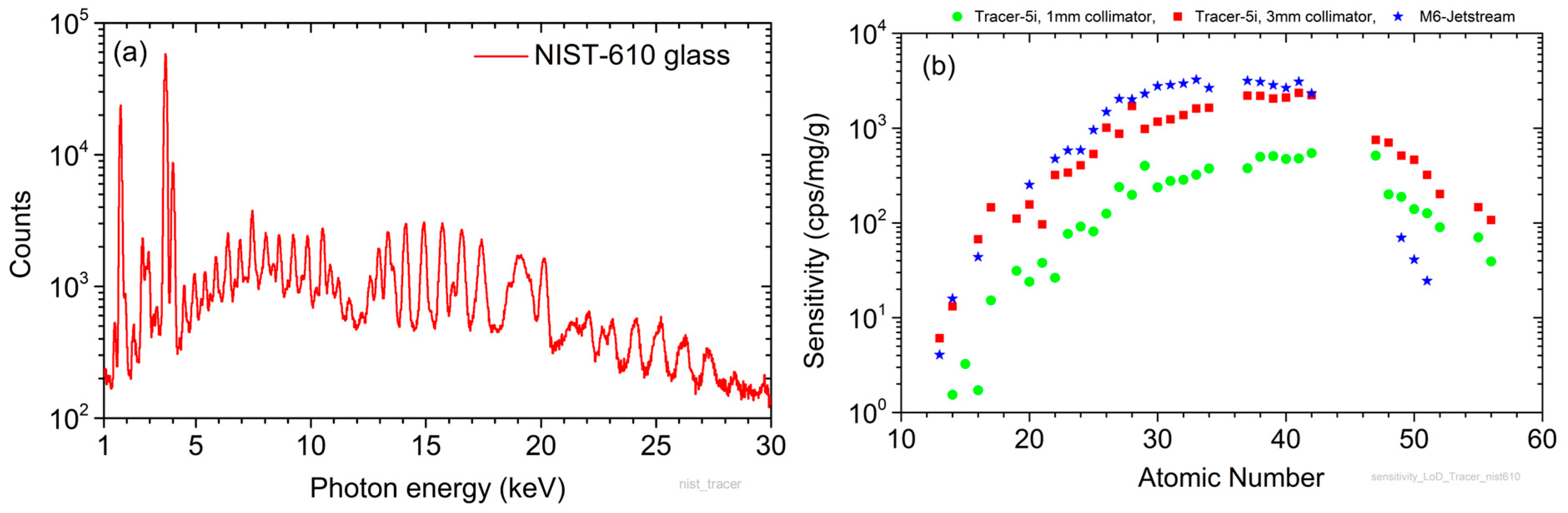
3. Analytical Capabilities of the Scanner
3.1. Sensitivity
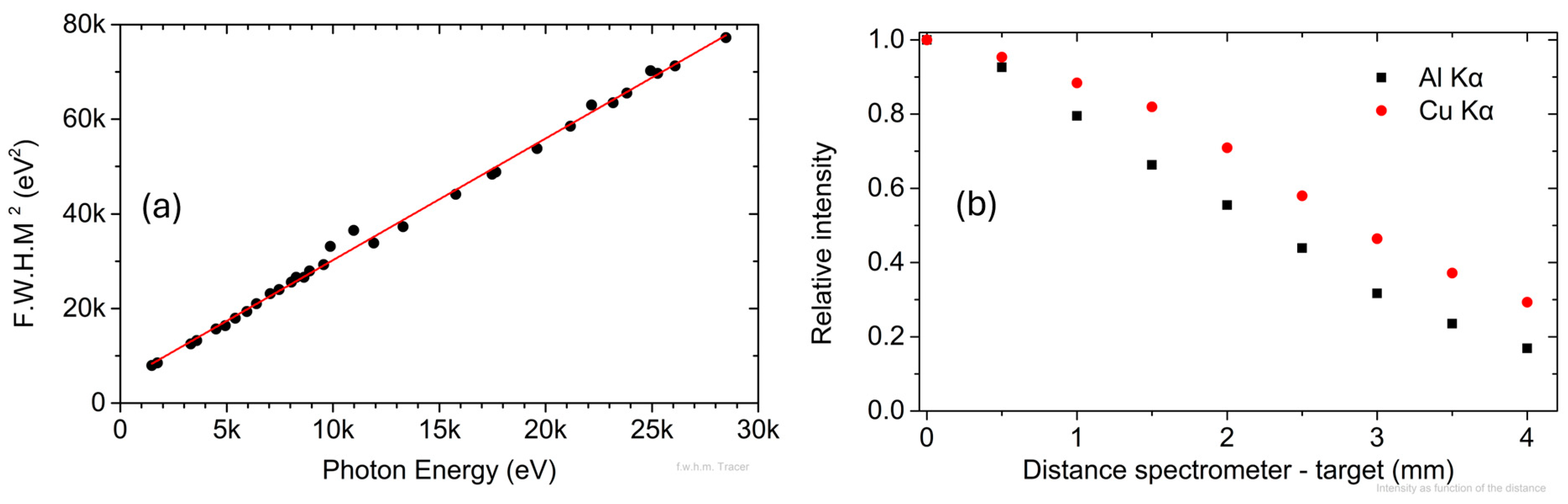
3.2. Energy Resolution
3.3. Intensity as a Function of the Distance
3.4. Beam Spot Size
4. Measurements and Discussion
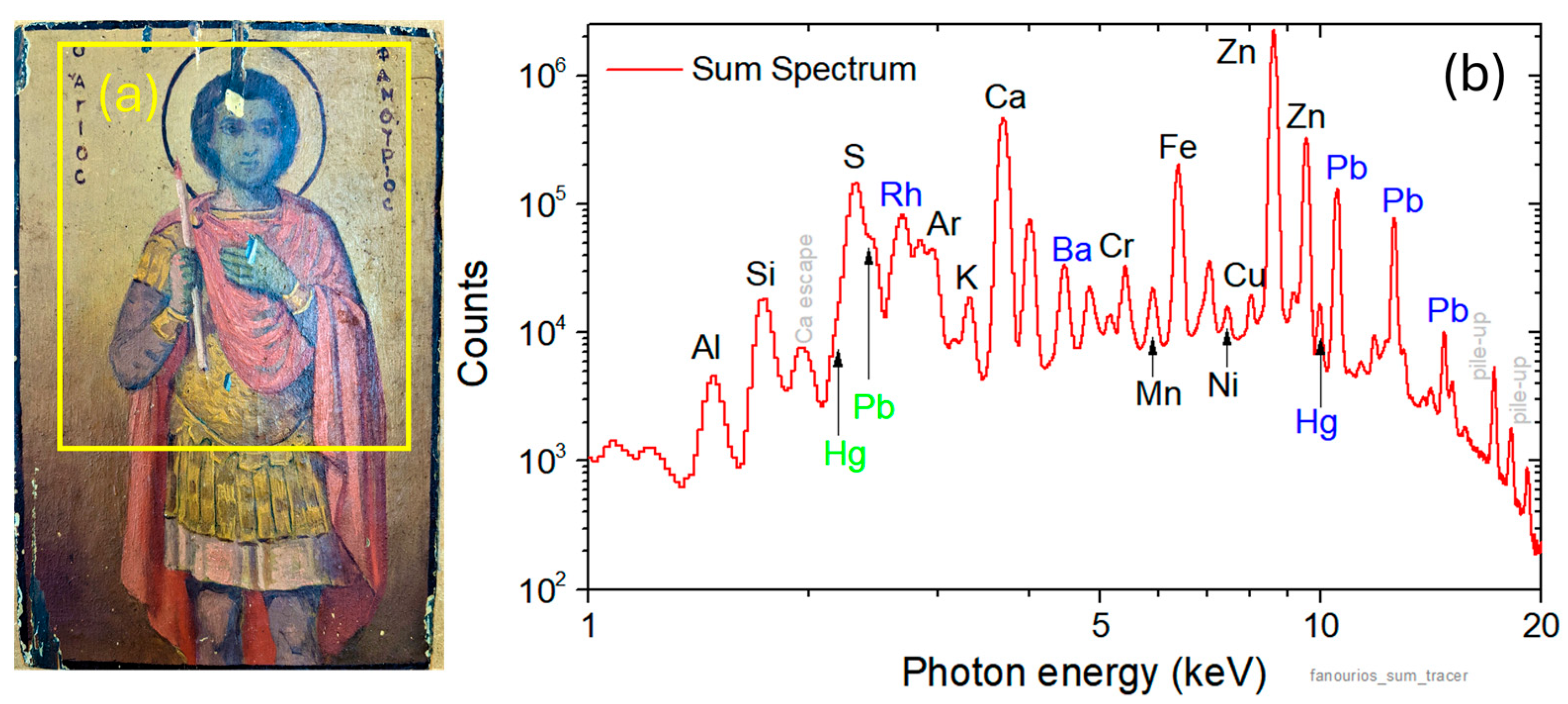
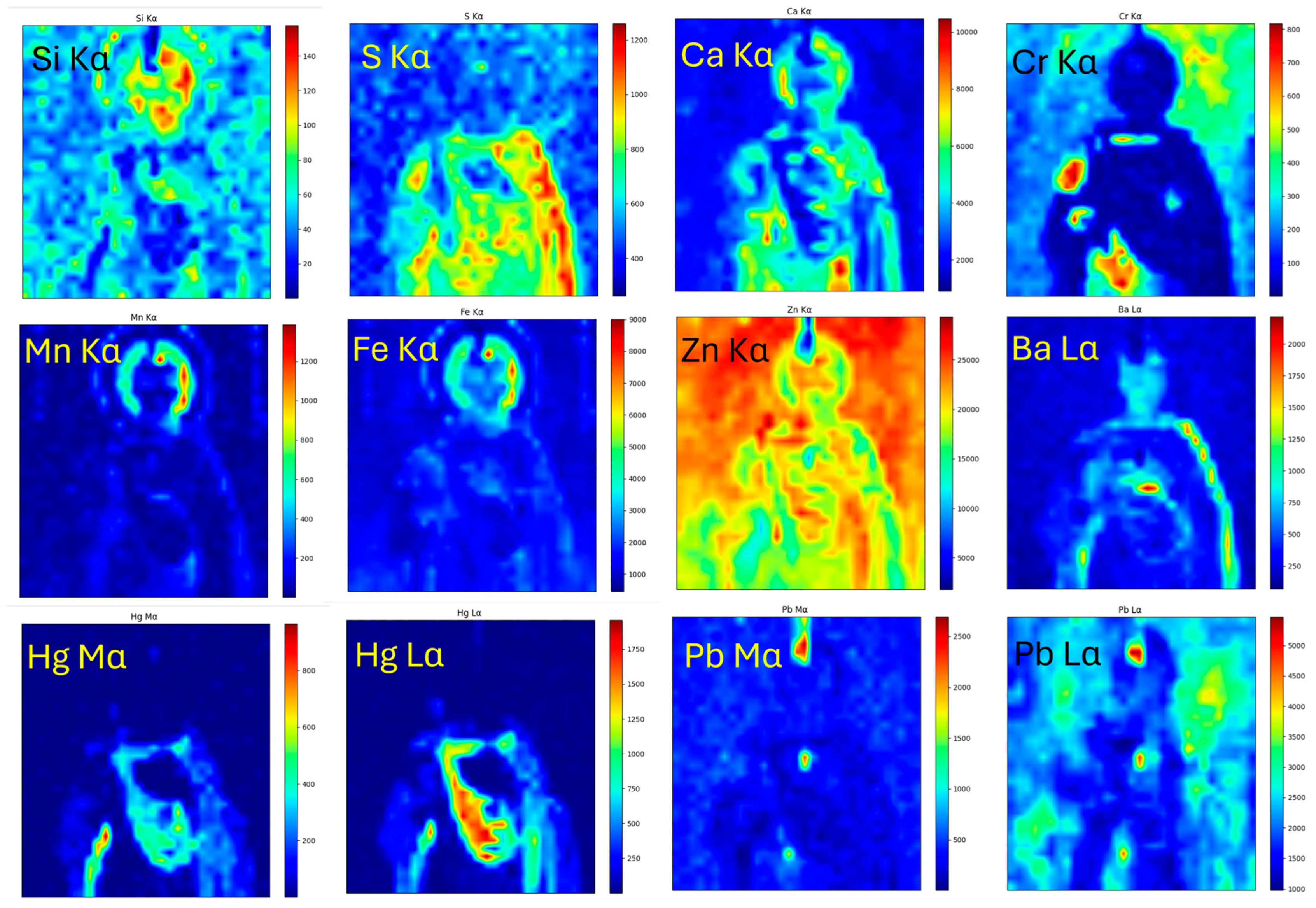
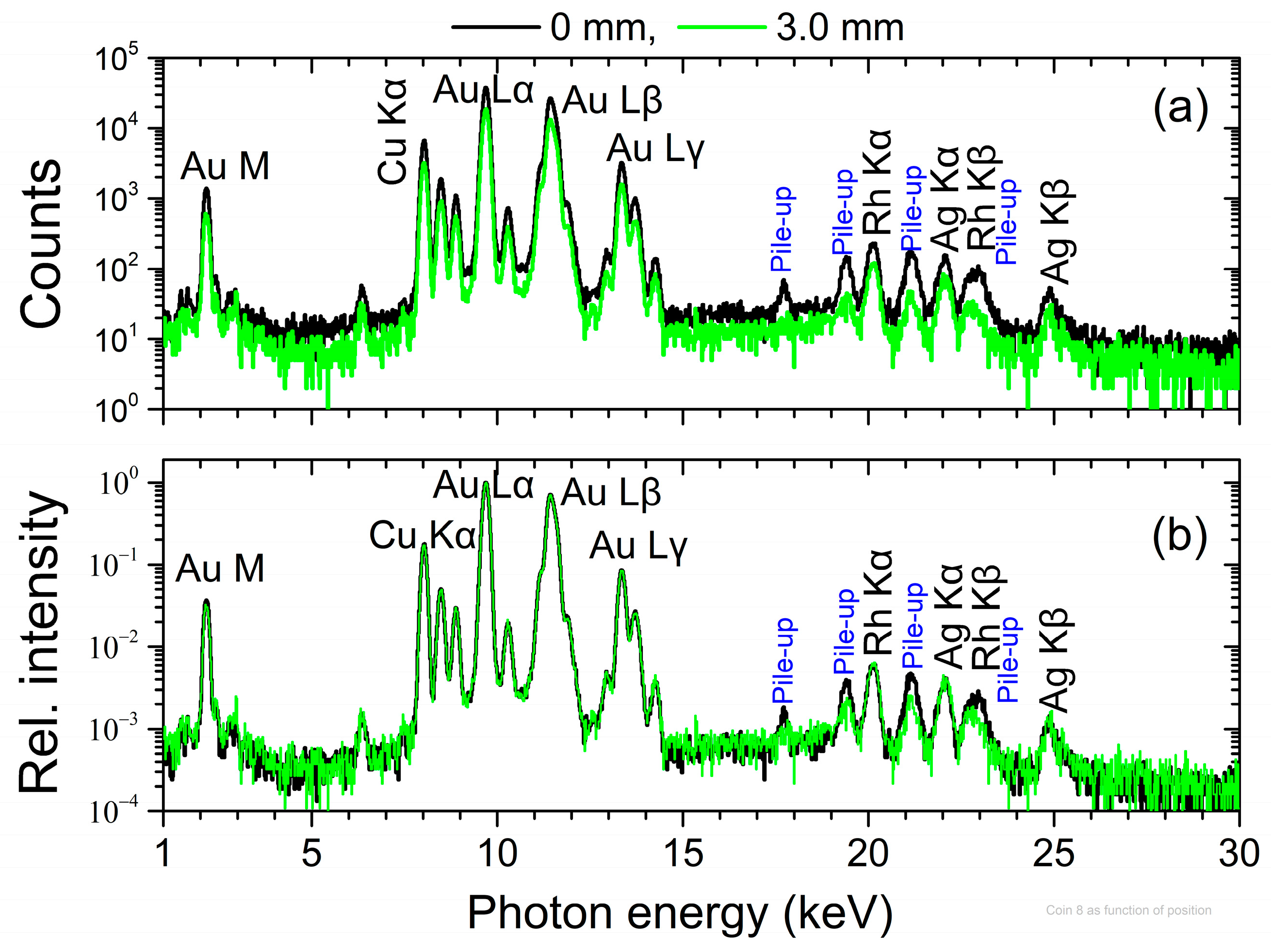
4.1. Imaging
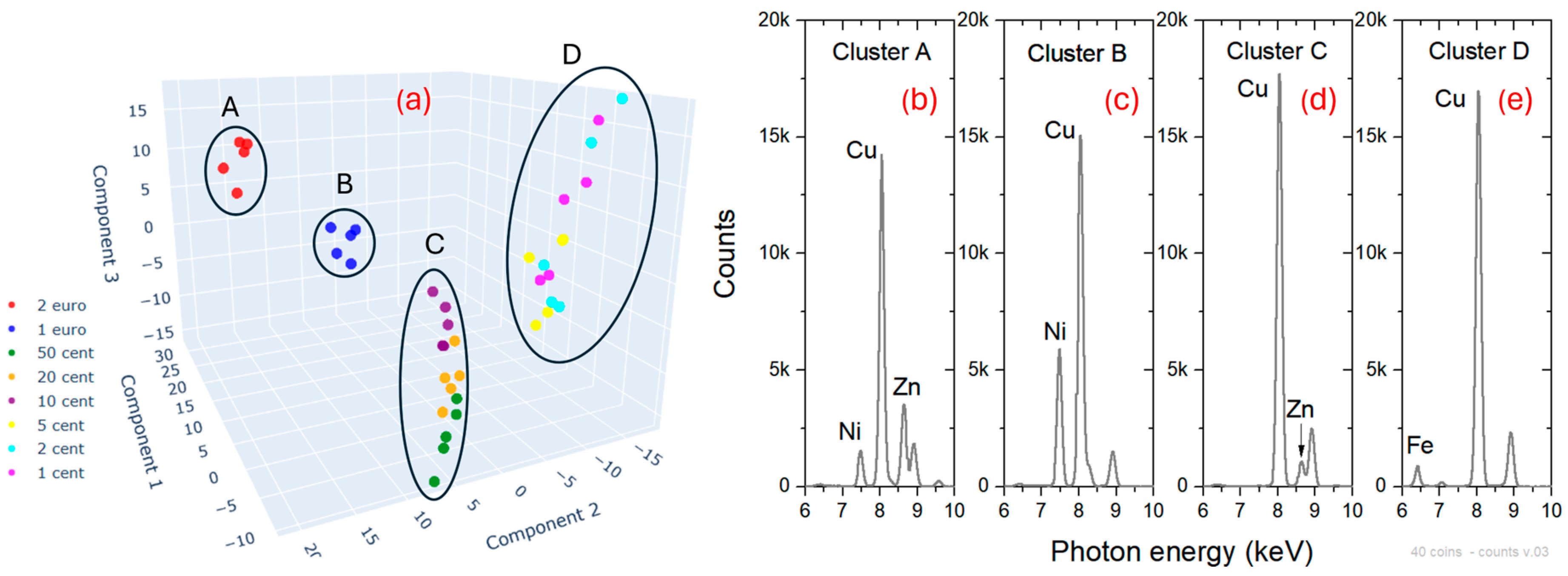
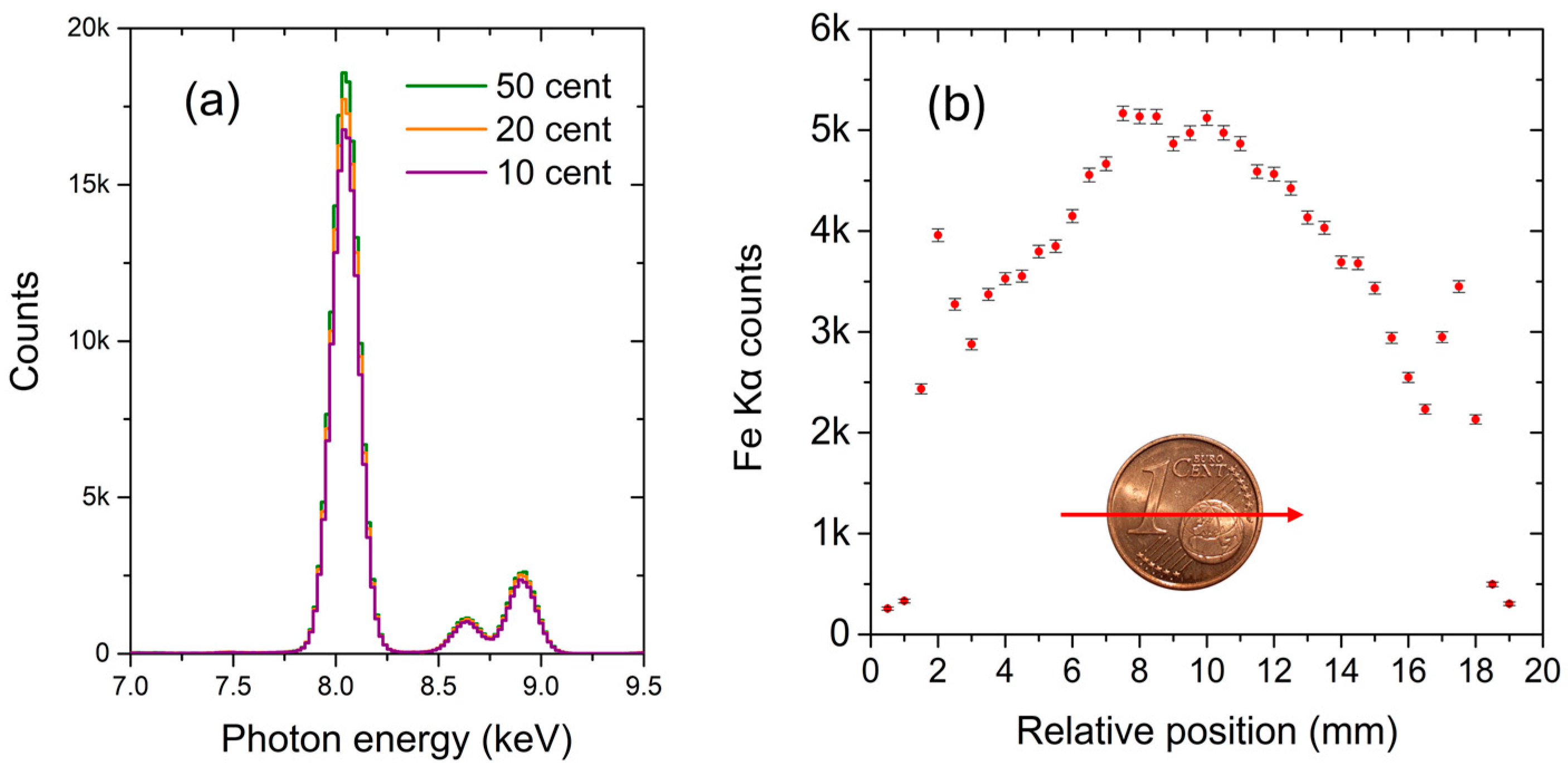
4.2. Object Classification
4.3. Quantitative Analysis during Scanning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfeld, M.; Pedroso, J.V.; van Eikema Hommes, M.; Van der Snickt, G.; Tauber, G.; Blaas, J.; Haschke, M.; Erler, K.; Dik, J.; Janssens, K. A Mobile Instrument for in Situ Scanning Macro-XRF Investigation of Historical Paintings. J. Anal. At. Spectrom. 2013, 28, 760–767. [Google Scholar] [CrossRef]
