Co-Pyrolysis of Mushroom Residue Blended with Pine Sawdust/Wheat Straw for Sustainable Utilization of Biomass Wastes: Thermal Characteristics, Kinetic/Thermodynamic Analysis, and Structure Evolution of Co-Pyrolytic Char
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Pyrolysis Experiments
2.3. Kinetic Method
2.4. Thermodynamic Parameter Calculation
2.5. Char Characterization
3. Results and Discussion
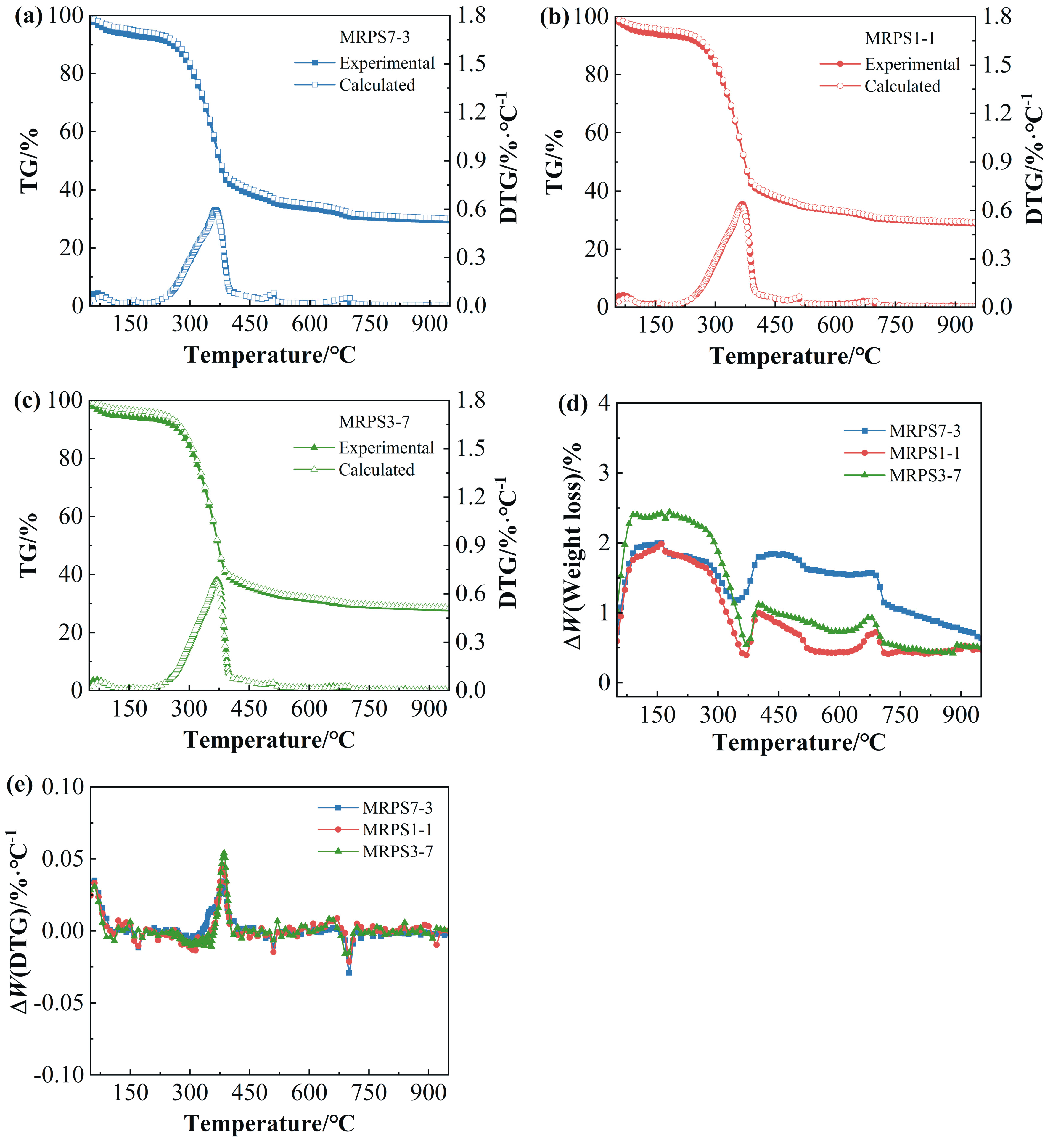
3.1. Thermal Characteristics
3.1.1. Individual Feedstocks
3.1.2. Blended Feedstocks
3.2. Interactions between MR and PS/WS
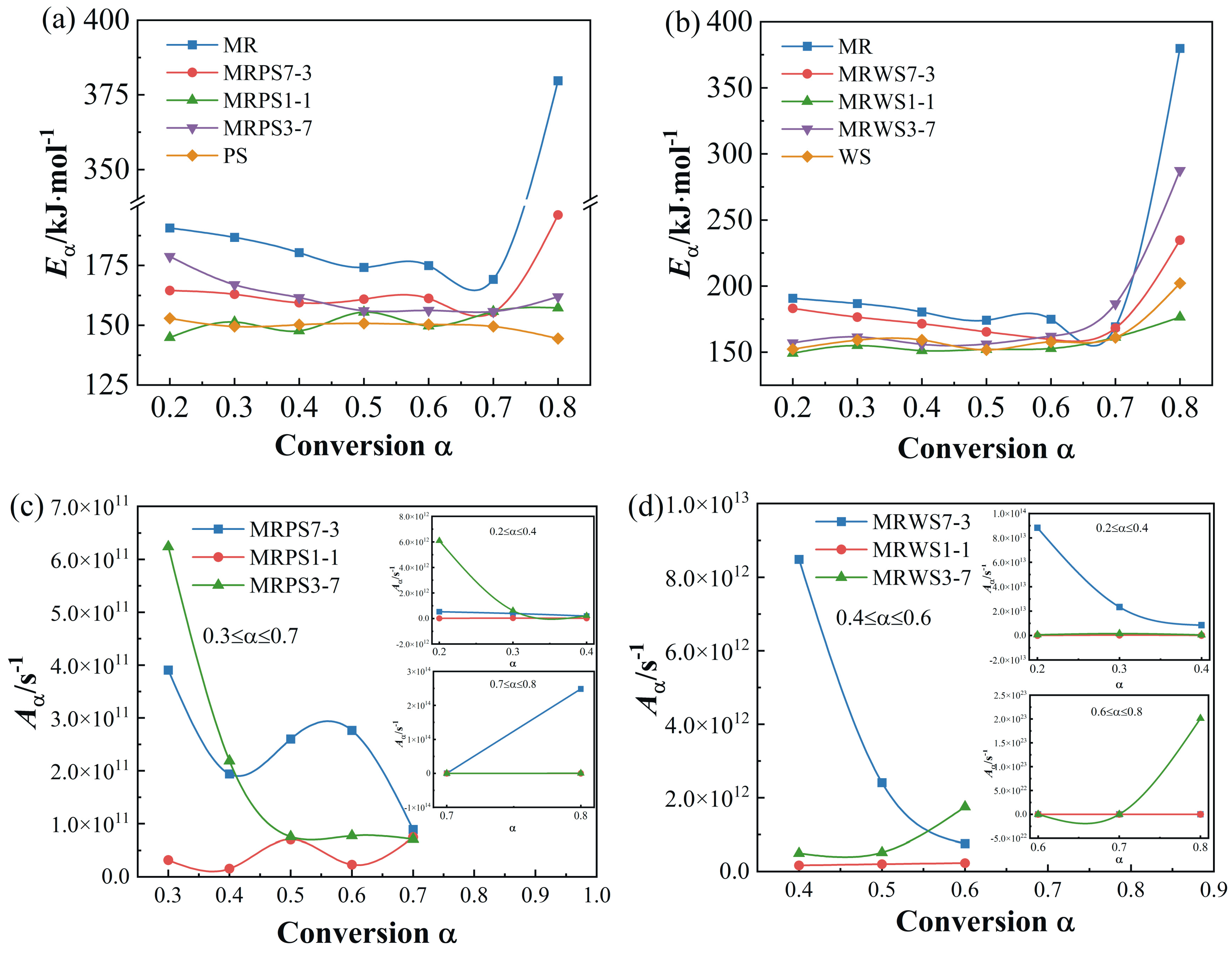
3.3. Kinetic Analysis
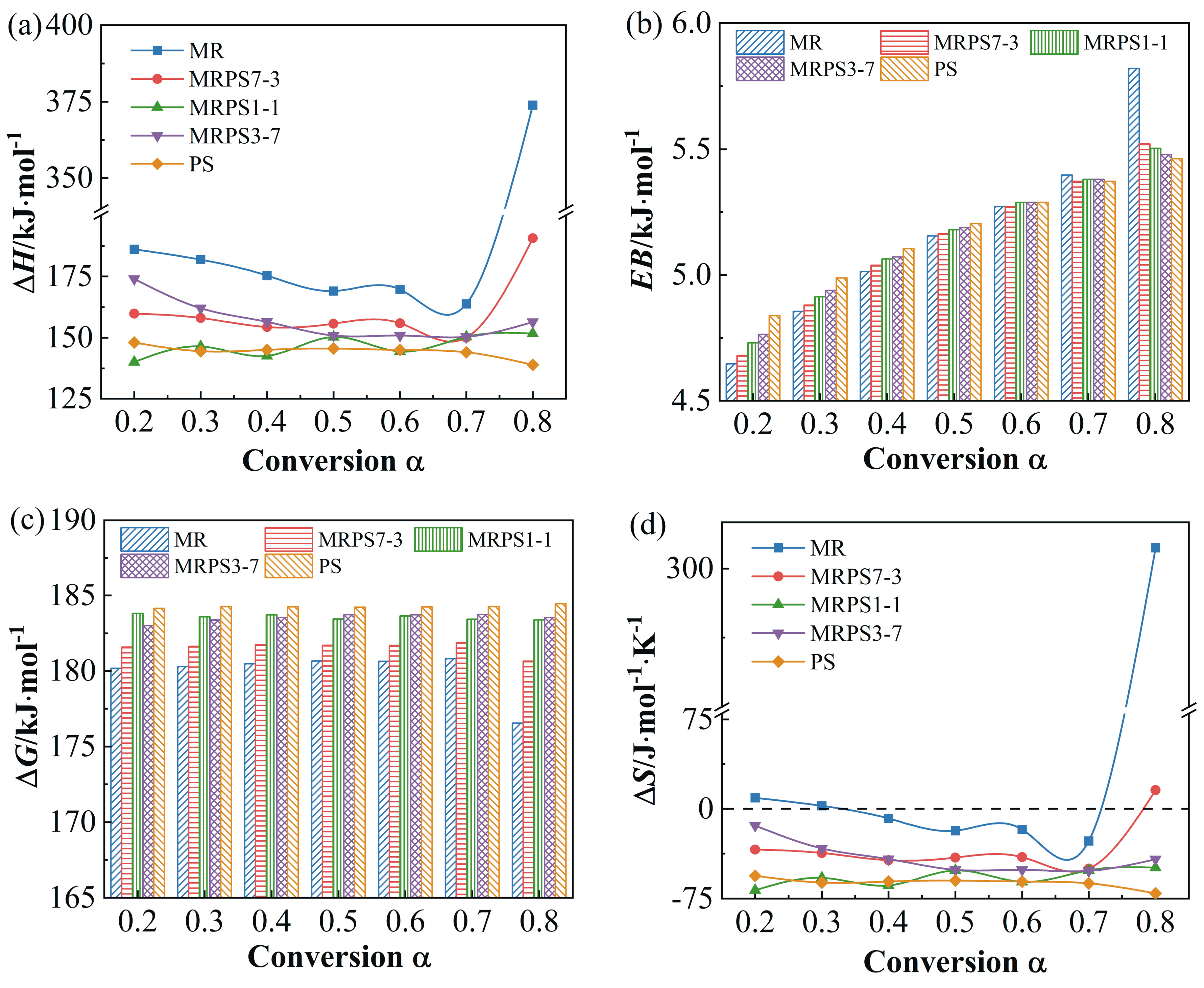
3.4. Thermodynamic Analysis
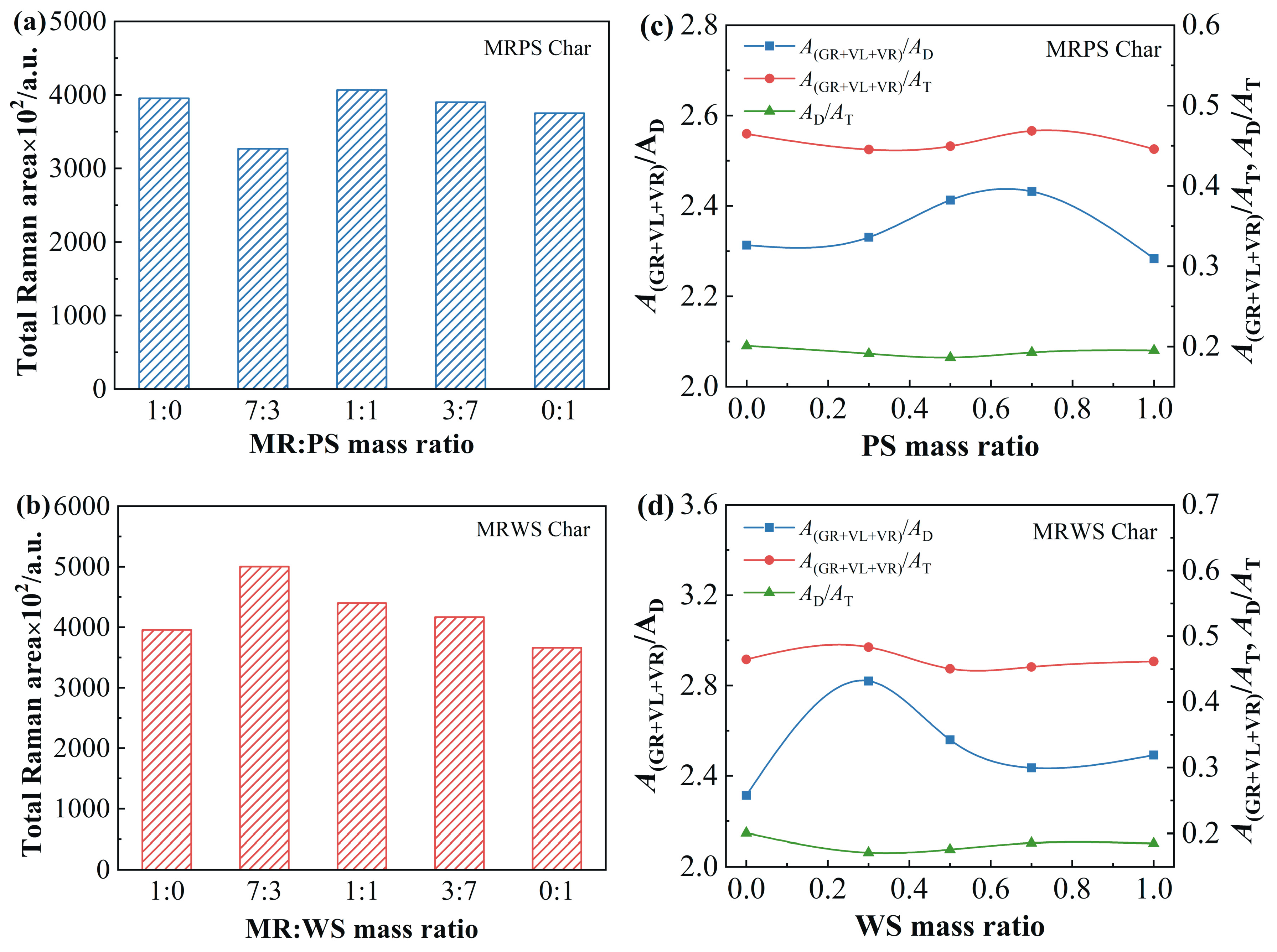
3.5. Structure Properties of Co-Pyrolytic Chars
3.5.1. Carbon Microcrystalline Structure
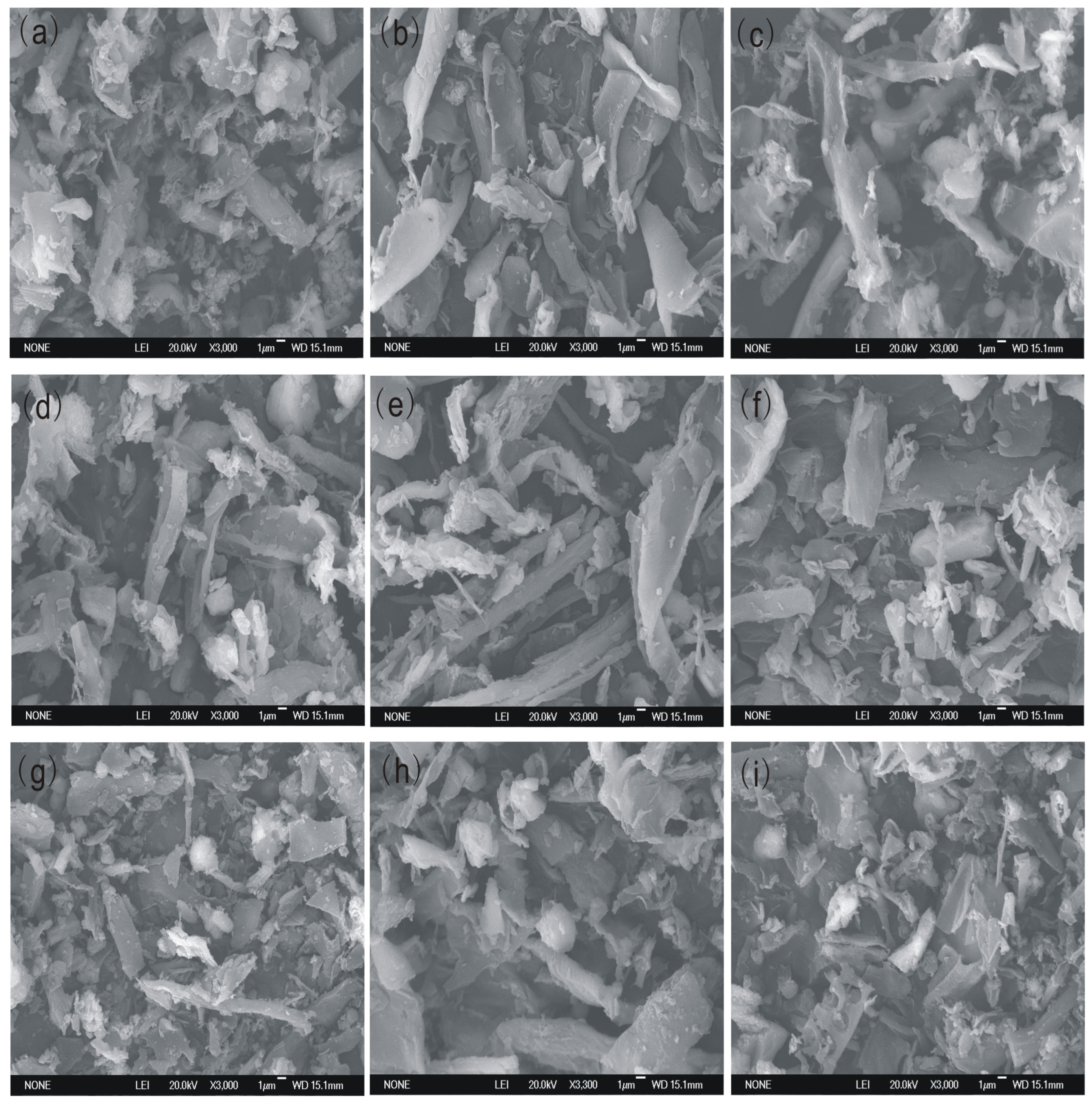
3.5.2. Surface Texture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, M.G.; Wang, Z.W.; Chen, G.F.; Zhang, M.J.; Sun, T.L.; Wang, Q.; Zhu, H.N.; Guo, S.H.; Chen, Y.; Zhu, Y.J.; et al. Synergistic effects and products distribution during Co-pyrolysis of biomass and plastics. J. Energy Inst. 2023, 111, 101392. [Google Scholar] [CrossRef]
- Kar, T.; Kaygusuz, Ö.; Güney, M.S.; Cuce, E.; Keles, S.; Shaik, S.; Owolabi, A.B.; Nsafon, B.E.K.; Ogunsua, J.M.; Huh, J.S. Fast Pyrolysis of Tea Bush, Walnut Shell, and Pine Cone Mixture: Effect of Pyrolysis Parameters on Pyrolysis Crop Yields. Sustainability 2023, 15, 13718. [Google Scholar] [CrossRef]
- Xu, Z.X.; Dou, R.; Gao, F.; Chen, Y.X.; Leng, L.J.; Osman, S.M.; Luque, R. Bio-adhesives derived from sewage sludge via hydrothermal carbonization: Influence of aqueous phase recycling. Chem. Eng. J. 2024, 490, 151685. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Li, C.; Zhang, L.J.; Lin, H.S.; Zhang, S.; Wang, Y.; Hu, S.; Xiang, J.; Hu, X. Influence of torrefaction with microwave and furnace heating on pyrolysis of poplar sawdust. Fuel Process. Technol. 2023, 245, 107696. [Google Scholar] [CrossRef]
- Thangaraj, B.; Wongyao, N.; Solomon, P.R.; Gupta, V.; Abdullah, A.; Abedrabbo, S.; Hassan, J. Synthesis of reduced graphene oxide from onion peel waste by single-stage pyrolysis, characterization and evaluation of its antibacterial activity. J. Environ. Chem. Eng. 2024, 12, 113474. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.F.; Lei, H.W.; Wang, C.X.; Zhao, Y.F.; Huo, E.G.; Lin, X.N.; Zhang, Q.F.; Qian, M.; Mateo, W.D.; et al. Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent mushroom compost and coal tailings for energy recovery: Comparison of thermal treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Qin, P.F.; Li, T.Y.; Liu, C.; Liang, Y.S.; Sun, H.B.; Chai, Y.Z.; Yang, T.Y.; Gong, X.M.; Wu, Z.B. Extraction and utilization of active substances from edible fungi substrate and residue: A review. Food Chem. 2023, 398, 133872. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, G.W.; Zhong, Z.P.; Ruan, R. Microwave-assisted catalytic fast pyrolysis of spent edible mushroom substrate for bio-oil production using surface modified zeolite catalyst. J. Anal. Appl. Pyrolysis 2017, 123, 92–98. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yu, H.D.; Lin, F.W.; Zhang, Z.M.; Yan, B.B.; Song, Y.J. Utilization of edible fungi residues towards synthesis of high-performance porous carbon for effective sorption of Cl-VOCs. Sci. Total Environ. 2020, 727, 138475. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chavez, A.M.; Mayer, L.; Alberto, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Li, J.L.; Pu, T.; Wang, Z.H.; Liu, T.Z. Thermal Behavior and Pyrolysis Kinetics of Mushroom Residue with the Introduction of Waste Plastics. Polymers 2023, 15, 3824. [Google Scholar] [CrossRef] [PubMed]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. Steam gasification of pyrolysis char from spent mushroom substrate. Biomass Bioenerg. 2019, 122, 336–342. [Google Scholar] [CrossRef]
