Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters
Abstract
:1. Introduction
2. Vinasse and Its Characterization
3. Main Fundamentals of Electrocoagulation
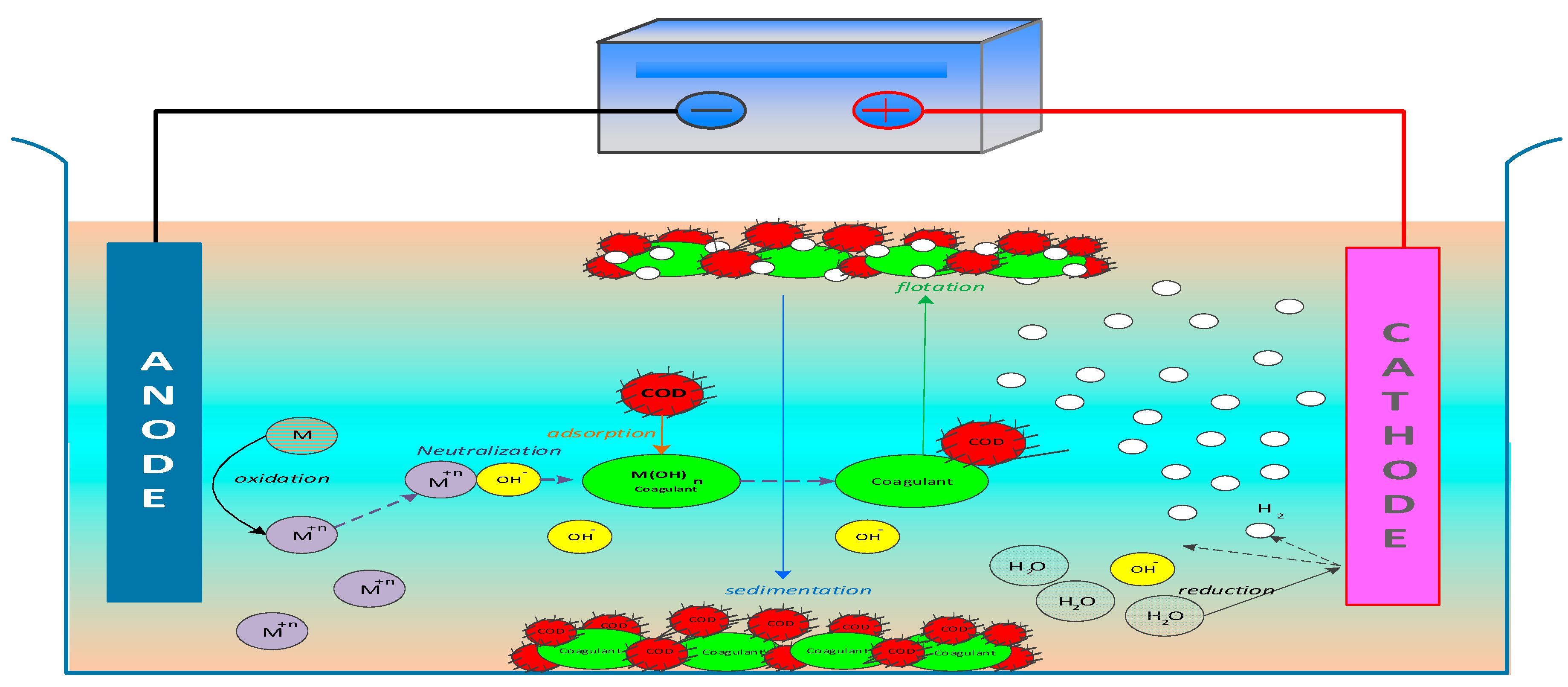
3.1. Mechanisms of Electrocoagulation
3.2. Types of Electrodes
3.3. Electrocoagulators of Reactors
3.4. Data Analysis
- Faraday’s Law
- I: current intensity (A).
- t: electrolysis time (s).
- V: volume of treated wastewater (m3).
- F: Faraday constant (96,487 C/mol).
- M: molar mass of electrode consumed (g/mol).
- z: electron transfer number.
- Energy consumption
- SEC: the specific energy consumption (kWh/kg COD removed).
- U: applied voltage (V).
- I: current intensity (A).
- t: electrolysis time (h).
- CODx: chemical oxygen demand before treatment (g/L) and CODy: chemical oxygen demand after treatment (g/L).
- Operating Cost
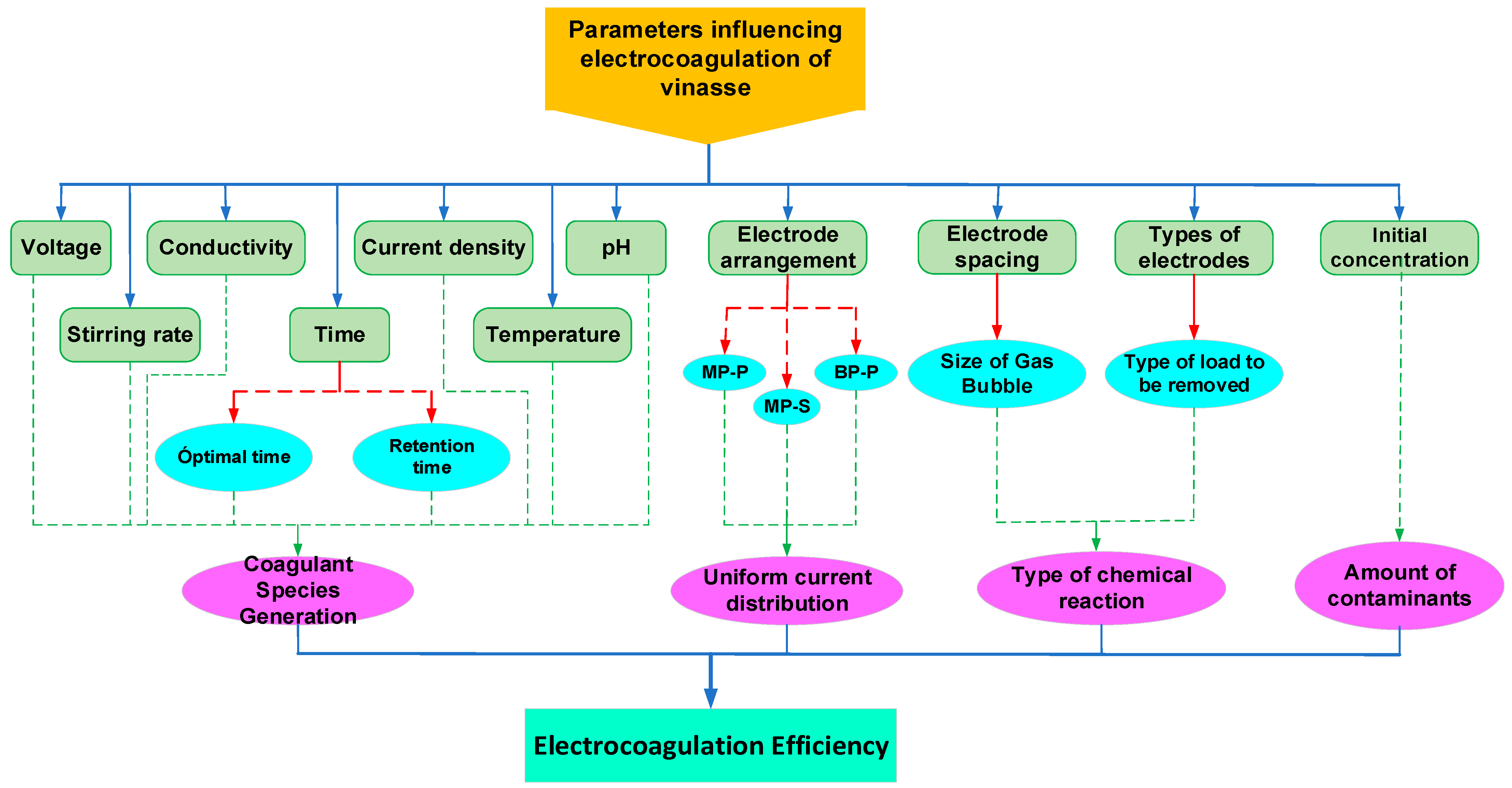
4. Main Operating Parameters
4.1. Influence of pH
4.2. Influence of Current Density
4.3. Influence of Electrolysis Time
4.4. Influence of Electrode Spacing
4.5. Influence of Electrode Type
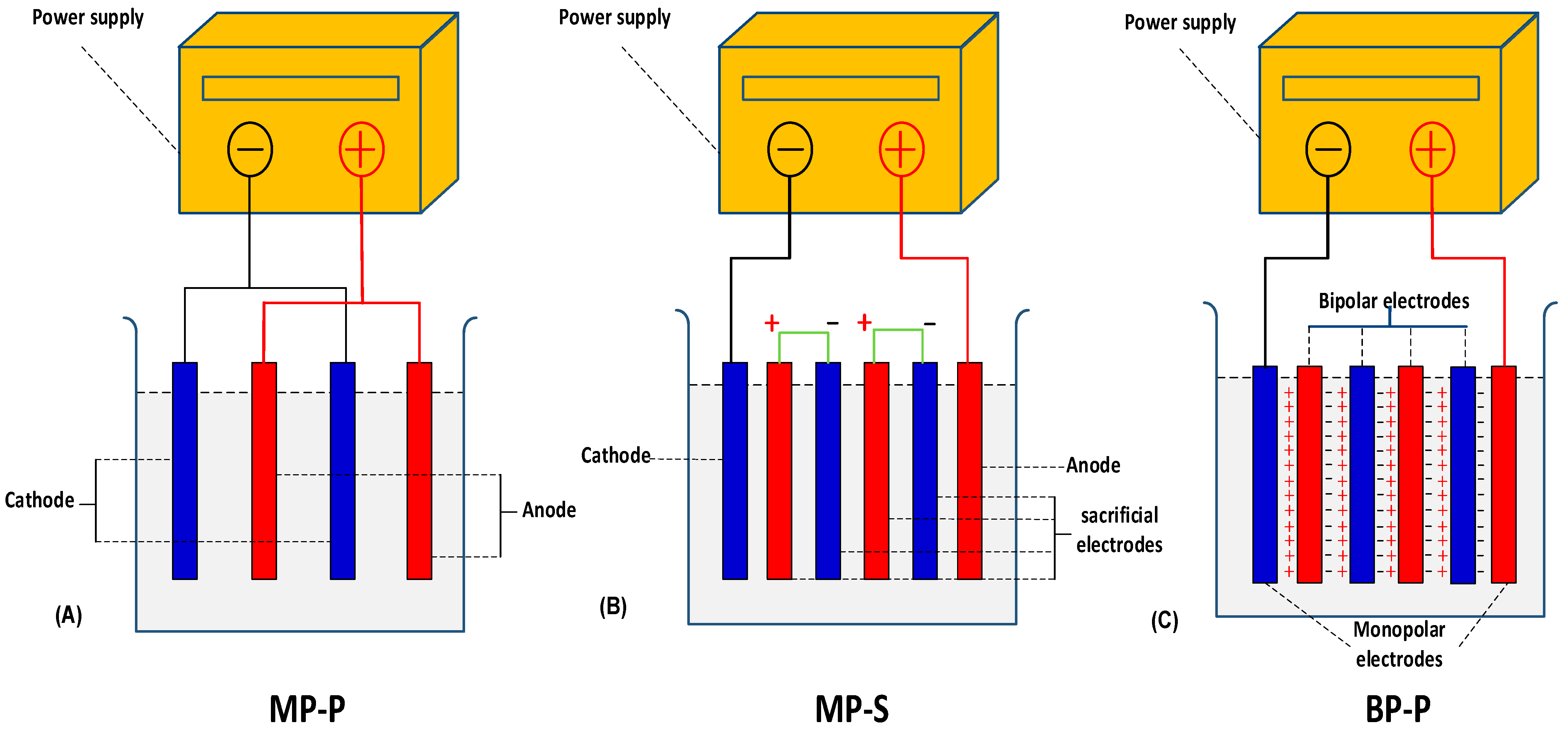
4.6. Influence of Mode of Electrode Connection
- Monopolar electrodes with parallel connection (MP-P)
- Monopolar electrodes with series connection (MP-S)
- Bipolar electrode with connection (BP)
4.7. Influence of Temperature
4.8. Influence of Stirring Speed
4.9. Influence of Initial Concentration
4.10. Influence of Conductivity
4.11. Influence of Voltage
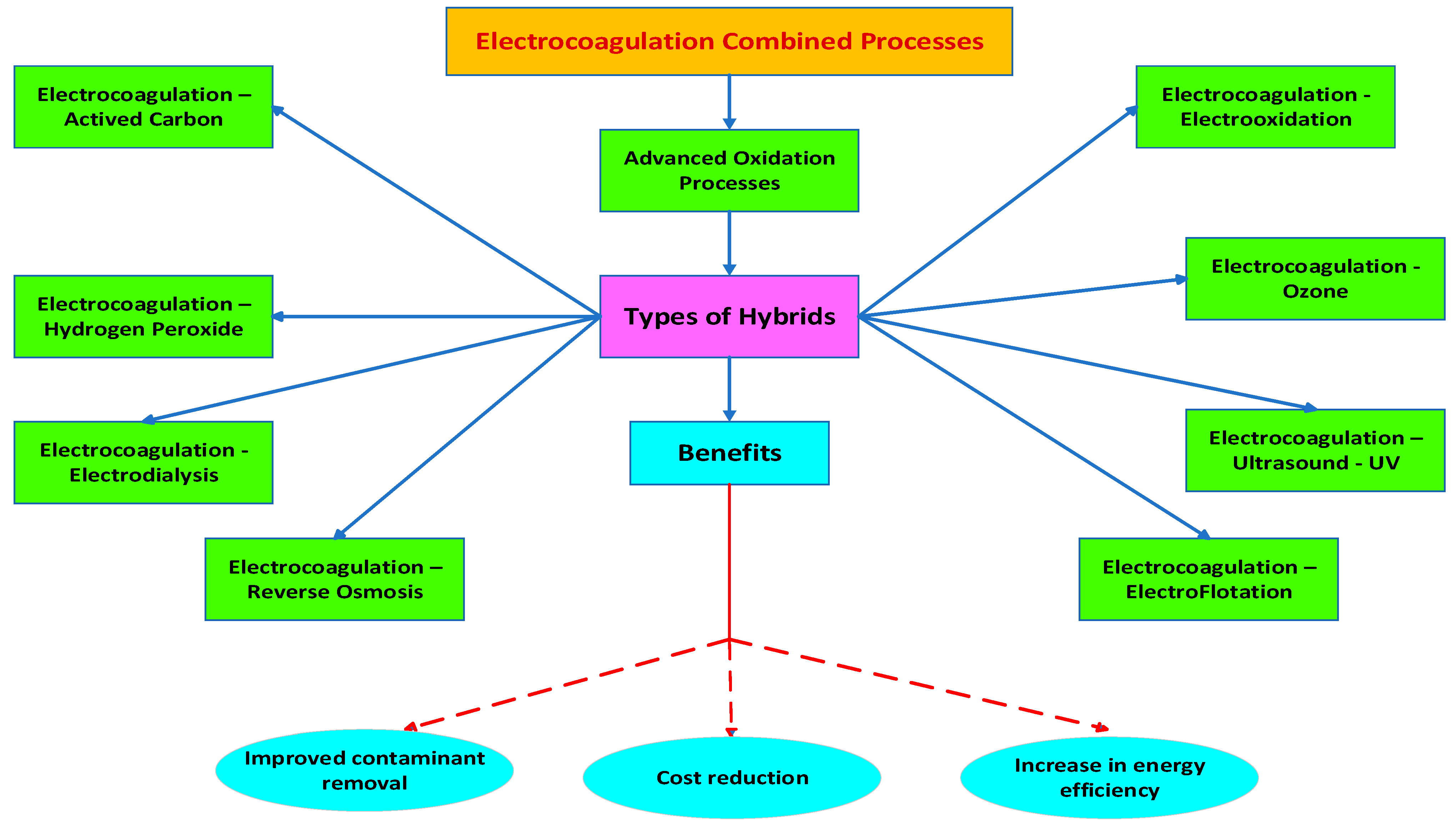
5. Hybrid Methods in Electrocoagulation
- Electrocoagulation–Adsorption
- b.
- Electrocoagulation–Electroflotation
- c.
- Electrocoagulation–Ozone
- d.
- Electrocoagulation–Hydrogen Peroxide
- e.
- Electrocoagulation–Electrooxidation
- f.
- Electrocoagulation–Ultrasound–UV
- g.
- Electrocoagulation–Electrodialysis
- h.
- Electrocoagulation–Reverse Osmosis (EC-RO)
6. Conclusions
7. Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclatures
| EC | electrocoagulation |
| COD | chemical oxygen demand (g/L) |
| chemical oxygen demand before treatment (g/L) | |
