Mitigating UV-Induced Degradation in Solar Panels through ZnO Nanocomposite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
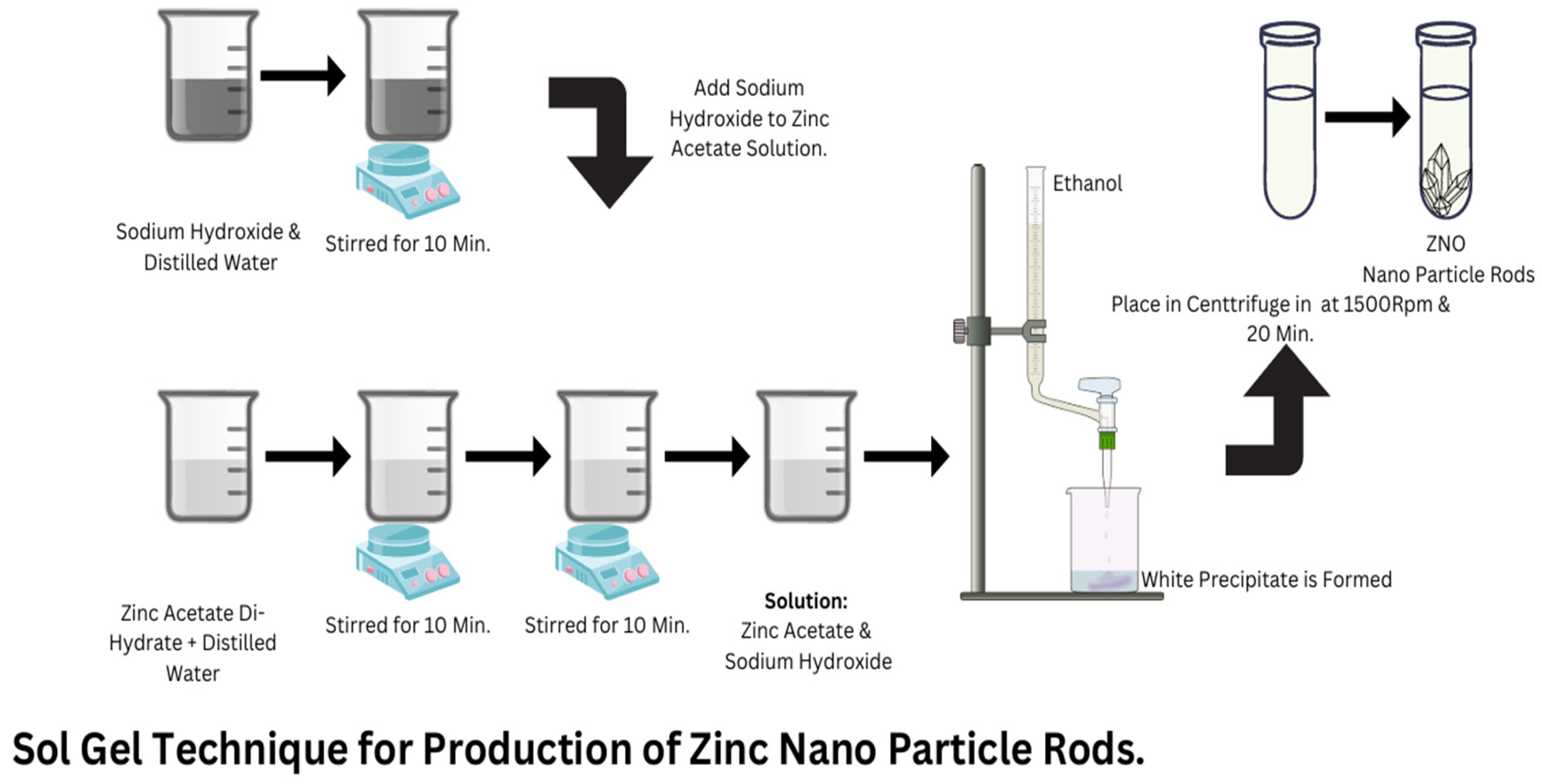
2.2.1. Synthesis of ZnO Nano-Powder
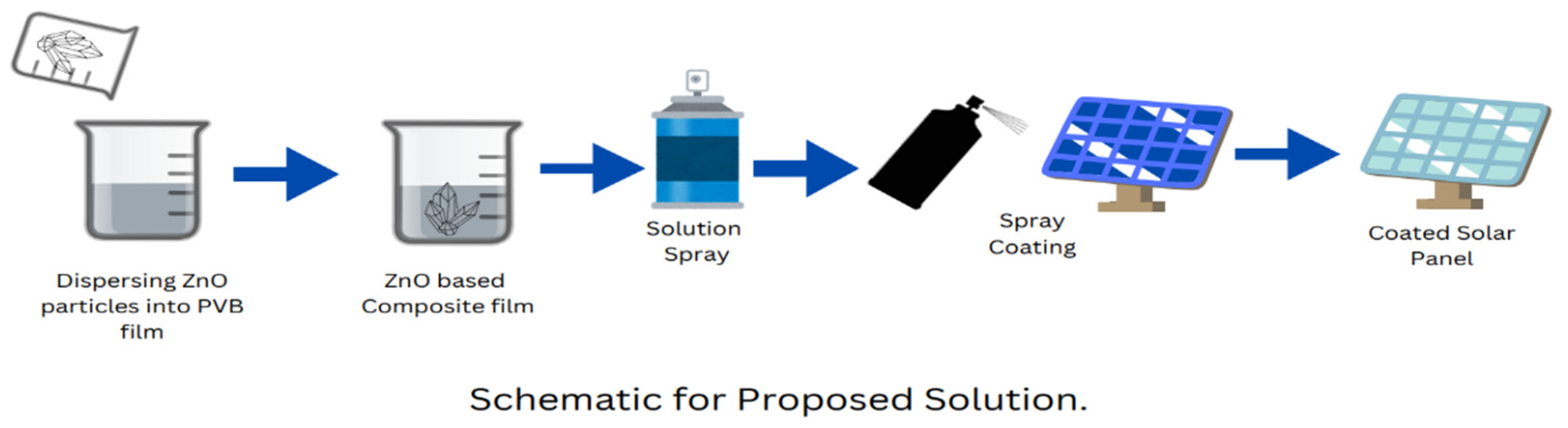
2.2.2. Synthesis of PVB/ZnO-Based Nanocomposite Films
3. Characterization Techniques
3.1. Scanning Electron Microscopy (SEM)
3.2. X-ray Diffraction (XRD)
3.3. Fourier-Transform Infrared (FTIR) Spectroscopic Analysis
3.4. Ultraviolet-Visible (UV-Vis) Spectroscopic Analysis
3.5. Contact Angle Measurements
3.6. Solar Panel Stability Testing
4. Results and Discussion
4.1. Morphological Analysis
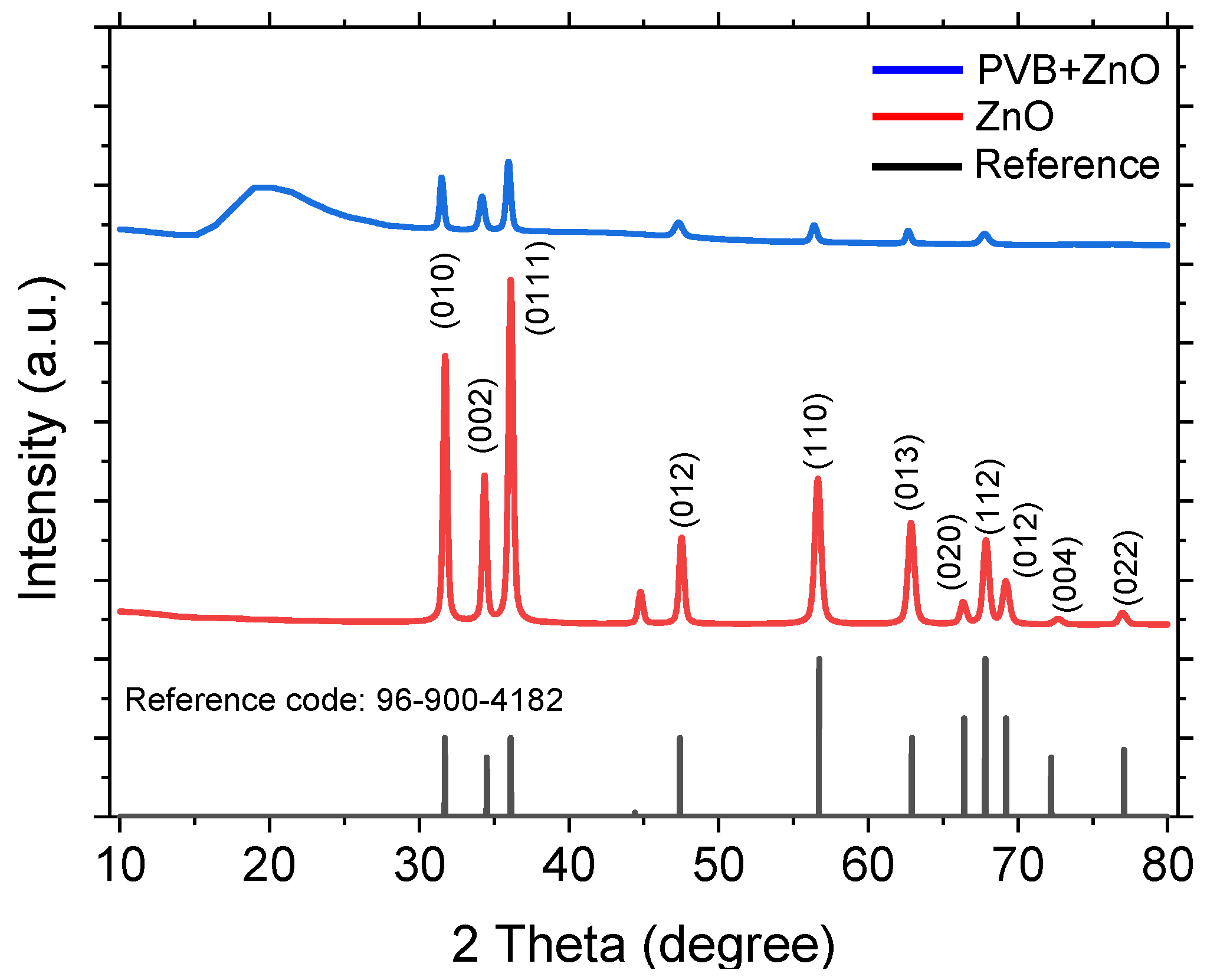
4.2. XRD X-ray Diffraction (XRD) Analysis
4.3. Fourier-Transform Infrared (FTIR) Spectroscopic Analysis
4.4. Ultraviolet-Visible Light (UV-Vis) Spectroscopic Analysis
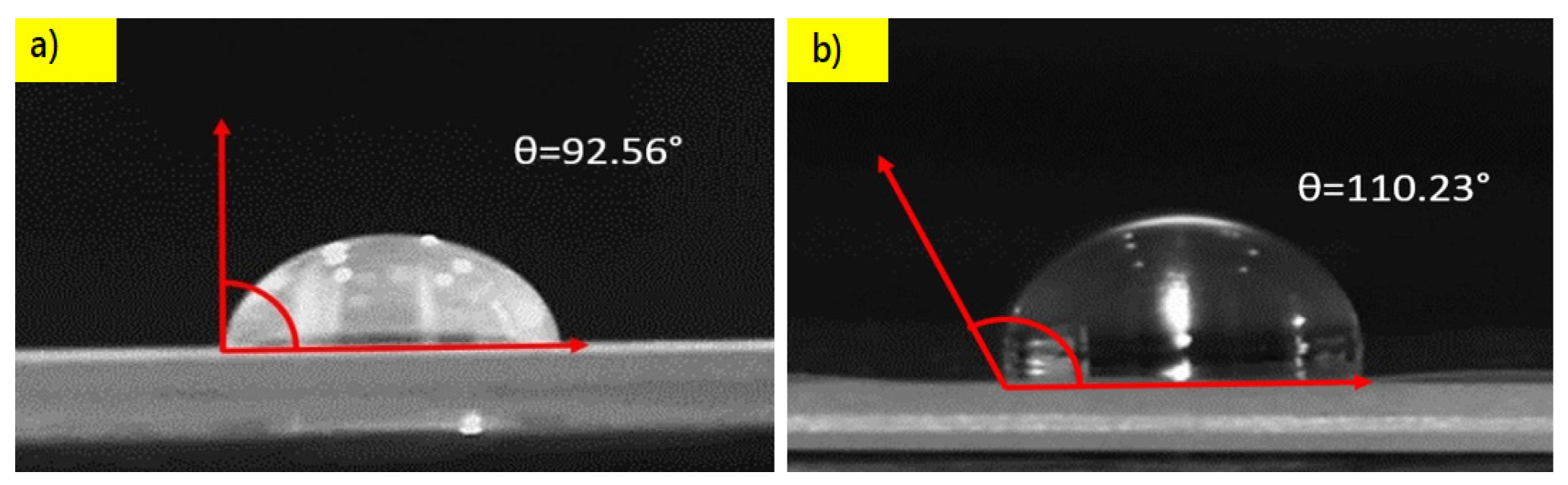
4.5. Contact Angle Measurements
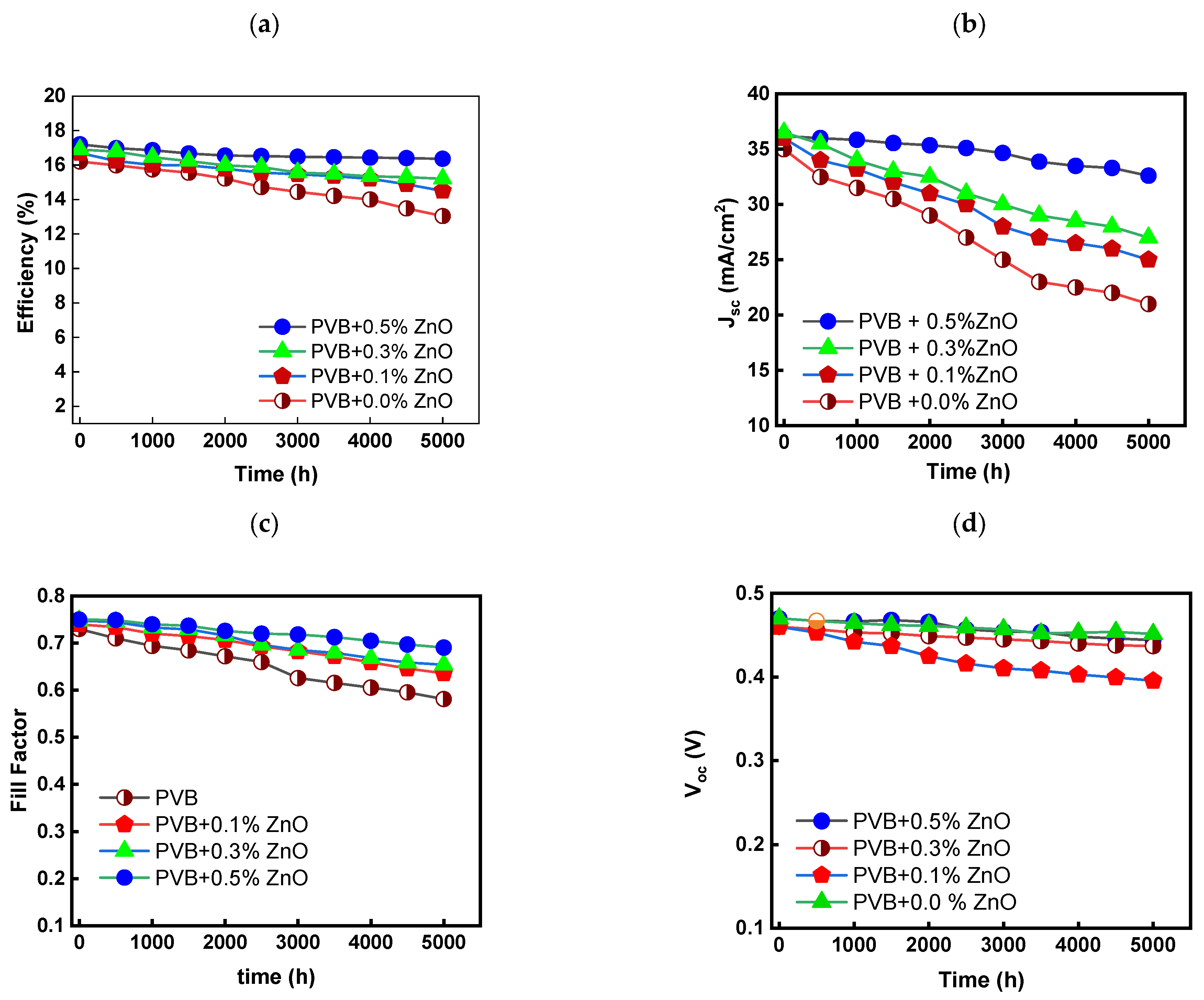
4.6. Solar Cell Performance (Sun Test)
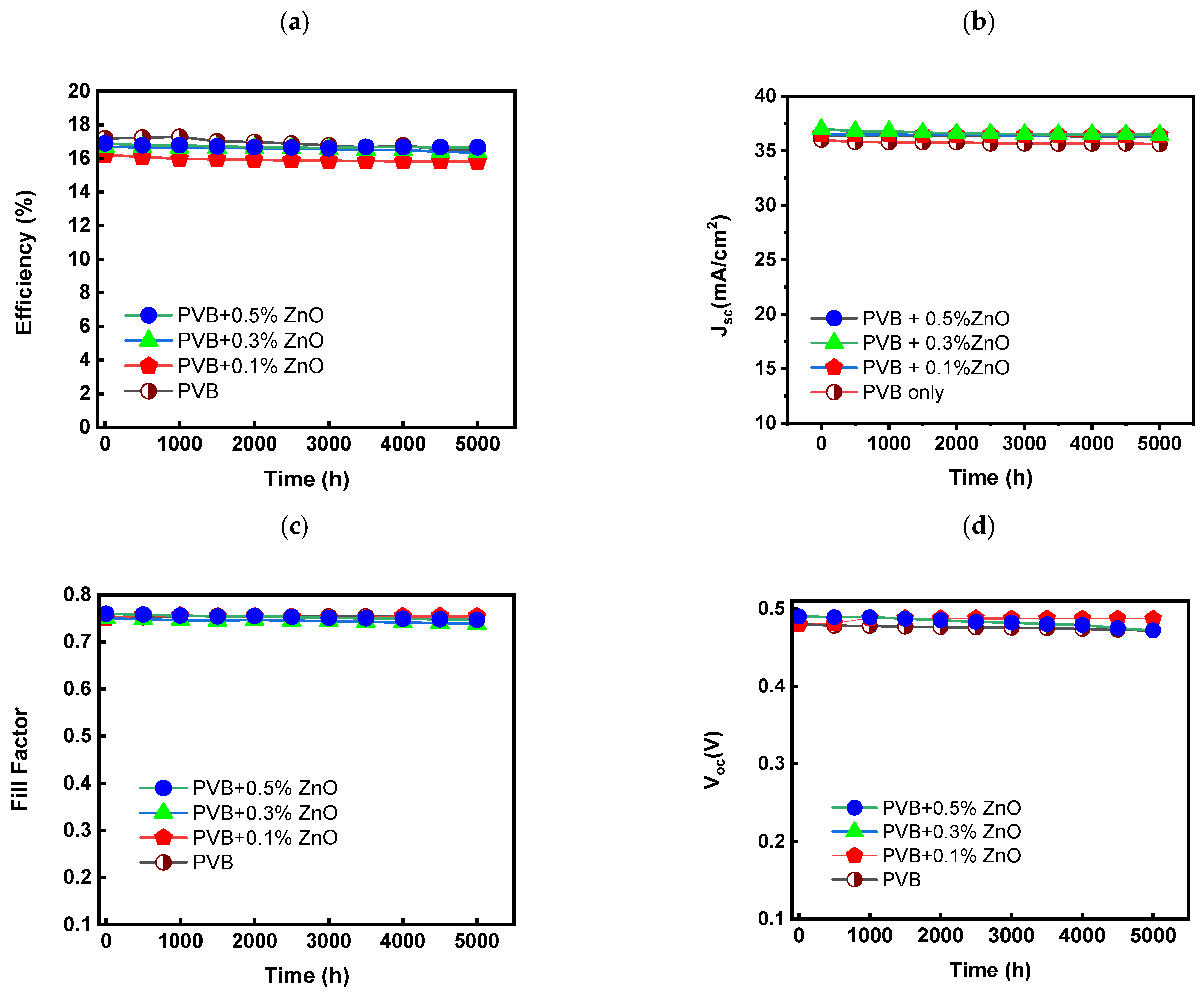
4.7. Solar Cell Performance (Damp Heat Test)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beauchamp, W.; Tuttle-Hart, T. UV/IR Reflecting Solar Cell Cover. U.S. Patent 5, 449, 413, 12 September 1995. [Google Scholar]
- Liu, W.-D.; Yin, L.-C.; Li, L.; Yang, Q.; Wang, D.-Z.; Li, M.; Shi, X.-L.; Liu, Q.; Bai, Y.; Gentle, I.; et al. Grain boundary re-crystallization and sub-nano regions leading to high plateau figure of merit for Bi2Te3 nanoflakes. Energy Environ. Sci. 2023, 16, 5123–5135. [Google Scholar] [CrossRef]
- Koehl, M.; Philipp, D.; Lenck, N.; Zundel, M. Development and application of a UV light source for PV-module testing. Reliab. Photovolt. Cells Modul. Compon. Syst. II 2009, 7412, 741202. [Google Scholar]
- Cao, M.; Ji, W.; Chao, C.; Li, J.; Dai, F.; Fan, X. Recent Advances in UV-Cured Encapsulation for Stable and Durable Perovskite Solar Cell Devices. Polymers 2023, 15, 3911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Wu, Z.; Luo, D.; Yu, H.Y.; Lu, Z.H. Review and perspective of materials for flexible solar cells. Mater. Rep. Energy 2021, 1, 100001. [Google Scholar] [CrossRef]
