Tribological Performance of ZnO Green Particles as Lubricating Oil Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation and Characterization
2.3. Synthesis of ZnO Particles
2.4. Characterization of ZnO Particles
2.5. Wear Test
3. Results and Discussion
3.1. Characterization of Moscato Seeds and Barbera GP Extract
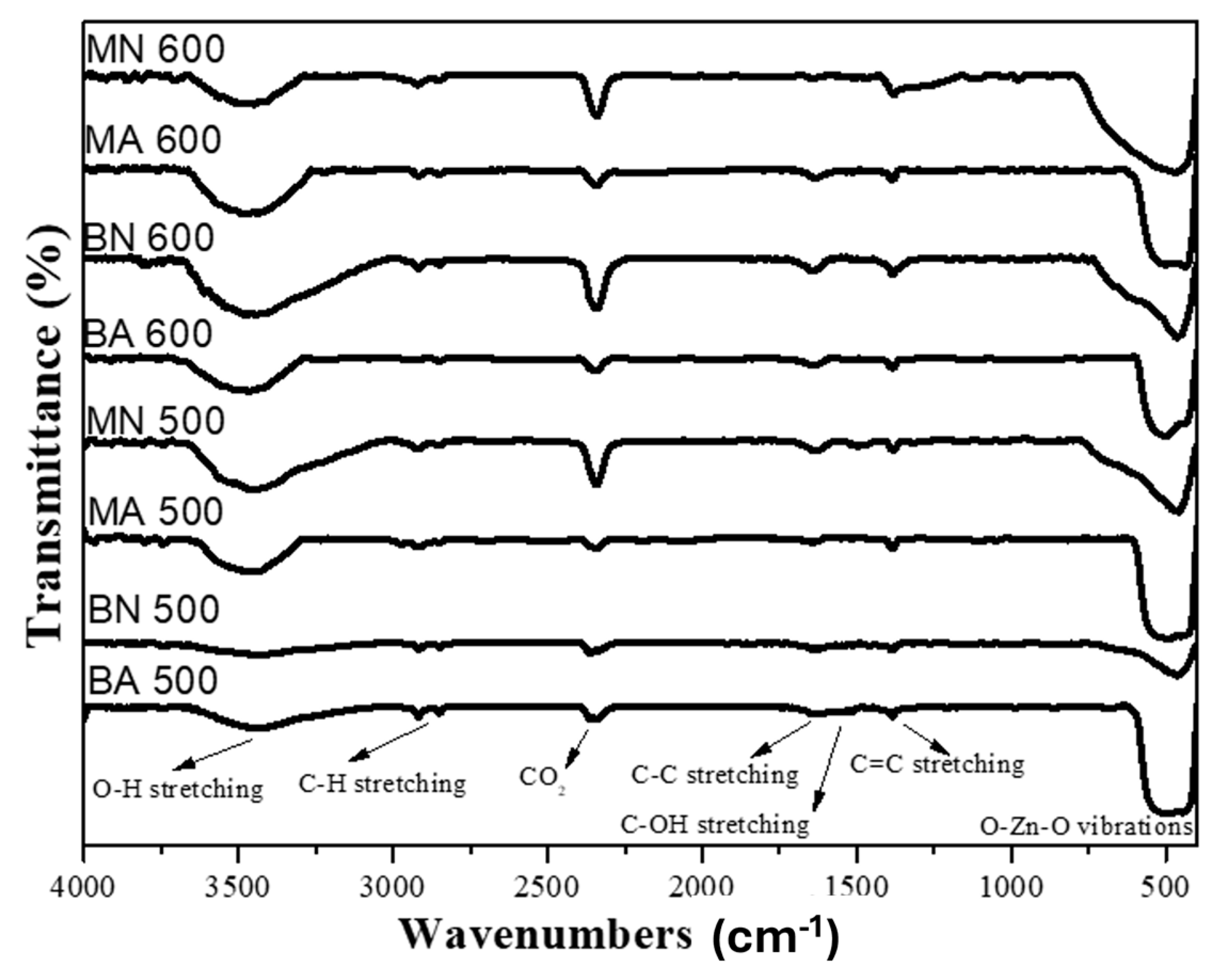
3.2. FTIR Analysis of ZnO Nanoparticles
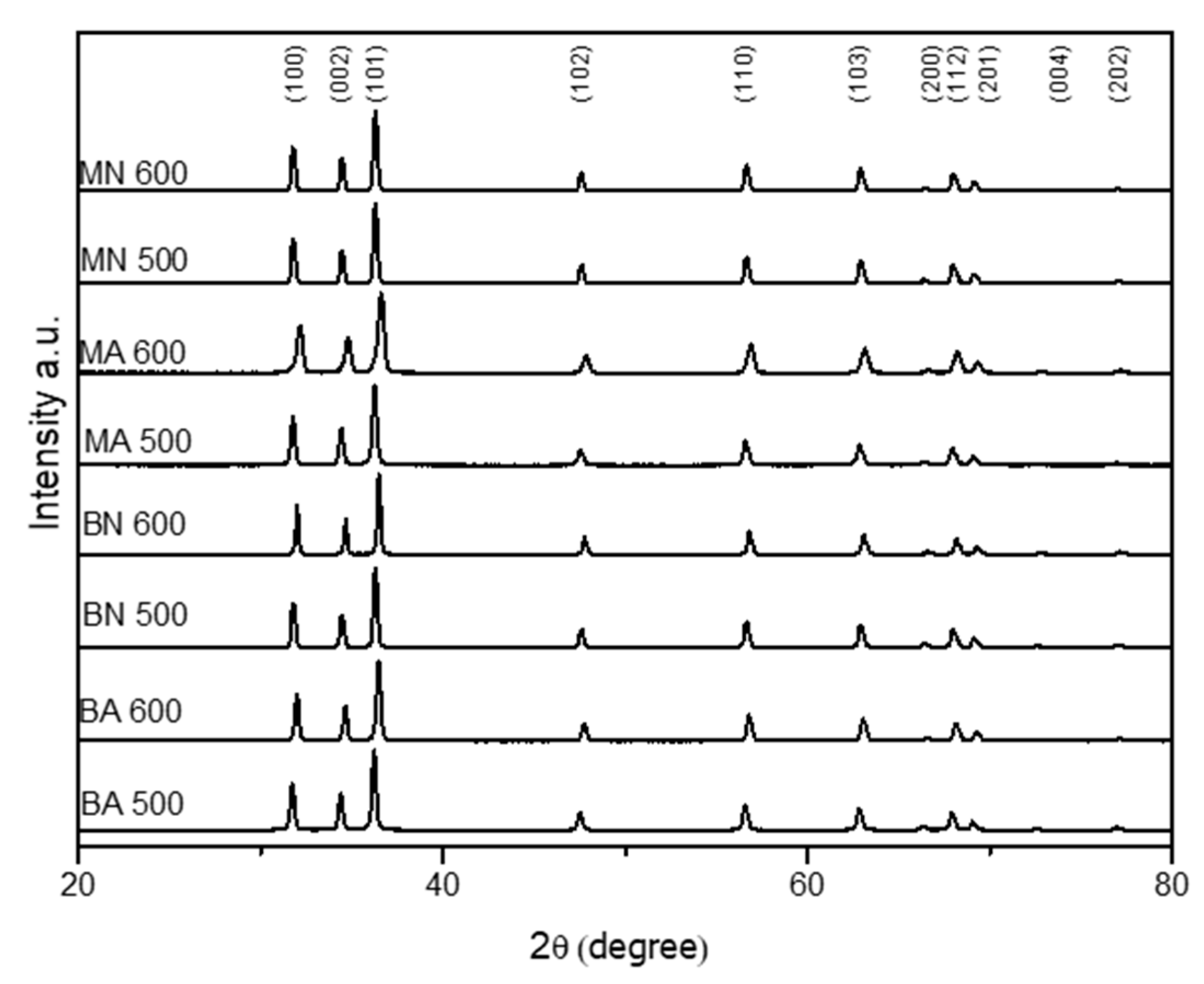
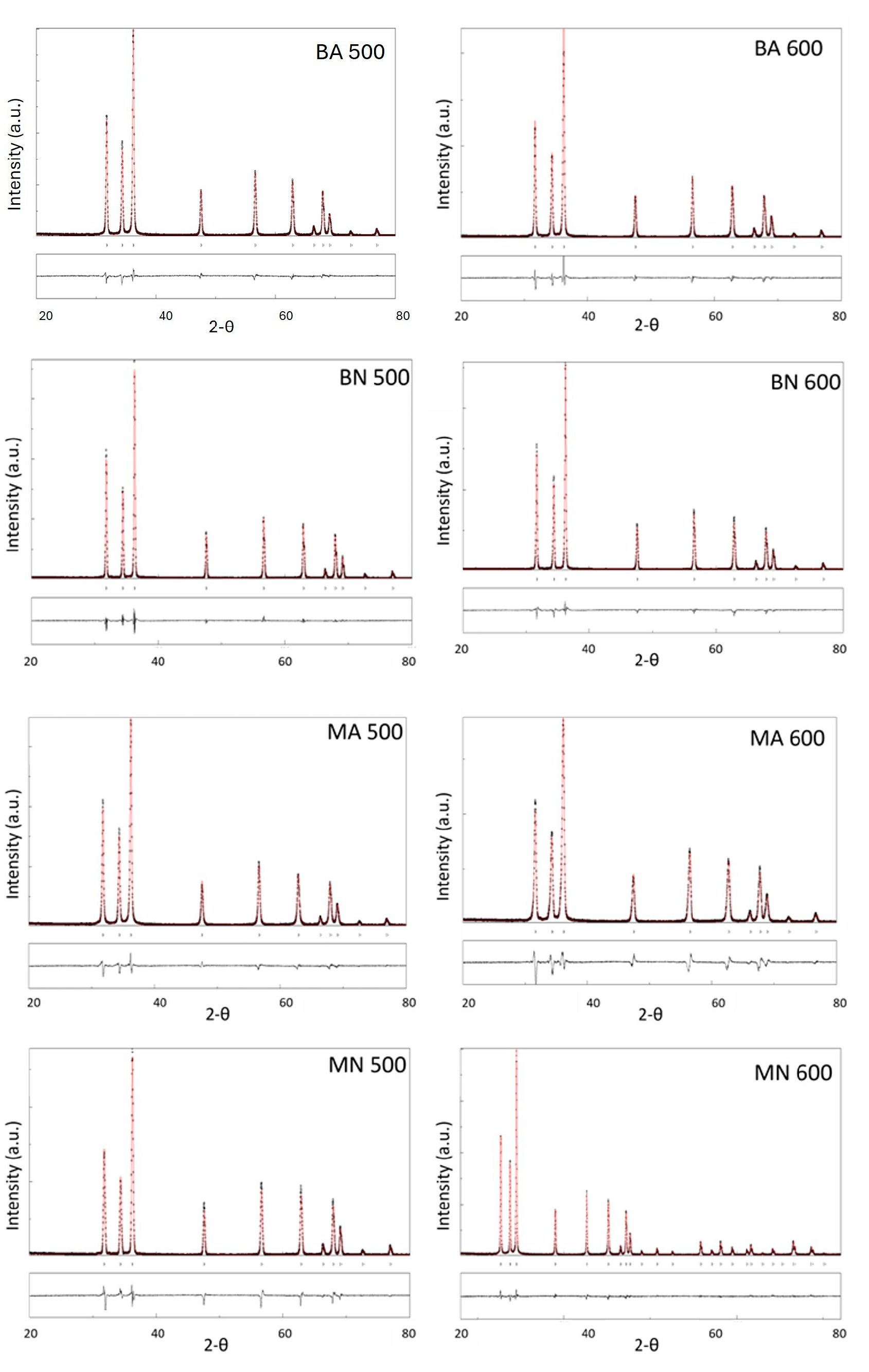
3.3. X-ray Diffraction Analysis of ZnO Particles
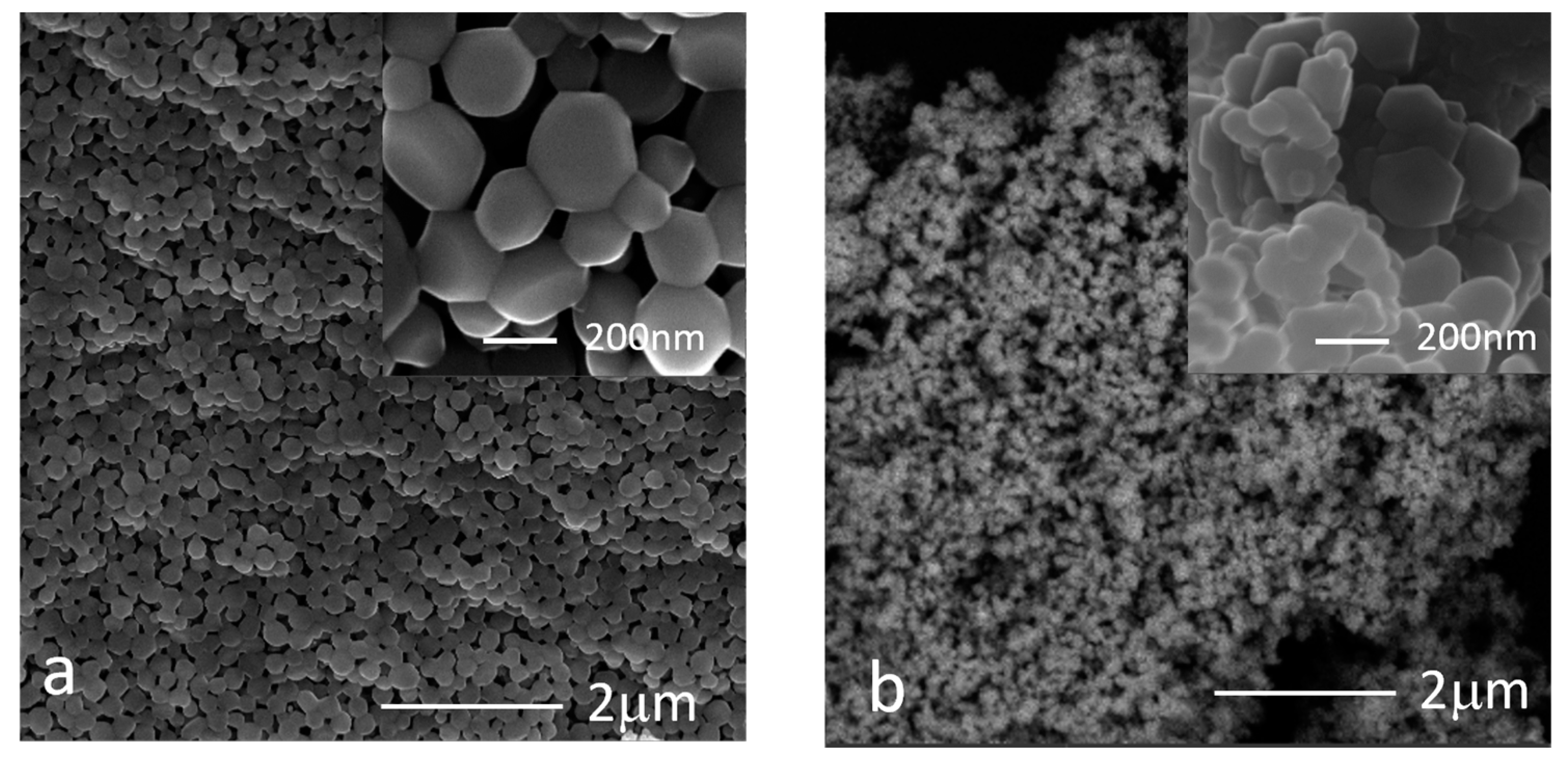
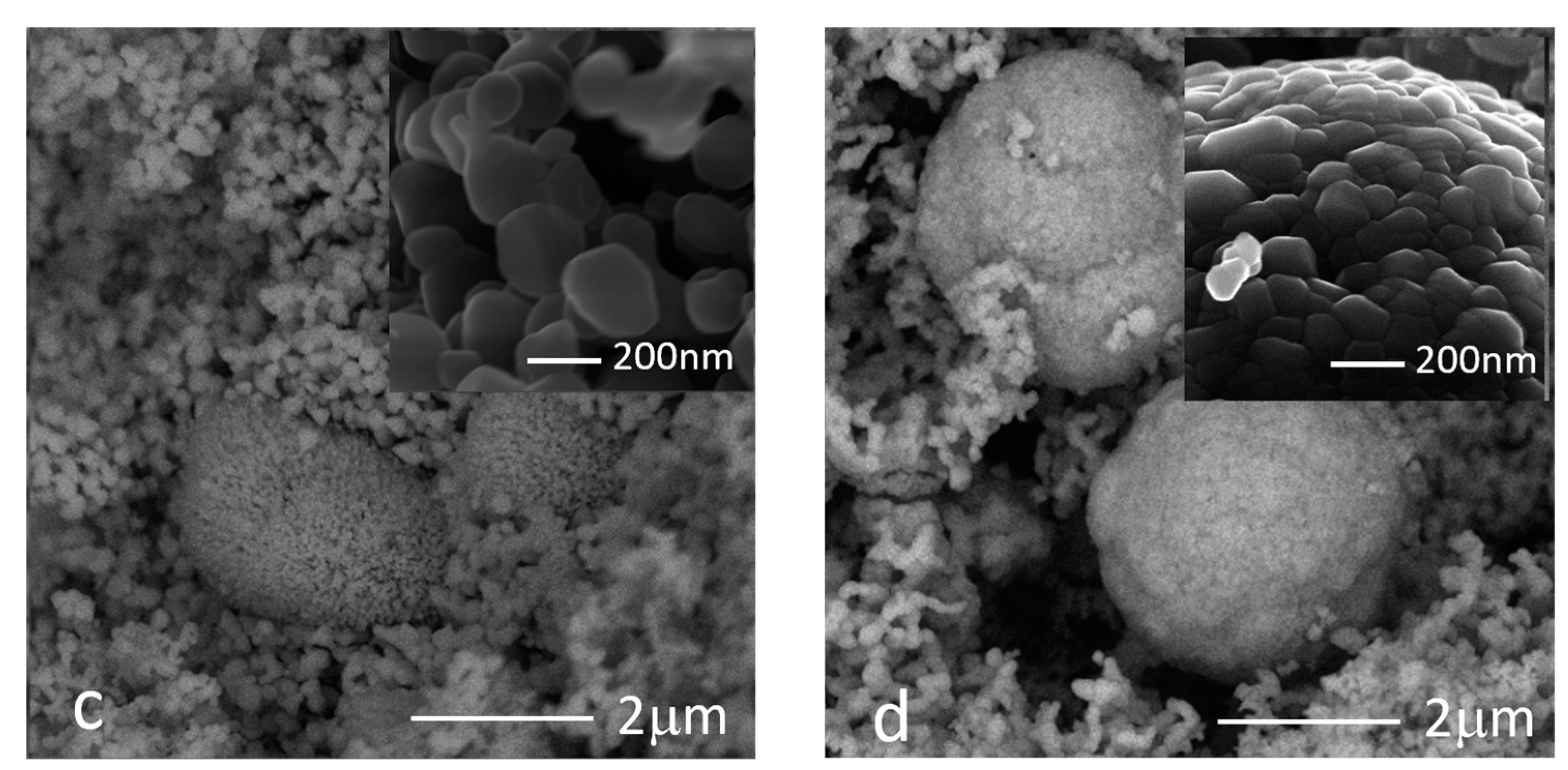
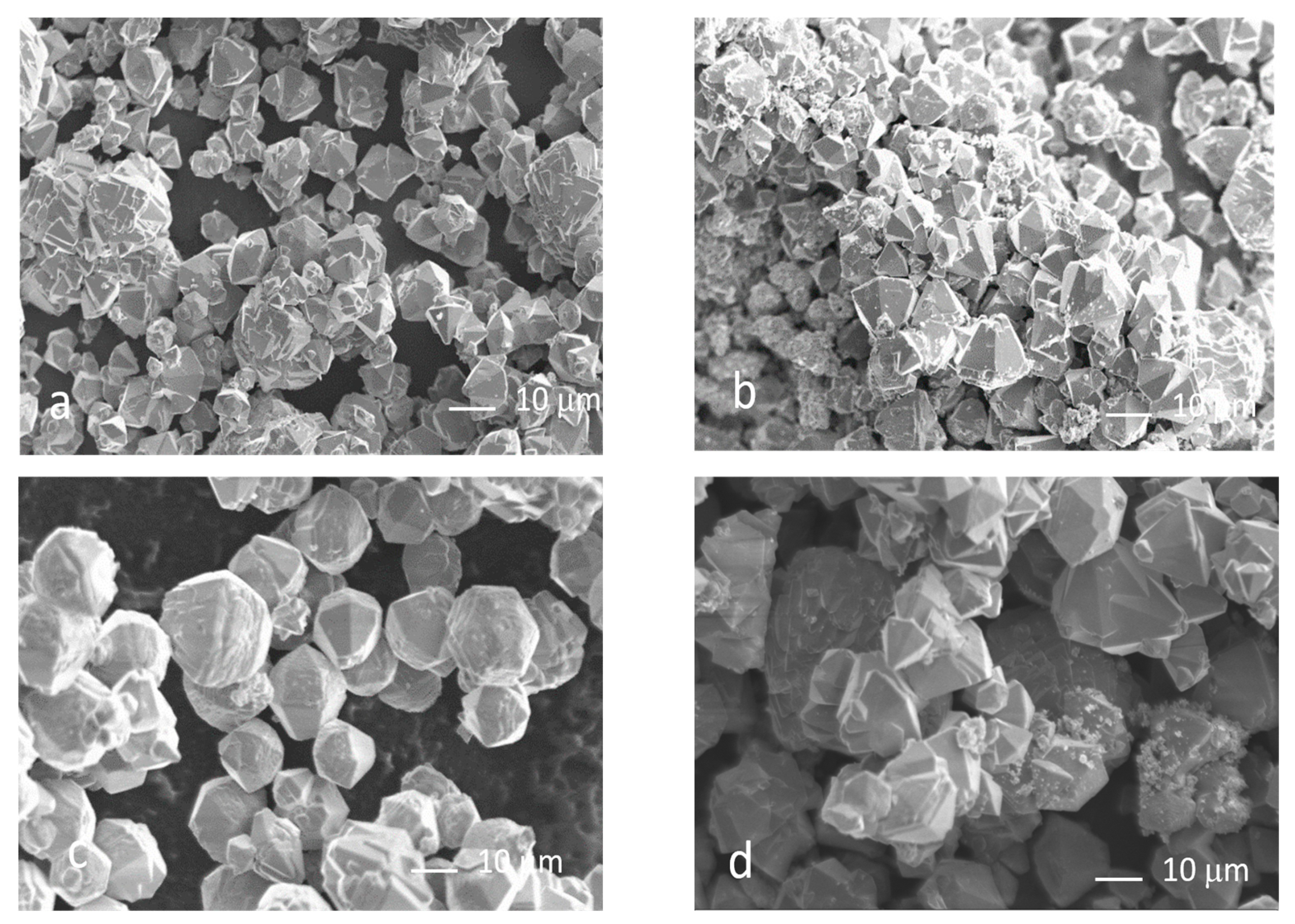
3.4. Morphological Analysis
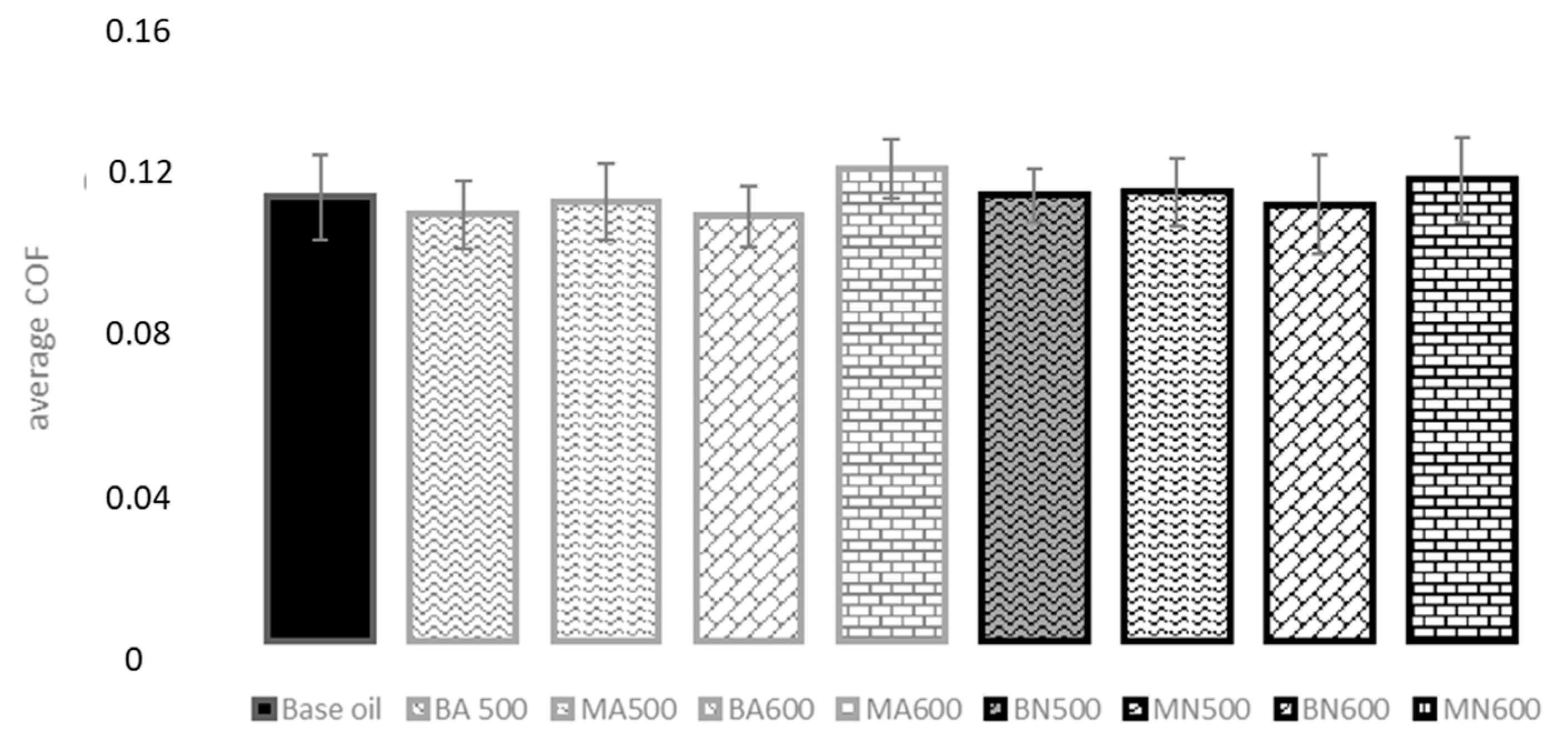
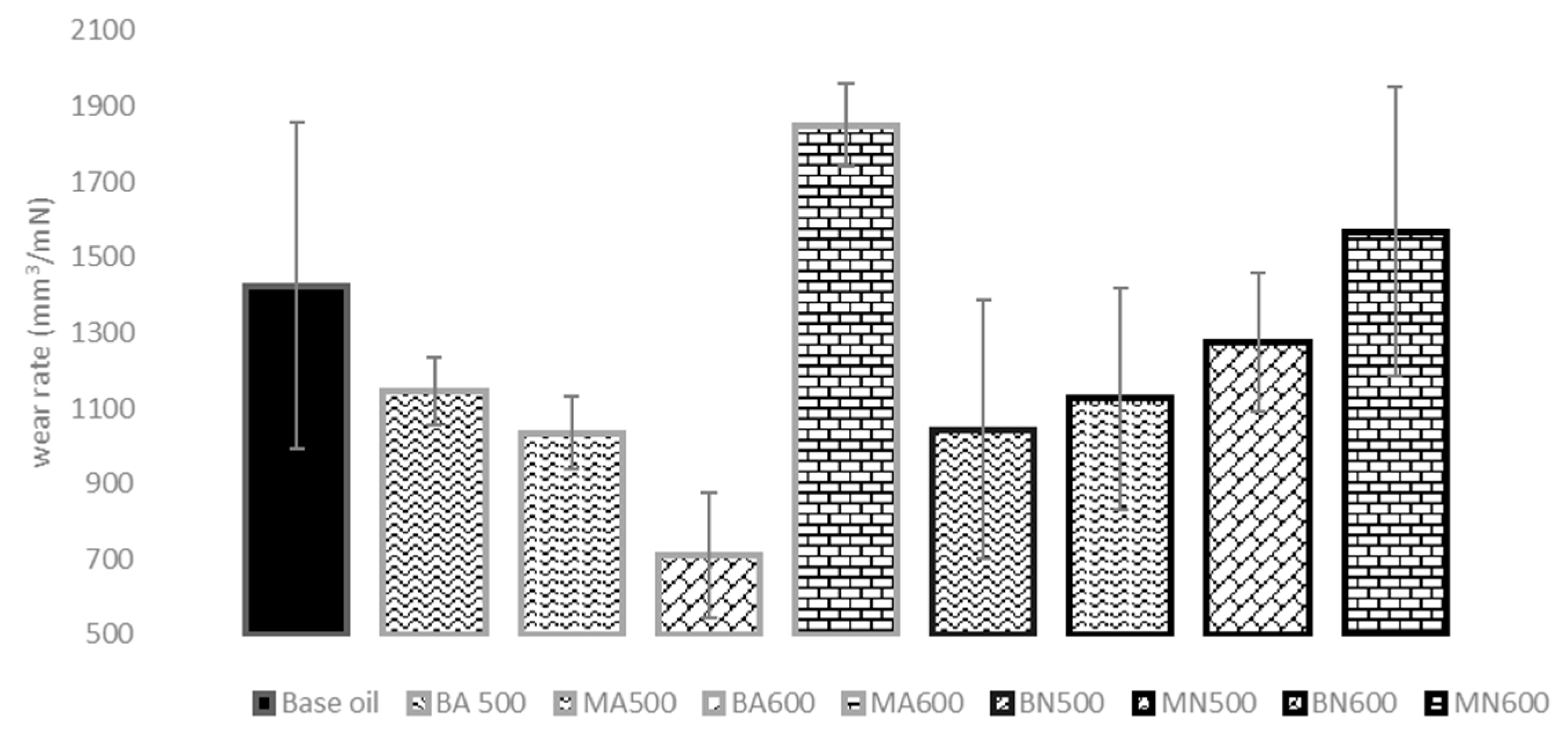
3.5. Tribological Tests
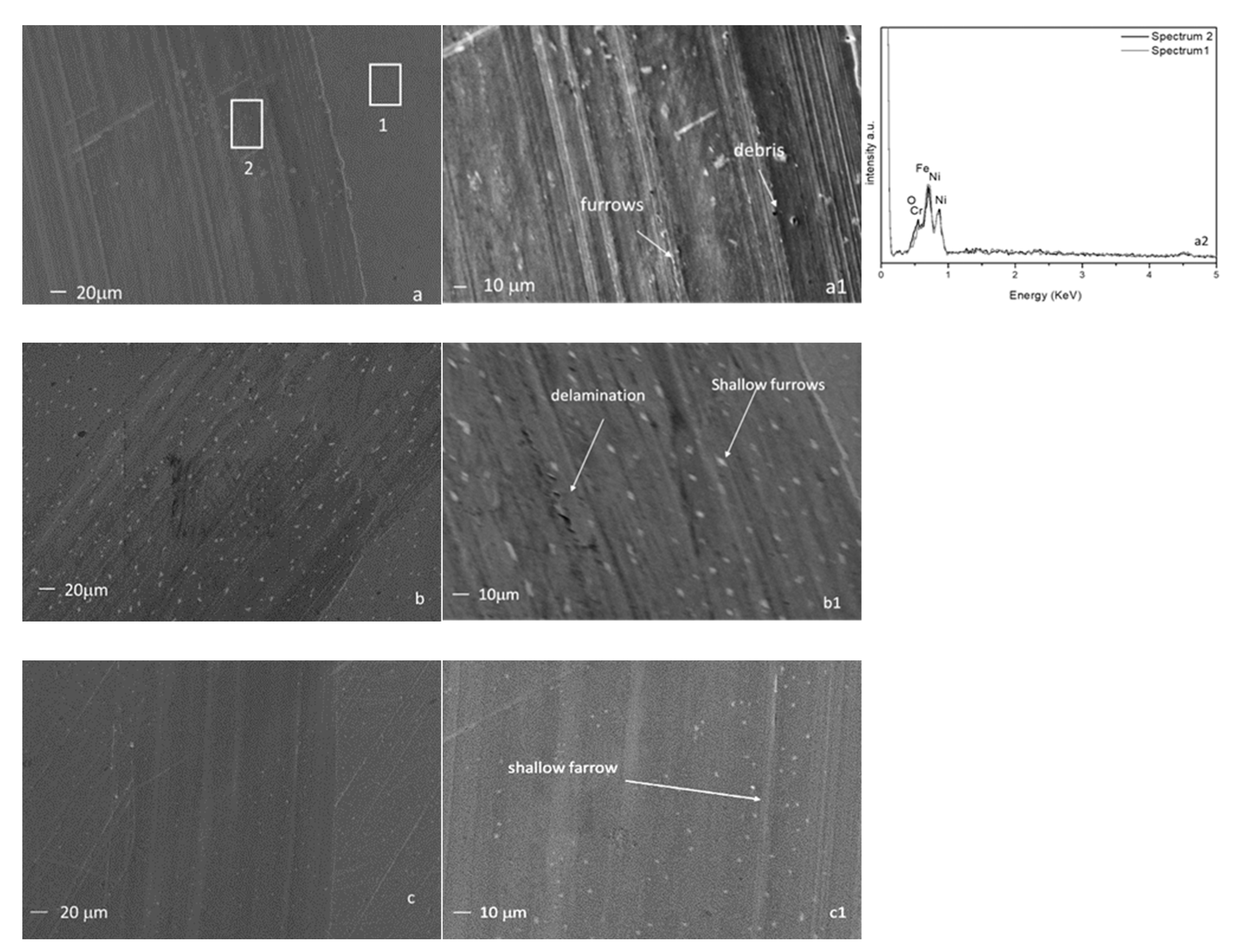
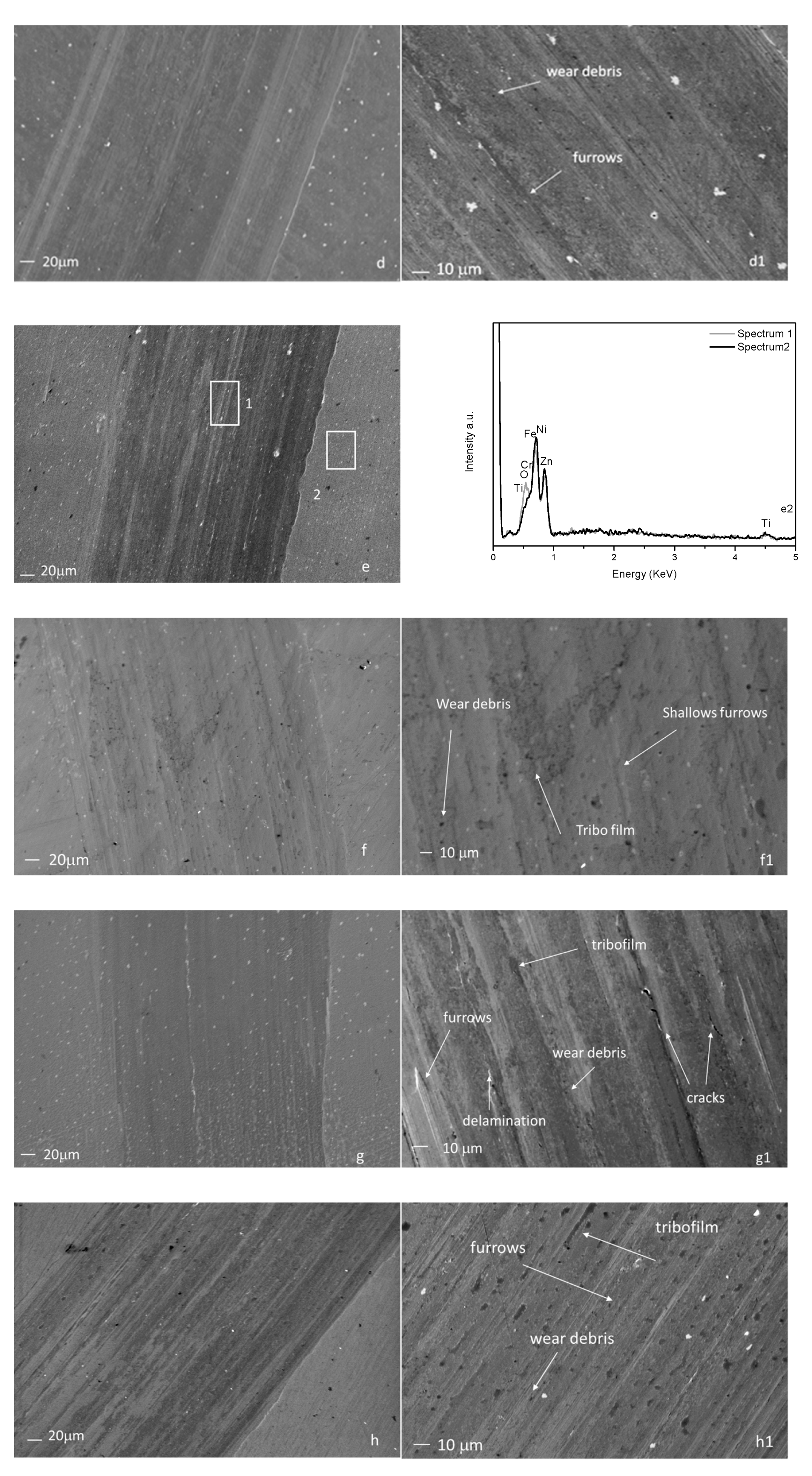
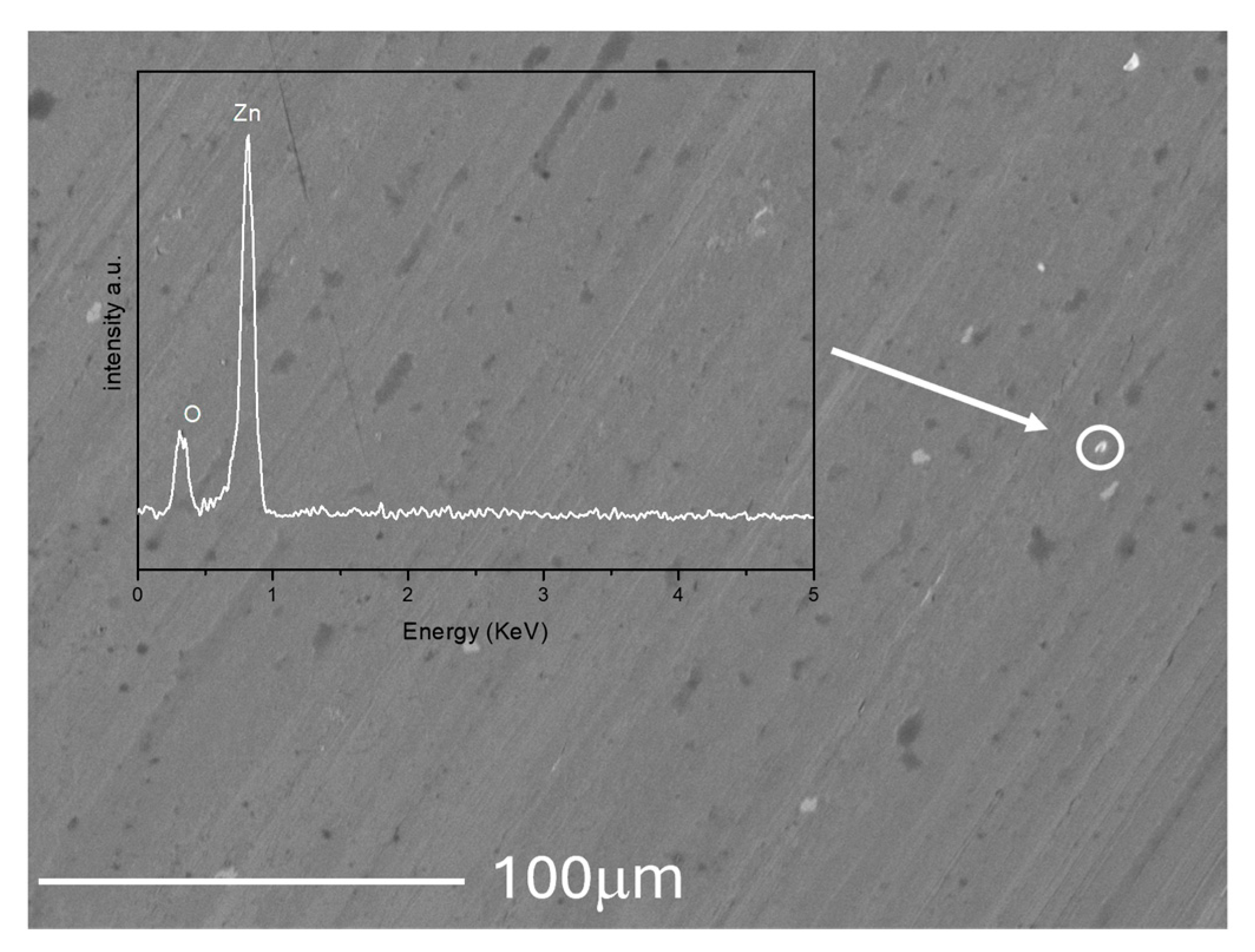
3.6. Worn Morphology
4. Conclusions
- Moscato extracts were richer in polyphenolic compounds than Barbera ones, likely due to the different winemaking technology and the natural distribution of the molecules in the berry. Barbera GP extracts contained anthocyanins, HCTA, and flavonols, while Moscato seeds extracts lacked these compounds. Barbera tannins had lower mDP, gallate subunits, and different proportions of (+)-catechin and (−)-epicatechin. FTIR analysis showed similar absorption bands for the two extracts, with slight differences mainly related to the presence of fatty acids and their glycerides in seeds extract;
- XRD analysis revealed that all the ZnO particles had a hexagonal structure with space group symmetry P63mc. The lattice parameters of ZnO particles were close to standard values, except for those obtained from Moscato seed extract and zinc acetate synthetized at 600 °C (MA600), which had higher values. Crystallite size increased with synthesis temperature, particularly in the case of BN500. Nitrate-based ZnO had higher crystallite size than acetate-based one. The 100/002 intensity ratio showed that the ZnO synthesised with Moscato seed extract led to a lower ratio than that synthesised with Barbera GP, indicating preferential growth along the c-axis;