| chemical oxygen demand after treatment (g/L) | |
| BOD | biochemical oxygen demand(g/L) |
| TS | total solids (g/L) |
| VS | volatile solid (g/L) |
| TSS | total suspended solids (g/L) |
| t | electrolysis time (s) |
| U | applied voltage (V) |
| I | current intensity (A) |
| F | Faraday constant (96,487 C/mol) |
| Z | electron transfer number |
References
- Khandegar, V.; Saroh, A.K. Treatment of Distillery Spentwash by Electrocoagulation. J. Clean Energy Technol. 2014, 2, 244–247. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef] [PubMed]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic model of electrocoagulation process for treating vinasse waste: Effect of initial pH. J. Environ. Chem. Eng. 2020, 8, 103756. [Google Scholar] [CrossRef]
- Dhote, L.; Kumar, S.; Singh, L.; Kumar, R. A systematic review on options for sustainable treatment and resource recovery of distillery sludge. Chemosphere 2021, 263, 128225. [Google Scholar] [CrossRef] [PubMed]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2006. [Google Scholar]
- Syaichurrozi, I.; Budiyono; Sumardiono, S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hakika, D.C.; Sarto, S.; Mindaryani, A.; Hidayat, M. Decreasing COD in sugarcane vinasse using the fenton reaction: The effect of processing parameters. Catalysts 2019, 9, 881. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.K.F.; De Castro, H.F. Vinasse treatment within the sugarcane-ethanol industry using ozone combined with anaerobic and aerobic microbial processes. Environments 2019, 6, 5. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Ribeiro, L.A.; Amaral, M.C.S. Efficiency of nutrients recovery from sugarcane vinasse treatment by different electrodialysis configurations and in sequential-batch operation. Sep. Purif. Technol. 2023, 311, 123295. [Google Scholar] [CrossRef]
- Duarte, F.P.; Silva, A.F.R.; Lange, L.C.; Amaral, M.C.S.; Neta, L.S.D.F.; Moravia, W.G. Vinasse processing by electrodialysis combined with nanofiltration: Emphasis on process optimization and environmental sustainability. Water Sci. Technol. 2023, 88, 2677–2693. [Google Scholar] [CrossRef]
