Experimental Investigation on the Damage Evolution of Thermally Treated Granodiorite Subjected to Rapid Cooling with Liquid Nitrogen
Abstract
1. Introduction
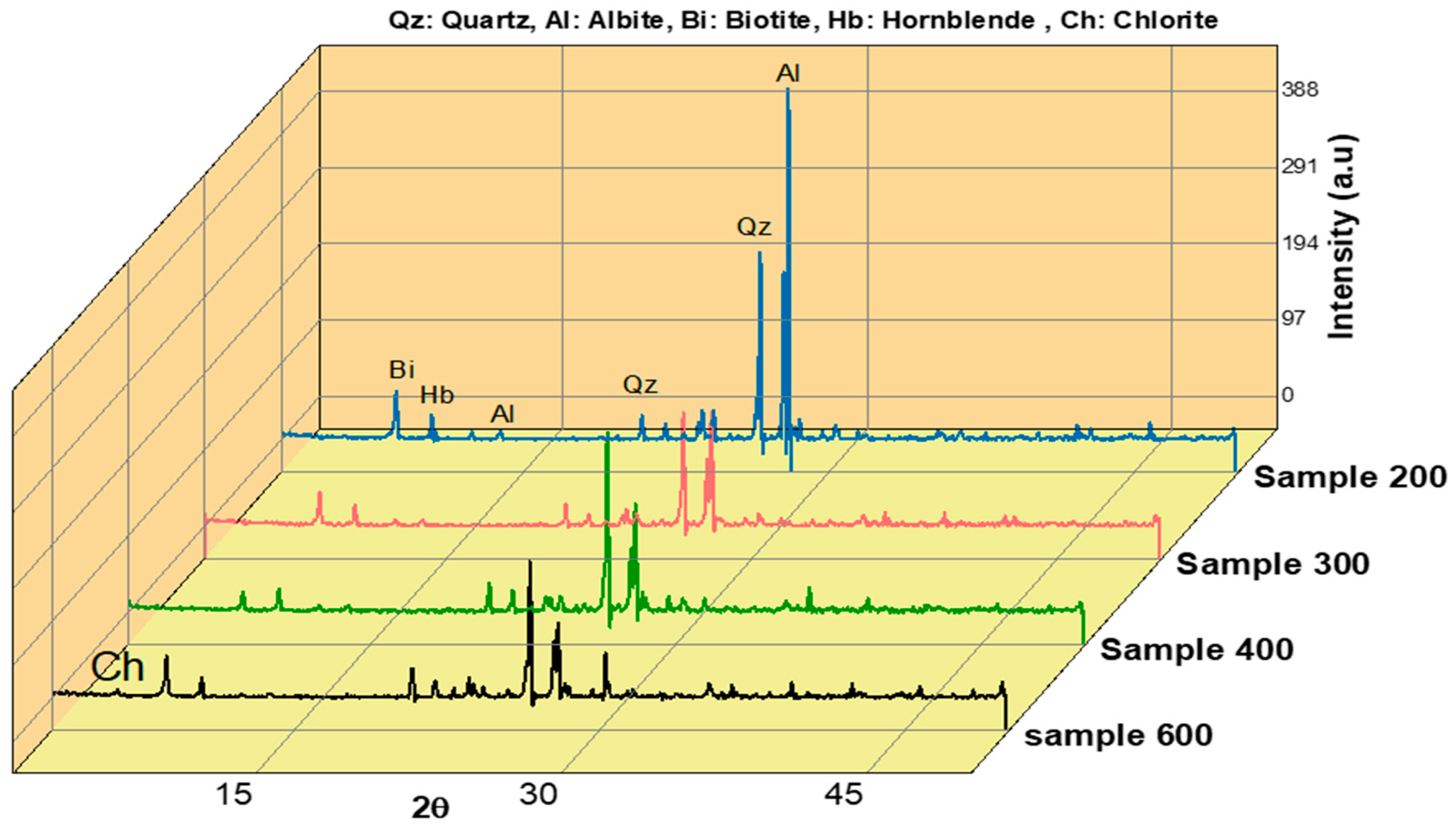
2. Materials and Methods
3. Results
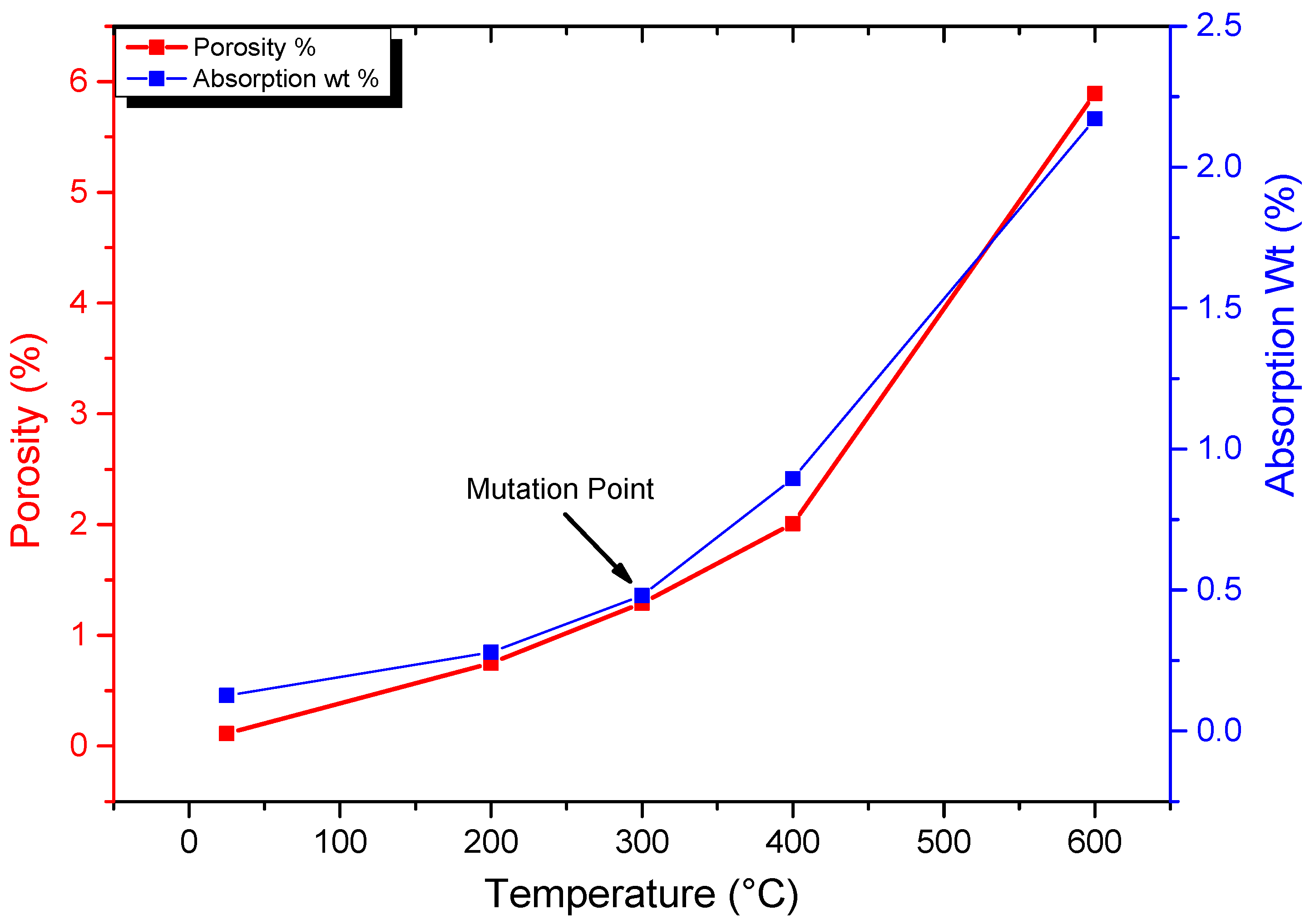
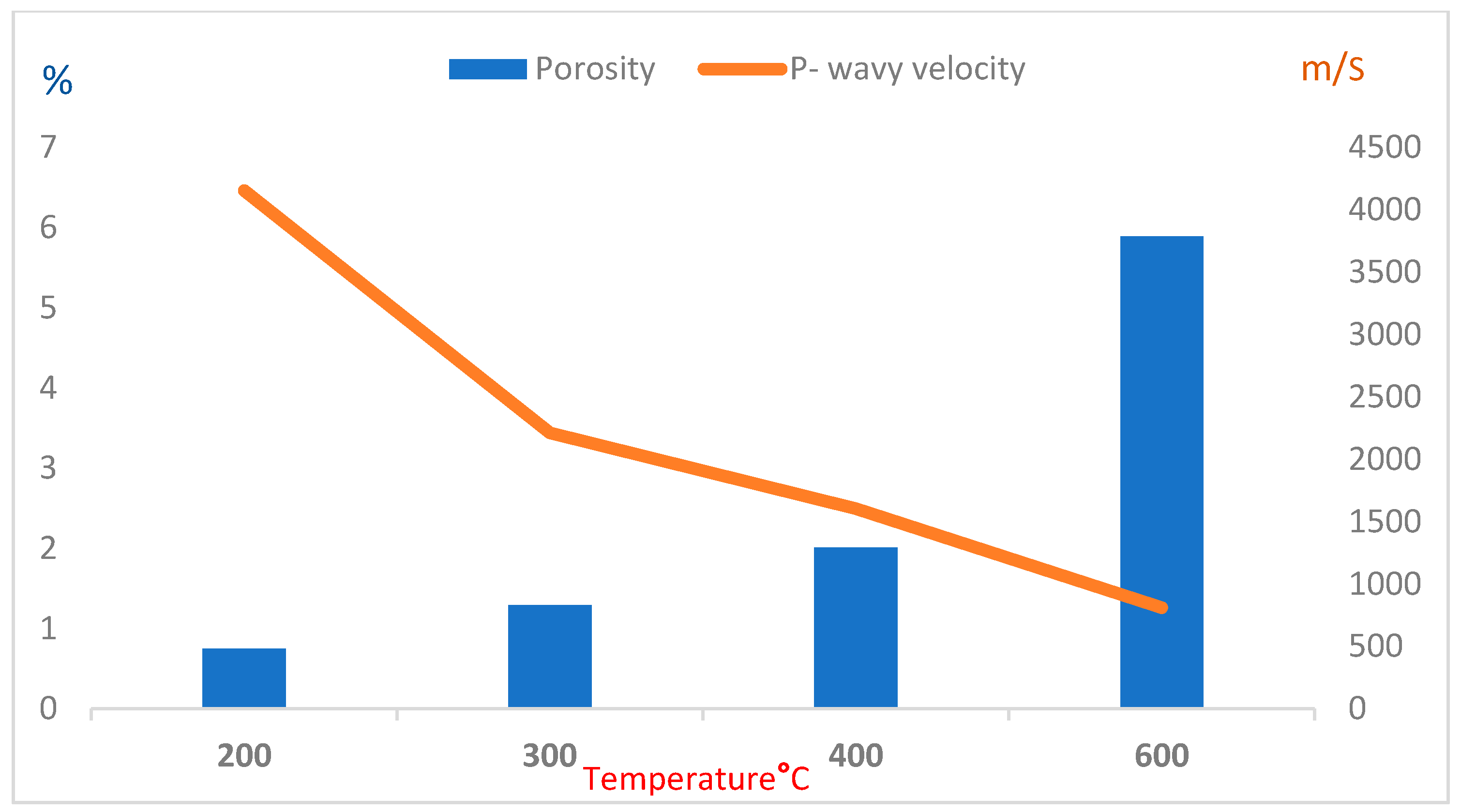
3.1. Porosity and Absorption Deterioration
3.2. Mass, Volume, and Density Behaviors
3.3. Ultrasonic Velocity
3.4. SEM Observation
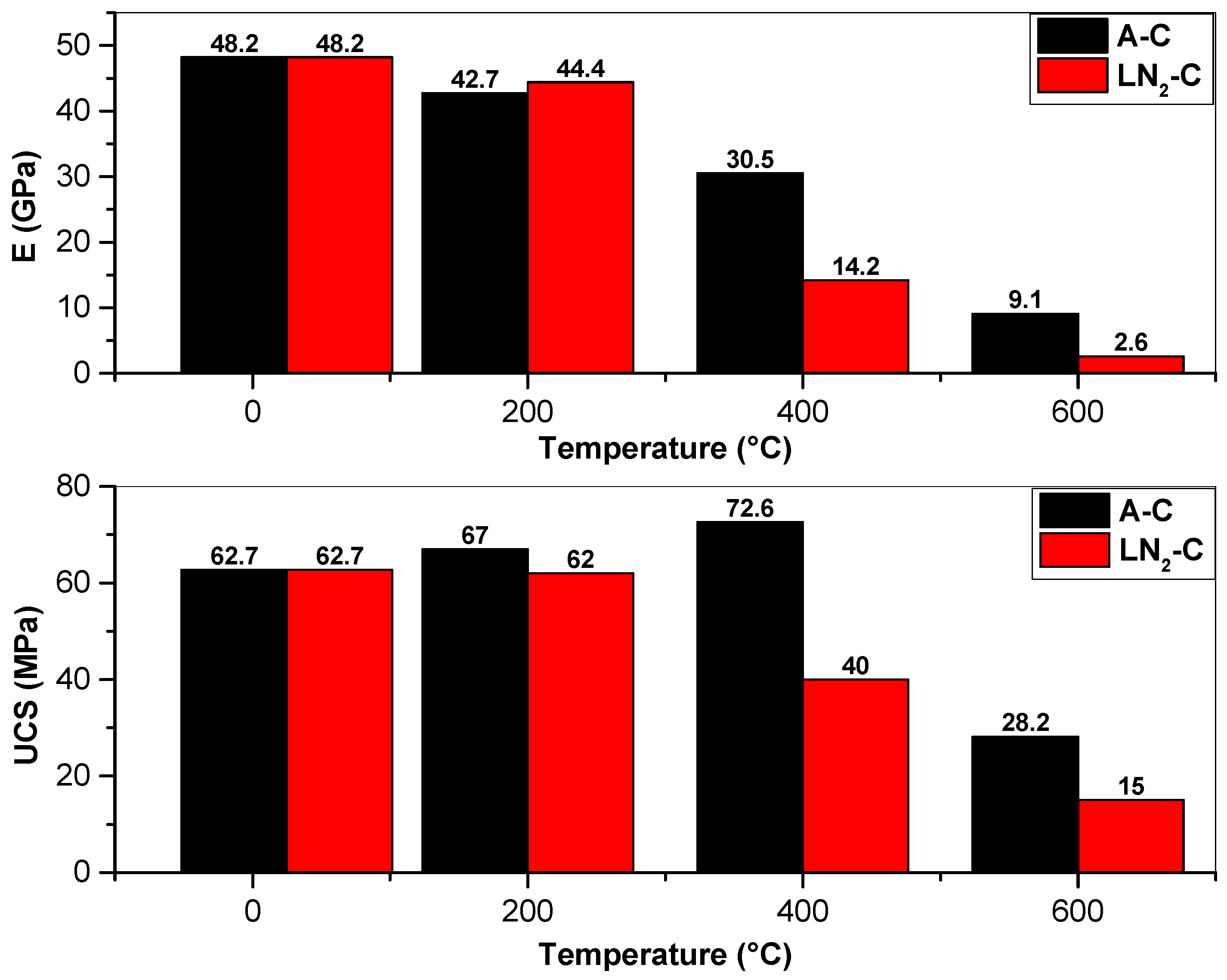
3.5. Mechanical Properties
3.5.1. UCS
3.5.2. E
3.5.3. Failure Modes
4. Discussion
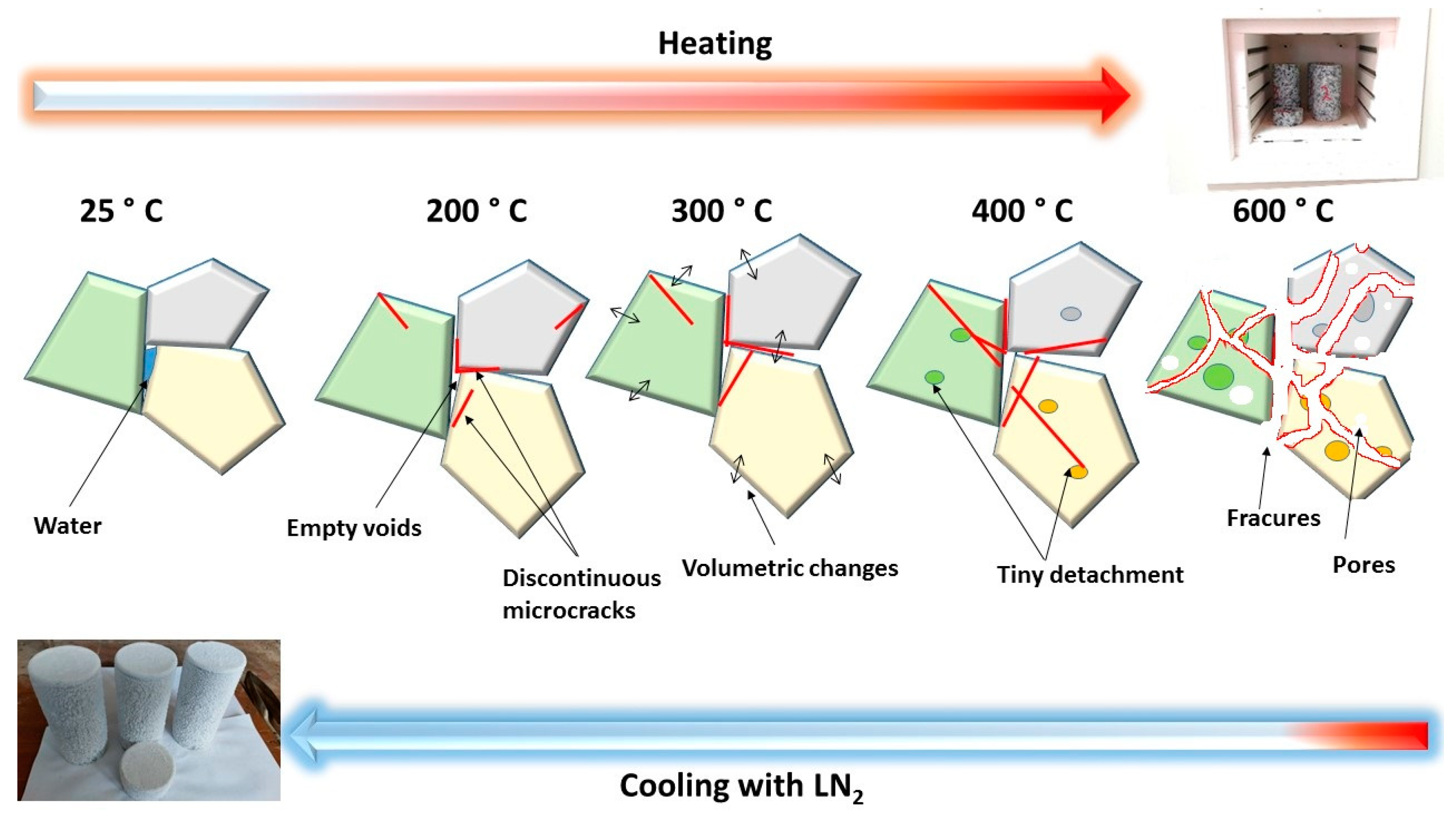
4.1. Microstructural Evaluation
4.2. Physical Property Responses
4.3. Mechanical Properties Responses
4.4. Thermal Damage Evolution of Vp and E
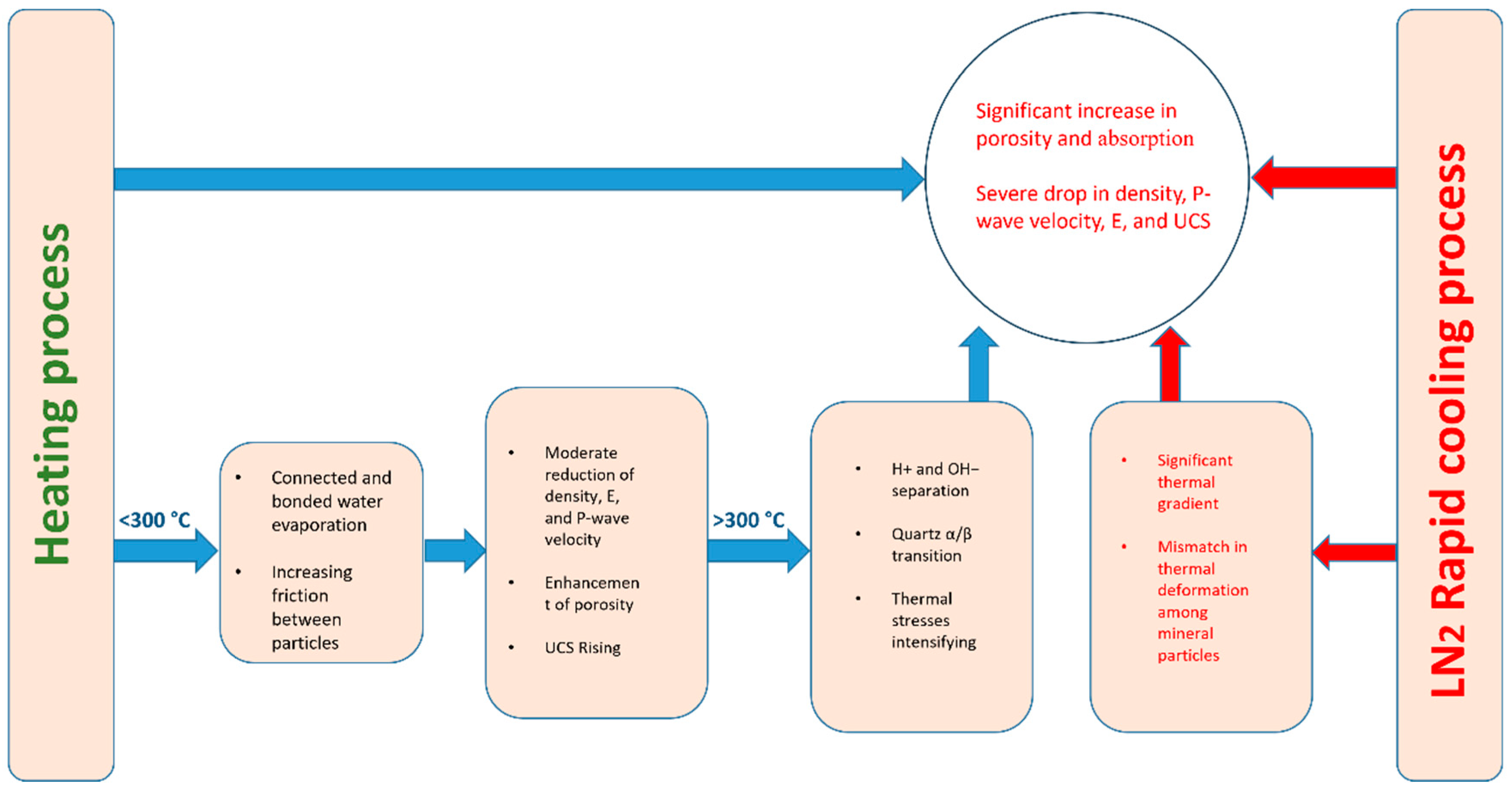
4.5. Liquid Nitrogen Cooling Impact Analysis
5. Conclusions
- (1)
- Up to 300 °C, the porosity, crack density, thermal damage, and density reduction ratio progressively rose before drastically increasing beyond this point. Similarly, the uniaxial compressive strength climbed to 300 °C, and then it drastically dropped linearly as the temperature rose. In contrast, there was a gradual reduction in the elastic modulus and P-wave velocity as the temperature reached 200 °C, followed by a significant decline. Hence, this study revealed the following two mutation temperatures in the evolution of granodiorite mechanical and physical properties: 200 and 300 °C. After that, all metrics massively deteriorated;
- (2)
- Microcrack initiation and propagation can be associated with two primary granodiorite failure types. At 200 °C, tiny boundary and transgranular microcracks were visible, which led to the axial splitting modes. On the other hand, the shear failure mode was produced by developing boundary and transgranular cracks at 300, 400, and 600 °C;
- (3)
- Microscopically, the granodiorite exhibits boundary and transgranular cracks; however, boundary cracking is the main form of failure under thermal loading. Also, the impact of quartz on granodiorite thermal degradation is significant. Thus, most boundary cracks disperse at the quartz’s borders. After being heated and cooled, granodiorite experiences many transgranular cracks at 600 °C; most occur in quartz;