- The precursor significantly affected size and shape of the ZnO particles. Indeed, particles obtained from nitrate were bipyramidal-shaped, with copresence of spherical nanoparticles when using Moscato, and around 10 to 20 microns in size, whereas those from acetate were quasi-spherical and between 50 and 200 nm in size;
- Regarding tribological results, the addition to oil of both nitrate and acetate-derived ZnO particles stabilized the friction coefficient. COF was slightly higher than the base oil for most sliding tests, particularly when MA600 was added to the oil. Less pronounced differences with other samples were observed. All the ZnO particles, except MA600 and MN600, improved the wear resistance compared to the standard oil. This behaviour appeared dependent on the morphological characteristics of the ZnO powders. Indeed, spherical nanoparticles, obtained using acetate, functioned both as ball bearings between friction surfaces and as fillers of the holes/defects created in the steel disc, inducing a repairing effect, then lowering the wear. In the case of microparticles, obtained from the nitrate salt, they functioned only as load bearing, being too big for acting as fillers. Indeed, the wear resistance of ZnO microparticles appeared lower if compared to ZnO nanoparticles. Furthermore, an uneven tribofilm was observed to develop.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Brandão, A.S.; Gonçalves, A.; Santos, J.M.R.C.A. Circular Bioeconomy Strategies: From Scientific Research to Commercially Viable Products. J. Clean. Prod. 2021, 295, 126407. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and in Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO Nanomaterials: Green Synthesis, Toxicity Evaluation and New Insights in Biomedical Applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Kumar, J.A.; Krithiga, T.; Manigandan, S.; Sathish, S.; Renita, A.A.; Prakash, P.; Prasad, B.S.N.; Kumar, T.R.P.; Rajasimman, M.; Hosseini-Bandegharaei, A.; et al. A Focus to Green Synthesis of Metal/Metal Based Oxide Nanoparticles: Various Mechanisms and Applications towards Ecological Approach. J. Clean. Prod. 2021, 324, 129198. [Google Scholar] [CrossRef]
- Ijaz, I.; Bukhari, A.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R.; hussain, S.; hussain, T.; bukhari, A.; naseer, y.; et al. Green Synthesis of Silver Nanoparticles Using Different Plants Parts and Biological Organisms, Characterization and Antibacterial Activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100704. [Google Scholar] [CrossRef]
- Xia, C.; Jin, X.; Garalleh, H.A.; Garaleh, M.; Wu, Y.; Hill, J.M.; Pugazhendhi, A. Optimistic and Possible Contribution of Nanomaterial on Biomedical Applications: A Review. Environ. Res. 2023, 218, 114921. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, M.; Sui, T.; Zheng, W.; Qiao, G.; Yan, S.; Liu, X. Role of Nanoparticle Materials as Water-Based Lubricant Additives for Ceramics. Tribol. Int. 2020, 142, 105978. [Google Scholar] [CrossRef]
- Ouyang, T.; Tang, W.; Pan, M.; Tang, J.; Huang, H. Friction-Reducing and Anti-Wear Properties of 3D Hierarchical Porous Graphene/Multi-Walled Carbon Nanotube in Castor Oil under Severe Condition: Experimental Investigation and Mechanism Study. Wear 2022, 498–499, 204302. [Google Scholar] [CrossRef]