- Saavedra, M.D.M.; Concha, V.O.C.; Bastos, R.G. Electrocoagulation treatment of sugarcane vinasse: Operating parameters and cost analysis by response surface methodology. J. Clean. Prod. 2024, 448, 141597. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Znad, H.; Hasan, M.N. Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Susree, M.; Asaithambi, P.; Saravanathamizhan, R.; Matheswaran, M. Studies on various mode of electrochemical reactor operation for the treatment of distillery effluent. J. Environ. Chem. Eng. 2013, 1, 552–558. [Google Scholar] [CrossRef]
- Dubey, S.; Rekhate, C.; Sharma, A.; Joshi, A.; Prajapati, A.K. Optimizing distillery effluent treatment through sono-electrocoagulation: A response surface methodology approach. Total Environ. Adv. 2024, 9, 200093. [Google Scholar] [CrossRef]
- Karmankar, S.B.; Sharma, A.; Ahirwar, R.C.; Mehra, S.; Pal, D.; Prajapati, A.K. Cost cutting approach of distillery effluent treatment using solar photovoltaic cell driven electrocoagulation: Comparison with conventional electrocoagulation. J. Water Process Eng. 2023, 54, 103982. [Google Scholar] [CrossRef]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Distillery spent wash: Treatment technologies and potential applications. J. Hazard. Mater. 2009, 163, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.S.D.; Neto, A.R.; Duda, R.M.; de Oliveira, R.A.; Boaventura, R.A.R.; Madeira, L.M. Combination of chemical coagulation, photo-Fenton oxidation and biodegradation for the treatment of vinasse from sugar cane ethanol distillery. J. Clean. Prod. 2017, 142, 3634–3644. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-surface oxygenation with hydrogen peroxide. J. Environ. Manag. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Ahmed, P. Bioremediation of Vinasse from Alcohol Distilleries by Indigenous Microorganisms Isolated from Contaminated Environments. Institute of Agroindustrial Technology of Northwestern Argentina (ITANOA). 2016. Available online: https://ri.conicet.gov.ar/handle/11336/85316 (accessed on 15 January 2024).
