Microplastic Distribution Characteristics Considering the Marine Environment Based on Surface Seawater Quality Parameters in Southern Sea of Korea, 2019
Abstract
:1. Introduction
2. Materials and Methods
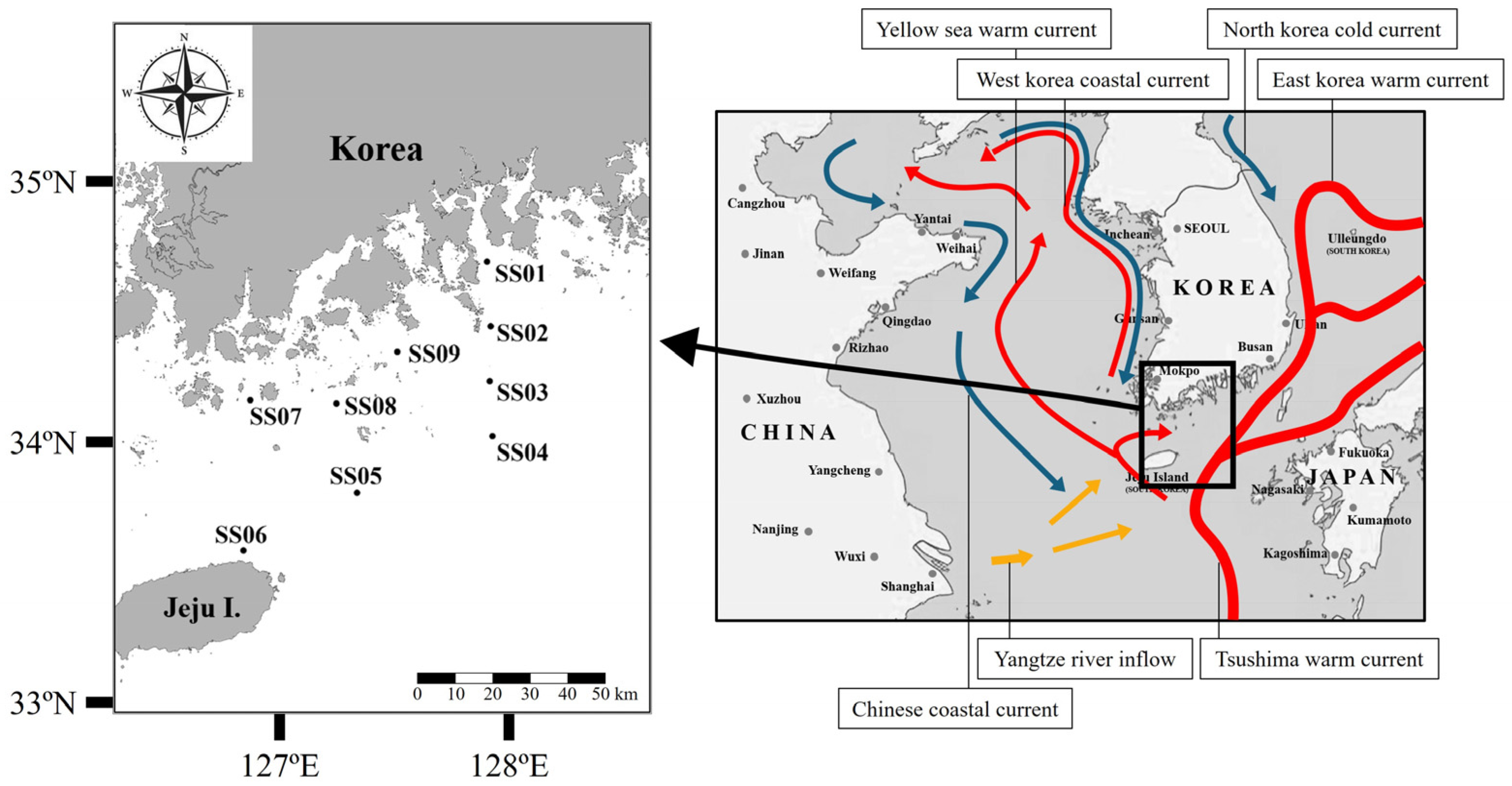
2.1. Study Area and Sampling Methods
2.2. Water Quality Parameters
2.3. Microplastics
2.3.1. Sample Collection
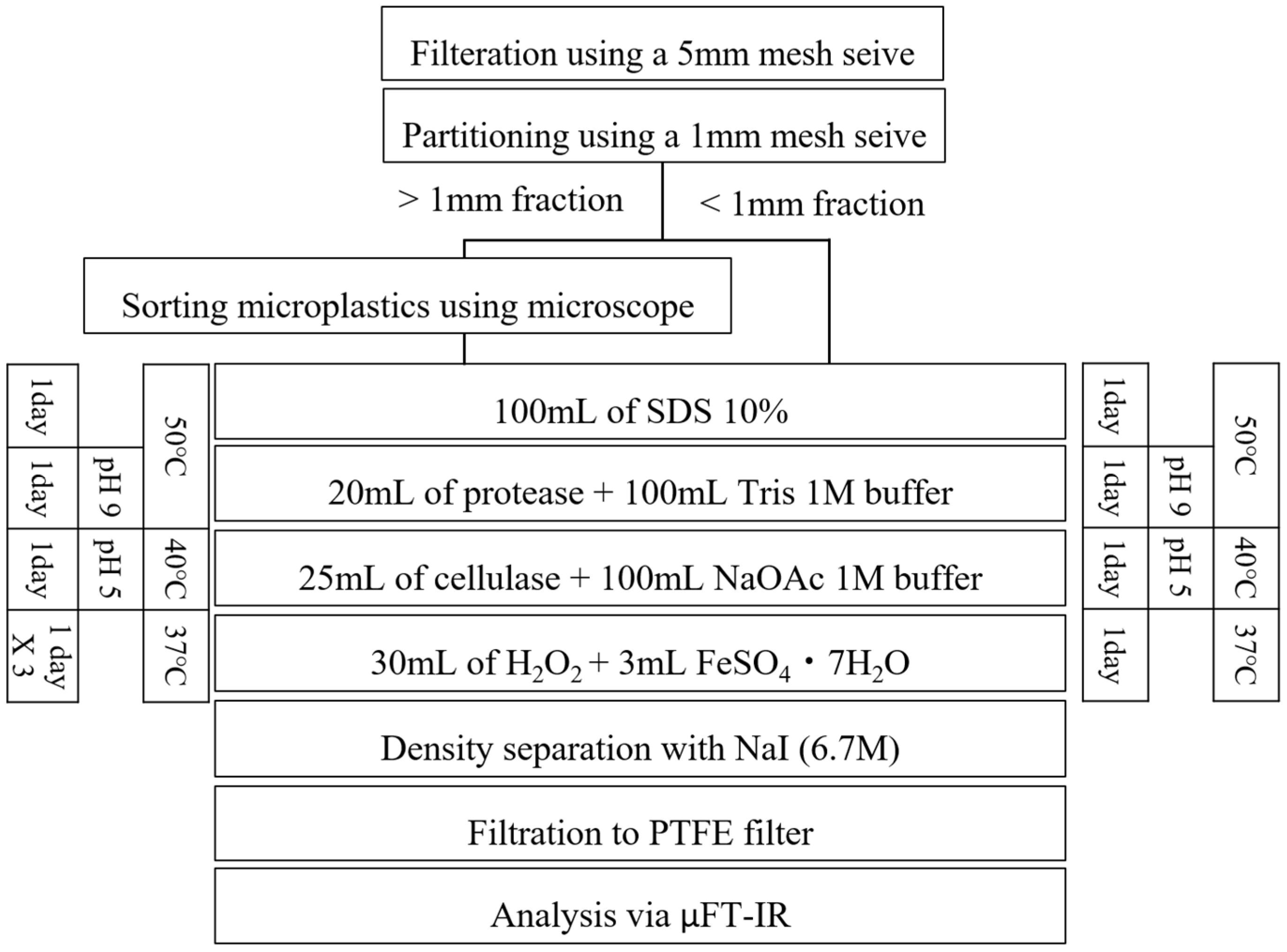
2.3.2. Pretreatment Procedures
2.3.3. Microplastic Polymer Identification