- Alves, S.M.; Mello, V.S.; Faria, E.A.; Camargo, A.P.P. Nanolubricants Developed from Tiny CuO Nanoparticles. Tribol. Int. 2016, 100, 263–271. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of Nanoparticles in Oil Lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Waqas, M.; Zahid, R.; Bhutta, M.U.; Khan, Z.A.; Saeed, A. A Review of Friction Performance of Lubricants with Nano Additives. Materials 2021, 14, 6310. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Tsui, W.C.; Liu, T.C. Experimental Analysis of Tribological Properties of Lubricating Oils with Nanoparticle Additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental Comparison between ZnO and MoS2 Nanoparticles as Additives on Performance of Diesel Oil-Based Nano Lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Maggirwar, R.; Datta, S.; Pathak, S.D. Analyses of Anti-Wear and Extreme Pressure Properties of Castor Oil with Zinc Oxide Nano Friction Modifiers. Appl. Surf. Sci. 2018, 449, 277–286. [Google Scholar] [CrossRef]
- Hernandez Battez, A.; Fernandez Rico, J.E.; Navas Arias, A.; Viesca Rodriguez, J.L.; Chou Rodriguez, R.; Diaz Fernandez, J.M. The Tribological Behaviour of ZnO Nanoparticles as an Additive to PAO6. Wear 2006, 261, 256–263. [Google Scholar] [CrossRef]
- Arumugam, S.; Baskar, S.; Sankaranarayanan, S.; Athreya, S.H.; Narayanan, N.L.; Prasad, S.S.D. Influence of Morphology of Anti-Wear Nano Additives on Tribological Behavior of Chemically Modified Rapeseed Oil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 390, 012017. [Google Scholar] [CrossRef]
- Guaita, M.; Panero, L.; Motta, S.; Mangione, B.; Bosso, A. Effects of High-Temperature Drying on the Polyphenolic Composition of Skins and Seeds from Red Grape Pomace. LWT 2021, 145, 111323. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Bosso, A.; Cassino, C.; Motta, S.; Panero, L.; Tsolakis, C.; Guaita, M. Polyphenolic Composition and In Vitro Antioxidant Activity of Red Grape Seeds as Byproducts of Short and Medium-Long Fermentative Macerations. Foods 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Ummarino, I.; Ferrandino, A.; Di Stefano, R.; Cravero, M.C. Evoluzione Dei Polifenoli Di Uve Di Biotipi Di Pinot Nero Durante La Maturazione. L’Enologo 2001, XXXVII, 71–82. [Google Scholar]