- Sun, Z.S.; Yang, X.T.; Zhang, B.; Yang, B.L.; Shang, J.X.; Wu, Z.Q. Product-oriented thermodynamic function construction of wheat straw and thermodynamic analysis for rapid pyrolysis. Fuel 2024, 358, 130285. [Google Scholar] [CrossRef]
- Huang, J.L.; Zhang, J.H.; Liu, J.Y.; Xie, W.M.; Kuo, J.H.; Chang, K.L.; Buyukada, M.; Evrendilek, F.; Sun, S.Y. Thermal conversion behaviors and products of spent mushroom substrate in CO2 and N2 atmospheres: Kinetic, thermodynamic, TG and Py-GC/MS analyses. J. Anal. Appl. Pyrolysis 2019, 139, 177–186. [Google Scholar] [CrossRef]
- Slezak, R.; Krzystek, L.; Ledakowicz, S. Thermogravimetric analysis coupled with mass spectrometry of spent mushroom substrate and its fractions. J. Anal. Appl. Pyrolysis 2018, 133, 1–8. [Google Scholar] [CrossRef]
- Wang, G.X.; Guo, B.W.; Song, Z.L.; Wang, W.L.; Mao, Y.P.; Sun, J.; Zhao, X.Q. Pyrolysis characteristics and kinetic analysis of edible fungus chaff. Trans. Chin. Soc. Agric. Eng. 2020, 36, 301–307. [Google Scholar]
- Koido, K.; Ogura, T.; Matsumoto, R.; Endo, K.; Sato, M. Spent mushroom substrate performance for pyrolysis, steam co-gasification, and ash melting. Biomass Bioenerg. 2021, 145, 105954. [Google Scholar] [CrossRef]
- Jiang, H.F.; Cheng, Z.Q.; Zhao, T.Q.; Liu, M.Z.; Zhang, M.Y.; Li, J.N.; Hu, M.J.; Zhang, L.; Li, J.F. Pyrolysis kinetics of spent lark mushroom substrate and characterization of bio-oil obtained from the substrate. Energy Conv. Manag. 2014, 88, 259–266. [Google Scholar] [CrossRef]
- Sarfraz, R.; Li, S.W.; Yang, W.H.; Zhou, B.Q.; Xing, S.H. Assessment of Physicochemical and Nutritional Characteristics of Waste Mushroom Substrate Biochar under Various Pyrolysis Temperatures and Times. Sustainability 2019, 11, 277. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.P.; Ding, K.; Xue, Z.Y. Catalytic fast pyrolysis of mushroom waste to upgraded bio-oil products via pre-coked modified HZSM-5 catalyst. Bioresour. Technol. 2016, 212, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, Z.P.; Song, Z.W.; Ding, K.; Deng, A.D. Modification and regeneration of HZSM-5 catalyst in microwave assisted catalytic fast pyrolysis of mushroom waste. Energy Conv. Manag. 2016, 123, 29–34. [Google Scholar] [CrossRef]
- Jiang, H.F.; Deng, S.H.; Chen, J.; Zhang, L.; Zhang, M.Y.; Li, J.N.; Li, S.; Li, J.F. Preliminary Study on Copyrolysis of Spent Mushroom Substrate as Biomass and Huadian Oil Shale. Energy Fuels 2016, 30, 6342–6349. [Google Scholar] [CrossRef]
- Jerzak, W.; Bieniek, A.; Magdziarz, A. Multifaceted analysis of products from the intermediate co-pyrolysis of biomass with Tetra Pak waste. Int. J. Hydrogen Energy 2023, 48, 11680–11694. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.A.; Mohanty, B.; Sawarkar, A.N. Critical insights into pyrolysis and co-pyrolysis of poplar and eucalyptus wood sawdust: Physico-chemical characterization, kinetic triplets, reaction mechanism, and thermodynamic analysis. Renew. Energy 2023, 210, 321–334. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Wang, S.Z.; Zhao, J.; Chen, L.; Meng, H.Y. Thermal behavior and char structure evolution of bituminous coal blends with edible fungi residue during co-pyrolysis. Energy Fuels 2014, 28, 1792–1801. [Google Scholar] [CrossRef]