- Romano, F.P.; Caliri, C.; Nicotra, P.; Di Martino, S.; Pappalardo, L.; Rizzo, F.; Santos, H.C. Real-Time Elemental Imaging of Large Dimension Paintings with a Novel Mobile Macro X-ray Fluorescence (MA-XRF) Scanning Technique. J. Anal. At. Spectrom. 2017, 32, 773–781. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Asvestas, A.; Gerodimos, T.; Anagnostopoulos, D.F. Revealing the Materials, Painting Techniques, and State of Preservation of a Heavily Altered Early 19th Century Greek Icon through MA-XRF. Heritage 2023, 6, 1903–1920. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Asvestas, A.; Gerodimos, T.; Tzima, A.; Papadopoulou, V.; Anagnostopoulos, D.F. MA-XRF Investigation of a 17th Century Icon by the Renowned Painter Theodoros Poulakis. J. Archaeol. Sci. Rep. 2024, 53, 104313. [Google Scholar] [CrossRef]
- Shugar, A.N.; Mass, J.L. Handheld XRF for Art and Archaeology; Leuven University Press: Leuven, Belgium, 2012; ISBN 978-90-5867-934-5. [Google Scholar]
- Clarke, M.L.; Gabrieli, F.; Rowberg, K.L.; Hare, A.; Ueda, J.; McCarthy, B.; Delaney, J.K. Imaging Spectroscopies to Characterize a 13th Century Japanese Handscroll, The Miraculous Interventions of Jizō Bosatsu. Herit. Sci. 2021, 9, 20. [Google Scholar] [CrossRef]
- Shugar, A.N. Handheld Macro-XRF Scanning: Development of Collimators for Sub-mm Resolution. In Proceedings of the Fourth International Symposium on Analytical Methods in Philately, Washington, DC, USA, 13–14 November 2020; pp. 13–20. [Google Scholar]
