Sustainable Use of the Fungus Aspergillus sp. to Simultaneously Generate Electricity and Reduce Plastic through Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
- a.
- Design and assembly of MFCs
- b.
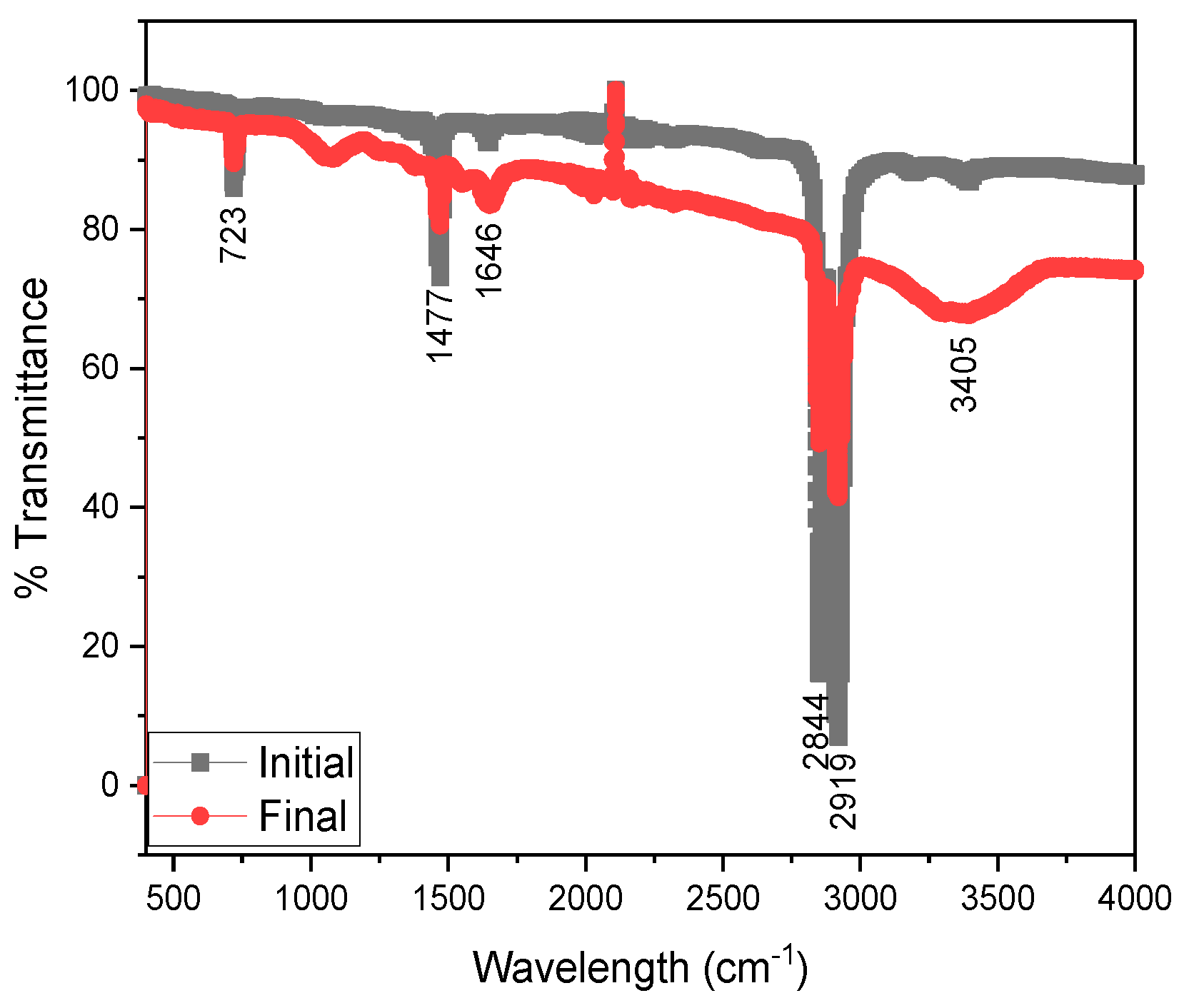
- Electrochemical and morphological tests.
- c.