- Huang, J.L.; Liu, J.Y.; Chang, K.L.; Buyukada, M.; Evrendilek, F. (Co-)pyrolytic performances and by-products of textile dyeing sludge and spent mushroom substrate. J. Clean. Prod. 2020, 261, 121195. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Jung, H.; Woo, S.H. Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour. Technol. 2017, 244, 1142–1149. [Google Scholar] [CrossRef]
- Jiang, H.F.; Zhang, M.Y.; Chen, J.; Li, S.; Shao, Y.F.; Yang, J.Q.; Li, J.F. Characteristics of bio-oil produced by the pyrolysis of mixed oil shale semi-coke and spent mushroom substrate. Fuel 2017, 200, 218–224. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, L.; Dong, L.; Qiu, P.H.; Wang, S.; Jiang, S.J.; Li, C.Z. Changes in char structure during the low-temperature pyrolysis in N-2 and subsequent gasification in air of Loy Yang brown coal char. Fuel 2018, 212, 187–192. [Google Scholar] [CrossRef]
- Chen, J.Z.; Lu, Z.M.; Jian, J.; Bao, Z.Y.; Cai, J.F.; Yao, S.C. Effect of torrefaction on yield, reactivity and physicochemical properties of pyrolyzed char from three major biomass constituents. J. Anal. Appl. Pyrolysis 2023, 173, 106104. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Yang, W.C.; Yang, B.L. Thermal characteristics and surface morphology of char during co-pyrolysis of low-rank coal blended with microalgal biomass: Effects of Nannochloropsis and Chlorella. Bioresour. Technol. 2018, 249, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.Y.; Wang, S.Z.; Chen, L.; Wu, Z.Q.; Zhao, J. Thermal behavior and the evolution of char structure during co-pyrolysis of platanus wood blends with different rank coals from northern China. Fuel 2015, 158, 602–611. [Google Scholar] [CrossRef]
- Wang, C.A.; Du, Y.B.; Che, D.F. Reactivities of coals and synthetic model coal under oxy-fuel conditions. Thermochim. Acta 2013, 553, 8–15. [Google Scholar] [CrossRef]
- Tariq, R.; Zaifullizan, Y.M.; Salema, A.A.; Abdulatif, A.; Ken, L.S. Co-pyrolysis and co-combustion of orange peel and biomass blends: Kinetics, thermodynamic, and ANN application. Renew. Energy 2022, 198, 399–414. [Google Scholar] [CrossRef]
- Meng, H.Y.; Wang, M.Z.; Wu, Z.Q.; Zhao, J.; Li, J.K.; Wang, S.Z. Physico-Chemical Structure and Gasification Performance of Co-Pyrolytic Char Produced by the Pyrolysis of Polyvinyl Chloride Blends with Two Rank Coals. ACS Omega 2022, 7, 32280–32291. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef]
- Lei, J.L.; Ye, X.F.; Wang, H.; Zhao, D.N. Insights into Pyrolysis Kinetics, Thermodynamics, and the Reaction Mechanism of Wheat Straw for Its Resource Utilization. Sustainability 2023, 15, 12536. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of thermodynamic parameters in the thermal decomposition of plastic waste-waste lube oil compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, L.P.; Hu, X.; Zhang, L.; Li, T.T.; Li, C.Z. Effects of the Particle Size and Gasification Atmosphere on the Changes in the Char Structure during the Gasification of Mallee Biomass. Energy Fuels 2018, 32, 7678–7684. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy 2018, 220, 408–418. [Google Scholar] [CrossRef]