2.3.4. Microplastic Size and Shape Measurement
2.3.5. QA/QC
2.4. Statistical Analysis
3. Results
3.1. Water Quality Parameter
3.2. Microplastics
3.2.1. Microplastic Abundance
3.2.2. Microplastic Polymers
3.2.3. Microplastic Size
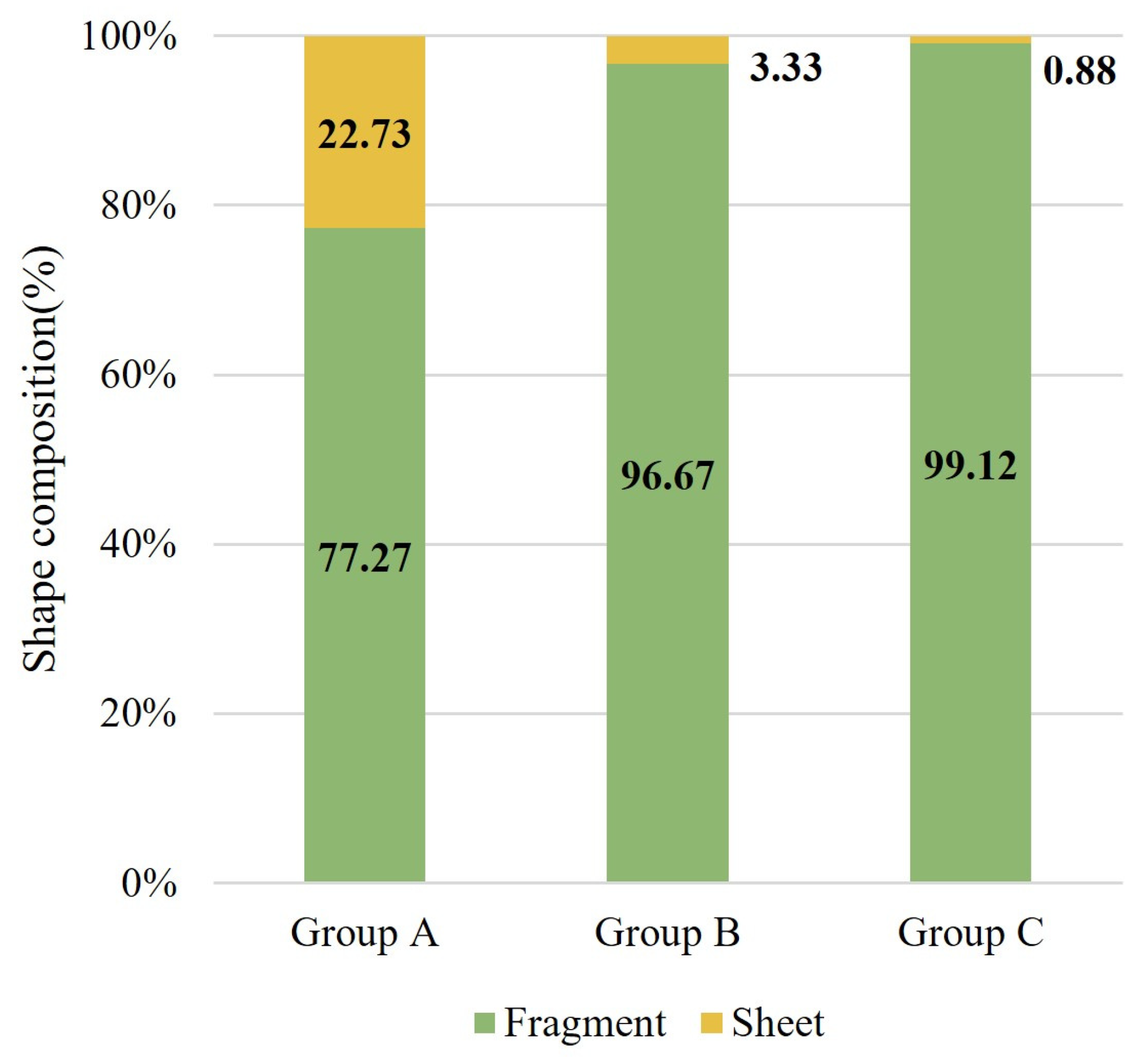
3.2.4. Microplastic Shapes
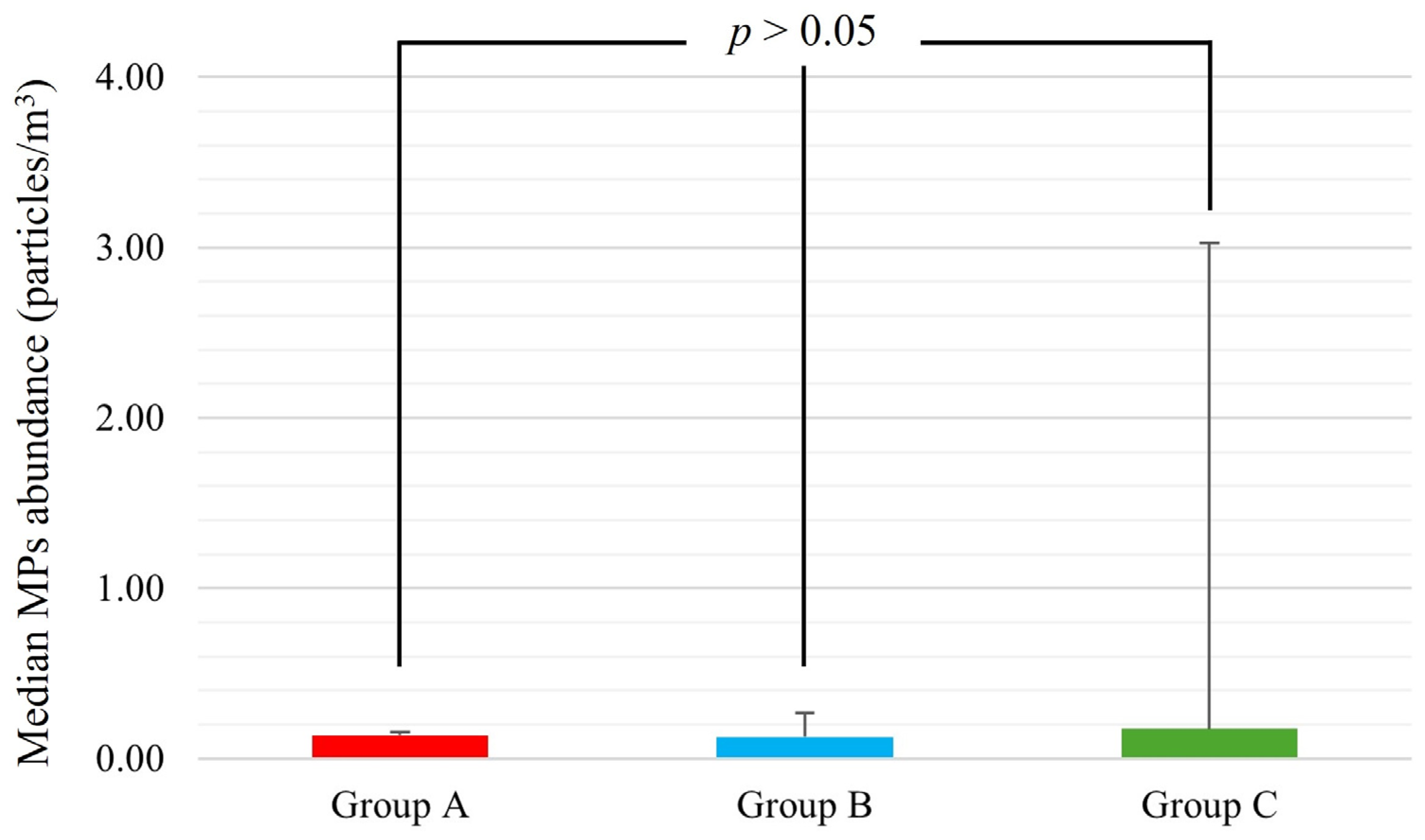
3.3. Principal Component Analysis and Cluster Analysis
4. Discussion
4.1. Sea Area Characteristics
4.2. Microplastic Characteristics
4.2.1. Microplastic Abundance
4.2.2. Microplastics Polymer
4.2.3. Microplastic Size
4.2.4. Microplastic Shape
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, C.; Baker, J.E.; Bamford, H.A. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, 9–11 September 2008; University of Washington Tacoma: Tacoma, WA, USA, 2009. Available online: https://repository.library.noaa.gov/view/noaa/2509 (accessed on 29 April 2024).