- (4)
- Compared to air cooling, LN2 cooling produces more notable changes in mechanical and physical characteristics, illustrating the cooling influence of liquid nitrogen on granodiorite’s properties. Nevertheless, raising the desired heating temperature for both cooling methods causes more severe damage.
- (5)
- To avoid or reduce possible geological hazards, it is crucial to consider the adverse effects of using LN2 for hydraulic fracturing stimulation, such as the weakening of host rocks’ mechanical properties.
6. Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gallup, D.L. Production Engineering in Geothermal Technology: A Review. Geothermics 2009, 38, 326–334. [Google Scholar] [CrossRef]
- Breede, K.; Dzebisashvili, K.; Liu, X.; Falcone, G. A Systematic Review of Enhanced (or Engineered) Geothermal Systems: Past, Present and Future. Geotherm. Energy 2013, 1, 4. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Hu, D.; Skoczylas, F.; Shao, J. Laboratory Investigation on Physical and Mechanical Properties of Granite after Heating and Water-Cooling Treatment. Rock Mech. Rock Eng. 2018, 51, 677–694. [Google Scholar] [CrossRef]
- Chen, Z.; Sha, S.; Xu, L.; Quan, J.; Rong, G.; Jiang, M. Damage Evaluation and Statistic Constitutive Model of High-Temperature Granites Subjected to Liquid Nitrogen Cold Shock. Rock Mech. Rock Eng. 2022, 55, 2299–2321. [Google Scholar] [CrossRef]
- Feng, Z.; Zhao, Y.; Zhou, A.; Zhang, N. Development Program of Hot Dry Rock Geothermal Resource in the Yangbajing Basin of China. Renew. Energy 2012, 39, 490–495. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Xi, B.; Wan, Z.; Yang, D.; Liang, W. Deformation and Instability Failure of Borehole at High Temperature and High Pressure in Hot Dry Rock Exploitation. Renew. Energy 2015, 77, 159–165. [Google Scholar] [CrossRef]
- Brook, B.W.; Alonso, A.; Meneley, D.A.; Misak, J.; Blees, T.; van Erp, J.B. Why Nuclear Energy Is Sustainable and Has to Be Part of the Energy Mix. Sustain. Mater. Technol. 2014, 1, 8–16. [Google Scholar] [CrossRef]