- Shugar, A.N.; Drake, B.L.; Kelley, G. Rapid Identification of Wood Species Using XRF and Neural Network Machine Learning. Sci. Rep. 2021, 11, 17533. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Flege, S.; Ensinger, W.; Wolf, F.; Weber, C.; Seeberg, S.; Sander, J.; Schultz, J.; Krekel, C.; Tagle, R.; et al. MA-XRF Investigation of the Altenberg Retable from 1330. X-ray Spectrom. 2018, 47, 215–222. [Google Scholar] [CrossRef]
- Handheld XRF Spectrometers TRACER 5. Available online: https://www.bruker.com/en/products-and-solutions/elemental-analyzers/handheld-xrf-spectrometers/TRACER-5.html (accessed on 10 May 2024).
- G-Code. Available online: https://en.wikipedia.org/wiki/G-code (accessed on 16 April 2024).
- Available online: https://github.com/pywinauto/pywinauto (accessed on 16 April 2024).
- ESP32 Series of Modules. Available online: https://www.espressif.com/en/products/modules/esp32 (accessed on 16 April 2024).
- FluidNC. Available online: https://github.com/bdring/FluidNC (accessed on 16 April 2024).
- wget 3.2. Available online: https://pypi.org/project/wget/#description (accessed on 30 April 2024).
- Standard Reference Material® 610. Available online: https://tsapps.nist.gov/srmext/certificates/610.pdf (accessed on 16 April 2024).
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A Multiplatform Code for the Analysis of Energy-Dispersive X-ray Fluorescence Spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- X-ray Interactions with Matter. Available online: https://henke.lbl.gov/optical_constants/gastrn2.html (accessed on 30 April 2024).
- Sitko, R.; Zawisza, B.; Malicka, E. Energy-Dispersive X-ray Fluorescence Spectrometer for Analysis of Conventional and Micro-446 Samples: Preliminary Assessment. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 436–441. [Google Scholar] [CrossRef]
- Gerodimos, T.; Patakiouta, I.V.; Papadakis, V.M.; Exarchos, D.; Asvestas, A.; Kenanakis, G.; Matikas, T.E.; Anagnostopoulos, D.F. Scanning Micro X-ray Fluorescence and Multispectral Imaging Fusion: A Case Study on Postage Stamps. J. Imaging 2024, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- 1951 USAF Resolution Test Chart. Available online: https://en.wikipedia.org/wiki/1951_USAF_resolution_test_chart (accessed on 3 May 2024).