- Rashidi, M.; Alavi, N.; Amereh, F.; Rafiee, M.; Amanidaz, N.; Partovi, K.; Mosanefi, S.; Bakhshoodeh, R. Biohydrogen production from co-digestion of sugarcane vinasse and bagasse using anaerobic dark fermentation. Bioresour. Technol. Rep. 2024, 25, 101793. [Google Scholar] [CrossRef]
- Barros, L.B.M.; Brasil, Y.L.; Silva, A.F.R.; Andrade, L.H.; Amaral, M.C.S. Potassium recovery from vinasse by integrated electrodialysis—Precipitation process: Effect of the electrolyte solutions. J. Environ. Chem. Eng. 2020, 8, 104238. [Google Scholar] [CrossRef]
- Chen, X.; Mao, H.; Cui, Y.; Jiang, Y.; Liu, J.; Zha, X.; Huang, L.; Shen, P. Multi-angle evaluation of the anaerobic digestion of Molasses vinasse using two different feeding patterns. Water Cycle 2023, 4, 170–178. [Google Scholar] [CrossRef]
- Torres, M.A.; Valdez, A.L.; Angelicola, M.V.; Raimondo, E.E.; Pajot, H.F.; Nieto-Peñalver, C.G. Vinasse as a substrate for inoculant culture and soil fertigation: Advancing the circular and green economy. Sci. Total Environ. 2023, 887, 164014. [Google Scholar] [CrossRef]
- Un, U.T.; Koparal, A.S.; Ogutveren, U.B. Electrocoagulation of vegetable oil refinery wastewater using aluminum electrodes. J. Environ. Manag. 2009, 90, 428–433. [Google Scholar] [CrossRef]
- Hu, Q.; He, L.; Lan, R.; Feng, C.; Pei, X. Recent advances in phosphate removal from municipal wastewater by electrocoagulation process: A review. Sep. Purif. Technol. 2023, 308, 122944. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Khalid, Z.B.; Gilhotra, V.; Emamjomeh, M.M. A critical review of state-of-the-art electrocoagulation technique applied to COD-rich industrial wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Jasim, M.A.; AlJaberi, F.Y.; Salman, A.D.; Alardhi, S.M.; Le, P.C.; Kulcsár, G.; Jakab, M. Studying the effect of reactor design on the electrocoagulation treatment performance of oily wastewater. Heliyon 2023, 9, e17794. [Google Scholar] [CrossRef]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Ming, C.K.; Arif, P.; Baharuddin, N.; Borhan, W.W.S.W.; Jalal, N.S.A.; Samsul, S.N.A.; Jitai, A.A.; et al. Treatment of tropical peat water in Sarawak peatlands nature reserve by utilising a batch electrocoagulation system. Sustain. Chem. Environ. 2023, 4, 100043. [Google Scholar] [CrossRef]
- Safwat, S.M. Treatment of real printing wastewater using electrocoagulation process with titanium and zinc electrodes. J. Water Process Eng. 2020, 34, 101137. [Google Scholar] [CrossRef]
- Jafari, E.; Malayeri, M.R.; Brückner, H.; Krebs, P. Impact of operating parameters of electrocoagulation-flotation on the removal of turbidity from synthetic wastewater using aluminium electrodes. Miner. Eng. 2023, 193, 108007. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Experiment and kinetic analysis of the effect of agitation speed on electrocoagulation process for the treatment of vinasse. J. Water Process Eng. 2022, 50, 103144. [Google Scholar] [CrossRef]
- Afsharnia, M.; Biglari, H.; Rasouli, S.S.; Karimi, A.; Kianmehr, M. Sono-electrocoagulation of fresh leachate from municipal solid waste; Simultaneous applying of iron and copper electrodes. Int. J. Electrochem. Sci. 2018, 13, 472–484. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Naghdali, Z.; Al-Qodah, Z.; Alizadeh, S.M.; Niaragh, E.K.; Malekmohammadi, S.; Nidheesh, P.V.; Roberts, E.P.; Sillanpää, M.; Emamjomeh, M.M. A systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Technol. Assess. 2021, 47, 101353. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.; Bilyeu, B.; Roa, G.; Bernal-Martinez, L. Physicochemical aspects of electrocoagulation. Sep. Purif. Rev. 2011, 40, 1–24. [Google Scholar] [CrossRef]
- Nemade, P.; Wagh, M.P.; Nemade, P.D. Treatment of Distillery Spent Wash by Using Chemical Coagulation (CC) and Electro-coagulation [EC]. Am. J. Environ. Prot. 2015, 3, 159–163. [Google Scholar] [CrossRef]
- Akbal, F.; Camci, S. Treatment of metal plating wastewater by electrocoagulation. Environ. Prog. Sustain. Energy 2012, 31, 340–350. [Google Scholar] [CrossRef]
- Patel, R.K.; Shankar, R.; Khare, P.; Mondal, P. Treatment of sugar processing industry wastewater using copper electrode by electrocoagulation: Performance and economic study. J. Indian Chem. Soc. 2022, 99, 100563. [Google Scholar] [CrossRef]
- Hussin, F.; Abnisa, F.; Issabayeva, G.; Aroua, M.K. Removal of lead by solar-photovoltaic electrocoagulation using novel perforated zinc electrode. J. Clean. Prod. 2017, 147, 206–216. [Google Scholar] [CrossRef]
- Trompette, J.L. On the specific limitations of titanium electrodes in the electrocoagulation process. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129196. [Google Scholar] [CrossRef]
- Jafari, E.; Malayeri, M.R.; Brückner, H.; Weimer, T.; Krebs, P. Innovative spiral electrode configuration for enhancement of electrocoagulation-flotation. J. Environ. Manag. 2023, 347, 119085. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Y.; Sun, W.; Xie, J.; Wang, M.; Guo, Z. A novel electrocoagulation process with centrifugal electrodes for wastewater treatment: Electrochemical behavior of anode and kinetics of heavy metal removal. Chemosphere 2023, 310, 136862. [Google Scholar] [CrossRef]
- Alkhatib, A.M.; Hawari, A.H.; Hafiz, M.A.; Benamor, A. A novel cylindrical electrode configuration for inducing dielectrophoretic forces during electrocoagulation. J. Water Process Eng. 2020, 35, 101195. [Google Scholar] [CrossRef]
- AlJaberi, F.Y. Desalination of groundwater by electrocoagulation using a novel design of electrodes. Chem. Eng. Process. Process Intensif. 2022, 174, 108864. [Google Scholar] [CrossRef]
- Alcocer-Meneses, P.; Cabrera-Salazar, A.B.; Medina-Collana, J.T.; Rosales-Huamani, J.A.; Franco-Gonzales, E.J.; Reyna-Mendoza, G.E. Effects of the Operational Parameters in a Coupled Process of Electrocoagulation and Advanced Oxidation in the Removal of Turbidity in Wastewater from a Curtember. Appl. Sci. 2022, 12, 8158. [Google Scholar] [CrossRef]
- Milla, A.; Medina, J. Electrocoagulator Equipment with Mobile Electrodes. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=PE277652041&docAn=2018002022 (accessed on 15 January 2024).