- Grüneberger, F.; Künniger, T.; Huch, A.; Zimmermann, T.; Arnold, M. Nanofibrillated cellulose in wood coatings: Dispersion and stabilization of ZnO as UV absorber. Prog. Org. Coat. 2015, 87, 112–121. [Google Scholar] [CrossRef]
- Jiamprasertboon, A.; Dixon, S.C.; Sathasivam, S.; Powell, M.J.; Lu, Y.; Siritanon, T.; Carmalt, C.J. Low-Cost One-Step Fabrication of Highly Conductive ZnO:Cl Transparent Thin Films with Tunable Photocatalytic Properties via Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Electron. Mater. 2019, 1, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Canto-Aguilar, E.J.; Rodríguez-Pérez, M.; García-Rodríguez, R.; Lizama-Tzec, F.I.; De Denko, A.T.; Osterloh, F.E.; Oskam, G. ZnO-based dye-sensitized solar cells: Effects of redox couple and dye aggregation. Electrochim. Acta 2017, 258, 396–404. [Google Scholar] [CrossRef]
- Parui, S.S.; Kumar, N.; Tiwari, P.; Tiwari, N.; Chauhan, R.N. Zinc oxide and cupric oxide based thin films for solar cell applications. Mater. Today Proc. 2019, 41, 233–236. [Google Scholar] [CrossRef]
- Chen, Y. Review of ZnO Transparent Conducting Oxides for solar applications. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012170. [Google Scholar] [CrossRef]
- Akhta, M.S.; Riaz, S.; Noor, R.; Naseem, S. Optical and structural properties of ZnO thin films for solar cell applications. Adv. Sci. Lett. 2013, 19, 834–838. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, A.; Virdi, A. Effect of single and double layer antireflection coating to enhance photovoltaic efficiency of silicon solar. J. Nano- Electron. Phys. 2017, 9, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Bouhafs, D.; Moussi, A.; Chikouche, A.; Ruiz, J.M. Design and simulation of antireflection coating systems for optoelectronic devices: Application to silicon solar cells. Sol. Energy Mater. Sol. Cells 1998, 52, 79–93. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mourad, A.H.I.; Pervez, H.; Surkatti, R. Recent developments in multifunctional coatings for solar panel applications: A review. Sol. Energy Mater. Sol. Cells 2019, 189, 75–102. [Google Scholar] [CrossRef]