- Nyambura, S.M.; Wang, J.F.; Li, H.; Feng, X.B.; Pan, X.J.; Li, B.H.; Ahmad, R.; Xu, J.L.; Bertrand, G.V.; Ndiithi, J.; et al. Microwave co-pyrolysis of kitchen food waste and rice straw for waste reduction and sustainable biohydrogen production: Thermo-kinetic analysis and evolved gas analysis. Sustain. Energy Technol. Assess. 2022, 52, 102072. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.M.; Xu, J.N.; Li, X.Y.; Xu, C.B. Co-pyrolysis behavior of fermentation residues with woody sawdust by thermogravimetric analysis and a vacuum reactor. Bioresour. Technol. 2017, 245, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Yuchi, W.; Bai, Z.Q.; Hou, R.R.; Feng, Z.H.; Guo, Z.X.; Kong, L.X.; Bai, J.; Meyer, B.; Li, W. Insight into the charging methods effects during clean recycling of plastic by co-pyrolysis with low-rank coal. J. Clean. Prod. 2022, 333, 130168. [Google Scholar] [CrossRef]
- Yuan, X.S.; He, T.; Cao, H.L.; Yuan, Q.X. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- Jerzak, W.; Gajek, M.; Magdziarz, A. Oat straw pyrolysis with ammonium chloride doping: Analysis of evolved gases, kinetic triplet, and thermodynamic parameters. Bioresour. Technol. 2023, 388, 129784. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Xu, F.F.; Jiang, Y.; Zong, P.J.; Wang, B.; Li, J.; Qiao, Y.Y.; Tian, Y.Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar] [CrossRef]
- Xu, Y.L.; Chen, B.L. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Keown, D.M.; Li, X.J.; Hayashi, J.I.; Li, C.Z. Characterization of the structural features of char from the pyrolysis of cane trash using Fourier transform—Raman spectroscopy. Energy Fuels 2007, 21, 1816–1821. [Google Scholar] [CrossRef]







| Material | MR | PS | WS |
|---|---|---|---|
| Proximate analysis/wt %, ad | |||
| Moisture | 8.24 | 7.86 | 7.15 |
| Ash | 7.79 | 1.40 | 17.12 |
| Volatile matter | 72.16 | 77.24 | 61.81 |
| Fixed carbon | 11.81 | 13.50 | 13.92 |
| Ultimate analysis/wt %, daf | |||
| Carbon | 47.92 | 50.03 | 47.10 |
| Hydrogen | 5.05 | 5.37 | 5.20 |
| Nitrogen | 0.85 | 0.20 | 1.56 |
| Sulfur | 0.20 | 0.03 | 0.57 |
| Oxygen c | 45.98 | 44.37 | 45.57 |
| H/C atomic ratio | 1.26 | 1.29 | 1.32 |
| Qgr,d/MJ·kg−1 | 17.10 | 20.47 | 16.64 |
| Parameters | MR | 30% PS | 50% PS | 70% PS | PS | 30% WS | 50% WS | 70% WS | WS |
|---|---|---|---|---|---|---|---|---|---|
| Tin/°C | 292 | 299 | 304 | 307 | 307 | 281 | 281 | 282 | 281 |
| T1/°C | 361 | 363 | 368 | 369 | 370 | 338 | 334 | 332 | 329 |
| R1/%·min−1 | 10.41 | 11.93 | 12.82 | 13.69 | 15.24 | 10.19 | 10.69 | 12.21 | 13.61 |
| T2/°C | 508 | 507 | 505 | 504 | 502 | 500 | 475 | ||
| R2/%·min−1 | 2.19 | 1.53 | 1.21 | 1.01 | 1.60 | 1.35 | 1.17 | ||
| T3/°C | 689 | 683 | 668 | 662 | 678 | 672 | 657 | ||
| R3/%·min−1 | 1.35 | 0.88 | 0.83 | 0.59 | 0.89 | 0.84 | 0.59 | ||
| Tmax/°C | 361 | 363 | 368 | 369 | 370 | 338 | 334 | 332 | 329 |
| Rmax/%·min−1 | 10.41 | 11.93 | 12.82 | 13.69 | 15.24 | 10.19 | 10.69 | 12.21 | 13.61 |
| ΔT1/2/°C | 91 | 84 | 81 | 79 | 76 | 95 | 88 | 62 | 55 |
| Di/10−7%·min−1·°C−3 | 10.85 | 13.09 | 14.15 | 15.30 | 17.65 | 11.29 | 12.94 | 21.03 | 26.77 |
| Char yield/% | 31.20 | 29.41 | 28.81 | 28.02 | 27.37 | 33.12 | 34.31 | 36.60 | 39.04 |
| Samples | α | Eα/kJ·mol−1 | Aα/s−1 | R2 | ||
|---|---|---|---|---|---|---|
| 10 °C·min−1 | 20 °C·min−1 | 40 °C·min−1 | ||||
| MR | 0.2 | 190.72 | 1.02 × 1014 | 9.75 × 1013 | 8.07 × 1013 | 0.9999 |
| 0.3 | 186.76 | 4.64 × 1013 | 4.51 × 1013 | 3.79 × 1013 | 0.9999 | |
| 0.4 | 180.38 | 1.30 × 1013 | 1.30 × 1013 | 1.12 × 1013 | 0.9999 | |
| 0.5 | 174.20 | 3.81 × 1012 | 3.88 × 1012 | 3.45 × 1012 | 0.9999 | |
| 0.6 | 174.96 | 4.44 × 1012 | 4.50 × 1012 | 3.99 × 1012 | 0.9998 | |
| 0.7 | 169.19 | 1.41 × 1012 | 1.46 × 1012 | 1.33 × 1012 | 0.9999 | |