- Ardhianto, R.; Anggrainy, A.D.; Samudro, G.; Triyawan, A.; Bagastyo, A.Y. A study of continuous-flow electrocoagulation process to minimize chemicals dosing in the full-scale treatment of plastic plating industry wastewater. J. Water Process Eng. 2024, 60, 105217. [Google Scholar] [CrossRef]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Taib, S.N.L.; Parabi, A.; Borhan, W.W.S.W.; Ming, C.K.; Parabi, A.S.L.; Jalal, N.S.A.; Baharuddin, N.; et al. Batch electrocoagulation system for the treatment of Borneo urban river in relation to the industrial zone. J. Environ. Chem. Eng. 2024, 12, 112514. [Google Scholar] [CrossRef]
- Jose, J.T.; Priya, K.L.; Chellappan, S.; Sreelekshmi, S.; Remesh, A.; Venkidesh, V.; Krishna, A.J.; Pugazhendhi, A.; Selvam, S.; Baiju, V.; et al. A hybrid electrocoagulation-biocomposite adsorption system for the decolourization of dye wastewater. Environ. Res. 2024, 252, 118759. [Google Scholar] [CrossRef]
- Genethliou, C.; Triantaphyllidou, I.E.; Chatzitheodorou, D.; Tekerlekopoulou, A.G.; Vayenas, D.V. Development of Hybrid Systems by Integrating an Adsorption Process with Natural Zeolite and/or Palygorskite into the Electrocoagulation Treatment of Sanitary Landfill Leachate. Sustainability 2023, 15, 8344. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M.; Myszograj, S.; Włodarczyk, M. Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies 2023, 16, 5591. [Google Scholar] [CrossRef]
- Rakhmania Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent Applications of the Electrocoagulation Process on Agro-Based Industrial Wastewater: A Review. Sustainability 2022, 14, 1985. [Google Scholar] [CrossRef]
- Patel, S.R.; Pathan, M.; Nayak, M.G.; Parikh, S.P.; Rajaraman, T.S.; Ambegaonkar, N.J.; Trivedi, J.B. Energy efficient electrocoagulation using brass electrode for simultaneous nickel and chromium removal from synthetic wastewater: Cost and parametric evaluation. Results Eng. 2024, 22, 102361. [Google Scholar] [CrossRef]
- Ansari, K.; Shrikhande, A.; Malik, M.A.; Alahmadi, A.A.; Alwetaishi, M.; Alzaed, A.N.; Elbeltagi, A. Optimization and Operational Analysis of Domestic Greywater Treatment by Electrocoagulation Filtration Using Response Surface Methodology. Sustainability 2022, 14, 15230. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, C.; Shin, E.-B.; Kim, S. Decolorization of Disperse and Reactive Dyes by Continuous Electrocoagulation Process. 2002. Available online: www.elsevier.com/locate/desal (accessed on 15 January 2024).
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Wark, M.; Mitchell, C.A. A Quantitative Comparison between Chemical Dosing and Electrocoagulation. Available online: www.elsevier.com/locate/colsurfa (accessed on 15 January 2024).
- Manikandan, S.; Saraswathi, R. Electrocoagulation technique for removing Organic and Inorganic pollutants (COD) from the various industrial effluents: An overview. Environ. Eng. Res. 2023, 28, 220231. [Google Scholar] [CrossRef]
- Janpoor, F.; Torabian, A.; Khatibikamal, V. Treatment of laundry waste-water by electrocoagulation. J. Chem. Technol. Biotechnol. 2011, 86, 1113–1120. [Google Scholar] [CrossRef]
- Sen, S.; Prajapati, A.K.; Bannatwala, A.; Pal, D. Electrocoagulation treatment of industrial wastewater including textile dyeing effluent—A review. Desalination Water Treat. 2019, 161, 21–34. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, H.A.; Kasiri, M.B. Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Tirado, L.; Gökkuş, Ö.; Brillas, E.; Sirés, I. Treatment of cheese whey wastewater by combined electrochemical processes. J. Appl. Electrochem. 2018, 48, 1307–1319. [Google Scholar] [CrossRef]
- Bayramoglu, M.; Eyvaz, M.; Kobya, M. Treatment of the textile wastewater by electrocoagulation. Economical evaluation. Chem. Eng. J. 2007, 128, 155–161. [Google Scholar] [CrossRef]
- Akter, S.; Suhan, M.B.K.; Islam, M.S. Recent advances and perspective of electrocoagulation in the treatment of wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100643. [Google Scholar] [CrossRef]
- Islam, S.M.D.-U. Electrocoagulation (EC) technology for wastewater treatment and pollutants removal. Sustain. Water Resour. Manag. 2019, 5, 359–380. [Google Scholar] [CrossRef]
- Kazeem, T.S.; Labaran, B.A.; Mohammed, T.; Essa, M.H.; Al-Suwaiyan, M.S.; Vohra, M.S. Treatment of Aqueous Selenocyanate Anions Using Electrocoagulation. Int. J. Electrochem. Sci. 2019, 14, 10538–10564. [Google Scholar] [CrossRef]
- Kobya, M.; Ulu, F.; Gebologlu, U.; Demirbas, E.; Oncel, M.S. Treatment of potable water containing low concentration of arsenic with electrocoagulation: Different connection modes and Fe-Al electrodes. Sep. Purif. Technol. 2011, 77, 283–293. [Google Scholar] [CrossRef]
- Ghosh, D.; Medhi, C.R.; Purkait, M.K. Treatment of fluoride containing drinking water by electrocoagulation using monopolar and bipolar electrode connections. Chemosphere 2008, 73, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Körbahti, B.K.; Artut, K. Electrochemical oil/water demulsification and purification of bilge water using Pt/Ir electrodes. Desalination 2010, 258, 219–228. [Google Scholar] [CrossRef]
- Helmy, E.; Hussein, M. Study on the Removal of Water Hardness by Electrocoagulation Technique. 2017. Available online: https://www.iscientific.org/wp-content/uploads/2019/09/1-IJCBS-17-12-1.pdf (accessed on 15 January 2024).