- Minemoto, T.; Mizuta, T.; Takakura, H.; Hamakawa, Y. Antireflective coating fabricated by chemical deposition of ZnO for spherical Si solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 191–194. [Google Scholar] [CrossRef]
- Sarkın, A.S.; Ekren, N.; Sağlam, Ş. A review of anti-reflection and self-cleaning coatings on photovoltaic panels. Sol. Energy 2020, 199, 63–73. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Yang, X.; Shi, J.; Yan, L.; Xu, L.; Wu, Z.; Chen, R.; Peng, J.; Kang, J.; et al. Damp-Heat-Stable, High-Efficiency, Industrial-Size Silicon Heterojunction Solar Cells. Joule 2020, 4, 913–927. [Google Scholar] [CrossRef]
- Karas, J.; Sinha, A.; Buddha, V.S.P.; Li, F.; Moghadam, F.; Tamizhmani, G.; Bowden, S.; Augusto, A. Damp Heat Induced Degradation of Silicon Heterojunction Solar Cells with Cu-Plated Contacts. IEEE J. Photovolt. 2020, 10, 153–158. [Google Scholar] [CrossRef]
- Stuckelberger, M.; Riesen, Y.; Despeisse, M.; Schüttauf, J.W.; Haug, F.J.; Ballif, C. Light-induced Vocincrease and decrease in high-efficiency amorphous silicon solar cells. J. Appl. Phys. 2014, 116, 094503. [Google Scholar] [CrossRef]
- Singh, V.P.; Das, D.; Rath, C. Studies on intrinsic defects related to Zn vacancy in ZnO nanoparticles. Mater. Res. Bull. 2013, 48, 682–686. [Google Scholar] [CrossRef]
- Nagarani, N.; Smgcw, P.; Vasu, V. Structural and Optical Characterization of ZnO thin films by Sol- Gel Method. J. Photonics Spintron. 2013, 2, 19–21. [Google Scholar]
- Bukhari, S.N.U.S.; Shah, A.A.; Bhatti, M.A.; Tahira, A.; Channa, I.A.; Shah, A.K.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S.; Ibhupoto, Z.H.; et al. Psyllium-Husk-Assisted Synthesis of ZnO Microstructures with Improved Photocatalytic Properties for the Degradation of Methylene Blue (MB). Nanomaterials 2022, 12, 3568. [Google Scholar] [CrossRef] [PubMed]
- Idiawati, R.; Mufti, N.; Taufiq, A.; Wisodo, H.; Laila, I.K.R.; Fuad, A.; Sunaryono. Effect of Growth Time on the Characteristics of ZnO Nanorods. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012050. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Wu, H.; Guo, S. Control of the formation of rod-like ZnO mesocrystals and their photocatalytic properties. CrystEngComm 2013, 15, 2608–2615. [Google Scholar] [CrossRef]
