Qualitative Production of Mixture Silage within a Sustainable Concept
Abstract
:1. Introduction
2. Materials and Methods
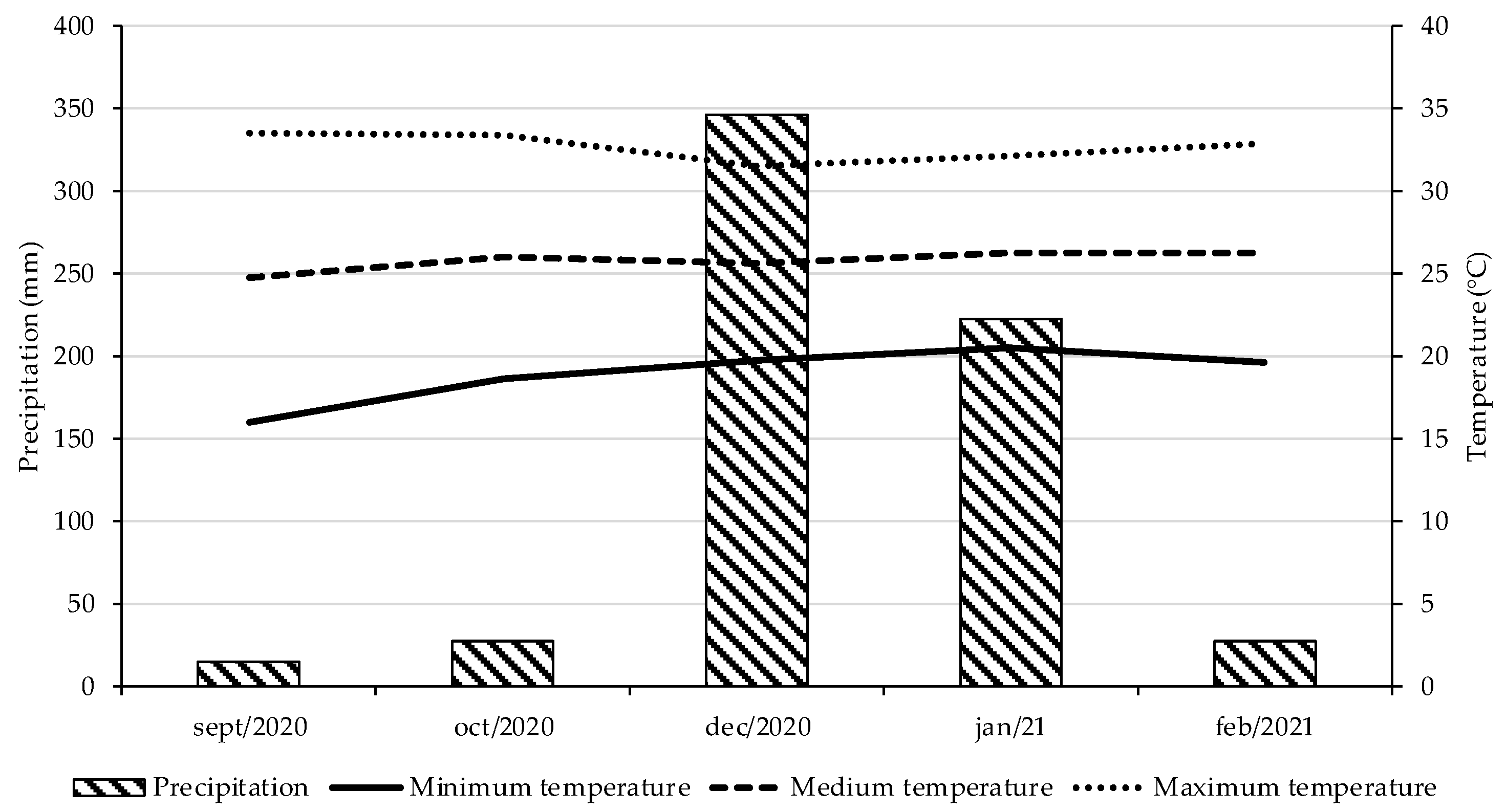
2.1. Experiment Location, Treatments, and Plant Cultivation
2.2. Harvesting and Ensiling
2.3. Chemical Analyses
2.4. Calculations of the Contribution and Proportion of Each Forage in the DM, CP, CF, NDF, ADF, EE, MM, Lignin, NDIN, ADIN, Cellulose, and Hemicellulose in the Silages
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moorby, J.M.; Fraserb, M.D. Review: New feeds and new feeding systems in intensive and semi-intensive forage-fed ruminant livestock systems. Animal 2021, 15, 100297. [Google Scholar] [CrossRef] [PubMed]
- Marques, K.O.; Jakelaitis, A.; Guimarães, K.C.; Pereira, L.S.; Cardoso, I.S.; Lima, S.F. Production, fermentation profile, and nutritional quality of silage from corn and soybean intercropping. Semin. Ciênc. Agrár. 2019, 40, 3143–3156. [Google Scholar] [CrossRef]
- Carpici, E.B. Nutritive values of soybean silages ensiled with maize at different rates. Legume Res. 2016, 39, 810–813. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gillani, S.S.M.; Moise, E.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; et al. Corn-soybean intercropping improved the nutritional quality of forage cultivated on Podzols in boreal climate. Plants 2021, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Bolson, D.C.; Jacovaci, F.A.; Gritti, V.C.; Bueno, A.V.I.; Daniel, J.L.P.; Nussio, L.G.; Jobim, C.C. Intercropped maize-soybean silage: Effects on forage yield, fermentation pattern and nutritional composition. Grassl. Sci. 2021, 68, 3–12. [Google Scholar] [CrossRef]