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L. Plastics in the marine environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A. Plastic Pollution, Unsustainable Environmental Problem? Industry and academic research must co-operate to short out the pervasive global problem of plastic waste. Sci. Rev. 2024, 1, 1–42. [Google Scholar]
- OECD. Global Plastics Outlook: Policy Scenarios to 2060; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Marine Microplastics: Abundance, Distribution, and Composition. Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–26. [Google Scholar] [CrossRef]
- do Sul JA, I.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 118, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Eriksen, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Fred-Ahmadu, O.H. Occurrence and distribution of microplastics-sorbed phthalic acid esters (PAEs) in coastal psammitic sediments of tropical Atlantic Ocean, Gulf of Guinea. Sci. Total Environ. 2020, 730, 139013. [Google Scholar] [CrossRef] [PubMed]
- Ramirez MM, B.; Caamal, R.D.; von Osten, J.R. Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Sci. Total Environ. 2019, 672, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic. Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasand, L. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Michida, Y.; Chavanich, S.; Chiba, S.; Cordova, M.R.; Cozsar Cabanas, A.; Glagani, F.; Hagmann, P.; Hinata, H.; Isobe, A.; Kershaw, P.; et al. Guidelines for Harmonizing Ocean Surface Microplastic Monitoring Method, Version 1.1; Ministry of the Environment, Japan: Tokyo, Japan, 2019. [CrossRef]
- Choi, M.H.; Seo, H.S.; Kim, D.S. A Study on the Distribution of Summer Water Temperatures of the Central Coast of the Southern Sea of Korea Using Numerical Experimentation. J. Korean Soc. Mar. Environ. Saf. 2017, 23, 83–90. [Google Scholar] [CrossRef]
- Yeh, S.W.; Kim, C.H. Recent warming in the Yellow/East China Sea during winter and the associated atmospheric circulation. Cont. Shelf Res. 2010, 30, 1428–1434. [Google Scholar] [CrossRef]
- Bae, S.W.; Kim, D.S. Understanding the flow properties by a numerical modeling in the South Sea of Korea. J. Korean Soc. Mar. Environ. Saf. 2012, 18, 295–307. [Google Scholar] [CrossRef]
- Lee, C.I.; Cho, K.D.; Choe, Y.K. Spatial Variation of the Polar Front in relation to the Tsushima Warm Current in the East sea. Korean Environ. Sci. Soc. 2003, 12, 943–948. [Google Scholar] [CrossRef]
- Jang, S.T.; Lee, J.; Kim, C.H.; Jang, C.; Jang, Y.S. Movement of Cold Water Mass in the Northern East China Sea in Summer. Korean Soc. Ocean. 2011, 16, 1–13. [Google Scholar] [CrossRef]
- Jung, S.W.; Park, J.K.; Jeong, D.H.; Lim, D.I. Seasonal Changes in Water Masses and Phytoplankton Communities in the Western Part of South Coastal Waters, Korea. Korean J. Environ. Biol. 2012, 30, 328–338. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, S.H.; Rho, H.K. A Study on the Temperature Fronts observed in the South—West Sea of Korea and the Northern Area of the East China Sea. Korean J. Fish. Aquat. Sci. 1998, 31, 695–706. [Google Scholar]
- Yoon, S.C.; Youn, S.H.; Whang, J.D.; Suh, Y.S.; Yoon, Y.Y. Long-term variation in ocean environmental conditions of the northern East China Sea. J. Korean Soc. Mar. Environ. Energy 2015, 18, 189–206. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Park, J.S.; Soh, H.Y.; Hwang, D.J. A Characteristics of Thermohaline Structure and Phytoplankton Community from Southwestern Parts of the East China Sea during Early Summer, 2004. Korean Soc. Mar. Environ. Energy 2005, 8, 100–110. [Google Scholar] [CrossRef]
- Lee, H.G.; Kang, T.W.; Oh, J.H.; Min, W.G.; Rho, H.S.; Kim, D.S. Distributional Characteristics of the Meiobenthic Community in Gamak Bay in the South Sea, Korea. Korean Assoc. Ocean. Sci. Technol. Soc. 2011, 34, 79–90. [Google Scholar] [CrossRef]
- Yoon, Y.H. Characteristics on spatial distributions of phytoplankton communities in relation to water masses in the western South Sea, Korea in early autumn 2021. Korean J. Environ. Biol. 2021, 39, 559–572. [Google Scholar] [CrossRef]
- Pravettoni, R.; Rekacewicz, P. Global Linkages—A Graphic Look at the Changing Arctic (Rev.1). 2019. Available online: https://www.grida.no/resources/13339 (accessed on 29 April 2024).
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; Fisheries and Aquaculture Technical Paper No. 615; FAO: Rome, Italy, 2017; Available online: https://www.proquest.com/docview/1932298451/abstract/C03F2A1029C94777PQ/1?accountid=27782&sourcetype=Scholarly%20Journals (accessed on 29 April 2024).
- Kumar, S.; Singh, E.; Mishra, R.; Kumar, A.; Caucci, S. Utilization of plastic wastes for sustainable environmental management: A review. ChemSusChem 2021, 14, 3985–4006. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kwon, O.Y.; Shim, W.J. Potential threat of microplastics to zooplanktivores in the surface waters of the Southern Sea of Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.Y.; Kang, J.H.; Shim, W.J.; Hong, S.H. Distributional Characteristics of Microplastics in the Coastal Waters in East Sea of Korea; The Korean Society of Oceanography: Jeju, Republic of Korea, 2015; pp. 228–230. Available online: https://sciwatch.kiost.ac.kr/handle/2020.kiost/25159 (accessed on 29 April 2024).
- Kwon, O.Y.; Kang, J.H.; Shim, W.J.; Hong, S.H. Distributional Characteristics of Microplastics in the Coastal Waters in South Sea, Korea; The Korean Society of Oceanography: Jeju, Republic of Korea, 2016; p. 246. Available online: https://sciwatch.kiost.ac.kr/handle/2020.kiost/24460 (accessed on 29 April 2024).
- Kwon, O.Y.; Kang, J.H.; Hong, S.H.; Shim, W.J. Spatial distribution of microplastic in the surface waters along the coast of Korea. Mar. Pollut. Bull. 2020, 155, 110729. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, M.; Wang, Z.; Jiang, L.; Fan, S.; Zheng, R.; Yu, X. Impacts of terrestrial input on the distribution characteristics of microplastics in the East China Sea characterized by chromophoric dissolved organic matter (CDOM) analysis. Sci. Total Environ. 2022, 838, 156599. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Min, B.K.; Jeong, H.H.; Ju, M.J.; Ko, U.; Dae, K.H.; Kim, H.J.; Cho, C.R.; Soh, H.Y.; Ishibashi, Y.; Cho, H.S. Baseline Study on Microplastic Distribution in the Open Surface Waters of the Korean Southwest Sea. Water 2023, 15, 2393. [Google Scholar] [CrossRef]
- Xiong, W.; Mei, X.; Mi, B.B.; Yang, H.; Han, Z.Z.; Zhang, Y.; Lü, W.C. Current status and cause analysis of microplastic pollution in sea areas in China. China Geol. 2022, 5, 160–170. [Google Scholar] [CrossRef]
- Horton, A.A.; Barnes, D.K. Microplastic pollution in a rapidly changing world: Implications for remote and vulnerable marine ecosystems. Sci. Total Environ. 2020, 738, 140349. [Google Scholar] [CrossRef] [PubMed]
- La Daana, K.K.; Officer, R.; Lyashevska, O.; Thompson, R.C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307–314. [Google Scholar] [CrossRef]
- Lusher, A. Microplastics in the marine environment: Distribution, interactions and effects. In Marine Anthropogenic Litter; Springer: Cham, Switzerland; Amsterdam, The Netherlands, 2015; pp. 245–307. [Google Scholar] [CrossRef]
- Korean Hydrographic and Oceanographic Agency. Schematic Mapas of Surface Currents in the Neighboring Seas of Korea. Available online: http://www.khoa.go.kr/SynapDocViewServer/viewer/doc.html?key=53b9a09d8cc861900190c6222ac80e35&convType=img&convLocale=ko_KR&contextPath=/SynapDocViewServer (accessed on 29 April 2024).
