Biochar Prepared from Steam-Exploded Bitter Melon Vine for the Adsorption of Methylene Blue from Aqueous Solution: Kinetics, Isotherm, Thermodynamics and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar from Bitter Melon Vine
2.2. Biochar Preparation Using Steam-Explosion-Assisted Pyrolysis
2.3. Characterization of Biochar from Bitter Melon Vine
2.4. Experiment of MB Adsorption on Biochar
2.4.1. Effect of pH on the Adsorption Performance of Biochar
2.4.2. Effect of Initial Dosage on the Adsorption Performance of Biochar
2.4.3. Adsorption Kinetics Experiment
2.4.4. Adsorption Thermodynamics Experiments
3. Results and Discussion
3.1. Characterization of Biochars
3.1.1. Elemental Compositions of the Biochars
3.1.2. Infrared Spectral Analysis
3.1.3. SEM Analysis
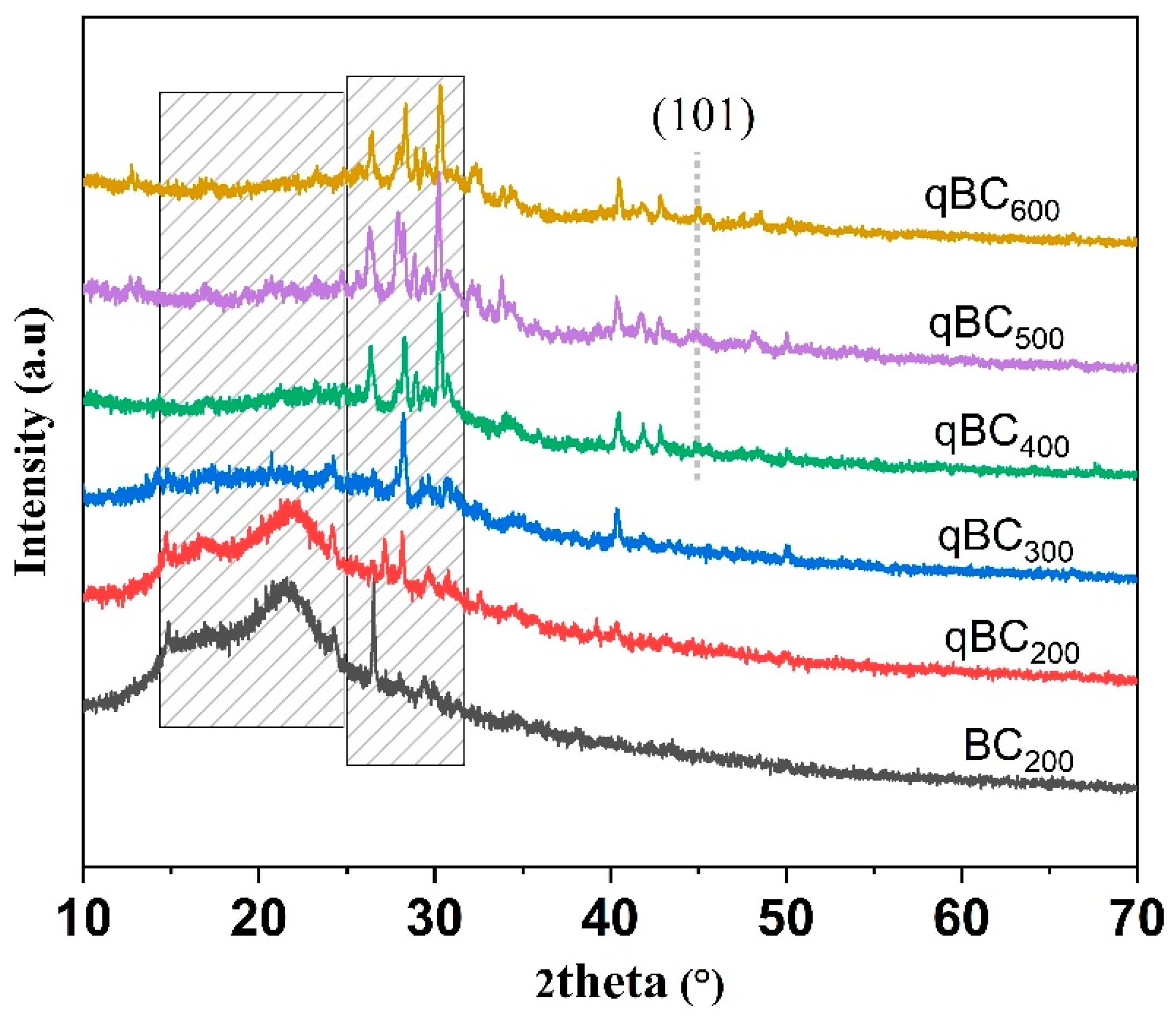
3.1.4. Crystal Structure Analysis of the Biochars
3.1.5. Specific Surface Area and Pore Size Analysis of the Biochars
3.1.6. Effect of Preparation Conditions on the Adsorption Performance of Biochar
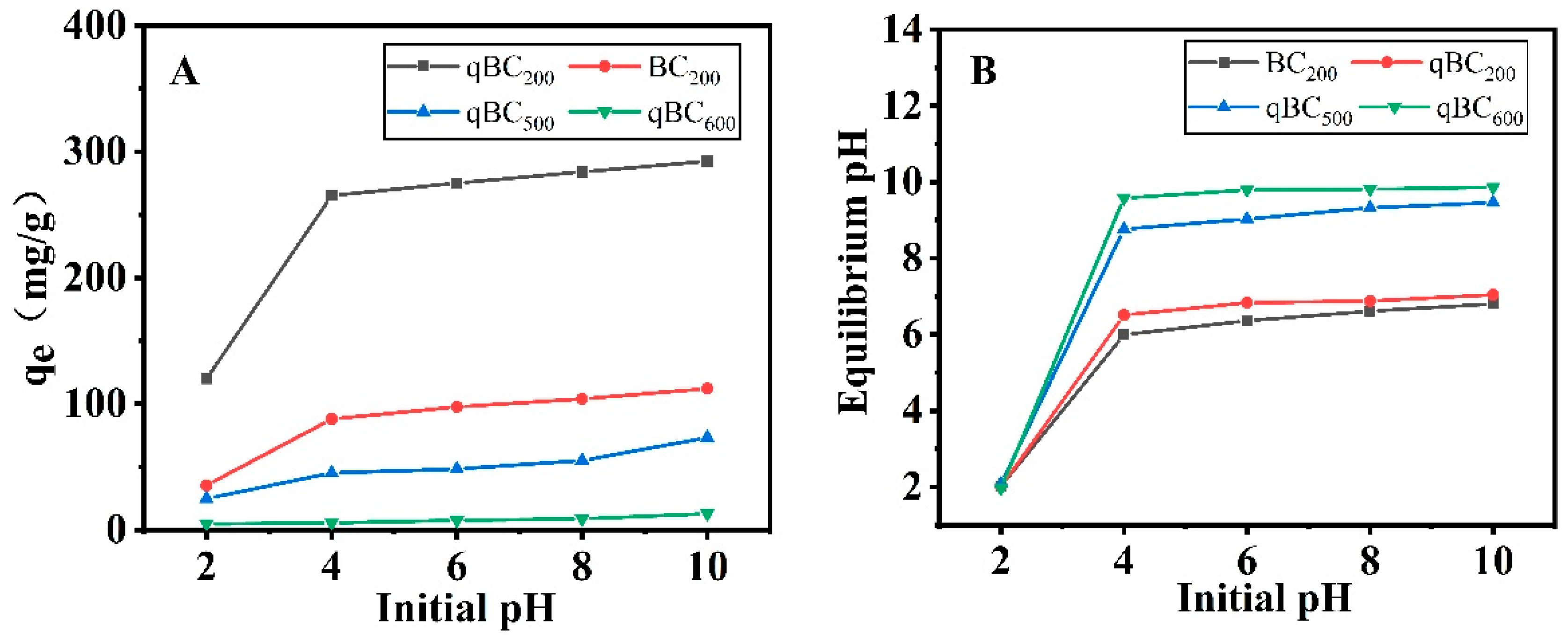
3.1.7. Effect of Initial Solution pH
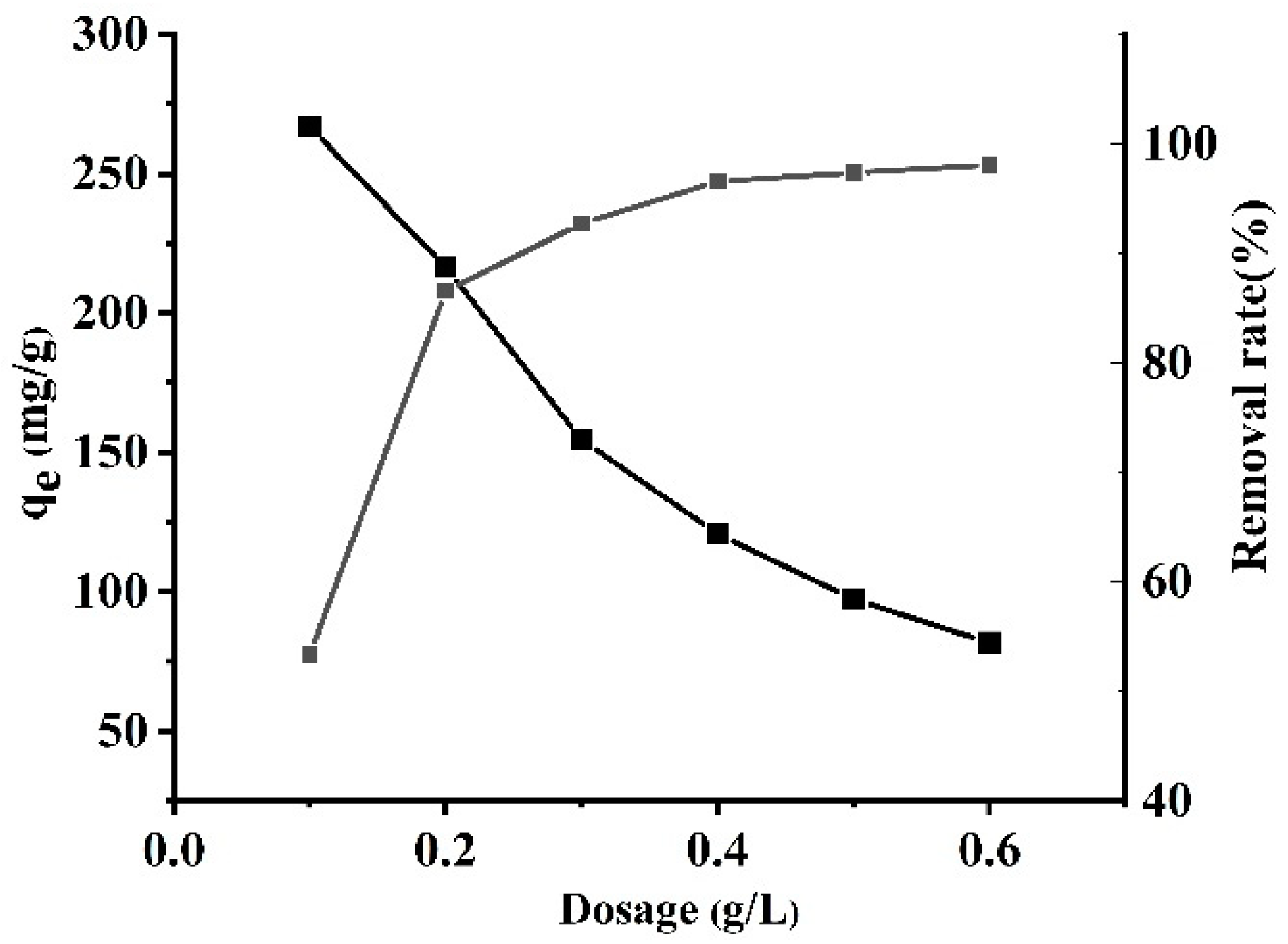
3.1.8. Effect of Initial Biochar Dosage
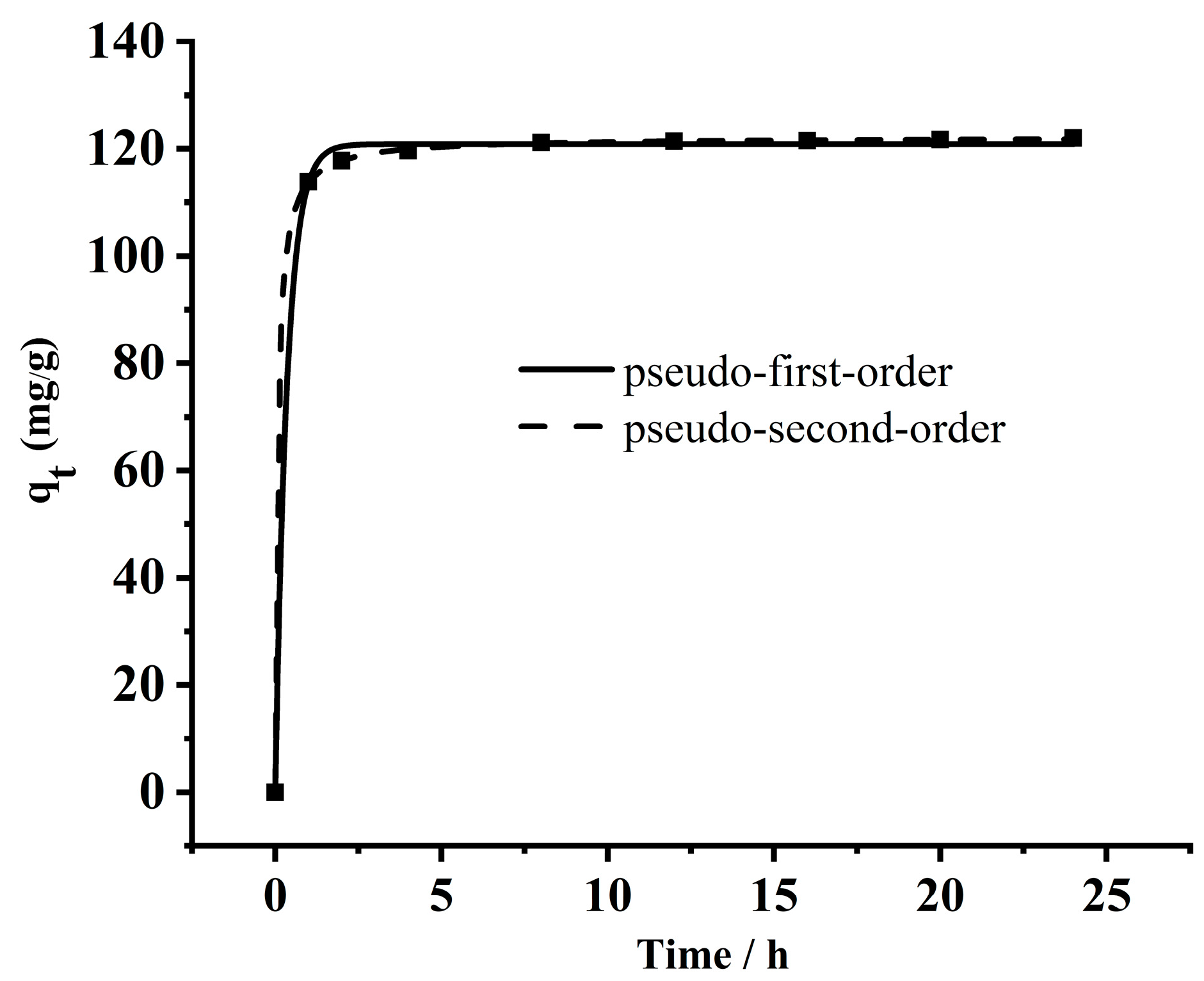
3.1.9. Adsorption Kinetics
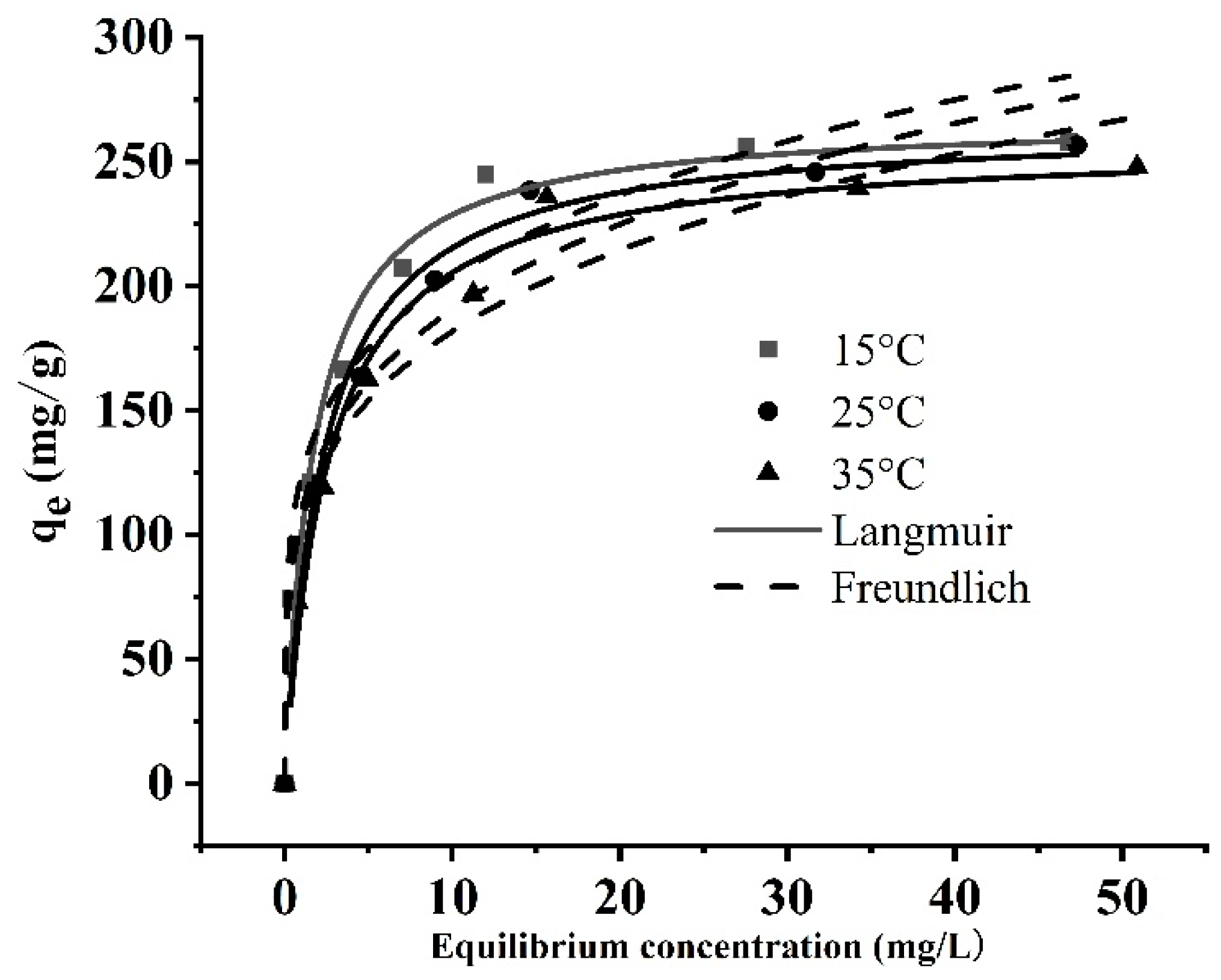
3.1.10. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, K.; Guo, R.; Wang, Z.; Huo, R.; Sun, Y.; Zhai, S.; Wang, J. Efficient Resource Utilization of Vegetable Waste. Chin. Agric. Sci. Bull. 2023, 39, 92–99. [Google Scholar]