- Xi, Z.; Yun, L.; Li, X.Z.; Huang, Z.; Lin, J.; Hua, X. Effects of Thermal Treatment on the Macroscopic Physical Properties and Microstructure of Beishan Fine-Grained Granite. Bull. Eng. Geol. Environ. 2022, 81, 190. [Google Scholar]
- Ferrero, A.M.; Marini, P. Experimental Studies on the Mechanical Behaviour of Two Thermal Cracked Marbles. Rock Mech. Rock Eng. 2001, 34, 57–66. [Google Scholar] [CrossRef]
- Freire-Lista, D.M. Thermal Stress-Induced Microcracking in Building Granite. Eng. Geol. 2016, 206, 83–93. [Google Scholar] [CrossRef]
- Zhang, R.R.; Jing, L.W.; Ma, Q.Y. Experimental Study on Thermal Damage and Energy Evolution of Sandstone after High Temperature Treatment. Shock Vib. 2018, 2018, 3845353. [Google Scholar] [CrossRef]
- Yang, S.-Q.Q.; Xu, P.; Li, Y.-B.B.; Huang, Y.-H.H. Experimental Investigation on Triaxial Mechanical and Permeability Behavior of Sandstone after Exposure to Different High Temperature Treatments. Geothermics 2017, 69, 93–109. [Google Scholar] [CrossRef]
- Ding, Q.L.; Ju, F.; Mao, X.B.; Ma, D.; Yu, B.Y.; Song, S.B. Experimental Investigation of the Mechanical Behavior in Unloading Conditions of Sandstone after High-Temperature Treatment. Rock Mech. Rock Eng. 2016, 49, 2641–2653. [Google Scholar] [CrossRef]
- Tripathi, A.; Gupta, N.; Singh, A.K.; Mohanty, S.P.; Rai, N.; Pain, A. Effects of Elevated Temperatures on the Microstructural, Physico-Mechanical and Elastic Properties of Barakar Sandstone: A Study from One of the World’s Largest Underground Coalmine Fire Region, Jharia, India. Rock Mech. Rock Eng. 2021, 54, 1293–1314. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Sun, C.; Xu, J.; Yang, S.; Li, J. On the Physical and Mechanical Responses of Egyptian Granodiorite after High-Temperature Treatments. Sustainability 2022, 14, 4632. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Khan, N.M.; Sun, C.; Xu, J.; Omar, A.A.; Mousa, B.G.; Abdelhamid, M.M.A.; Zaki, M.M. Prediction of Strength Parameters of Thermally Treated Egyptian Granodiorite Using Multivariate Statistics and Machine Learning Techniques. Mathematics 2022, 10, 4523. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Omar, A.A.; Abdel Latif, M.L.; Sun, C.; Xu, J. Thermal-Induced Microstructure Deterioration of Egyptian Granodiorite and Associated Physico-Mechanical Responses. Materials 2024, 17, 1305. [Google Scholar] [CrossRef] [PubMed]