- Ministry of Oceans and Fisheries. Official Standard Measurement Methods for Marine Environment. Available online: https://www.law.go.kr/admRulLsInfoP.do?admRulSeq=2000000109042#AJAX (accessed on 29 April 2024).
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter and Microplastics in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: London, UK, 2019; 130p. [Google Scholar] [CrossRef]
- Chae, D.H.; Kim, I.S.; Song, Y.K.; Kim, S.W.; Kim, S.K. Development of Analytical Method for Microplastics in Seawater. Korean Soc. Oceanogr. 2014, 19, 88–98. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef] [PubMed]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Microplastic pollution focused on sources, distribution, contaminant interactions, analytical methods, and wastewater removal strategies: A review. Int. J. Environ. Res. Public Health 2022, 19, 5610. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Kochleus, C.; Bänsch-Baltruschat, B.; Brennholt, N.; Reifferscheid, G. Sampling techniques and preparation methods for microplastic analyses in the aquatic environment—A review. TrAC Trends Anal. Chem. 2019, 113, 84–92. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Löder, M.G.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; et al. Enzymatic purification of microplastics in environmental samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef] [PubMed]
- Konechnaya, O.; Lüchtrath, S.; Dsikowitzky, L.; Schwarzbauer, J. Optimized microplastic analysis based on size fractionation, density separation and μ-FTIR. Water Sci. Technol. 2020, 81, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Jeong, H.H.; Kusano, T.; Addai-arhin, S.; Nugraha, W.C.; Novirsa, R.; Dinh, Q.P.; Shirosaki, T.; Fujita, E.; Kameda, Y.; Cho, H.S.; et al. Differences in microplastic distributions on the surface freshwater collected using 100–and 355–μm meshes. Environ. Monit. Contam. Res. 2022, 2, 22–34. [Google Scholar] [CrossRef]
- Walton, W.H. Feret‘s statistical diameter as a measure of particle size. Nature 1948, 162, 329–330. [Google Scholar] [CrossRef]
- Espiritu, E.Q.; Dayrit SA, S.; Coronel AS, O.; Paz NS, C.; Ronquillo PI, L.; Castillo VC, G.; Enriquez, E.P. Assessment of quantity and quality of microplastics in the sediments, waters, oysters, and selected fish species in key sites along the bombong estuary and the coastal waters of ticalan in San Juan, Batangas. Philipp. J. Sci. 2019, 148, 789–816. [Google Scholar]
- Jeong, H.H.; Choi, S.D.; Cho, H.S. Characteristics of Hypoxic Water Mass Occurrence in the Northwestern Gamak Bay, Korea, 2017. J. Korean Soc. Mar. Environ. Saf. 2021, 27, 708–720. [Google Scholar] [CrossRef]
- Zeinalzadeh, K.; Rezaei, E. Determining spatial and temporal changes of surface water quality using principal component analysis. J. Hydrol. Reg. Stud. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Wu, G.; Li, J.; Luo, W. Spatial distribution, source apportionment, and assessment of marine water quality parameters in the Bohai Sea, China. Mar. Pollut. Bull. 2023, 195, 115526. [Google Scholar] [CrossRef] [PubMed]
- Hammoumi, D.; Al-Aizari, H.S.; Alaraidh, I.A.; Okla, M.K.; Assal, M.E.; Al-Aizari, A.R.; Moshab, M.S.; Chakiri, S.; Bejjaji, Z. Seasonal Variations and Assessment of Surface Water Quality Using Water Quality Index (WQI) and Principal Component Analysis (PCA): A Case Study. Sustainability 2024, 16, 5644. [Google Scholar] [CrossRef]
- Haag, I.; Westrich, B. Processes governing river water quality identified by principal component analysis. Hydrol. Process. 2002, 16, 3113–3130. [Google Scholar] [CrossRef]
- Jeong, H.; Kang, Y.; Cho, H. C, N, and P mass balances in the bottom seawater–surface sediment Interface in the reducing environment due to anoxic water of Gamak Bay, Korea. Water 2022, 14, 2244. [Google Scholar] [CrossRef]
- Heo, M.Y.; Choi, Y.C. Formation and characteristics of ocean fronts at the East China Sea in southwestern sea area from Jeju Island, summer. J. Korean Soc. Mar. Environ. Energy 2004, 7, 64–69. [Google Scholar]
- Jang, S.T.; Lee, J.H.; Hong, C.S. Mixing of sea waters in the northern part of the East China Sea in Summer. Korean Soc. Ocean. 2007, 12, 390–399. [Google Scholar]
- Hur, H.B.; Jacobs, G.A.; Teague, W.J. Monthly variations of water masses in the Yellow and East China Seas, November 6, 1998. J. Oceanogr. 1999, 55, 171–184. [Google Scholar] [CrossRef]
- Hyun, K.H.; Pang, I.C.; Lee, J.H.; Rho, H.K. Water Mass Analysis in the East China Sea in Summer and the Northward Route of the Tsushima Current. Mar. Sci. Inst. Jeju Natl. Univ. 1997, 21, 85–101. [Google Scholar]
- Cho, K.D.; Yun, J.H. In Summer, the Origin of Tsushima Warm Current Water in Western Channel of the Korea Strait (1)—On the Water in Surface Layer. Korean Soc. Fish. Ocean Technol. 1990, 26, 184–191. [Google Scholar]
- Min, S.H.; Kim, D.H.; Yoon, H.J. Temporal and Spatial Analysis of SST in the Northeast Asian Seas Using NOAA/AVHRR data. Korea Inst. Inf. Commun. Eng. 2010, 14, 2818–2826. [Google Scholar] [CrossRef]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef] [PubMed]
- Kaberi, H.; Tsangaris, C.; Zeri, C.; Mousdis, G.; Papadopoulos, A.; Streftaris, N. Microplastics along the shoreline of a Greek island (Kea isl., Aegean Sea): Types and densities in relation to beach orientation, characteristics and proximity to sources. In Proceedings of the 4th International Conference on Environmental Management, Engineering, Planning and Economics (CEMEPE) and SECOTOX Conference, Mykonos Island, Greece, 24–28 June 2013; pp. 197–202. Available online: https://www.researchgate.net/publication/258437407 (accessed on 29 April 2024).