- Modirshahla, N.; Behnajady, M.A.; Mohammadi-Aghdam, S. Investigation of the effect of different electrodes and their connections on the removal efficiency of 4-nitrophenol from aqueous solution by electrocoagulation. J. Hazard. Mater. 2008, 154, 778–786. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Bharti, M.; Das, P.P.; Purkait, M.K. A review on the treatment of water and wastewater by electrocoagulation process: Advances and emerging applications. J. Environ. Chem. Eng. 2023, 11, 111558. [Google Scholar] [CrossRef]
- Kessentini, I.; Mousser, H.; Zouari, S.; Bargui, M. Removal of Copper from Aqueous Solution Using Electrocoagulation: Importance of Stirring Effect. Surf. Eng. Appl. Electrochem. 2019, 55, 210–218. [Google Scholar] [CrossRef]
- Aljaberi, F.Y. Studies of autocatalytic electrocoagulation reactor for lead removal from simulated wastewater. J. Environ. Chem. Eng. 2018, 6, 6069–6078. [Google Scholar] [CrossRef]
- Eskibalci, M.F.; Ozkan, M.F. Comparison of conventional coagulation and electrocoagulation methods for dewatering of coal preparation plant. Min. Eng. 2018, 122, 106–112. [Google Scholar] [CrossRef]
- Benekos, A.K.; Zampeta, C.; Argyriou, R.; Economou, C.N.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Tekerlekopoulou, A.G.; Vayenas, D.V. Treatment of table olive processing wastewaters using electrocoagulation in laboratory and pilot-scale reactors. Process Saf. Environ. Prot. 2019, 131, 38–47. [Google Scholar] [CrossRef]
- Ankoliya, D.; Mudgal, A.; Sinha, M.K.; Patel, V.; Patel, J. Application of electrocoagulation process for the treatment of dairy wastewater: A mini review. Mater. Today Proc. 2023, 77, 117–124. [Google Scholar] [CrossRef]
- Chou, W.L. Removal and adsorption characteristics of polyvinyl alcohol from aqueous solutions using electrocoagulation. J. Hazard. Mater. 2010, 177, 842–850. [Google Scholar] [CrossRef] [PubMed]
- El-Ashtoukhy, E.S.Z.; Amin, N.K.; Fouad, Y.O. Treatment of real wastewater produced from Mobil car wash station using electrocoagulation technique. Environ. Monit. Assess. 2015, 187, 628. [Google Scholar] [CrossRef]
- Mouedhen, G.; Feki, M.; Wery, M.D.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater. 2008, 150, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Mechanistic models of electrocoagulation kinetics of pollutant removal in vinasse waste: Effect of voltage. J. Water Process Eng. 2020, 36, 101312. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M.; Darsono, N.; Timuda, G.E.; Khaerudini, D.S. Evolution of solid contents in vinasse waste during electrocoagulation process at various current densities: Experimental and kinetic analyses. J. Water Process Eng. 2023, 53, 103758. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrochemical treatment of distillery spent wash using aluminum and iron electrodes. Chin. J. Chem. Eng. 2012, 20, 439–443. [Google Scholar] [CrossRef]
- Dubey, S.; Joshi, A.; Parmar, N.; Rekhate, C.; Amitesh; Prajapati, A.K. Process optimization of electrocoagulation reactor for treatment of distillery effluent using aluminium electrode: Response surface methodology approach. Chem. Data Collect. 2023, 45–101023. [Google Scholar] [CrossRef]
- David, C.; Karpagaraj, A.; Thangavelu, A. Degradation of distillery effluent by twisted-type Iron electrodes: Experimental with ANN approach. Int. J. Environ. Anal. Chem. 2022, 102, 6121–6133. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Chaudhari, P.K. Electrochemical treatment of rice grain-based distillery effluent: Chemical oxygen demand and colour removal. Environ. Technol. 2014, 35, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Farhadi, K.; Ghaneian, M.T.; Ehrampoush, M.H.; Jambarsang, S.; Salmani, M.H.; Ahmadzadeh Kokya, T. Development of Circulating Electrocoagulation as a Novel Technique for the Treatment of Raw Vinasse Effluents of Ethanol Production Industries. Anal. Bioanal. Chem. Res. 2023, 10, 319–327. [Google Scholar]
- Syaichurrozi, I.; Sarto, S.; Sediawan, W.B.; Hidayat, M. Effect of current and initial ph on electrocoagulation in treating the distillery spent wash with very high pollutant content. Water 2021, 13, 11. [Google Scholar] [CrossRef]
- GilPavas, E.; Correa-Sanchez, S. Assessment of the optimized treatment of indigo-polluted industrial textile wastewater by a sequential electrocoagulation-activated carbon adsorption process. J. Water Process Eng. 2020, 36, 101306. [Google Scholar] [CrossRef]
- El houda, M.N.; Chabani, M.; Bouafia-Chergui, S.; Touil, A. Removal of chemical oxygen demand from real petroleum refinery wastewater through a hybrid approach: Electrocoagulation and adsorption. Chem. Eng. Process. Process Intensif. 2024, 196, 109680. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Milyutina, A.; Desyatov, A.; Kolesnikov, V. Electroflotation recovery of highly dispersed carbon materials from aqueous solutions of electrolyte. Sep. Purif. Technol. 2019, 209, 73–78. [Google Scholar] [CrossRef]
- Bellebia, S.; Kacha, S.; Bouberka, Z.; Bouyakoub, A.Z.; Derriche, Z. Color Removal from Acid and Reactive Dye Solutions by Electrocoagulation and Electrocoagulation/Adsorption Processes. Water Environ. Res. 2009, 81, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Chowdhury, Z.; Sen, D.; Bhattacharjee, C. Electric field assisted membrane separation for oily wastewater with a novel and cost-effective electrocoagulation and electroflotation enhanced membrane module (ECEFMM). Chem. Eng. Process. Process Intensif. 2020, 151, 107918. [Google Scholar] [CrossRef]
- Zuo, Q.; Chen, X.; Li, W.; Chen, G. Combined electrocoagulation and electroflotation for removal of fluoride from drinking water. J. Hazard. Mater. 2008, 159, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, P.; Susree, M.; Saravanathamizhan, R.; Matheswaran, M. Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalination 2012, 297, 1–7. [Google Scholar] [CrossRef]
- Song, S.; Yao, J.; He, Z.; Qiu, J.; Chen, J. Effect of operational parameters on the decolorization of C.I. Reactive Blue 19 in aqueous solution by ozone-enhanced electrocoagulation. J. Hazard. Mater. 2008, 152, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Qodah, Z.; Al-Shannag, M. On the Performance of Free Radicals Combined Electrocoagulation Treatment Processes. Sep. Purif. Rev. 2019, 48, 143–158. [Google Scholar] [CrossRef]