| 0.8 | 379.73 | 1.50 × 1030 | 7.20 × 1029 | 2.60 × 1029 | 0.9049 | |
| Avg. | 207.99 | 2.14 × 1029 | 1.03 × 1029 | 3.72 × 1028 | ||
| MRPS7-3 | 0.2 | 164.54 | 5.27 × 1011 | 5.29 × 1011 | 4.69 × 1011 | 0.9942 |
| 0.3 | 162.99 | 3.87 × 1011 | 3.91 × 1011 | 3.49 × 1011 | 0.9953 | |
| 0.4 | 159.41 | 1.90 × 1011 | 1.94 × 1011 | 1.76 × 1011 | 0.9998 | |
| 0.5 | 160.91 | 2.56 × 1011 | 2.60 × 1011 | 2.35 × 1011 | 0.9988 | |
| 0.6 | 161.22 | 2.72 × 1011 | 2.77 × 1011 | 2.49 × 1011 | 0.9999 | |
| 0.7 | 155.41 | 8.55 × 1010 | 8.89 × 1010 | 8.22 × 1010 | 0.9974 | |
| 0.8 | 196.15 | 2.81 × 1014 | 2.48 × 1014 | 1.90 × 1014 | 0.9638 | |
| Avg. | 165.80 | 4.03 × 1013 | 3.57 × 1013 | 2.74 × 1013 | ||
| MRPS1-1 | 0.2 | 144.87 | 9.93 × 109 | 8.98 × 109 | 8.44 × 109 | 0.9960 |
| 0.3 | 151.37 | 3.63 × 1010 | 3.18 × 1010 | 2.89 × 1010 | 0.9999 | |
| 0.4 | 147.68 | 1.74 × 1010 | 1.55 × 1010 | 1.44 × 1010 | 0.9987 | |
| 0.5 | 155.50 | 8.27 × 1010 | 7.08 × 1010 | 6.32 × 1010 | 0.9999 | |
| 0.6 | 149.69 | 2.60 × 1010 | 2.29 × 1010 | 2.11 × 1010 | 0.9988 | |
| 0.7 | 155.77 | 8.72 × 1010 | 7.46 × 1010 | 6.66 × 1010 | 0.9999 | |
| 0.8 | 157.25 | 1.17 × 1011 | 9.94 × 1010 | 8.81 × 1010 | 0.9998 | |
| Avg. | 151.73 | 5.38 × 1010 | 4.63 × 1010 | 4.15 × 1010 | ||
| MRPS3-7 | 0.2 | 178.80 | 5.99 × 1012 | 6.09 × 1012 | 4.63 × 1012 | 0.9877 |
| 0.3 | 167.00 | 5.88 × 1011 | 6.24 × 1011 | 5.03 × 1011 | 0.9953 | |
| 0.4 | 161.58 | 2.02 × 1011 | 2.19 × 1011 | 1.81 × 1011 | 0.9962 | |
| 0.5 | 156.09 | 6.85 × 1010 | 7.56 × 1010 | 6.45 × 1010 | 0.9969 | |
| 0.6 | 156.22 | 7.03 × 1010 | 7.75 × 1010 | 6.61 × 1010 | 0.9990 | |
| 0.7 | 155.77 | 6.43 × 1010 | 7.11 × 1010 | 6.07 × 1010 | 0.9999 | |
| 0.8 | 161.93 | 2.17 × 1011 | 2.34 × 1011 | 1.94 × 1011 | 0.9974 | |
| Avg. | 162.49 | 1.03 × 1012 | 1.06 × 1012 | 8.14 × 1011 | ||
| PS | 0.2 | 152.90 | 3.47 × 1010 | 3.88 × 1010 | 3.38 × 1010 | 0.9998 |
| 0.3 | 149.51 | 1.78 × 1010 | 2.02 × 1010 | 1.78 × 1010 | 0.9998 | |
| 0.4 | 150.21 | 2.04 × 1010 | 2.31 × 1010 | 2.03 × 1010 | 0.9987 | |
| 0.5 | 150.80 | 2.30 × 1010 | 2.59 × 1010 | 2.27 × 1010 | 0.9998 | |
| 0.6 | 150.34 | 2.10 × 1010 | 2.37 × 1010 | 2.08 × 1010 | 0.9999 | |
| 0.7 | 149.48 | 1.77 × 1010 | 2.00 × 1010 | 1.77 × 1010 | 0.9998 | |
| 0.8 | 144.43 | 6.53 × 109 | 7.53 × 109 | 6.83 × 109 | 0.9998 | |
| Avg. | 149.67 | 2.02 × 1010 | 2.27 × 1010 | 2.00 × 1010 | ||
| Samples | α | Eα/kJ·mol−1 | Aα/s−1 | R2 | ||
|---|---|---|---|---|---|---|
| 10 °C·min−1 | 20 °C·min−1 | 40 °C·min−1 | ||||
| MR | 0.2 | 190.72 | 1.02 × 1014 | 9.75 × 1013 | 8.07 × 1013 | 0.9999 |
| 0.3 | 186.76 | 4.64 × 1013 | 4.51 × 1013 | 3.79 × 1013 | 0.9999 | |
| 0.4 | 180.38 | 1.30 × 1013 | 1.30 × 1013 | 1.12 × 1013 | 0.9999 | |
| 0.5 | 174.20 | 3.81 × 1012 | 3.88 × 1012 | 3.45 × 1012 | 0.9999 | |
| 0.6 | 174.96 | 4.44 × 1012 | 4.50 × 1012 | 3.99 × 1012 | 0.9998 | |
| 0.7 | 169.19 | 1.41 × 1012 | 1.46 × 1012 | 1.33 × 1012 | 0.9999 | |
| 0.8 | 379.73 | 1.50 × 1030 | 7.20 × 1029 | 2.60 × 1029 | 0.9049 | |
| Avg. | 207.99 | 2.14 × 1029 | 1.03 × 1029 | 3.72 × 1028 | ||
| MRWS7-3 | 0.2 | 183.13 | 9.46 × 1013 | 8.83 × 1013 | 5.61 × 1013 | 0.9845 |
| 0.3 | 176.55 | 2.43 × 1013 | 2.33 × 1013 | 1.54 × 1013 | 0.9709 | |
| 0.4 | 171.56 | 8.68 × 1012 | 8.49 × 1012 | 5.77 × 1012 | 0.9761 | |
| 0.5 | 165.35 | 2.41 × 1012 | 2.41 × 1012 | 1.70 × 1012 | 0.9802 | |
| 0.6 | 159.63 | 7.37 × 1011 | 7.55 × 1011 | 5.51 × 1011 | 0.9834 | |
| 0.7 | 168.18 | 4.32 × 1012 | 4.28 × 1012 | 2.97 × 1012 | 0.9833 | |
| 0.8 | 234.74 | 3.83 × 1018 | 2.92 × 1018 | 1.36 × 1018 | 0.9195 | |