- Zanine, A.M.; Sene, O.A.; Ferreira, D.J.; Parente, H.N.; Parente, M.O.M.; Pinho, R.M.A.; Santos, E.M.; Nascimento, T.V.C.; Lima, A.G.V.O.; Perazzo, A.F.; et al. Fermentative profile, losses and chemical composition of silage soybean genotypes amended with sugarcane levels. Sci. Rep. 2020, 10, 21064. [Google Scholar] [CrossRef]
- Serbester, U.; Akkaya, M.R.; Yucel, C.; Gorgulu, M. Comparison of yield, nutritive value, and in vitro digestibility of monocrop and intercropped corn-soybean silages cut at two maturity stages. Ital. J. Anim. Sci. 2015, 14, 66–70. [Google Scholar] [CrossRef]
- Iqbal, N.; Hussain, S.; Ahmed, Z.; Yang, F.; Wang, X.; Liu, W.; Yong, T.; Du, J.; Shu, K.; Yang, W.; et al. Comparative analysis of maize–soybean strip intercropping systems: A review. Plant Prod. Sci. 2019, 22, 131–142. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture; Natural Resources Conservation Service: Washington, WA, USA, 2014; 359p. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Van Raij, B.; Cantarela, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; Instituto Agronômico e Fundação IAC: Campinas, SP, Brazil, 1997; 285p. [Google Scholar]
- Association of Official Analytical Chemists—AOAC. Official Method of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990; Volume 1, pp. 69–88. [Google Scholar]
- Wiles, P.G.; Gray, I.K.; Kissling, R.C. Routine analysis of protein by Kjeldahl and Dumas methods: Review and interlaboratory study using dairy products. J. AOAC Int. 1998, 81, 620–632. [Google Scholar] [CrossRef]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; Editora UFV: Viçosa, MG, Brazil, 2002; 235p. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Goering, H.; Keith, H.; Van Soest, P.J. Forage Fiber Analyses: Apparatus, Reagents, Procedures, and Some Applications; USDA-ARS: Washington, DC, USA, 1970; 379p.