- Pedrotti, M.L.; Petit, S.; Elineau, A.; Bruzaud, S.; Crebassa, J.C.; Dumontet, B.; Martí, E.; Gorsky, G.; Cózar, A. Changes in the floating plastic pollution of the Mediterranean Sea in relation to the distance to land. PLoS ONE 2016, 11, e0161581. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, X.; Zhang, Y.; Zhu, B.; Wu, H. Adsorption behavior of aged polystyrene microplastics (PSMPs) for manganese in water: Critical role of hydrated functional zone surrounding the microplastic surface. J. Environ. Chem. Eng. 2022, 10, 109040. [Google Scholar] [CrossRef]
- Statistics Korea, General Survey of Agriculture and Fisheries. Fishery Value and Farm Area by Food Species. 2020. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1FS15121 (accessed on 29 April 2024).
- National Institute of Environmental Research. National Pollution Survey Report. (11-1480523-000429-10); NIER: Paris, France, 2021. [Google Scholar]
- Lee, J.M.; Jang, Y.C.; Hong, S.Y.; Lee, J.S.; Kim, K.S.; Choi, H.J.; Hong, S.W. A Study on the Annual Inflow and Its Control of Styrofoam Buoy Debris in Oyster Aquaculture Farm in Gyeongnam. Korea Ocean. Policy Res. 2016, 31, 55–79. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Kang, J.H.; Shim, W.J.; Hong, S.H. Distribution of Microplastics in the Yellow and South Sea; The Korean Society of Oceanography: Jeju, Republic of Korea, 2018; p. 1. Available online: https://sciwatch.kiost.ac.kr/handle/2020.kiost/22952 (accessed on 29 April 2024).
- Korea Meteorological Administration, Weather Data Open Portal. National Climate Data Quality Analysis Annual Report. Available online: https://data.kma.go.kr/stcs/grnd/grndRnList.do?pgmNo=69 (accessed on 29 April 2024).
- Eo, S.E.; Hong, S.H.; Song, Y.K.; Han, G.M.; Seo, S.; Shim, W.J. Prevalence of small high-density microplastics in the continental shelf and deep sea waters of East Asia. Water Res. 2021, 200, 117238. [Google Scholar] [CrossRef]
- Iwasaki, S.; Isobe, A.; Kako, S.I.; Uchida, K.; Tokai, T. Fate of microplastics and mesoplastics carried by surface currents and wind waves: A numerical model approach in the Sea of Japan. Mar. Pollut. Bull. 2017, 121, 85–96. [Google Scholar] [CrossRef]
- Chen, Q.; Reisser, J.; Cunsolo, S.; Kwadijk, C.; Kotterman, M.; Proietti, M.; Slat, B.; Ferrari, F.F.; Schwarz, A.; Levivier, A.; et al. Pollutants in plastics within the North Pacific subtropical gyre. Environ. Sci. Technol. 2018, 52, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W.; Havea, P.H.; Balogun, A.L.; Boenecke, J.; Maharaj, A.A.; Ha’apio, M.; Hemstock, S.L. Plastic debris on Pacific Islands: Ecological and health implications. Sci. Total Environ. 2019, 670, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, A.V.; Hong, S.; Burgess, H.K.; Lim, S.; Dettloff, K. Towards a North Pacific long-term monitoring program for ocean plastic pollution: A systematic review and recommendations for shorelines. Environ. Pollut. 2022, 310, 119862. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Not, C. Variations in the spatial distribution of expanded polystyrene marine debris: Are Asian’s coastlines more affected? Environ. Adv. 2023, 11, 100342. [Google Scholar] [CrossRef]
- Ferrándiz-Mas, V.; García-Alcocel, E. Durability of expanded polystyrene mortars. Constr. Build. Mater. 2013, 46, 175–182. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Han, G.M.; Shim, W.J. Rapid production of micro-and nanoplastics by fragmentation of expanded polystyrene exposed to sunlight. Environ. Sci. Technol. 2020, 54, 11191–11200. [Google Scholar] [CrossRef] [PubMed]
- Davis, W., III; Murphy, A.G. Plastic in surface waters of the Inside Passage and beaches of the Salish Sea in Washington State. Mar. Pollut. Bull. 2015, 97, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Björkroth, F.; Karlsson, T.; Hassellöv, M. Nanofragmentation of expanded polystyrene under simulated environmental weathering (thermooxidative degradation and hydrodynamic turbulence). Front. Mar. Sci. 2021, 7, 578178. [Google Scholar] [CrossRef]
- Eo, S.E.; Hong, S.H.; Song, Y.K.; Lee, J.; Lee, J.; Shim, W.J. Abundance, composition, and distribution of microplastics larger than 20 μm in sand beaches of South Korea. Environ. Pollut. 2018, 238, 894–902. [Google Scholar] [CrossRef]
- Yu, X.; Huang, W.; Wang, Y.; Wang, Y.; Cao, L.; Yang, Z.; Dou, S. Microplastic pollution in the environment and organisms of Xiangshan Bay, East China Sea: An area of intensive mariculture. Water Res. 2022, 212, 118117. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Ma, L.; Chen, S.; Zeng, J.; Dong, H.; Yang, H.; Bai, H.; Liu, R.; Huang, W. A comprehensive evaluation of microplastic pollution in the Xiangshan Bay of China with special reference to seasonal variation. Sci. Total Environ. 2023, 873, 162350. [Google Scholar] [CrossRef] [PubMed]
- De Mora, S.J.; Stewart, C.; Phillips, D. Sources and rate of degradation of tri (n-butyl) tin in marine sediments near Auckland, New Zealand. Mar. Pollut. Bull. 1995, 30, 50–57. [Google Scholar] [CrossRef]
- Kim, H.J. Distribution Characteristics of Organotin Compounds in Sediments inside Harbors of Jeju Island. Doctoral Dissertation, Jeju National University, Jeju, Republic of Korea, 2002. Available online: https://oak.jejunu.ac.kr/handle/2020.oak/18006 (accessed on 29 April 2024).