- Ji, C.; Kong, C.; Mei, Z.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Biotechnol. Appl. Bioc. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- Igalavithana, A.; Lee, S.E.; Lee, Y.; Tsang, D.; Rinklebe, J.; Kwon, E.; Ok, Y. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Igalavithana, A.; Kwon, E.; Vithanage, M.; Rinklebe, J.; Moon, D.; Meers, E.; Tsang, D.; Ok, Y. Soil lead immobilization by biochars in short-term laboratory incubation studies. Environ. Int. 2019, 127, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Khan, M. Microwave assisted convective drying of bitter gourd: Drying kinetics and effect on ascorbic acid, total phenolics and antioxidant activity. J. Food Meas. Charact. 2019, 13, 2481–2490. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Liu, S.; Zhou, J.; Ke, L.; Rao, P. Increase in the free radical scavenging capability of bitter gourd by a heat-drying process. Food Funct. 2013, 4, 1850–1855. [Google Scholar]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of Torrefied Biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhan, Y.; Zhu, L. Reduced carbon sequestration potential of biochar in acidic soil. Sci. Total Environ. 2016, 572, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis temperature-dependent carbon retention and stability of biochar with participation of calcium: Implications to carbon sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Zou, X.; Debiagi, P.; Amjed, M.; Zhai, M.; Faravelli, T. Impact of high-temperature biomass pyrolysis on biochar formation and composition. J. Anal. Appl. Pyrolysis 2024, 179, 106463. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.; Mašek, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tang, J.; Chen, J.; Zhang, Q. Remediation of cadmium-contaminated soil with biochar simultaneously improves biochar′s recalcitrance. Environ. Pollut. 2020, 256, 113436. [Google Scholar] [CrossRef] [PubMed]