- Motta, S.; Guaita, M.; Petrozziello, M.; Panero, L.; Bosso, A. Effect of Reductive Pressing on the Concentration of Reduced Glutathione and Phenols in the Musts of Four Italian Cultivars. Am. J. Enol. Vitic. 2014, 65, 471–478. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C. Metodi per Lo Studio Dei Polifenoli Dell’uva. Riv. Vitic. Enol. 1991, 2, 37–45. [Google Scholar]
- Zum Gahr, K.-H.; Wahl, R.; Wauthier, K. Experimental Study of the Effect of Microtexturing on Oil Lubricated Ceramic/Steel Friction Pairs. Wear 2009, 267, 1241–1251. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Varasteh, M.; Deng, Z.; Hwang, H.; Kim, Y.J.; Wong, G.B. Quantitative Determination of Polymorphic Impurity by X-ray Powder Diffractometry in an OROS® Formulation. Int. J. Pharm. 2009, 366, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gautier di Confiengo, G.; Faga, M.G.; La Parola, V.; Magnacca, G.; Paganini, M.C.; Testa, M.L. Microwave Approach and Thermal Decomposition: A Sustainable Way to Produce ZnO Nanoparticles with Different Chemo-Physical Properties. Mater. Chem. Phys. 2024, 321, 129485. [Google Scholar] [CrossRef]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef]
- Dutta, S.; Chattopadhyay, S.; Sarkar, A.; Chakrabarti, M.; Sanyal, D.; Jana, D. Role of Defects in Tailoring Structural, Electrical and Optical Properties of ZnO. Prog. Mater. Sci. 2009, 54, 89–136. [Google Scholar] [CrossRef]
- Goswami, M.; Adhikary, N.C.; Bhattacharjee, S. Effect of Annealing Temperatures on the Structural and Optical Properties of Zinc Oxide Nanoparticles Prepared by Chemical Precipitation Method. Optik 2018, 158, 1006–1015. [Google Scholar] [CrossRef]