- Chong, X.; Li, L.; Yan, X.; Hu, D.; Li, H.; Wang, Y. Synthesis, characterization and room temperature photoluminescence properties of Al doped ZnO nanorods. Phys. E Low-Dimens. Syst. Nanostructures 2012, 44, 1399–1405. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.G.; Ucer, K.B.; Williams, R.T. Photoluminescence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi Curr. Top. Solid State Phys. 2006, 3, 3577–3581. [Google Scholar]
- Abbasi, H.Y.; Habib, A.; Tanveer, M. Synthesis and characterization of nanostructures of ZnO and ZnO/Graphene composites for the application in hybrid solar cells. J. Alloys Compd. 2017, 690, 21–26. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band Gap Narrowing and Widening of ZnO Nanostructures and Doped Materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Chandrashekar, B.N.; Sadasivuni, K.K.; Yamani, Z.H.; Cheng, C.; Byrappa, K. Heterogeneous growth mechanism of ZnO nanostructures and the effects of their morphology on optical and photocatalytic properties. CrystEngComm 2017, 19, 3299–3312. [Google Scholar] [CrossRef]
- Al-Hardan, N.H.; Hamid, M.A.A.; Ahmed, N.M.; Jalar, A.; Shamsudin, R.; Othman, N.K.; Keng, L.K.; Mohammed, S.M. A Study on the UV Photoresponse of Hydrothermally Grown Zinc Oxide Nanorods with Different Aspect Ratios. IEEE Sens. J. 2015, 15, 6811–6818. [Google Scholar] [CrossRef]
- Ulianova, V.; Zazerin, A.; Pashkevich, G.; Bogdan, O.; Orlov, A. High-performance ultraviolet radiation sensors based on zinc oxide nanorods. Sens. Actuators A Phys. 2015, 234, 113–119. [Google Scholar] [CrossRef]
- Lopez-Garcia, A.J.; Bauer, A.; Rubio, R.F.; Payno, D.; Li-Kao, Z.J.; Kazim, S.; Hariskos, D.; Izquierdo-Roca, V.; Saucedo, E.; Pérez-Rodríguez, A. UV-Selective Optically Transparent Zn(O,S)-Based Solar Cells. Sol. RRL 2020, 4, 2000470. [Google Scholar] [CrossRef]