- Aborisade, M.A.; Oba, B.T.; Kumar, A.; Liu, J.S.; Chen, D.Y.; Okimiji, O.P.; Zhao, L. Remediation of metal toxicity and alleviation of toxic metals-induced oxidative stress in Brassica chinensis L using biochar-iron nanocomposites. Plant Soil 2023, 493, 629–645. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Feng, A.X.; Zheng, X.H.; Oba, B.T.; Kumar, A.; Battamo, A.Y.; Kavwenje, S.; Liu, J.S.; Chen, D.; Okimiji, O.P.; et al. Carbothermal reduction synthesis of eggshell-biochar modified with nanoscale zerovalent iron/activated carbon for remediation of soil polluted with lead and cadmium. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100726. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Cui, Y.; Xue, Z.; Ba, Y. Slow pyrolysis polygeneration of bamboo Phyllostachys pubescens: Product yield prediction and biochar formation mechanism. Bioresour. Technol. 2018, 9, 19065–19078. [Google Scholar] [CrossRef]
- Kopecký, M.; Kolář, L.; Konvalina, P.; Strunecký, O.; Teodorescu, F.; Mráz, P.; Peterka, J.; Váchalová, R.; Bernas, J.; Bartoš, P.; et al. Modified Biochar-A Tool for Wastewater Treatment. Energies 2020, 13, 5270. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Okwuosa, U.C.; Ross, A.B. Recovery of phosphate with chemically modified biochars. J. Environ. Chem. Eng. 2016, 4, 1156–1165. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Raud, M.; Krennhuber, K.; Jäger, A.; Kikas, T. Nitrogen explosive decompression pre-treatment: An alternative to steam explosion. Energy 2019, 177, 175–182. [Google Scholar] [CrossRef]