- Robertson, J.B.; Van Soest, P.J. The detergent system of analysis. In The Analysis of Dietary Fiber in Food; James, W.P.T., Theander, O., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 123–158. [Google Scholar]
- Cappelle, E.R.; Valadares, S.C.; Silva, J.F.C.; Cecon, P.R. Estimativas do valor energético a partir de características químicas e bromatológicas dos alimentos. Rev. Bras. Zootec. 2001, 30, 1837–1856. [Google Scholar] [CrossRef]
- Hall, M.B. Neutral Detergent-Soluble Carbohydrates: Nutritional Relevance and Analysis; University of Florida Extension–Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2000; 77p. [Google Scholar]
- Pryce, J.D. A modification of the Barker-Summeson method for the determination of lactic acid. Analyst 1969, 94, 1151–1152. [Google Scholar] [CrossRef]
- Kung, L.; Grieve, D.B.; Thomas, J.W.; Huber, J.T. Added ammonia or microbial inoculants for fermentation and nitrogenous compounds of alfalfa ensiled at various percents of dry matter. J. Dairy Sci. 1984, 67, 299–306. [Google Scholar] [CrossRef]
- Famme, P.; Knudsen, J. Direct gas chromatographic determination of short–chain (C2-C4) volatile fatty acids in aqueous solutions. Comp. Biochem. Physiol. 1984, 77, 617–618. [Google Scholar] [CrossRef]
- Supelco. Analyzing Fatty Acids by Packed Column Gas Chromatography; Sigma-Aldrich Co.: Bellefonte, PA, USA, 1998; 12p. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide 2009, 2nd ed.; SAS Institute Inc.: Cary, FL, USA, 2009; 176p. [Google Scholar]
- Castro-Montoya, J.M.; Dickhoefer, U. The nutritional value of tropical legume forages fed to ruminants as affected by their growth habit and fed form: A systematic review. Anim. Feed Sci. Technol. 2020, 269, 114641. [Google Scholar] [CrossRef]
- Lima, M.H.M.; Pires, D.A.A.; Moura, M.M.A.; Costa, R.F.; Rodrigues, J.A.S.; Alves, K.A. Nutritional characteristics of Sorghum hybrids hay (Sorghum sudanense vs. Sorghum bicolor). Acta Sci. Anim. Sci. 2017, 39, 229–234. [Google Scholar] [CrossRef]
- Andrade, C.A.O.; Borghi, E.; Bortolon, L.; Bortolon, E.S.O.; Camargo, F.P.; Avanzi, J.C.; Guarda, V.D.A.; Cunha, M.K.; Silva, R.R.; Fidelis, R.R. Forage production and bromatological composition of forage species intercropped with soybean. J. Agric. Sci. 2020, 12, 84–94. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, Y.; Shi, Y.; Shen, H.; Hu, H.; Luo, Y.; Xu, L.; Kang, J.; Xing, A.; Wang, S.; et al. Yield and quality properties of silage maize and their influencing factors in China. Sci. China Life Sci. 2022, 65, 1655–1666. [Google Scholar] [CrossRef]
- Kustantinah, K.; Suhartanto, B.; Indarto, E.; Zulfa, I.H.; Atmojo, F.A. Degradation of nitrogen fraction in Kacang goats feed supplementation Calliandra calothyrsus substituted soybean meal. Key Eng. Mater. 2020, 840, 118–123. [Google Scholar] [CrossRef]