- Chandio, A.D.; Pato, A.H.; Channa, I.A.; Gilani, S.J.; Shah, A.A.; Ashfaq, J.; Buledi, J.A.; Chandio, I.A.; Jumah, M.N. Bin Exploring the Heterocatalytic Proficiencies of ZnO Nanostructures in the Simultaneous Photo-Degradation of Chlorophenols. Sustainability 2022, 14, 14562. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Nasser, M. UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Khoirunnisa, A.R.; Joni, I.M.; Panatarani, C.; Rochima, E.; Praseptiangga, D. UV-screening, transparency and water barrier properties of semi refined iota carrageenan packaging film incorporated with ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 3–8. [Google Scholar]
- Vyas, S. A short review on properties and applications of zinc oxide based thin films and devices: ZnO as a promising material for applications in electronics, optoelectronics, biomedical and sensors. Johns. Matthey Technol. Rev. 2020, 64, 202–218. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, G.; Cheng, F.; Lei, H.; Wang, W.; Qin, P.; Zhou, H. Hybrid graphene-ZnO nanocomposites as electron acceptor in polymer-based bulk-heterojunction organic photovoltaics. J. Phys. D. Appl. Phys. 2012, 45, 455103. [Google Scholar] [CrossRef]
- Kenanakis, G.; Vernardou, D.; Koudoumas, E.; Katsarakis, N. Growth of c-axis oriented ZnO nanowires from aqueous solution: The decisive role of a seed layer for controlling the wires’ diameter. J. Cryst. Growth 2009, 311, 4799–4804. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Scharfe, B.; Feroze, S.; Forberich, K.; Lipovšek, B.; Brabec, C.J.; Egelhaaf, H.J. Solution processed oxygen and moisture barrier based on glass flakes for encapsulation of organic (opto-) electronic devices. Flex. Print. Electron. 2021, 6, 25006. [Google Scholar] [CrossRef]
- Dosmailov, M.; Leonat, L.N.; Patek, J.; Roth, D.; Bauer, P.; Scharber, M.C.; Sariciftci, N.S.; Pedarnig, J.D. Transparent conductive ZnO layers on polymer substrates: Thin film deposition and application in organic solar cells. Thin Solid Film. 2015, 591, 97–104. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Poxson, D.J.; Kim, Y.S.; Mont, F.W.; Kim, J.K.; Schubert, E.F.; Lin, S.