- Balme, M.R.; Rocchi, V.; Jones, C.; Sammonds, P.R.; Meredith, P.G.; Boon, S. Fracture Toughness Measurements on Igneous Rocks Using a High-Pressure, High-Temperature Rock Fracture Mechanics Cell. J. Volcanol. Geotherm. Res. 2004, 132, 159–172. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Q.; Dong, Z.; Ge, Z.; Wang, S.; Xu, C. A Study on Thermal Damage Mechanism of Sandstone Based on Thermal Reaction Kinetics. Geomech. Geophys. Geo-Energy Geo-Resour. 2021, 7, 64. [Google Scholar] [CrossRef]
- Sundberg, J.; Back, P.-E.; Christiansson, R.; Hökmark, H.; Ländell, M.; Wrafter, J. Modelling of Thermal Rock Mass Properties at the Potential Sites of a Swedish Nuclear Waste Repository. Int. J. Rock Mech. Min. Sci. 2009, 46, 1042–1054. [Google Scholar] [CrossRef]
- Feng, G.; Wang, X.; Wang, M.; Kang, Y. Experimental Investigation of Thermal Cycling Effect on Fracture Characteristics of Granite in a Geothermal-Energy Reservoir. Eng. Fract. Mech. 2020, 235, 107180. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Z.; Oterkus, E.; Oterkus, S.; Xu, H. Geohazard Mechanics Research on the Effects of Heating and Cooling Processes on the Mechanical Properties of Yellow Rust Granite. Geohazard Mech. 2023, 1, 231–243. [Google Scholar] [CrossRef]
- Hou, P.; Liang, X.; Zhang, Y.; He, J.; Gao, F.; Liu, J. 3D Multi-Scale Reconstruction of Fractured Shale and Influence of Fracture Morphology on Shale Gas Flow. Nat. Resour. Res. 2021, 30, 2463–2481. [Google Scholar] [CrossRef]
- Riahi, A.; Pettitt, W.; Damjanac, B.; Varun; Blanksma, D. Numerical Modeling of Discrete Fractures in a Field-Scale FORGE EGS Reservoir. Rock Mech. Rock Eng. 2019, 52, 5245–5258. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Y.; Yu, Z.; Hu, Z. Investigation on Reservoir Stimulation Characteristics in Hot Dry Rock Geothermal Formations of China during Hydraulic Fracturing. Rock Mech. Rock Eng. 2021, 54, 3817–3845. [Google Scholar] [CrossRef]