- Mo, S.W. The Behavioral Analysis of the Trading Volumes of Gwangyang Port: Comparison with Incheon and Pyeongtaek-Dangjin Port. Korea Port Econ. Assoc. 2012, 28, 111–125. [Google Scholar]
- Turner, A. Marine pollution from antifouling paint particles. Mar. Pollut. Bull. 2010, 60, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Watermann, B.T.; Daehne, B.; Sievers, S.; Dannenberg, R.; Overbeke, J.C.; Klijnstra, J.W.; Heemken, O. Bioassays and selected chemical analysis of biocide-free antifouling coatings. Chemosphere 2005, 60, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Miao, J.; Li, Y.; Li, Y.; Wang, X.; Pan, L.; Li, Y.; Wu, J.; Lin, Y. Toxic effect of p-chloroaniline and butyl acrylateon Nannochloropsis Oculata based on water samples from two sea areas. Environ. Toxicol. Pharmacol. 2021, 83, 103582. [Google Scholar] [CrossRef] [PubMed]
- Alzieu, C. Biological Effects of Tributyltin on Marine Organisms in Tributyltin: Case Study of an Environmental Contaminant; De Mora, S., Ed.; Cambridge Environmental Chemistry Series; Cambridge University Press: Cambridge, UK, 1996; pp. 167–211. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Ohji, M.; Takeuchi, I.; Takahashi, S.; Tanabe, S.; Miyazaki, N. Differences in the acute toxicities of tributyltin between the Caprellidea and the Gammaridea (Crustacea: Amphipoda). Mar. Pollut. Bull. 2002, 44, 16–24. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Kajiwara, T.; Padermshoke, A.; Van Nguyen, T.; Feng, S.; Mokudai, H.; Masaki, T.; Takigawa, M.; Van Nguyen, T.; Masunaga, H.; et al. Environmental Degradation of nylon, poly (ethylene terephthalate)(PET), and poly (vinylidene fluoride)(PVDF) fishing line fibers. ACS Appl. Polym. Mater. 2023, 5, 4427–4436. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Sambrook Smith, G.H. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef]
- Phuong, N.N.; Poirier, L.; Lagarde, F.; Kamari, A.; Zalouk-Vergnoux, A. Microplastic abundance and characteristics in French Atlantic coastal sediments using a new extraction method. Environ. Pollut. 2018, 243, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are we underestimating microplastic contamination in aquatic environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Onink, V.; Kaandorp, M.L.; van Sebille, E.; Laufkötter, C. Influence of particle size and fragmentation on large-scale microplastic transport in the Mediterranean Sea. Environ. Sci. Technol. 2022, 56, 15528–15540. [Google Scholar] [CrossRef]
- Shamskhany, A.; Li, Z.; Patel, P.; Karimpour, S. Evidence of microplastic size impact on mobility and transport in the marine environment: A review and synthesis of recent research. Front. Mar. Sci. 2021, 8, 760649. [Google Scholar] [CrossRef]
- Hu, L.; Fu, J.; Wang, S.; Xiang, Y.; Pan, X. Microplastics generated under simulated fire scenarios: Characteristics, antimony leaching, and toxicity. Environ. Pollut. 2021, 269, 115905. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Kojima, F.; Miwa, Y.; Ito, N.; Nihei, Y.; Fukunaga, Y.; Yashima, Y.; Itsubo, N. Rapid sampling of suspended and floating microplastics in challenging riverine and coastal water environments in Japan. Water 2020, 12, 1903. [Google Scholar] [CrossRef]