- Chen, Y.; Jyoti, N.; Hyun-U, K.; Kim, J. Effect of Annealing Temperature on the Characteristics of ZnO Thin Films. J. Phys. Chem. Solids 2012, 73, 1259–1263. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Farias, A.C.; Marcilio, N.R.; Bueno, J.M.C. The Catalytic Behavior of Zinc Oxide Prepared from Various Precursors and by Different Methods. Mater. Res. Bull. 2005, 40, 2089–2099. [Google Scholar] [CrossRef]
- Sherin, L.; Sohail, A.; Amjad, U.-S.; Mustafa, M.; Jabeen, R.; Ul-Hamid, A. Facile Green Synthesis of Silver Nanoparticles Using Terminalia Bellerica Kernel Extract for Catalytic Reduction of Anthropogenic Water Pollutants. Colloid. Interface Sci. Commun. 2020, 37, 100276. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green Route to Synthesize Zinc Oxide Nanoparticles Using Leaf Extracts of Cassia Fistula and Melia Azadarach and Their Antibacterial Potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef] [PubMed]
- Nava, O.J.; Soto-Robles, C.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Olivas, A.; Luque, P.A. Fruit Peel Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Bandeira, M.; Possan, A.L.; Pavin, S.S.; Raota, C.S.; Vebber, M.C.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Crespo, J.S. Mechanism of Formation, Characterization and Cytotoxicity of Green Synthesized Zinc Oxide Nanoparticles Obtained from Ilex Paraguariensis Leaves Extract. Nano-Struct. Nano-Objects 2020, 24, 100532. [Google Scholar] [CrossRef]
- Han, X.; Thrush, S.J.; Zhang, Z.; Barber, G.C.; Qu, H. Tribological Characterization of ZnO Nanofluids as Fastener Lubricants. Wear 2021, 468–469, 203592. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, F.; Barber, G.C.; Zou, Q.; Wang, J.; Guo, S.; Yuan, Y.; Jiang, Q. Role of Nano-Sized Materials as Lubricant Additives in Friction and Wear Reduction: A Review. Wear 2022, 490–491, 204206. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Qin, B.; Xing, D.; Guo, Y.; Fan, R. Investigation of the Mending Effect and Mechanism of Copper Nano-Particles on a Tribologically Stressed Surface. Tribol. Lett. 2004, 17, 961–966. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Qingping, C.; Turkson, R.F.; Bicheng, C. Improving the Tribological Characteristics of Piston Ring Assembly in Automotive Engines Using Al2O3 and TiO2 Nanomaterials as Nano-Lubricant Additives. Tribol. Int. 2016, 103, 540–554. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Liu, A.; Wu, C.; Li, W. Tribological Performance of Silicone Oil Based Al2O3 Nano Lubricant for an Mg Alloy Subjected to Sliding at Elevated Temperatures. Tribol. Int. 2022, 175, 107779. [Google Scholar] [CrossRef]




| Sample Name | Extract | Zinc Salt Precursor | Calcination Temperature |
|---|---|---|---|
| BA500 | Barbera | Zinc acetate | 500 °C |
| BA600 | Barbera | Zinc acetate | 600 °C |
| BN500 | Barbera | Zinc nitrate | 500 °C |
| BN600 | Barbera | Zinc nitrate | 600 °C |
| MA500 | Moscato | Zinc acetate | 500 °C |
| MA600 | Moscato | Zinc acetate | 600 °C |
| MN500 | Moscato | Zinc nitrate | 500 °C |
| MN600 | Moscato | Zinc nitrate | 600 °C |
| Fe | C | Si | Mn | Cr | Ni | P | S | W | Co |
|---|---|---|---|---|---|---|---|---|---|
| Bal. | 0.4–0.5 | 2.7–3.3 | ≤0.8 | 8–10 | ≤0.6 | ≤0.22 | ≤0.03 | - | - |
| Polyphenolic Category | Polyphenolic Profile | Barbera | Moscato |
|---|---|---|---|
| Total polyphenols (GAE) | 219.6 ± 4.98 | 540.0 ± 2.71 | |
| Total anthocyanins | 25.9 ± 2.05 | - | |
| Condensed tannins (polymeric flavan-3-ols) | Condensed tannins | 63.2 ± 4.21 | 340.1 ± 8.86 |
| mDP | 3.8 ± 0.05 | 4.4 ± 0.06 | |
| gallates % | 14.9 ± 0.26 | 20.1 ± 0.06 | |
| (+)-catechin % | 32.4 ± 0.42 | 20.4 ± 0.03 | |
| (−)-epicatechin % | 67.6 ± 0.42 | 79.6 ± 0.03 | |
| monomeric flavan-3-ols | (+)-catechin | 1.8 ± 0.05 | 15.6 ± 0.03 |
| (−)-epicatechin | 1.4 ± 0.05 | 10.4 ± 0.27 | |
| dimeric flavan-3-ols | dimer B1 | 0.4 ± 0.02 | 2.5 ± 0 |
| dimer B2 | 1.4 ± 0.01 | 2.0 ± 0.13 | |
| gallic acid | 2.3 ± 0.04 | 3.1 ± 0.01 | |
| ∑HCTA | 2.6 ± 0.04 | - | |
| ∑flavonols | 5.4 ± 0.05 | - |