- King, G.E. Hydraulic Fracturing 101: What Every Representative, Environmentalist, Regulator, Reporter, Investor, University Researcher, Neighbor and Engineer Should Know about Estimating Frac Risk and Improving Frac Performance in Unconventional Gas and Oil Wells. In Proceedings of the SPE Hydraulic Fracturing Technology Conference and Exhibition, The Woodlands, TX, USA, 6–8 February 2012; SPE: Kuala Lumpur, Malaysia, 2012; p. SPE-152596. [Google Scholar]
- Aguilera, R.F.; Ripple, R.D.; Aguilera, R. Link between Endowments, Economics and Environment in Conventional and Unconventional Gas Reservoirs. Fuel 2014, 126, 224–238. [Google Scholar] [CrossRef]
- Cai, C.; Li, G.; Huang, Z.; Tian, S.; Shen, Z.; Fu, X. Experiment of Coal Damage Due to Super-Cooling with Liquid Nitrogen. J. Nat. Gas Sci. Eng. 2015, 22, 42–48. [Google Scholar] [CrossRef]
- Zhou, Z.; Cai, X.; Li, X.; Cao, W.; Du, X. Dynamic Response and Energy Evolution of Sandstone under Coupled Static–Dynamic Compression: Insights from Experimental Study into Deep Rock Engineering Applications. Rock Mech. Rock Eng. 2020, 53, 1305–1331. [Google Scholar] [CrossRef]
- Kumari, W.G.P.; Ranjith, P.G.; Perera, M.S.A.; Chen, B.K. Experimental Investigation of Quenching Effect on Mechanical, Microstructural and Flow Characteristics of Reservoir Rocks: Thermal Stimulation Method for Geothermal Energy Extraction. J. Pet. Sci. Eng. 2018, 162, 419–433. [Google Scholar] [CrossRef]
- Kumari, W.G.P.; Ranjith, P.G.; Perera, M.S.A.; Chen, B.K.; Abdulagatov, I.M. Temperature-Dependent Mechanical Behaviour of Australian Strathbogie Granite with Different Cooling Treatments. Eng. Geol. 2017, 229, 31–44. [Google Scholar] [CrossRef]
- Shao, S.; Wasantha, P.L.P.; Ranjith, P.G.; Chen, B.K. Effect of Cooling Rate on the Mechanical Behavior of Heated Strathbogie Granite with Different Grain Sizes. Int. J. Rock Mech. Min. Sci. 2014, 70, 381–387. [Google Scholar] [CrossRef]
- Hou, P.; Gao, F.; Gao, Y.; Yang, Y.; Cai, C. Changes in Breakdown Pressure and Fracture Morphology of Sandstone Induced by Nitrogen Gas Fracturing with Different Pore Pressure Distributions. Int. J. Rock Mech. Min. Sci. 2018, 109, 84–90. [Google Scholar] [CrossRef]