| Avg. | 179.88 | 5.47 × 1017 | 4.17 × 1017 | 1.95 × 1017 | ||
| MRWS1-1 | 0.2 | 149.19 | 1.24 × 1011 | 1.11 × 1011 | 1.04 × 1011 | 0.9955 |
| 0.3 | 155.00 | 4.18 × 1011 | 3.65 × 1011 | 3.32 × 1011 | 0.9985 | |
| 0.4 | 151.20 | 1.88 × 1011 | 1.68 × 1011 | 1.55 × 1011 | 0.9986 | |
| 0.5 | 152.10 | 2.27 × 1011 | 2.02 × 1011 | 1.86 × 1011 | 0.9998 | |
| 0.6 | 152.75 | 2.61 × 1011 | 2.30 × 1011 | 2.12 × 1011 | 0.9987 | |
| 0.7 | 161.29 | 1.56 × 1012 | 1.32 × 1012 | 1.16 × 1012 | 0.9935 | |
| 0.8 | 176.48 | 3.73 × 1013 | 2.93 × 1013 | 2.40 × 1013 | 0.9213 | |
| Avg. | 156.86 | 5.73 × 1012 | 4.53 × 1012 | 3.74 × 1012 | ||
| MRWS3-7 | 0.2 | 157.07 | 6.44 × 1011 | 6.22 × 1011 | 5.87 × 1011 | 0.9997 |
| 0.3 | 161.54 | 1.64 × 1012 | 1.56 × 1012 | 1.44 × 1012 | 0.9980 | |
| 0.4 | 155.97 | 5.12 × 1011 | 4.96 × 1011 | 4.70 × 1011 | 0.9985 | |
| 0.5 | 156.17 | 5.34 × 1011 | 5.17 × 1011 | 4.90 × 1011 | 0.9999 | |
| 0.6 | 162.13 | 1.86 × 1012 | 1.75 × 1012 | 1.62 × 1012 | 0.9998 | |
| 0.7 | 186.60 | 3.08 × 1014 | 2.62 × 1014 | 2.16 × 1014 | 0.9953 | |
| 0.8 | 287.37 | 3.68 × 1023 | 2.01 × 1023 | 1.06 × 1023 | 0.9904 | |
| Avg. | 180.98 | 5.26 × 1022 | 2.87 × 1022 | 1.51 × 1022 | ||
| WS | 0.2 | 152.31 | 2.81 × 1011 | 2.75 × 1011 | 2.63 × 1011 | 0.9638 |
| 0.3 | 159.17 | 1.19 × 1012 | 1.13 × 1012 | 1.05 × 1012 | 0.9901 | |
| 0.4 | 159.20 | 1.20 × 1012 | 1.14 × 1012 | 1.06 × 1012 | 0.9853 | |
| 0.5 | 151.77 | 2.51 × 1011 | 2.46 × 1011 | 2.36 × 1011 | 0.9878 | |
| 0.6 | 157.79 | 8.91 × 1011 | 8.51 × 1011 | 7.94 × 1011 | 0.9962 | |
| 0.7 | 161.00 | 1.75 × 1012 | 1.65 × 1012 | 1.52 × 1012 | 0.9748 | |
| 0.8 | 202.17 | 9.83 × 1015 | 7.72 × 1015 | 5.89 × 1015 | 0.9920 | |
| Avg. | 163.35 | 1.41 × 1015 | 1.10 × 1015 | 8.42 × 1014 | ||
| Samples | α | 10 °C·min−1 | 20 °C·min−1 | 40 °C·min−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ||
| MR | 0.2 | 186.15 | 180.06 | 9.78 | 186.07 | 180.18 | 9.29 | 186.00 | 181.08 | 7.57 |
| 0.3 | 181.99 | 180.17 | 2.92 | 181.90 | 180.29 | 2.54 | 181.82 | 181.19 | 0.97 | |
| 0.4 | 175.46 | 180.35 | −7.86 | 175.37 | 180.48 | −8.06 | 175.28 | 181.38 | −9.41 | |
| 0.5 | 169.15 | 180.53 | −18.30 | 169.05 | 180.66 | −18.32 | 168.95 | 181.57 | −19.45 | |
| 0.6 | 169.79 | 180.51 | −17.23 | 169.69 | 180.64 | −17.26 | 169.58 | 181.55 | −18.43 | |
| 0.7 | 163.90 | 180.68 | −26.97 | 163.79 | 180.81 | −26.84 | 163.68 | 181.73 | −27.81 | |
| 0.8 | 373.99 | 176.50 | 317.44 | 373.91 | 176.55 | 311.22 | 373.89 | 177.36 | 302.73 | |
| Avg. | 202.92 | 179.83 | - | 202.83 | 179.94 | - | 202.74 | 180.84 | - | |
| MRPS | 0.2 | 159.93 | 181.14 | −34.03 | 159.86 | 181.58 | −34.15 | 159.76 | 182.79 | −35.32 |
| 7-3 | 0.3 | 158.19 | 181.19 | −36.90 | 158.11 | 181.63 | −36.98 | 158.00 | 182.85 | −38.10 |
| 0.4 | 154.47 | 181.30 | −43.06 | 154.37 | 181.75 | −43.04 | 154.26 | 182.97 | −44.01 | |
| 0.5 | 155.85 | 181.26 | −40.78 | 155.75 | 181.70 | −40.80 | 155.63 | 182.92 | −41.84 | |
| 0.6 | 156.06 | 181.25 | −40.42 | 155.95 | 181.69 | −40.46 | 155.83 | 182.90 | −41.51 | |
| 0.7 | 150.15 | 181.44 | −50.21 | 150.04 | 181.88 | −50.06 | 149.91 | 183.10 | −50.90 | |
| 0.8 | 190.70 | 180.23 | 16.79 | 190.63 | 180.65 | 15.68 | 190.50 | 181.84 | 13.27 | |
| Avg. | 160.76 | 181.12 | - | 160.67 | 181.55 | - | 160.56 | 182.77 | - | |
| MRPS | 0.2 | 140.25 | 182.12 | −67.08 | 140.14 | 183.81 | −68.12 | 140.05 | 185.32 | −68.79 |
| 1-1 | 0.3 | 146.56 | 181.89 | −56.61 | 146.46 | 183.58 | −57.90 | 146.35 | 185.08 | −58.85 |
| 0.4 | 142.73 | 182.02 | −62.94 | 142.62 | 183.71 | −64.09 | 142.51 | 185.22 | −64.89 | |