- Campos, F.P.D.; Nussio, L.G.; Sarmento, P.; Daniel, J.L.P.; Lima, C.G.D. Effects of addition of different sources and doses of sugars on in vitro digestibilities of dry matter, fibre and cell wall monosaccharides of corn silage in ruminants. Animal 2020, 14, 1667–1675. [Google Scholar] [CrossRef]
- Costa, P.M.; Villela, S.D.J.; Leonel, F.D.P.; Araújo, S.A.D.C.; Araújo, K.G.; Ruas, J.R.M.; Coelho, F.S.; Andrade, V.R. Intercropping of corn, brachiaria grass and leguminous plants: Productivity, quality and composition of silages. Rev. Bras. Zootec. 2012, 41, 2144–2149. [Google Scholar] [CrossRef]
- Soe Htet, M.N.; Hai, J.B.; Bo, P.T.; Gong, X.W.; Liu, C.J.; Dang, K.; Tian, L.X.; Soomro, R.N.; Aung, K.L.; Feng, B.L. Evaluation of nutritive values through comparison of forage yield and silage quality of mono-cropped and intercropped maize-soybean harvested at two maturity stages. Agriculture 2021, 11, 452. [Google Scholar] [CrossRef]
- Detmann, E.; Valadares Filho, S.C. On the estimation of non-fibrous carbohydrates in feeds and diets. Arq. Bras. Med. Vet. Zootec. 2010, 62, 980–984. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Oliveira, J.F.A.; Jakelaitis, A.; Cabral Filho, S.L.S.; Silva, C.J.D.; Guimarães, K.C.; Pereira, L.S.; Sousa, G.D.; Oliveira, G.S.D. Silage quality from intercropping corn and soybean managed with inoculant Azospirillum brasilense and nitrogen fertilization. Rev. Bras. Saude Prod. Anim. 2021, 22, e2122092021. [Google Scholar] [CrossRef]
- Mota, A.D.S.; Rocha Júnior, V.R.; Souza, A.S.; Reis, S.T.; Tomich, T.R.; Caldeira, L.A.; Menezes, G.C.C.; Costa, M.D. Perfil de fermentação e perdas na ensilagem de diferentes frações da parte aérea de quatro variedades de mandioca. Rev. Bras. Zootec. 2011, 40, 1466–1473. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, Y.; Xin, Y.; Chen, C.; Du, Z.; Li, X.; Zhong, J.; Tahir, M.; Kang, B.; Jiang, D.; et al. Silage quality and output of different maize–soybean strip intercropping patterns. Fermentation 2022, 8, 174. [Google Scholar] [CrossRef]
- Macêdo, A.J.D.S.; Santos, E.M. Princípios básicos para produção de silagem. Arq. Ciênc. Vet. Zool. UNIPAR 2019, 22, 147–156. [Google Scholar] [CrossRef]
- Batista, K.; Giacomini, A.A.; Gerdes, L.; Mattos, W.T.D.; Otsuk, I.P. Potential interaction of soybean-grass intercropping with residual nitrogen for a no-tillage system implementation. Acta Sci. Agron. 2024, 46, e62944. [Google Scholar] [CrossRef]
| Parameters | Soy | AGG | CG | S + AGG | S + CG |
|---|---|---|---|---|---|
| DM (g kg−1) | 200.00 | 255.90 | 196.58 | 259.90 | 199.70 |
| CP (g kg−1) | 174.40 | 133.40 | 131.25 | 135.01 | 149.70 |
| CF (g kg−1) | 351.80 | 434.70 | 369.60 | 463.50 | 359.20 |
| NDF (g kg−1) | 719.50 | 765.00 | 724.15 | 820.20 | 709.10 |
| ADF (g kg−1) | 622.20 | 735.30 | 675.40 | 765.10 | 653.20 |
| EE (g kg−1) | 7.10 | 14.00 | 12.20 | 6.10 | 8.90 |
| MM (g kg−1) | 78.20 | 91.10 | 83.80 | 75.30 | 73.60 |
| NDIN (g kg−1) | 234.80 | 411.40 | 196.80 | 319.90 | 192.70 |
| ADIN (g kg−1) | - | 93.80 | 58.70 | 185.00 | 45.00 |
| Lignin (g kg−1) | 193.60 | 181.40 | 115.89 | 221.30 | 123.10 |