- Das, P.P.; Mondal, P.; Sinha, A.; Biswas, P.; Sarkar, S.; Purkait, M.K. Integrated ozonation assisted electrocoagulation process for the removal of cyanide from steel industry wastewater. Chemosphere 2021, 263, 128370. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Zhang, Z.; Zhang, J.; Li, S. The addition of hydrogen peroxide in the electrocoagulation treatment for improving toxic organic matters removal: A comparative study. Sep. Sci. Technol. 2017, 52, 1404–1411. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.Y.; Demir, A. A comparative study of electrocoagulation and electro-Fenton for food industry wastewater treatment: Multiple response optimization and cost analysis. Sep. Sci. Technol. 2018, 53, 2727–2740. [Google Scholar] [CrossRef]
- Asfaha, Y.G.; Tekile, A.K.; Zewge, F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean. Eng. Technol. 2021, 4, 100261. [Google Scholar] [CrossRef]
- Turan, N.B. The application of hybrid electrocoagulation–electrooxidation system for the treatment of dairy wastewater using different electrode connections. Sep. Sci. Technol. 2020, 56, 1788–1801. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; He, F.; Mao, Y.; Chen, Z.; Wang, Y.; Cai, Z. Membrane distillation combined with electrocoagulation and electrooxidation for the treatment of landfill leachate concentrate. Sep. Purif. Technol. 2022, 291, 120936. [Google Scholar] [CrossRef]
- Guvenc, S.Y.; Varank, G.; Can-Güven, E.; Ercan, H.; Yaman, D.; Saricam, E.; Türk, O.K. Application of the hybrid electrocoagulation–electrooxidation process for the degradation of contaminants in acidified biodiesel wastewater. J. Electroanal. Chem. 2022, 926, 116933. [Google Scholar] [CrossRef]
- Asaithambi, P.; Yesuf, M.B.; Govindarajan, R.; Hariharan, N.M.; Thangavelu, P.; Alemayehu, E. Distillery industrial wastewater (DIW) treatment by the combination of sono (US), photo (UV) and electrocoagulation (EC) process. J. Environ. Manag. 2022, 320, 115926. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep. Purif. Rev. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Deghles, A.; Kurt, U. Treatment of tannery wastewater by a hybrid electrocoagulation/electrodialysis process. Chem. Eng. Process. Process Intensif. 2016, 104, 43–50. [Google Scholar] [CrossRef]
- Gardeshi, M.E.; Arab, H.; Drogui, P. Hybrid process combining electrocoagulation and electrodialysis for chloride ions removal from runoff water loaded with road de-icing salts: Statistical optimization by response surface methodology. J. Water Process Eng. 2024, 58, 104830. [Google Scholar] [CrossRef]
- Nurjanah, I.; Chang, T.-T.; You, S.-J.; Huang, C.-Y.; Sean, W.-Y. Reverse osmosis integrated with renewable energy as sustainable technology: A review. Desalination 2024, 581, 117590. [Google Scholar] [CrossRef]
- Kukizaki, M.; Goto, M. Demulsification of water-in-oil emulsions by permeation through Shirasu-porous-glass (SPG) membranes. J. Membr. Sci. 2008, 322, 196–203. [Google Scholar] [CrossRef]
- Bhattacharya, P.K.; Jayan, R.; Bhattacharjee, C. A combined biological and membrane-based treatment of prehydrolysis liquor from pulp mill. Sep. Purif. Technol. 2005, 45, 119–130. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, H.; Srivastava, V.C.; Singh, S.; Lo, S.L. Treatment of biologically treated distillery spent wash employing electrocoagulation and reverse-osmosis treatment train. Environ. Technol. 2022, 43, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Y. EC and EF processes for the treatment of alcohol distillery wastewater. Sep. Purif. Technol. 2007, 53, 135–140. [Google Scholar] [CrossRef]
- Kannan, N.; Karthikeyan, G.; Tamilselvan, N. Comparison of treatment potential of electrocoagulation of distillery effluent with and without activated Areca catechu nut carbon. J. Hazard. Mater. 2006, 137, 1803–1809. [Google Scholar] [CrossRef]
- Aziz, A.R.A.; Asaithambi, P.; Daud, W.M.A.B.W. Combination of electrocoagulation with advanced oxidation processes for the treatment of distillery industrial effluent. Process Saf. Environ. Prot. 2016, 99, 227–235. [Google Scholar] [CrossRef]
| Characteristics | Units | Values | ||
|---|---|---|---|---|
| [9,20,22] | [22,23,24] | [22,25,26] | ||
| pH | - | 4.0–4.5 | 3.8–5 | 3.5–4 |
| TS | mg/L | 59,000–82,000 | 48,000–62,000 | 142,150 ± 4600 |
| VS | mg/L | 38,000–66,000 | 42,800–50,300 | 102,430 ± 1900 |
| TSS | mg/L | 2400–5000 | 23,000–31,500 | - |
| COD | mg/L | 100,000–150,000 | 60,000–72,000 | 155,833 ± 8065 |
| BOD | mg/L | 35,000–50,000 | 25,000–50,500 | 18,160 ± 1378 |
| Conductivity | mS/cm | 8.3 ± 0.7 | 12.1 ± 2.3 | 9.49 ± 0.02 |
| Nitrogen—NH3 | mg/L | 1660–4200 | <1.00 | 12,209.52–2018.27 |
| Total phosphorus | mg/L | 225–308 | - | 260.80–16.76 |
| Potassium | mg/L | 9600–15,475 | - | - |
| Iron | mg/L | 1550–1800 | 6.7–9.0 | 190–204 |
| Sulfates | mg/L | 2100–2300 | - | - |
| Calcium | mg/L | 2300–2500 | - | 329–371 |
| Magnesium | mg/L | 220–250 | <1.00 | 598–609 |
| Types | References |
|---|---|
| Electrocoagulator with spiral electrode configuration. | [44] |
| Electrocoagulator with centrifugal electrodes. | [45] |
| Electrocoagulator with cylindrical electrode. | [46] |
| Electrocoagulator with cylindrical anode with multiple fins. | [47] |
| Electrocoagulator with integrated advanced oxidation system. | [48] |
| Electrocoagulator equipment with mobile electrodes. | [49] |
| Continuous flow electrocoagulator. | [50] |
| Discontinuous flow electrocoagulator. | [51] |
| Hybrid electrocoagulation–adsorption system. | [52,53] |
| Type of Wastewater | Initial Parameters | Electrode Specifications | Operating Conditions | Treatment Efficiency | R |
|---|---|---|---|---|---|
| Vinasse from distillery wastewater | COD: 100.16 g/L | n = 2; type anode: Fe Dimensions: 20 cm, 3 cm, 3 mm; area ≈ 28.5 cm2 | pH: 4.3; 5; 6; current: 2.38; 2.66; 2.98 A:10 V; time: 1 h; 200 RPM | COD: 4.83; 8.59; 13.96% pH: 4.90; 5.85; 7.50 | [3] |
| Distillery vinasse residues | TS: 91.89 g/L TSS: 11.78 g/L; TDS: 80.11 g/L; | n = 2; type: Fe Dimensions: 2, 0.3, 0.03 dm; area ≈ 0.636 dm2; distance: 0.5 dm | Current density: 3.9; 4.72; 5.5 A/dm2; volume: 1 L; pH: 4.4; time: 8 h; voltage: 10.4; 11.8; 15.1 V | TS: 97.5; 90.5; 79.44 g/L TSS: 10.3; 9.11; 2.29 g/L TDS: 87.29; 81.40; 77.15 pH: 7.3; 7.3; 7.5 | [89] |