| 0.5 | 150.43 | 181.75 | −50.18 | 150.32 | 183.43 | −51.65 | 150.20 | 184.94 | −52.77 | |
| 0.6 | 144.53 | 181.95 | −59.95 | 144.40 | 183.64 | −61.20 | 144.29 | 185.14 | −62.08 | |
| 0.7 | 150.51 | 181.74 | −50.04 | 150.39 | 183.43 | −51.52 | 150.27 | 184.93 | −52.66 | |
| 0.8 | 151.87 | 181.69 | −47.78 | 151.75 | 183.38 | −49.33 | 151.62 | 184.87 | −50.52 | |
| Avg. | 146.70 | 181.88 | - | 146.58 | 183.57 | - | 146.47 | 185.07 | - | |
| MRPS | 0.2 | 174.10 | 182.92 | −13.99 | 174.04 | 183.00 | −13.96 | 173.94 | 184.78 | −16.43 |
| 3-7 | 0.3 | 162.14 | 183.27 | −33.53 | 162.06 | 183.37 | −33.18 | 161.95 | 185.16 | −35.15 |
| 0.4 | 156.60 | 183.45 | −42.60 | 156.51 | 183.54 | −42.10 | 156.39 | 185.34 | −43.85 | |
| 0.5 | 151.00 | 183.63 | −51.77 | 150.90 | 183.73 | −51.12 | 150.78 | 185.53 | −52.64 | |
| 0.6 | 151.04 | 183.62 | −51.71 | 150.93 | 183.72 | −51.06 | 150.81 | 185.52 | −52.59 | |
| 0.7 | 150.51 | 183.64 | −52.58 | 150.39 | 183.74 | −51.93 | 150.27 | 185.54 | −53.43 | |
| 0.8 | 156.56 | 183.43 | −42.65 | 156.45 | 183.53 | −42.17 | 156.32 | 185.32 | −43.94 | |
| Avg. | 157.42 | 183.42 | - | 157.33 | 183.52 | - | 157.21 | 185.31 | - | |
| PS | 0.2 | 148.16 | 184.05 | −56.87 | 148.06 | 184.15 | −56.11 | 147.96 | 185.95 | −57.45 |
| 0.3 | 144.63 | 184.17 | −62.65 | 144.52 | 184.27 | −61.80 | 144.41 | 186.07 | −63.01 | |
| 0.4 | 145.22 | 184.15 | −61.67 | 145.11 | 184.24 | −60.85 | 145.00 | 186.04 | −62.08 | |
| 0.5 | 145.71 | 184.12 | −60.86 | 145.60 | 184.22 | −60.06 | 145.48 | 186.02 | −61.32 | |
| 0.6 | 145.17 | 184.14 | −61.75 | 145.05 | 184.24 | −60.93 | 144.93 | 186.04 | −62.18 | |
| 0.7 | 144.23 | 184.17 | −63.28 | 144.11 | 184.27 | −62.44 | 143.98 | 186.07 | −63.66 | |
| 0.8 | 139.10 | 184.35 | −71.70 | 138.97 | 184.45 | −70.72 | 138.83 | 186.26 | −71.73 | |
| Avg. | 144.60 | 184.16 | - | 144.49 | 184.26 | - | 144.37 | 186.06 | - | |
| Samples | α | 10 °C·min−1 | 20 °C·min−1 | 40 °C·min−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ΔH/ kJ·mol−1 | ΔG/ kJ·mol−1 | ΔS/ J·mol−1·K−1 | ||
| MR | 0.2 | 186.15 | 180.06 | 9.78 | 186.07 | 180.18 | 9.29 | 186.00 | 181.08 | 7.57 |
| 0.3 | 181.99 | 180.17 | 2.92 | 181.90 | 180.29 | 2.54 | 181.82 | 181.19 | 0.97 | |
| 0.4 | 175.46 | 180.35 | −7.86 | 175.37 | 180.48 | −8.06 | 175.28 | 181.38 | −9.41 | |
| 0.5 | 169.15 | 180.53 | −18.30 | 169.05 | 180.66 | −18.32 | 168.95 | 181.57 | −19.45 | |
| 0.6 | 169.79 | 180.51 | −17.23 | 169.69 | 180.64 | −17.26 | 169.58 | 181.55 | −18.43 | |
| 0.7 | 163.90 | 180.68 | −26.97 | 163.79 | 180.81 | −26.84 | 163.68 | 181.73 | −27.81 | |
| 0.8 | 373.99 | 176.50 | 317.44 | 373.91 | 176.55 | 311.22 | 373.89 | 177.36 | 302.73 | |
| Avg. | 202.92 | 179.83 | - | 202.83 | 179.94 | - | 202.74 | 180.84 | - | |
| MRWS | 0.2 | 178.62 | 173.04 | 9.30 | 178.56 | 173.29 | 8.62 | 178.47 | 175.53 | 4.66 |
| 7-3 | 0.3 | 171.84 | 173.23 | −2.31 | 171.79 | 173.48 | −2.77 | 171.68 | 175.72 | −6.41 |
| 0.4 | 166.70 | 173.37 | −11.12 | 166.64 | 173.62 | −11.43 | 166.52 | 175.87 | −14.83 | |
| 0.5 | 160.38 | 173.55 | −21.99 | 160.30 | 173.81 | −22.10 | 160.18 | 176.06 | −25.21 | |
| 0.6 | 154.55 | 173.73 | −32.01 | 154.47 | 173.99 | −31.94 | 154.33 | 176.25 | −34.77 | |
| 0.7 | 162.97 | 173.47 | −17.53 | 162.88 | 173.72 | −17.74 | 162.75 | 175.97 | −20.98 | |
| 0.8 | 229.18 | 171.81 | 95.75 | 229.14 | 172.03 | 93.44 | 229.01 | 174.23 | 86.94 | |
| Avg. | 174.89 | 173.17 | - | 174.82 | 173.42 | - | 174.71 | 175.66 | - | |
| MRWS | 0.2 | 144.70 | 171.86 | −45.86 | 144.60 | 173.09 | −46.93 | 144.52 | 174.16 | −47.65 |