- Liang, X.; Hou, P.; Xue, Y.; Yang, X.; Gao, F.; Liu, J. A Fractal Perspective on Fracture Initiation and Propagation of Reservoir Rocks under Water and Nitrogen Fracturing. Fractals 2021, 29, 2150189. [Google Scholar] [CrossRef]
- Hou, P.; Xue, Y.; Gao, F.; Dou, F.; Su, S.; Cai, C.; Zhu, C. Effect of Liquid Nitrogen Cooling on Mechanical Characteristics and Fracture Morphology of Layer Coal under Brazilian Splitting Test. Int. J. Rock Mech. Min. Sci. 2022, 151, 105026. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Song, H.; Zhang, S.; Cheng, Z.; Li, R.; Wen, H.; Huang, P.; Dai, X. Variations of Physical and Mechanical Properties of Heated Granite after Rapid Cooling with Liquid Nitrogen. Rock Mech. Rock Eng. 2019, 52, 2123–2139. [Google Scholar] [CrossRef]
- Li, Q.; Yin, T.; Li, X.; Zhang, S. Effects of Rapid Cooling Treatment on Heated Sandstone: A Comparison between Water and Liquid Nitrogen Cooling. Bull. Eng. Geol. Environ. 2020, 79, 313–327. [Google Scholar] [CrossRef]
- Kim, K.; Kemeny, J.; Nickerson, M. Effect of Rapid Thermal Cooling on Mechanical Rock Properties. In Proceedings of the Rock Mechanics and Rock Engineering, Vigo, Spain, 26–28 May 2014; American Rock Mechanics Association: Alexandria, VA, USA, 2014; Volume 47, pp. 2005–2019. [Google Scholar]
- Zhang, H.; Huang, Z.; Zhang, S.; Yang, Z.; Mclennan, J.D. Improving Heat Extraction Performance of an Enhanced Geothermal System Utilizing Cryogenic Fracturing. Geothermics 2020, 85, 101816. [Google Scholar] [CrossRef]
- Yang, R.; Huang, Z.; Shi, Y.; Yang, Z.; Huang, P. Laboratory Investigation on Cryogenic Fracturing of Hot Dry Rock under Triaxial-Confining Stresses. Geothermics 2019, 79, 46–60. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, Y.; Tang, X. The Influences of Heating and Uniaxial Loading on Granite Subjected to Liquid Nitrogen Cooling. Eng. Geol. 2020, 271, 105614. [Google Scholar] [CrossRef]
- Su, S.; Hou, P.; Gao, F.; Liang, X.; Ding, R.; Cai, C. Changes in Mechanical Properties and Fracture Behaviors of Heated Marble Subjected to Liquid Nitrogen Cooling. Eng. Fract. Mech. 2022, 261, 108256. [Google Scholar] [CrossRef]