- Tokai, T.; Uchida, K.; Kuroda, M.; Isobe, A. Mesh selectivity of neuston nets for microplastics. Mar. Pollut. Bull. 2021, 165, 112111. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Estelmann, A.; Kowalski, N.; Glockzin, M.; Waniek, J.J. Sinking velocity of sub-millimeter microplastic. Mar. Pollut. Bull. 2019, 139, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in 29 Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.P.; Neumann, C.A.; Berger, M.L.; Shaw, S.D. Grab vs. neuston tow net: A microplastic sampling performance comparison and possible advances in the field. Anal. Methods 2017, 9, 1446–1453. [Google Scholar] [CrossRef]
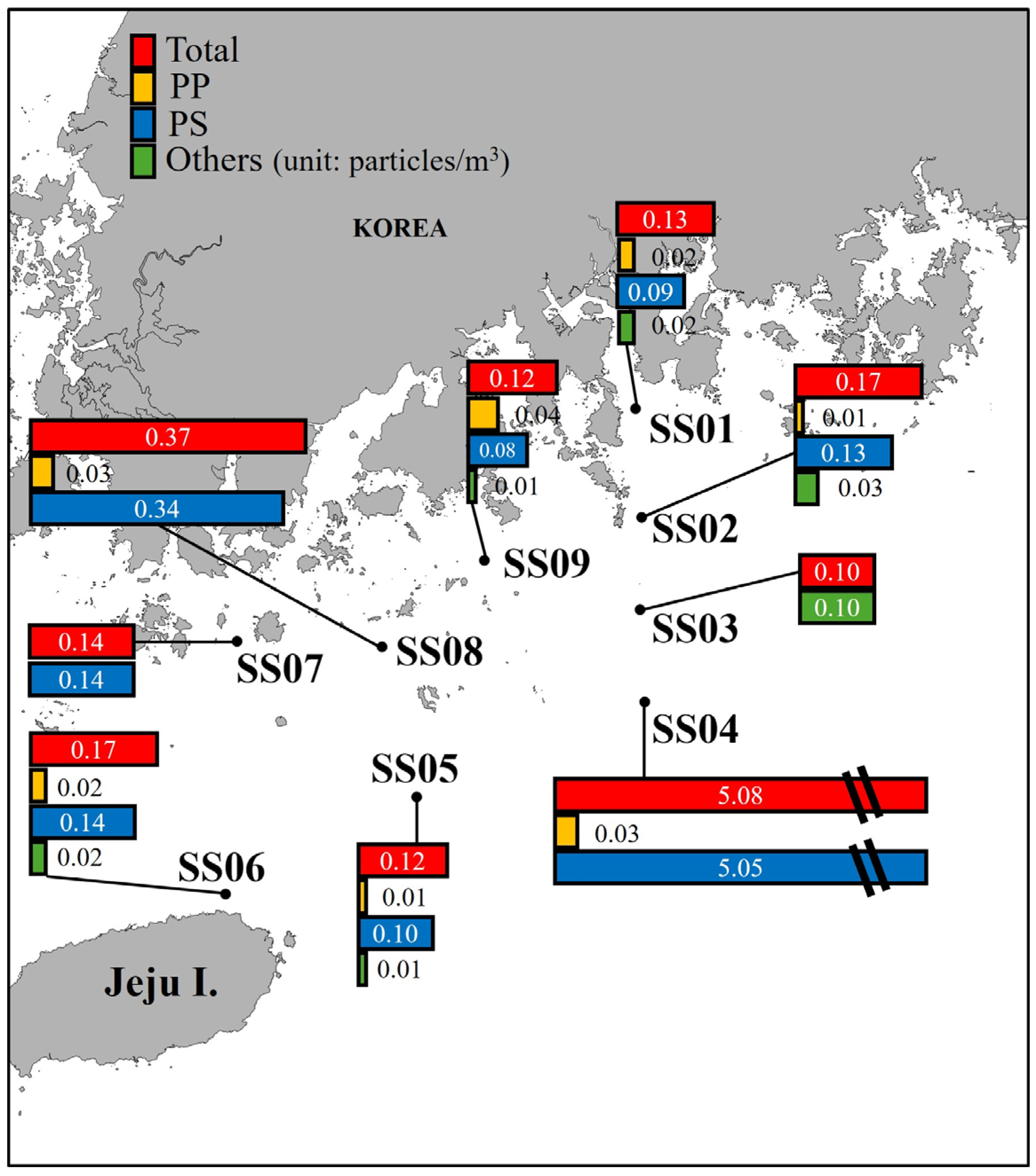






| Sampling Point | MPs Abundance (Particles/m3) * | MPs Size (%) | MPs Shape (%) ** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PP | PE | PS | Nylon | PVC | Butyl Acrylate | Total | 0.1–0.5 mm | 0.5–1 mm | 1–2 mm | 2–3 mm | 3–4 mm | 4–5 mm | Fragment | Sheet | |
| SS01 | 0.02 | ND | 0.09 | 0.01 | 0.01 | ND | 0.13 | 94.44 | 5.56 | ND | ND | ND | ND | 83.33 | 16.67 |
| SS02 | 0.01 | ND | 0.13 | ND | ND | 0.03 | 0.17 | 56.52 | ND | 8.70 | 13.04 | 21.74 | ND | 78.26 | 21.74 |
| SS03 | ND | 0.02 | ND | ND | ND | 0.08 | 0.10 | 15.38 | ND | 7.69 | 23.08 | 15.38 | 38.46 | 100.00 | ND |
| SS04 | 0.03 | ND | 5.05 | ND | ND | ND | 5.08 | 0.51 | ND | 5.74 | 26.64 | 36.37 | 30.74 | 99.80 | 0.20 |
| SS05 | 0.01 | 0.01 | 0.10 | ND | ND | ND | 0.12 | 45.83 | ND | 4.17 | 33.33 | 12.50 | 4.17 | 87.50 | 12.50 |
| SS06 | 0.02 | 0.02 | 0.14 | ND | ND | ND | 0.17 | 96.97 | ND | 3.03 | ND | ND | ND | 87.88 | 12.12 |
| SS07 | ND | ND | 0.14 | ND | ND | ND | 0.14 | 100.00 | ND | ND | ND | ND | ND | 33.33 | 66.67 |
| SS08 | 0.03 | ND | 0.34 | ND | ND | ND | 0.37 | 1.89 | ND | 3.77 | 20.75 | 33.96 | 39.62 | 100.00 | ND |
| SS09 | 0.04 | ND | 0.08 | 0.01 | ND | ND | 0.12 | 46.15 | ND | ND | 15.38 | 15.38 | 23.08 | 100.00 | ND |
| Min | ND | ND | ND | ND | ND | ND | 0.10 | 0.51 | ND | ND | ND | ND | ND | 33.33 | ND |
| Max | 0.04 | 0.02 | 5.05 | 0.01 | 0.01 | 0.08 | 5.08 | 100.00 | 5.56 | 8.70 | 33.33 | 36.37 | 39.62 | 100.00 | 66.67 |
| Mean | 0.02 | 0.02 | 0.76 | 0.01 | 0.01 | 0.06 | 0.71 | 50.86 | 0.62 | 3.69 | 14.69 | 15.04 | 15.12 | 85.57 | 14.43 |
| SD | 0.01 | 0.01 | 1.64 | 0.01 | 0.00 | 0.03 | 1.64 | 39.86 | 1.85 | 3.29 | 12.49 | 13.89 | 17.63 | 21.28 | 21.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; Jeong, H.H.; Kim, J.H.; Min, B.K.; Cho, C.R.; Soh, H.Y.; Ishibashi, Y.; Cho, H.S. Microplastic Distribution Characteristics Considering the Marine Environment Based on Surface Seawater Quality Parameters in Southern Sea of Korea, 2019. Sustainability 2024, 16, 6272. https://doi.org/10.3390/su16156272
Kim KY, Jeong HH, Kim JH, Min BK, Cho CR, Soh HY, Ishibashi Y, Cho HS. Microplastic Distribution Characteristics Considering the Marine Environment Based on Surface Seawater Quality Parameters in Southern Sea of Korea, 2019. Sustainability. 2024; 16(15):6272. https://doi.org/10.3390/su16156272
Chicago/Turabian StyleKim, Ki Yoon, Hui Ho Jeong, Ji Hoo Kim, Byeong Kyu Min, Chon Rae Cho, Ho Young Soh, Yasuhiro Ishibashi, and Hyeon Seo Cho. 2024. "Microplastic Distribution Characteristics Considering the Marine Environment Based on Surface Seawater Quality Parameters in Southern Sea of Korea, 2019" Sustainability 16, no. 15: 6272. https://doi.org/10.3390/su16156272