| Standard ZnO (JCPDF: 36-1451) | BA 500 | BA 600 | BN 500 | BN 600 | MA 500 | MA 600 | MN 500 | MN 600 |
|---|---|---|---|---|---|---|---|---|
| 1.29 | 1.29 | 1.09 | 1.06 | 1.04 | 1.02 | 1.02 | 1.02 | 1.01 |
| D (nm) | ε | a (Å) | c (Å) | Rwp (%) | Rp (%) | χ2 | |
|---|---|---|---|---|---|---|---|
| BA 500 | 65.61 | 2.876 × 10−4 | 3.251 | 5.208 | 12.526 | 6.77 | 1.834 |
| BA 600 | 70.76 | 2.180 × 10−4 | 3.252 | 5.209 | 9.548 | 7.33 | 1.915 |
| BN 500 | 96.7 | 2.030 × 10−4 | 3.249 | 5.206 | 8.819 | 6.57 | 1.921 |
| BN 600 | 155.62 | 7.387 × 10−4 | 3.252 | 5.210 | 9.993 | 5.95 | 1.962 |
| MA 500 | 54.18 | 2.189 × 10−4 | 3.250 | 5.208 | 8.417 | 5.98 | 1.625 |
| MA 600 | 58.24 | 3.289 × 10−4 | 3.256 | 5.218 | 12.701 | 10.03 | 1.781 |
| MN 500 | 90.4 | 2.006 × 10−4 | 3.250 | 5.207 | 11.577 | 8.87 | 1.985 |
| MN 600 | 105.59 | 1.964 × 10−4 | 3.249 | 5.206 | 13.031 | 6.98 | 1.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautier di Confiengo, G.; Malusà, E.; Guaita, M.; Motta, S.; Di Maro, M.; Faga, M.G. Tribological Performance of ZnO Green Particles as Lubricating Oil Additives. Sustainability 2024, 16, 6810. https://doi.org/10.3390/su16166810
Gautier di Confiengo G, Malusà E, Guaita M, Motta S, Di Maro M, Faga MG. Tribological Performance of ZnO Green Particles as Lubricating Oil Additives. Sustainability. 2024; 16(16):6810. https://doi.org/10.3390/su16166810
Chicago/Turabian StyleGautier di Confiengo, Giovanna, Eligio Malusà, Massimo Guaita, Silvia Motta, Mattia Di Maro, and Maria Giulia Faga. 2024. "Tribological Performance of ZnO Green Particles as Lubricating Oil Additives" Sustainability 16, no. 16: 6810. https://doi.org/10.3390/su16166810
APA StyleGautier di Confiengo, G., Malusà, E., Guaita, M., Motta, S., Di Maro, M., & Faga, M. G. (2024). Tribological Performance of ZnO Green Particles as Lubricating Oil Additives. Sustainability, 16(16), 6810. https://doi.org/10.3390/su16166810