| Vinasse waste from the bioethanol industry | COD:113.70 g/L | n = 2; type: Fe Dimensions: 2 × 0.3 × 0.03 dm Active area: 0.95 × 0.3 × 0.06 dm; distance: 0.55 dm | Agitation speed: 0, 250, 500 rpm; volume: 1 L; T: 301.65°K; pH: 4.1 Voltage: 12.6; 12.3; 12.4 V | pH: 6.2; 6.7; 7 COD: 67.62% | [35] |
| Distillery spent wash | COD: 120 g/L | n = 2; type: Al-Al, Fe-Fe, Al-Fe; dimensions: 150 × 32 × 1 in mm; active area: 50 mm × 32 mm; distance: 3 cm | Volume: 300 mL; time: 2 h; pH: 3 Current density: 0.187 A cm2; Agitation speed: 500 rpm | COD: with Al-Al: 73.3% with Al-Fe: 60% whit Fe-Fe: 46.6% | [90] |
| Distillery effluent | COD: 5.15 g/L BOD: 0.8 g/L Conductivity: 5.9 mS/cm | Type: Al | Time: 93 min pH: 3.5–9.5 Current density: 44.5–225.5 A/m2 | COD: 85.1%; color: 79.4% pH: 8; current density: 135 A/m2 | [91] |
| Vinasse residues | COD: 100.16 g/L | Type: Fe Dimensions: 0.2 m × 0.03 m × 0.003 m; distance: 0.055 m | Voltage: 7.5–12.5 V 200 rpm; pH: 6 Temperature: 105–110 °C | COD: 83.17 kg/m3 | [88] |
| Distillery effluent | pH: 4.8 COD: 140 g/L Conductivity: 32 mS/cm | Type: Fe Dimensions: 10 cm × 2.5 cm × 0.5 cm Distance: 2 cm | Agitation speed: 100 RPM; Time 2 h; pH: 3–9 Intensity: 0.5; 1; 1.5; 1.9 A | pH: 4.8; COD: 19.87%, 51.67%; color: 83.75% | [92] |
| Distillery wastewater effluent | COD: 13.8 g/L | n = 4; type: Al Dimensions: 8 cm × 7 cm; area: 56 cm2 Distance: 2.0 cm | Voltage: 0–30 V; volume: 1.5 dm3 Time: 2 h; I: 0–5 A; D.C: 89.3 A/m2 | COD: 93%; pH: 8; color: 87%, light yellow | [93] |
| Treatment of sugarcane vinasse | Turbidity, 2440 ± 400 NTU; total dissolved solids, 6810 ± 840 mg/L; total suspended solids, 5200 ± 300 mg/L | Aluminum electrodes (125 × 80 × 2 mm) connected in parallel monopolar mode | Electrode spacing (1 cm), current density 6.1 mA cm−2; 430 rpm; time 3 h | 98.8% NTU; % total dissolved solids; total suspended solids, 20% and 96% | [11] |
| Vinasse effluents | COD 46,550 mg/L; turbidity 697 NTU | Anode of aluminum, steel cathode (60 mm × 50 mm × 2 mm) | 1 A, pH 7 and time 45 min; constant circulation flow rate of 10 mL s−1 | COD removal 80.8%; turbidity removal 73.10% | [94] |
| Distillery spent wash | COD 112,948.5; 27.2 mS cm−1; pH 4.4; total solid 92,624.25 mg L−1 | Electrodes (iron plates) with dimensions of length, width and thickness of 20.3 and 3 mm | Currents (2.5, 3 and 3.5 A) and initial pH (4.4, 5.0 and 7.0); 500 rpm; time 6 h | COD removal (74.9%) | [95] |
| Method | Initial Parameters | Electrode Specifications | Operating Conditions | Treatment Efficiency | R |
|---|---|---|---|---|---|
| Electrocoagulation and Electro-Fenton | Turbidity: 34.90 NTU COD: 4750 mg/L | n = 2 Type: Fe Area ≈ 100 cm2 Distance: 3 mm | Electrocoagulation: pH: 4–5.15; current density: 10; 15; 20 mA/cm2; 180 min | Electrocoagulation: COD: 11.1% and 14.3% (15 y 20 mA/cm2) | [120] |
| Electro-Fenton: pH: 4–5.15; current density: 30; 40; 50 mA/cm2; 180 min | Electro-Fenton: COD: 92.6%, 88.7% | ||||
| Electrocoagulation with and without activated carbon of Areca catechu nuts | COD: 18,868.8 mg/L BOD: 11,653.2 mg/L | Monopolar electrodes Type: Fe, Al Dimensions: 104 mm, 25 mm, 6 mm Distance: 28 mm | T: 30 °C; time: 60 min; voltage: 30 V; intensity: 2 A; current density: 182 A/m2 slow agitation | No activated carbon, pH = 5.96 Color: colorless; turbidity: 3.85 NTU; COD: 4618.8 mg/L; BOD: 3565 mg/L With activated carbon, pH = 6.41; Color: colorless; turbidity: 2.7 NTU; COD: 3754.1; BOD: 1199.7 mg/L | [121] |
| Ozone-assisted electrocoagulation | COD: 80,000–90,000 mg/L BOD: 7000–8000 mg/L | Type: iron Dimensions: 9 cm × 5 cm Area: 45 cm2 Electrode Distance: 1 cm | pH: 2–10; current density: 3 A/dm2; T: 30 °C; O3 generator: 2 g/h; stirring speed: 10,000 rpm; stirring time: 15 min | COD: 83% Color: 100% | [102] |
| Electrocoagulation with advanced oxidation processes | COD: 8500 mg/L BOD: 3000 mg/L | Type: iron Distance: 2 cm Dimensions: 10 cm × 10 cm × 0.1 cm | pH: 2–10; current density: 0.10–0.20 A/dm2; reaction time: 240 min; O3 generator: 2 g/h | COD: 94% BOD: 92% Color: 100% | [122] |
| Combination of sound (US), photography process (UV) and electrocoagulation (EC) | BOD: 7000–8000 mg/L COD: 80,000–90,000 mg/L | Type: Fe and Al Dimensions: 0.1 cm × 10 cm × 15 cm Direct current | UV + EC process: Current density: 0.75 A/dm2 Distance: 0.75 cm; pH: 7 US + UV+EC process: Current density: 0.07 + 0.2 A Time: 4 h; Fuente UV: 8–32 W | US + UV + EC process COD: 95.63% Color: 100% | [112] |
| Electrocoagulation powered by photovoltaic cells | COD:4252 mg/L BOD: 918 mg/L Conductivity: 5.9 mS/cm | Type: Fe and Al Distance: 2 cm Dimension: 50 mm × 60 mm | Reaction time: 60 min Current density: 24.9 mA/cm2 | COD: 94.9% Color: 81.3% pH: 7 | [17] |
| Reverse osmosis–electrocoagulation | ph:7.8–8 COD:15,000–16,000 mg/L | Type: stainless steel Area ≈ 0.636 dm2 Distance: 2.2 cm | Current density: 154.32 A/m2 A/dm2 pH: 7.8; time: 135 min | COD: 98% Color: 99.2% TDS: 98.5% | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Collana, J.T.; Ayllon Ormeño, M.; Julca Meza, C.; Moreyra Cuadros, G.; Carrasco Venegas, L.A.; Ancieta Dextre, C.A.; Rodríguez Taranco, O.J.; Avelino Carhuaricra, C.; Diaz Bravo, P.; Montaño Pisfil, J.A. Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters. Sustainability 2024, 16, 6383. https://doi.org/10.3390/su16156383
Medina Collana JT, Ayllon Ormeño M, Julca Meza C, Moreyra Cuadros G, Carrasco Venegas LA, Ancieta Dextre CA, Rodríguez Taranco OJ, Avelino Carhuaricra C, Diaz Bravo P, Montaño Pisfil JA. Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters. Sustainability. 2024; 16(15):6383. https://doi.org/10.3390/su16156383
Chicago/Turabian StyleMedina Collana, Juan Taumaturgo, Marisol Ayllon Ormeño, Caroline Julca Meza, Gonzalo Moreyra Cuadros, Luis Américo Carrasco Venegas, Carlos Alejandro Ancieta Dextre, Oscar Juan Rodríguez Taranco, Carmen Avelino Carhuaricra, Pablo Diaz Bravo, and Jorge Alberto Montaño Pisfil. 2024. "Processes Coupled to Electrocoagulation for the Treatment of Distillery Wastewaters" Sustainability 16, no. 15: 6383. https://doi.org/10.3390/su16156383