-Y. Realization of a near-perfect antireflection coating for silicon solar energy utilization. Opt. Lett. 2008, 33, 2527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ahmad, I.; Leung, T.; Lin, J.; Chen, W.; Liu, F.; Ng, A.M.C.; Zhang, Y.; Djurišić, A.B. Encapsulation and Stability Testing of Perovskite Solar Cells for Real Life Applications. ACS Mater. Au 2021, 2, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Hayat, Z.; Zhang, D.D.; Li, M.Y.; Hu, S.; Wu, Q.; Cao, Y.F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- de Primo, J.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal From Wastewater. Front. Chem. 2020, 8, 571790. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Bhatti, M.A.; Tahira, A.; Chandio, A.D.; Channa, I.A.; Sahito, A.G.; Chalangar, E.; Willander, M.; Nur, O.; Ibupoto, Z.H. Facile synthesis of copper doped ZnO nanorods for the efficient photo degradation of methylene blue and methyl orange. Ceram. Int. 2020, 46, 9997–10005. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-ray Microanalysis; Springer US: New York, NY, USA, 1981. [Google Scholar]
- Lupan, O.; Chow, L.; Ono, L.K.; Cuenya, B.R.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Synthesis and characterization of ag- or sb-doped zno nanorods by a facile hydrothermal route. J. Phys. Chem. C 2010, 114, 12401–12408. [Google Scholar] [CrossRef]
- Polsongkram, D.; Chamninok, P.; Pukird, S.; Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Effect of synthesis conditions on the growth of ZnO nanorods via hydrothermal method. Phys. B Condens. Matter 2008, 403, 3713–3717. [Google Scholar] [CrossRef]
- Chae, D.W.; Kim, B.C. Characterization on polystyrene/zinc oxide nanocomposites prepared from solution mixing. Polym. Adv. Technol. 2005, 16, 846–850. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Almani, K.F.; Shah, A.A.; Tahira, A.; Channa, I.A.; Aftab, U.; Ibupoto, M.H.; Mirjat, A.N.; Aboelmaaref, A.; Nafady, A.; et al. Renewable and eco-friendly ZnO immobilized onto dead sea sponge floating materials with dual practical aspects for enhanced photocatalysis and disinfection applications. Nanotechnology 2022, 34, 035602. [Google Scholar] [CrossRef] [PubMed]