- Wang, C.; Lin, M.; Yang, Q.; Fu, C.; Guo, Z. The Principle of Steam Explosion Technology and Its Application in Food Processing By-Products. Foods 2023, 12, 3307. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, L.; Chen, H.; Zhao, X. Improving storage property of wheat bran by steam explosion. Int. J. Food Sci. Technol. 2021, 56, 287–292. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Pu, Y.; Li, R.; Jia, Y.; Amirsalar, K.; Sui, W. Physicochemical properties and enzymatic digestibility of steam-exploded maize stalk under temperature-pressure decoupling. Trans. CSAE 2022, 38, 239–246. [Google Scholar]
- He, X.; Li, W.; Xia, X.; Lei, L.; Zhao, J.; Ming, J. Study on physicochemical properties and structure of insoluble dietary fiber from Tartary buckwheat bran pretreated by steam explosion. Food Ferment. Ind. 2020, 46, 47–53. [Google Scholar]
- Yu, G.; Guo, T.; Huang, Q.; Shi, X.; Zhou, X. Preparation of high-quality concentrated fragrance flaxseed oil by steam explosion pretreatment technology. Food Sci. Nutr. 2020, 8, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Q.; Rizwan, M.; Zhao, X.; Li, G. Steam explosion of crop straws improves the characteristics of biochar as a soil amendment. J. Integr. Agric. 2019, 18, 1486–1495. [Google Scholar] [CrossRef]
- Yao, C.; Wang, L.; Chen, H. The difference of enzymatic hydrolysis performance of long fibers and short fibers fractionated by steam explosion classification. Chin. J. Process Eng. 2023, 23, 1488–1496. [Google Scholar]
- Islam, T.; Li, Y.; Cheng, H. Biochars and Engineered Biochars for Water and Soil Remediation: A Review. Sustainability 2021, 13, 9932. [Google Scholar] [CrossRef]
- Shakya, A.; Vithanage, M.; Agarwal, T. Influence of pyrolysis temperature on biochar properties and Cr(VI) adsorption from water with groundnut shell biochars: Mechanistic approach. Environ. Res. 2022, 215, 114243. [Google Scholar] [CrossRef]