- Daniilia, S.; Bikiaris, D.; Burgio, L.; Gavala, P.; Clark, R.J.H.; Chryssoulakis, Y. An Extensive Non-Destructive and Micro-Spectroscopic Study of Two Post-Byzantine Overpainted Icons of the 16th Century. J. Raman Spectrosc. 2002, 33, 807–814. [Google Scholar] [CrossRef]
- Cortea, I.M.; Ratoiu, L.; Chelmuș, A.; Mureșan, T. Unveiling the original layers and color palette of 18th century overpainted Transylvanian icons by combined X-ray radiography, hyperspectral imaging, and spectroscopic spot analysis. X-ray Spectrom. 2022, 51, 26–42. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Beltsios, K.G. Pigments—Iron-based red, yellow, and brown ochres. Archaeol. Anthropol. Sci. 2022, 14, 35. [Google Scholar] [CrossRef]
- Helwig, K. Iron oxide pigments: Natural and synthetic. In Artists Pigments: A Handbook of Their History and Characteristics; Berrie, B.H., Ed.; National Gallery of Art: Washington, DC, USA, 2007; Volume 4, pp. 1–37. [Google Scholar]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Rogge, C.E.; Epley, B.A. Behind the Bocour Label: Identification of Pigments and Binders in Historic Bocour Oil and Acrylic Paints. J. Am. Inst. Conserv. 2017, 56, 15–42. [Google Scholar] [CrossRef]
- Shilstein, S.S.; Shalev, S. Making Sense out of Cents: Compositional Variations in European Coins as a Control Model for Archaeometallurgy. J. Archaeol. Sci. 2011, 38, 1690–1698. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Technical Characteristics of Euro coins. Available online: https://www.bcl.lu/en/Banknotes-and-Coins/billets_pieces/car_pieces/car_tec/index.html (accessed on 28 April 2024).
- Common Sides of Euro Coins. Available online: https://economy-finance.ec.europa.eu/euro/euro-coins-and-notes/euro-coins/common-sides-euro-coins_en (accessed on 24 April 2024).
- Electroplating: How the U.S. Mint Makes a Penny. Available online: https://www.comsol.com/blogs/electroplating-u-s-mint-makes-penny/ (accessed on 28 April 2024).
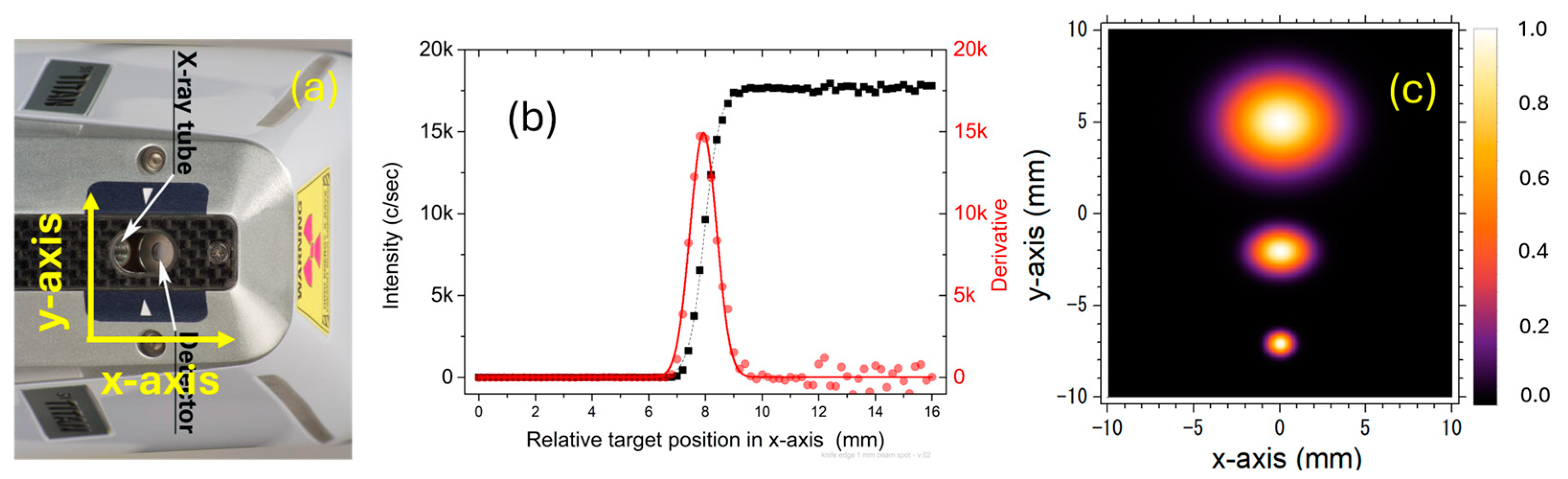
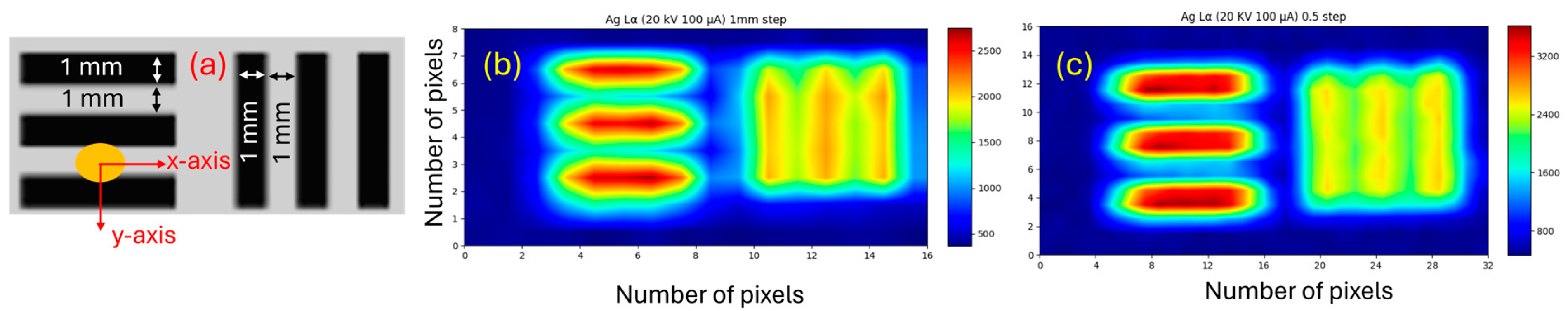

| Collimator | Beam Spot FWHM (mm) | Beam Spot FWTM (mm) | ||
|---|---|---|---|---|
| x-axis | y-axis | x-axis | y-axis | |
| “1 mm” | 1.1 ± 0.1 | 0.9 ± 0.1 | 2.0 ± 0.2 | 1.6 ± 0.2 |