- Ujjan, Z.A.; Bhatti, M.A.; Shah, A.A.; Tahira, A.; Shaikh, N.M.; Kumar, S.; Mugheri, A.Q.; Medany, S.S.; Nafady, A.; Alnjiman, F.; et al. Simultaneous doping of sulfur and chloride ions into ZnO nanorods for improved photocatalytic properties towards degradation of methylene blue. Ceram. Int. 2022, 48, 5535–5545. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Almaani, K.F.; Shah, A.A.; Tahira, A.; Chandio, A.D.; Mugheri, A.Q.; Bhatti, A.L.; Waryani, B.; Medany, S.S.; Nafady, A.; et al. Low Temperature Aqueous Chemical Growth Method for the Doping of W into ZnO Nanostructures and Their Photocatalytic Role in the Degradration of Methylene Blue. J. Clust. Sci. 2022, 33, 1445–1456. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Leontie, L.; Doroftei, C.; Carlescu, A.; Bulai, G.; Mothudi, B.; Zorkani, I.; Jorio, A. Microstructural, FTIR and Raman spectroscopic study of Rare earth doped ZnO nanostructures. Mater. Today Proc. 2021, 53, 319–323. [Google Scholar] [CrossRef]
- Djelloul, A.; Aida, M.S.; Bougdira, J. Photoluminescence, FTIR and X-ray diffraction studies on undoped and Al-doped ZnO thin films grown on polycrystalline α-alumina substrates by ultrasonic spray pyrolysis. J. Lumin. 2010, 130, 2113–2117. [Google Scholar] [CrossRef]
- Scarano, D.; Spoto, G.; Bordiga, S.; Zecchina, A.; Lamberti, C. Lateral interactions in CO adlayers on prismatic ZnO faces: A FTIR and HRTEM study. Surf. Sci. Lett. 1992, 276, A11–A12. [Google Scholar] [CrossRef]
- Raja, K.; Ramesh, P.S.; Geetha, D. Structural, FTIR and photoluminescence studies of Fe doped ZnO nanopowder by co-precipitation method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, S.; Gopalakrishnan, R. Structural, FTIR and photoluminescence studies of Cu doped ZnO nanopowders by co-precipitation method. Opt. Mater. 2012, 34, 1946–1953. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.; Yao, T. ZnO as a novel photonic material for the UV region. Mater. Sci. Eng. B 2000, 75, 190–198. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Zaiser, M.; Brabec, C.J.; Egelhaaf, H.-J. Thin Film Encapsulation of Organic Solar Cells by Direct Deposition of Polysilazanes from Solution. Adv. Energy Mater. 2019, 9, 1900598. [Google Scholar] [CrossRef]