- Wang, C.; Lin, M.; Li, Y.; Guo, Z. Improvement of soluble dietary fiber quality in Tremella fuciformis stem by steam explosion technology: An evaluation of structure and function. Food Chem. 2024, 437, 137867. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H. Phenolic foam prepared by lignin from a steam-explosion derived biorefinery of corn stalk. Chin. J. Biotechnol. 2014, 30, 901–910. [Google Scholar]
- Zhang, L.; Shi, L.; Wang, T.; Li, D.; Mao, Z. Conversion of wheat stalk lignocellulose during the process of steam explosion. Trans. CSAE 2008, 24, 195–199. [Google Scholar]
- Guo, C.; Zou, J.; Yang, J.; Wang, K.; Song, S. Surface characterization of maize-straw-derived biochar and their sorption mechanism for Pb2+ and methylene blue. PLoS ONE 2020, 15, e0244938. [Google Scholar]
- Eldeeb, T.M.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; El-Nemr, M.A.; Hassaan, M.A.; Ragab, S.; Osibote, O.A.; El Nemr, A. Adsorption of methylene blue (MB) dye on ozone, purified and sonicated sawdust biochars. Biomass Convers. Biorefin. 2024, 14, 9361–9383. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, Z.; Chen, B.; Liu, G.; Zhao, J. Analysis of XRD Spectral Structure and Carbonization of the Biochar Preparation. Spectrosc. Spec. Anal. 2016, 36, 355–3359. [Google Scholar]
- Zhu, Z.; Li, Y.; Wang, X. Machine learning prediction of biochar yield and carbon contents in biochar based on biomass characteristics and pyrolysis conditions. Bioresour. Technol. 2019, 288, 121527. [Google Scholar] [CrossRef] [PubMed]
- Frikha, K.; Limousy, L.; Arif, M.; Thevenin, N.; Ruidavets, L.; Zbair, M.; Bennici, S. Exhausted Grape Marc Derived Biochars: Effect of Pyrolysis Temperature on the Yield and Quality of Biochar for Soil Amendment. Sustainability 2021, 13, 11187. [Google Scholar] [CrossRef]