| Cellulose (g kg−1) | 428.60 | 553.90 | 559.50 | 543.80 | 530.00 |
| Hemicellulose (g kg−1) | 97.30 | 29.70 | 48.70 | 55.20 | 56.00 |
| IVDDM (g g−1) | 0.580 | 0.516 | 0.502 | 0.515 | 0.509 |
| IVDOM (g g−1) | 0.592 | 0.523 | 0.507 | 0.524 | 0.519 |
| TDN (g kg−1) | 538.50 | 427.30 | 421.10 | 407.60 | 443.30 |
| NFC (g kg−1) | 20.90 | - | 48.61 | - | 58.80 |
| Parameters | Means | Standard Error | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Soy | S + AGG | S + CG | Soy | S + AGG | S + CG | ||
| DM (g kg−1) | 179.40 b | 257.80 a | 191.20 b | 10.00 | 12.30 | 10.00 | 0.0013 |
| CP (g kg−1) | 139.00 a | 116.30 b | 154.30 a | 5.30 | 4.30 b | 4.30 | 0.0051 |
| CF (g kg−1) | 364.30 b | 449.30 a | 369.60 b | 11.30 | 13.80 | 11.30 | 0.0016 |
| NDF (g kg−1) | 662.90 b | 711.90 a | 648.80 b | 8.50 | 8.50 | 8.50 | 0.0002 |
| ADF (g kg−1) | 630.30 ab | 681.20 a | 604.60 b | 13.60 | 13.60 | 13.60 | 0.0140 |
| EE (g kg−1) | 26.40 a | 17.10 b | 25.30 a | 0.80 | 1.00 | 8.30 | 0.0005 |
| MM (g kg−1) | 81.40 b | 82.90 b | 119.80 a | 4.10 | 4.10 | 5.00 | 0.0029 |
| NDIN (g kg−1) | 84.50 b | 209.80 a | 93.30 b | 3.30 | 5.30 | 3.30 | 0.0001 |
| ADIN (g kg−1) | 69.90 b | 119.70 a | 68.30 b | 4.70 | 5.80 | 4.70 | 0.0003 |
| Lignin (g kg−1) | 96.50 b | 162.40 a | 148.10 ab | 10.80 | 10.80 | 10.80 | 0.0123 |
| Cellulose (g kg−1) | 531.40 a | 534.90 a | 446.50 b | 59.00 | 59.00 | 59.00 | 0.0035 |
| Hemicellulose (g kg−1) | 416.00 b | 223.00 c | 518.00 a | 35.00 | 43.00 | 35.00 | 0.0366 |
| IVDDM (g g−1) | 0.543 | 0.541 | 0.543 | 0.0004 | 0.0006 | 0.0004 | 0.9957 |
| IVDOM (g g−1) | 0.551 | 0.547 | 0.555 | 0.0024 | 0.0037 | 0.0024 | 0.8634 |
| TDN (g kg−1) | 504.60 | 467.30 | 515.20 | 14.10 | 14.10 | 14.10 | 0.0731 |
| NFC (g kg−1) | 85.10 | 69.50 | 62.80 | 12.60 | 12.60 | 12.60 | 0.4040 |
| Parameters | Means | Standard Error | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Soy | S + AGG | S + CG | Soy | S + AGG | S + CG | ||
| DM losses (%) | 2.24 b | 6.52 a | 1.15 b | 0.26 | 0.32 | 0.26 | 0.0001 |
| Density (kg m−3) | 591.85 b | 554.14 b | 661.36 a | 10.88 | 13.33 | 10.88 | 0.0002 |
| pH value | 5.52 a | 4.97 b | 4.63 b | 0.08 | 0.08 | 0.08 | 0.0001 |
| Lactic acid (mg mL−1) | 0.889 | 1.099 | 0.922 | 0.08 | 0.08 | 0.08 | 0.2091 |
| Acetic acid (mM) | 21.41 | 21.09 | 17.42 | 1.93 | 1.93 | 1.93 | 0.2624 |
| Propionic acid (mM) | 0.014 a | 0.011 ab | 0.010 b | 0.00 | 0.00 | 0.00 | 0.007 |
| Butyric acid (mM) | 1.218 | 1.154 | 1.170 | 0.10 | 0.10 | 0.10 | 0.8617 |
| Ammonia-N (% of total N) | 4.88 a | 4.08 ab | 3.262 b | 0.22 | 0.22 | 0.22 | 0.0008 |
| Soybeans | Grasses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Means | Standard Error | p-Value | Means | Standard Error | p-Value | ||||
| S + AGG | S + CG | S + AGG | S + CG | S + AGG | S + CG | S + AGG | S + CG | |||
| DM (%) | 3.19 b | 12.75 a | 0.07 | 0.07 | 0.0001 | 96.81 a | 87.25 b | 0.07 | 0.07 | 0.0001 |
| CP (%) | 5.00 b | 15.39 a | 0.10 | 0.10 | 0.0001 | 94.99 a | 84.62 b | 0.10 | 0.10 | 0.0001 |