- Qin, L.; Zhai, C.; Liu, S.; Xu, J. Factors Controlling the Mechanical Properties Degradation and Permeability of Coal Subjected to Liquid Nitrogen Freeze-Thaw. Sci. Rep. 2017, 7, 3675. [Google Scholar] [CrossRef] [PubMed]
- Read, H.; Hegemier, G.A. Strain Softening of Rock, Soil and Concrete—A Review Article. Mech. Mater. 1984, 3, 271–294. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Hao, S.; Geng, J.; Lv, C. Experimental Study on the Variation of Physical and Mechanical Properties of Rock after High Temperature Treatment. Appl. Therm. Eng. 2016, 98, 1297–1304. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Li, R.; Zhang, S.; Wen, H.; Huang, P.; Dai, X.; Zhang, C. Investigation on the Damage of High-Temperature Shale Subjected to Liquid Nitrogen Cooling. J. Nat. Gas Sci. Eng. 2018, 57, 284–294. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, H.; Wu, X.; Li, G.; Yang, R.; Li, R.; Zhang, C. Experimental Study on Cracking of High Temperature Granite Using Liquid Nitrogen. Zhongguo Shiyou Daxue Xuebao (Ziran Kexue Ban) 2019, 43, 68–76. [Google Scholar]
- Wu, X.; Huang, Z.; Cheng, Z.; Zhang, S.; Song, H.; Zhao, X. Effects of Cyclic Heating and LN2-Cooling on the Physical and Mechanical Properties of Granite. Appl. Therm. Eng. 2019, 156, 99–110. [Google Scholar] [CrossRef]
- Cha, M.; Alqahtani, N.B.; Yao, B.; Yin, X.; Kneafsey, T.J.; Wang, L.; Wu, Y.-S.; Miskimins, J.L. Cryogenic Fracturing of Wellbores under True Triaxial-Confining Stresses: Experimental Investigation. SPE J. 2018, 23, 1271–1289. [Google Scholar] [CrossRef]
- El-Taher, A.; Uosif, M.A.M.; Orabi, A.A. Natural Radioactivity Levels and Radiation Hazard Indices in Granite from Aswan to Wadi El-Allaqi Southeastern Desert, Egypt. Radiat. Prot. Dosim. 2007, 124, 148–154. [Google Scholar] [CrossRef]
- Streckeisen, A. Classification and Nomenclature of Volcanic Rocks, Lamprophyres, Carbonatites, and Melilitic Rocks: Recommendations and Suggestions of the IUGS Subcommission on the Systematics of Igneous Rocks. Geology 1979, 7, 331–335. [Google Scholar] [CrossRef]
- ASTM D7012-14e1; Test Method for Compressive Strength and Elastic Moduli of Intact Rock Core Specimens under Varying States of Stress and Temperatures. ASTM: West Conshohocken, PA, USA, 2010; pp. 1–8.