- Mihiretu, G.; Chimphango, A.; Görgens, J. Steam explosion pre-treatment of alkali-impregnated lignocelluloses for hemicelluloses extraction and improved digestibility. Bioresour. Technol. 2019, 294, 122121. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit. Assess. 2019, 191, 735. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Wen, X.; Yan, C.; Sun, N.; Luo, T.; Zhou, S.; Luo, W. A biomass cationic adsorbent prepared from corn stalk: Lowcost material and high adsorption capacity. J. Polym. Environ. 2018, 26, 1642–1651. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. 2018, 6, 2335–2343. [Google Scholar] [CrossRef]
- Na, L.; Zhang, L.; Zhang, F.; Hua, R. Calculation of Adsorption Thermodynamic Parameters at Solid liquid Interfaces. Mater. Rev. B Res. Pap. 2020, 34, 22030–22035. [Google Scholar]
- Cimirro, N.; Lima, E.; Cunha, M.; Thue, P.; Grimm, A.; Reis, G.; Rabiee, N.; Saeb, M.; Keivaniment, F.; Habibzadeh, S. Removal of diphenols using pine biochar. Kinetics, equilibrium, thermodynamics, and mechanism of uptake. J. Mol. Liq. 2022, 364, 119979. [Google Scholar] [CrossRef]
- Wang, K.F.; Peng, N.; Sun, J.T.; Lu, G.N.; Chen, M.Q.; Deng, F.C.; Dou, R.N.; Nie, L.J.; Zhong, Y.M. Synthesis of silica-composited biochars from alkali-fused fly ash and agricultural wastes for enhanced adsorption of methylene blue. Sci. Total Environ. 2020, 729, 139055. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Liu, J.X.; Wang, T. Co-pyrolysis of sewage sludge and grape dreg to produce efficient and low-cost biochar for methylene blue removal: Adsorption performance and characteristics. Desalin. Water Treat. 2022, 248, 277–287. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Nag, S.; Dahiya, A.; Pandey, P.; Arora, M.; Babu, J.N. Effect of Pyrolysis Temperature on Mechanistic Transformation for Adsorption of Methylene Blue on Leached Rice-Straw Biochar. Clean Soil Air Water 2022, 50, 2100108. [Google Scholar] [CrossRef]