| CF (%) | 3.58 b | 12.80 a | 0.06 | 0.06 | 0.0001 | 96.42 a | 87.20 b | 0.06 | 0.06 | 0.0001 |
| NDF (%) | 3.25 b | 10.91 a | 0.16 | 0.16 | 0.0001 | 96.72 a | 89.27 b | 0.06 | 0.06 | 0.0001 |
| ADF (%) | 3.07 b | 10.65 a | 0.13 | 0.13 | 0.0001 | 96.93 a | 89.35 b | 0.13 | 0.13 | 0.0001 |
| EE (%) | 5.77 b | 19.18 a | 0.72 | 0.72 | 0.0001 | 94.23 a | 80.82 b | 0.72 | 0.72 | 0.0001 |
| MM (%) | 5.56 b | 18.08 a | 0.13 | 0.13 | 0.0001 | 94.44 a | 81.92 b | 0.13 | 0.13 | 0.0001 |
| Lignin (%) | 3.91 b | 16.97 a | 0.28 | 0.28 | 0.0001 | 96.09 a | 83.03 b | 0.78 | 0.78 | 0.0001 |
| NDIN (%) | 0.81 b | 5.25 a | 0.20 | 0.20 | 0.0001 | 99.19 a | 94.75 b | 0.20 | 0.20 | 0.0001 |
| ADIN (%) | 2.61 b | 13.77 a | 0.78 | 0.78 | 0.0001 | 97.39 a | 86.23 b | 0.17 | 0.17 | 0.0001 |
| Cellulose (%) | 2.81 b | 8.95 a | 0.08 | 0.08 | 0.0001 | 97.19 a | 91.05 b | 0.08 | 0.08 | 0.0001 |
| Hemicellulose (%) | 8.01 b | 14.56 a | 0.59 | 0.59 | 0.0015 | 91.99 a | 85.44 b | 0.59 | 0.59 | 0.0015 |
| Soybeans | Grasses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Means | Standard Error | p-Value | Means | Standard Error | p-Value | ||||
| S + AGG | S + CG | S + AGG | S + CG | S + AGG | S + CG | S + AGG | S + CG | |||
| DM (%) | 0.83 b | 2.32 a | 0.04 | 0.04 | 0.0001 | 25.45 a | 15.97 b | 0.07 | 0.07 | 0.0001 |
| CP (%) | 0.49 b | 2.24 a | 0.09 | 0.09 | 0.0001 | 9.27 b | 12.30 a | 0.56 | 0.56 | 0.0028 |
| CF (%) | 1.67 b | 4.54 a | 0.09 | 0.09 | 0.0001 | 44.91 a | 30.93 b | 1.15 | 1.15 | 0.0001 |
| NDF (%) | 2.43 b | 7.15 a | 0.10 | 0.10 | 0.0001 | 72.53 a | 58.36 b | 1.50 | 1.50 | 0.0001 |
| ADF (%) | 2.22 b | 6.57 a | 0.09 | 0.09 | 0.0001 | 70.16 a | 55.20 b | 1.66 | 1.66 | 0.0001 |
| EE (%) | 0.10 b | 0.53 a | 0.02 | 0.02 | 0.0001 | 1.57 b | 2.11 a | 0.11 | 0.11 | 0.0036 |
| MM (%) | 0.46 b | 1.47 a | 0.13 | 0.13 | 0.0001 | 7.79 | 7.38 | 0.71 | 0.71 | 0.6541 |
| Lignin (%) | 0.75 b | 1.66 a | 0.11 | 0.14 | 0.0006 | 20.30 a | 8.61 b | 1.25 | 0.97 | 0.0001 |
| NDIN (%) | 0.16 b | 0.46 a | 0.01 | 0.01 | 0.0001 | 18.62 a | 8.69 b | 0.28 | 0.28 | 0.0012 |
| ADIN (%) | 0.32 b | 0.86 a | 0.01 | 0.01 | 0.0001 | 11.97 a | 5.86 b | 0.58 | 0.58 | 0.0002 |
| Cellulose (%) | 1.50 b | 4.59 a | 0.19 | 0.19 | 0.0001 | 51.76 | 46.68 | 2.25 | 2.25 | 0.1072 |
| Hemicellulose (%) | 0.20 b | 0.55 a | 0.01 | 0.01 | 0.0001 | 2.38 b | 4.21 a | 0.22 | 0.22 | 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, K.; de Campos, F.P. Qualitative Production of Mixture Silage within a Sustainable Concept. Sustainability 2024, 16, 6398. https://doi.org/10.3390/su16156398
Batista K, de Campos FP. Qualitative Production of Mixture Silage within a Sustainable Concept. Sustainability. 2024; 16(15):6398. https://doi.org/10.3390/su16156398
Chicago/Turabian StyleBatista, Karina, and Fábio Prudêncio de Campos. 2024. "Qualitative Production of Mixture Silage within a Sustainable Concept" Sustainability 16, no. 15: 6398. https://doi.org/10.3390/su16156398