| 1-1 | 0.3 | 150.33 | 171.67 | −36.04 | 150.23 | 172.90 | −37.34 | 150.14 | 173.96 | −38.30 |
| 0.4 | 146.39 | 171.79 | −42.90 | 146.28 | 173.02 | −44.05 | 146.18 | 174.09 | −44.87 | |
| 0.5 | 147.18 | 171.76 | −41.52 | 147.07 | 172.99 | −42.70 | 146.96 | 174.06 | −43.56 | |
| 0.6 | 147.72 | 171.74 | −40.57 | 147.60 | 172.97 | −41.78 | 147.50 | 174.04 | −42.66 | |
| 0.7 | 156.11 | 171.47 | −25.95 | 155.99 | 172.70 | −27.53 | 155.89 | 173.76 | −28.73 | |
| 0.8 | 170.98 | 171.03 | −0.09 | 170.82 | 172.24 | −2.35 | 170.76 | 173.29 | −4.07 | |
| Avg. | 151.91 | 171.62 | - | 151.80 | 172.85 | - | 151.71 | 173.91 | - | |
| MRWS | 0.2 | 152.58 | 171.60 | −32.13 | 152.50 | 172.21 | −32.58 | 152.41 | 172.98 | −33.23 |
| 3-7 | 0.3 | 156.88 | 171.47 | −24.64 | 156.79 | 172.07 | −25.25 | 156.69 | 172.84 | −26.07 |
| 0.4 | 151.18 | 171.64 | −34.55 | 151.09 | 172.25 | −34.96 | 150.98 | 173.02 | −35.59 | |
| 0.5 | 151.28 | 171.63 | −34.37 | 151.18 | 172.24 | −34.80 | 151.07 | 173.01 | −35.43 | |
| 0.6 | 157.15 | 171.45 | −24.15 | 157.05 | 172.05 | −24.79 | 156.94 | 172.82 | −25.64 | |
| 0.7 | 181.47 | 170.76 | 18.09 | 181.39 | 171.35 | 16.59 | 181.28 | 172.09 | 14.83 | |
| 0.8 | 281.90 | 168.63 | 191.28 | 281.84 | 169.17 | 186.18 | 281.76 | 169.87 | 180.71 | |
| Avg. | 176.06 | 171.03 | - | 175.98 | 171.62 | - | 175.88 | 172.38 | - | |
| WS | 0.2 | 147.81 | 170.81 | −39.04 | 147.75 | 171.44 | −39.33 | 147.64 | 172.23 | −39.91 |
| 0.3 | 154.52 | 170.60 | −27.28 | 154.45 | 171.22 | −27.85 | 154.34 | 172.00 | −28.66 | |
| 0.4 | 154.44 | 170.59 | −27.43 | 154.36 | 171.22 | −27.99 | 154.24 | 172.00 | −28.82 | |
| 0.5 | 146.92 | 170.83 | −40.58 | 146.84 | 171.46 | −40.88 | 146.72 | 172.24 | −41.43 | |
| 0.6 | 152.87 | 170.64 | −30.16 | 152.78 | 171.26 | −30.70 | 152.66 | 172.05 | −31.46 | |
| 0.7 | 155.98 | 170.54 | −24.72 | 155.90 | 171.16 | −25.34 | 155.77 | 171.94 | −26.25 | |
| 0.8 | 196.82 | 169.42 | 46.51 | 196.72 | 170.02 | 44.33 | 196.63 | 170.78 | 41.97 | |
| Avg. | 158.48 | 170.49 | - | 158.40 | 171.11 | - | 158.29 | 171.89 | - | |
| MR Blending Ratio | MRPS Char | MRWS Char | ||
|---|---|---|---|---|
| Ds | R2 | Ds | R2 | |
| 1 | 1.68 | 0.9921 | 1.68 | 0.9921 |
| 0.7 | 1.69 | 0.9921 | 1.83 | 0.9915 |
| 0.5 | 1.69 | 0.9921 | 1.63 | 0.9924 |
| 0.3 | 1.74 | 0.9918 | 1.76 | 0.9918 |
| 0 | 1.65 | 0.9923 | 1.67 | 0.9921 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Yang, H.; Wu, Z.; Li, D.; Wang, Z.; Wang, D.; Wang, H.; Li, H.; Li, J. Co-Pyrolysis of Mushroom Residue Blended with Pine Sawdust/Wheat Straw for Sustainable Utilization of Biomass Wastes: Thermal Characteristics, Kinetic/Thermodynamic Analysis, and Structure Evolution of Co-Pyrolytic Char. Sustainability 2024, 16, 6677. https://doi.org/10.3390/su16156677
Meng H, Yang H, Wu Z, Li D, Wang Z, Wang D, Wang H, Li H, Li J. Co-Pyrolysis of Mushroom Residue Blended with Pine Sawdust/Wheat Straw for Sustainable Utilization of Biomass Wastes: Thermal Characteristics, Kinetic/Thermodynamic Analysis, and Structure Evolution of Co-Pyrolytic Char. Sustainability. 2024; 16(15):6677. https://doi.org/10.3390/su16156677
Chicago/Turabian StyleMeng, Haiyu, Heng Yang, Zhiqiang Wu, Danting Li, Zhe Wang, Dongqi Wang, Hui Wang, Huaien Li, and Jiake Li. 2024. "Co-Pyrolysis of Mushroom Residue Blended with Pine Sawdust/Wheat Straw for Sustainable Utilization of Biomass Wastes: Thermal Characteristics, Kinetic/Thermodynamic Analysis, and Structure Evolution of Co-Pyrolytic Char" Sustainability 16, no. 15: 6677. https://doi.org/10.3390/su16156677