| Biochars | Elemental Composition (%) | O/C | H/C | (N + O)/C | S/C | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | C (%) | H (%) | O (%) | S (%) | |||||
| qBC | 2.90 | 38.25 | 4.61 | 36.20 | 0.17 | 0.95 | 0.12 | 1.02 | 0.0044 |
| qBC200 | 3.10 | 43.08 | 4.44 | 31.87 | 0.18 | 0.74 | 0.10 | 0.811 | 0.0042 |
| qBC600 | 2.14 | 40.50 | 2.08 | 25.02 | 0.31 | 0.62 | 0.051 | 0.671 | 0.0076 |
| BC0 | 2.61 | 40.97 | 5.21 | 41.65 | 0.07 | 1.02 | 0.13 | 1.080 | 0.0017 |
| BC200 | 2.10 | 42.08 | 4.74 | 39.46 | 0.09 | 0.94 | 0.11 | 0.987 | 0.0021 |
| Biochar | BET/(m2·g−1) | Total Pore Volume/cm3·g−1 | Average Pore Size/nm |
|---|---|---|---|
| BC200 | 3.0699 | 0.0026 | 3.5029 |
| qBC200 | 4.2130 | 0.0052 | 3.9629 |
| Biochar | qBC | qBC200 | qBC300 | qBC400 | qBC500 | qBC600 | BC | BC200 | BC300 | BC400 | BC500 | BC600 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| yield (%) | _ | 76.64 | 46.5 | 39.18 | 33.3 | 29.19 | _ | 86.22 | 49.6 | 26.42 | 20.54 | 18.67 |
| pH | 6.73 | 6.70 | 7.05 | 7.58 | 8.28 | 8.88 | 6.57 | 6.65 | 6.85 | 6.87 | 7.28 | 7.29 |
| q (mg/g) | 85.02 | 253.20 | 116.00 | 76.21 | 13.04 | 5.30 | 85.02 | 81.23 | 96.06 | 59.09 | 51.68 | 42.84 |
| Adsorbents | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 | R2 | qe (mg/g) | k2 | R2 | |
| MB | 120.84 | 2.810 | 0.9990 | 122.14 | 0.112 | 0.9999 |
| T(K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF | 1/n | R2 | |
| 298 | 267.72 | 0.5841 | 0.9560 | 123.66 | 0.22 | 0.9526 |
| 308 | 265.23 | 0.4314 | 0.9821 | 109.79 | 0.24 | 0.9602 |
| 318 | 258.01 | 0.3901 | 0.9749 | 104.81 | 0.24 | 0.9568 |