- Abdul Jabbar, G.A.H.; Saeed, A.A.; AL-Kadhemy, M.F.H. Impact of ZnO Nanoparticle on the Structural and Optical Properties of Poly(vinyl alcohol) Film. Al-Mustansiriyah J. Sci. 2022, 33, 153–161. [Google Scholar] [CrossRef]
- Moon, T.H.; Jeong, M.C.; Lee, W.; Myoung, J.M. The fabrication and characterization of ZnO UV detector. Appl. Surf. Sci. 2005, 240, 280–285. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Aono, M. ZnO-based ultraviolet photodetectors. Sensors 2010, 10, 8604–8634. [Google Scholar] [CrossRef] [PubMed]
- Lü, N.; Lü, X.; Jin, X.; Lü, C. Preparation and characterization of UV-curable ZnO/polymer nanocomposite films. Polym. Int. 2007, 56, 138–143. [Google Scholar] [CrossRef]
- Aberle, A.G. Thin-film solar cells. Thin Solid Films 2009, 517, 4706–4710. [Google Scholar] [CrossRef]
- Omazic, A.; Oreski, G.; Halwachs, M.; Eder, G.C.; Hirschl, C.; Neumaier, L.; Pinter, G.; Erceg, M. Relation between degradation of polymeric components in crystalline silicon PV module and climatic conditions: A literature review. Sol. Energy Mater. Sol. Cells 2019, 192, 123–133. [Google Scholar] [CrossRef]
- Theelen, M.; Beyeler, K.; Steijvers, H.; Barreau, N. Stability of CIGS solar cells under illumination with damp heat and dry heat: A comparison. Sol. Energy Mater. Sol. Cells 2017, 166, 262–268. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaffar, A.; Channa, I.A.; Chandio, A.D. Mitigating UV-Induced Degradation in Solar Panels through ZnO Nanocomposite Coatings. Sustainability 2024, 16, 6538. https://doi.org/10.3390/su16156538
Ghaffar A, Channa IA, Chandio AD. Mitigating UV-Induced Degradation in Solar Panels through ZnO Nanocomposite Coatings. Sustainability. 2024; 16(15):6538. https://doi.org/10.3390/su16156538
Chicago/Turabian StyleGhaffar, Abdul, Iftikhar Ahmed Channa, and Ali Dad Chandio. 2024. "Mitigating UV-Induced Degradation in Solar Panels through ZnO Nanocomposite Coatings" Sustainability 16, no. 15: 6538. https://doi.org/10.3390/su16156538
APA StyleGhaffar, A., Channa, I. A., & Chandio, A. D. (2024). Mitigating UV-Induced Degradation in Solar Panels through ZnO Nanocomposite Coatings. Sustainability, 16(15), 6538. https://doi.org/10.3390/su16156538