- El-Ramly, M.F.; Akaad, M.K. The Basement Complex in the Central Eastern Desert, between Latitudes 24 30′and 25 40′. Geol. Surv. Cairo 1960, 8, 35. [Google Scholar]
- Sabatakakis, N.; Koukis, G.; Tsiambaos, G.; Papanakli, S. Index Properties and Strength Variation Controlled by Microstructure for Sedimentary Rocks. Eng. Geol. 2008, 97, 80–90. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, X.; Gao, H.; An, R.; Yan, L. Engineering Geological Characterization of Micaceous Residual Soils Considering Effects of Mica Content and Particle Breakage. Eng. Geol. 2023, 327, 107367. [Google Scholar] [CrossRef]
- Nasseri, M.H.B.; Tatone, B.S.A.; Grasselli, G.; Young, R.P. Fracture Toughness and Fracture Roughness Interrelationship in Thermally Treated Westerly Granite. Pure Appl. Geophys. 2009, 166, 801–822. [Google Scholar] [CrossRef]
- Chaki, S.; Takarli, M.; Agbodjan, W.P. Influence of Thermal Damage on Physical Properties of a Granite Rock: Porosity, Permeability and Ultrasonic Wave Evolutions. Constr. Build. Mater. 2008, 22, 1456–1461. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Xu, J.; Omar, A.A.; Haoran, H.; Zaki, M.M. Micro to Macro-Cracking Mechanism in Thermally Treated Granodiorite Followed by Different Cooling Techniques. Int. J. Fract. 2023, 9, 161–180. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.; Qian, H. Effects of High Temperature Thermal Treatment on the Physical Properties of Clay. Environ. Earth Sci. 2016, 75, 610. [Google Scholar] [CrossRef]
- Clark, S.P., Jr. Handbook of Physical Constants; Geological Society of America: Boulder, CO, USA, 1966; Volume 97. [Google Scholar]
- Sun, Q.; Zhang, W.; Xue, L.; Zhang, Z.; Su, T. Thermal Damage Pattern and Thresholds of Granite. Environ. Earth Sci. 2015, 74, 2341–2349. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Sun, C.; Xu, J.; Sen, Y.; Li, J.; Ismael, M.; Elkarmoty, M. Macroscopic and Microscopic Research on Egyptian Granodiorite Behavior Exposed to the Various Heating and Cooling Strategies. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 1–22. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Bader, S.; Elkarmoty, M.; Ismael, M. Damage Evolution of Granodiorite after Heating and Cooling Treatments. Minerals 2021, 11, 779. [Google Scholar] [CrossRef]
- Shen, Y.J.; Hou, X.; Yuan, J.Q.; Wang, S.F.; Zhao, C.H. Thermal Cracking Characteristics of High-Temperature Granite Suffering from Different Cooling Shocks. Int. J. Fract. 2020, 225, 153–168. [Google Scholar] [CrossRef]
- Rao, G.M.N.; Murthy, C.R.L. Dual Role of Microcracks: Toughening and Degradation. Can. Geotech. J. 2001, 38, 427–440. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yin, G.Z.; Chen, L.J. Experimental Study on Effect of Temperature on Sandstone Damage. Chin. J. Rock Mech. Eng. 2009, 28, 2784–2788. [Google Scholar]












| Temperature | 200 °C | 300 °C | 400 °C | 600 °C |
|---|---|---|---|---|
| Failure Mode |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomah, M.E.; Wang, E.; Omar, A.A. Experimental Investigation on the Damage Evolution of Thermally Treated Granodiorite Subjected to Rapid Cooling with Liquid Nitrogen. Sustainability 2024, 16, 6396. https://doi.org/10.3390/su16156396
Gomah ME, Wang E, Omar AA. Experimental Investigation on the Damage Evolution of Thermally Treated Granodiorite Subjected to Rapid Cooling with Liquid Nitrogen. Sustainability. 2024; 16(15):6396. https://doi.org/10.3390/su16156396
Chicago/Turabian StyleGomah, Mohamed Elgharib, Enyuan Wang, and Ahmed A. Omar. 2024. "Experimental Investigation on the Damage Evolution of Thermally Treated Granodiorite Subjected to Rapid Cooling with Liquid Nitrogen" Sustainability 16, no. 15: 6396. https://doi.org/10.3390/su16156396
APA StyleGomah, M. E., Wang, E., & Omar, A. A. (2024). Experimental Investigation on the Damage Evolution of Thermally Treated Granodiorite Subjected to Rapid Cooling with Liquid Nitrogen. Sustainability, 16(15), 6396. https://doi.org/10.3390/su16156396