| Biochar | Pyrolytic Temperature (°C) | Qe (mg/g) | References |
|---|---|---|---|
| Sawdust ozone biochar | 500 | 200 | [46] |
| Sewage sludge and grape dreg biochar | 500 | 30.98 | [47] |
| Rice straw biochar | 400 | 26.87 | [48] |
| Orange peels biochar | 800 | 476 | [40] |
| Banana biochar | 800 | 390 | [40] |
| Silica-composited biochars from rice straw | 700 | 131.58 | [46] |
| Silica-composited biochars from swine manure | 700 | 143.76 | [46] |
| Bitter melon vine biochar | 200 | 267.72 | This work |
| T (K) | K0 | lnK0 | ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/mol·K) |
|---|---|---|---|---|---|
| 298 | 1.87 × 105 | 12.14 | −30.07 | −15.84 | 47.51 |
| 308 | 1.38 × 105 | 11.84 | −30.30 | ||
| 318 | 1.25 × 105 | 11.74 | −31.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jia, H.; Jiang, L.; Mou, Z.; Zhang, B.; Zhang, Z.; Chen, Y. Biochar Prepared from Steam-Exploded Bitter Melon Vine for the Adsorption of Methylene Blue from Aqueous Solution: Kinetics, Isotherm, Thermodynamics and Mechanism. Sustainability 2024, 16, 7278. https://doi.org/10.3390/su16177278
Li X, Jia H, Jiang L, Mou Z, Zhang B, Zhang Z, Chen Y. Biochar Prepared from Steam-Exploded Bitter Melon Vine for the Adsorption of Methylene Blue from Aqueous Solution: Kinetics, Isotherm, Thermodynamics and Mechanism. Sustainability. 2024; 16(17):7278. https://doi.org/10.3390/su16177278
Chicago/Turabian StyleLi, Xia, Hongyu Jia, Lihua Jiang, Zhengwei Mou, Bo Zhang, Zihui Zhang, and Yan Chen. 2024. "Biochar Prepared from Steam-Exploded Bitter Melon Vine for the Adsorption of Methylene Blue from Aqueous Solution: Kinetics, Isotherm, Thermodynamics and Mechanism" Sustainability 16, no. 17: 7278. https://doi.org/10.3390/su16177278