| “3 mm” | 2.5 ± 0.1 | 1.70 ± 0.05 | 4.6 ± 0.2 | 3.1 ± 0.1 |
| “8 mm” | 4.9 ± 0.1 | 3.75 ± 0.05 | 8.9 ± 0.2 | 6.8 ± 0.1 |
| Distance | Element | Alloy 1 | Alloy 2 | Alloy 3 | Alloy 4 | Alloy 5 | Alloy 6 | Alloy 7 | Alloy 8 |
|---|---|---|---|---|---|---|---|---|---|
| “0 mm” | Cu | 7.4 (1) | 47.2 (2) | 47.4 (2) | 8.7 (1) | 27.4 (1) | 10.0 (1) | 8.1 (1) | 8.2 (1) |
| Zn | — | — | 6.2 (1) | — | 6.8 (1) | — | — | — | |
| Ag | 92.6 (3) | 20.8 (2) | 7.0 (1) | 29.1 (2) | 8.3 (1) | 0.35 (5) | 0.39 (5) | 0.82 (5) | |
| Au | — | 31.9 (2) | 39.3 (2) | 61.9 (3) | 57.5 (3) | 89.6 (3) | 91.5 (1) | 91.0 (1) | |
| “3 mm” | Cu | 7.5(1) | 47.1 (2) | 47.4 (2) | 8.9 (1) | 27.3 (2) | 10.0 (1) | 8.3 (1) | 8.2 (1) |
| Zn | — | — | 5.6 (1) | — | 6.4 (1) | — | — | — | |
| Ag | 92.5 (4) | 21.2 (2) | 7.3 (1) | 29.7 (3) | 8.5 (2) | 0.35 (7) | 0.45 (5) | 0.94 (6) | |
| Au | — | 31.7 (2) | 39.7 (3) | 61.4 (3) | 57.8 (4) | 89.6 (4) | 91.2 (1) | 90.8 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asvestas, A.; Chatzipanteliadis, D.; Gerodimos, T.; Mastrotheodoros, G.P.; Tzima, A.; Anagnostopoulos, D.F. Real-Time Elemental Analysis Using a Handheld XRF Spectrometer in Scanning Mode in the Field of Cultural Heritage. Sustainability 2024, 16, 6135. https://doi.org/10.3390/su16146135
Asvestas A, Chatzipanteliadis D, Gerodimos T, Mastrotheodoros GP, Tzima A, Anagnostopoulos DF. Real-Time Elemental Analysis Using a Handheld XRF Spectrometer in Scanning Mode in the Field of Cultural Heritage. Sustainability. 2024; 16(14):6135. https://doi.org/10.3390/su16146135
Chicago/Turabian StyleAsvestas, Anastasios, Demosthenis Chatzipanteliadis, Theofanis Gerodimos, Georgios P. Mastrotheodoros, Anastasia Tzima, and Dimitrios F. Anagnostopoulos. 2024. "Real-Time Elemental Analysis Using a Handheld XRF Spectrometer in Scanning Mode in the Field of Cultural Heritage" Sustainability 16, no. 14: 6135. https://doi.org/10.3390/su16146135
APA StyleAsvestas, A., Chatzipanteliadis, D., Gerodimos, T., Mastrotheodoros, G. P., Tzima, A., & Anagnostopoulos, D. F. (2024). Real-Time Elemental Analysis Using a Handheld XRF Spectrometer in Scanning Mode in the Field of Cultural Heritage. Sustainability, 16(14), 6135. https://doi.org/10.3390/su16146135