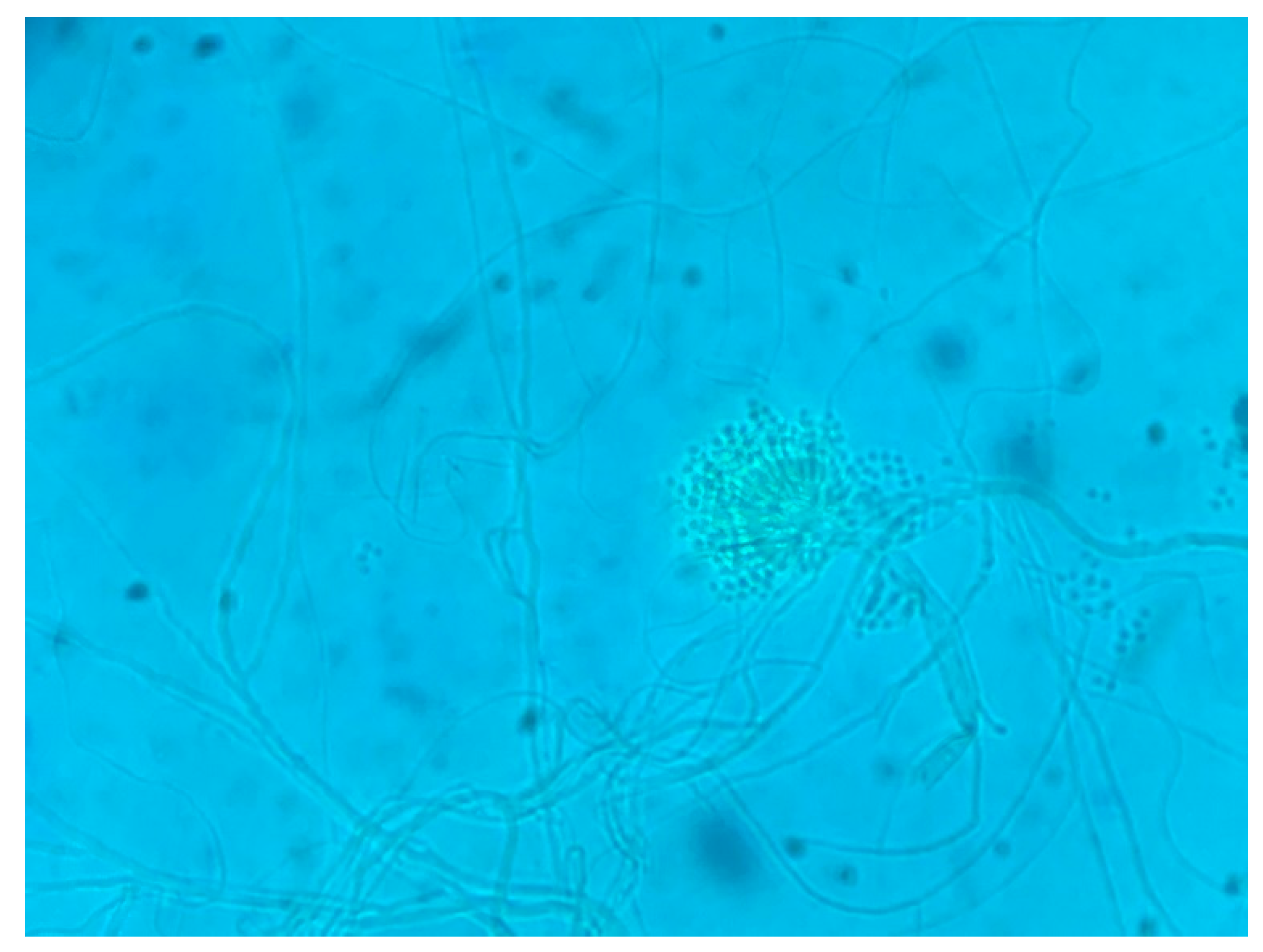
- Collection of samples, isolation, and selection of Aspergillus sp.
- d.
- Microbial fuel cell operation
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to wealth: Chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jing, Y.; Wang, Y.; Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 2022, 6, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, H.; Jiang, X.; Polaczyk, P.; Xiao, R.; Zhang, M.; Huang, B. The utilization of waste plastics in asphalt pavements: A review. Clean. Mater. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Xu, S.; Han, Z.; Yuan, K.; Qin, P.; Zhao, W.; Lin, T.; Zhou, T.; Huang, F. Upcycling chlorinated waste plastics. Nat. Rev. Methods Primers 2023, 3, 44. [Google Scholar] [CrossRef]
- Khan, M.T.; Shah, I.A.; Hossain, M.F.; Akther, N.; Zhou, Y.; Khan, M.S.; Al-Shaeli, M.; Bacha, M.S.; Ihsanullah, I. Personal protective equipment (PPE) disposal during COVID-19: An emerging source of microplastic and microfiber pollution in the environment. Sci. Total Environ. 2023, 860, 160322. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, I.; Khan, M.T.; Hossain, M.F.; Bilal, M.; Ali Shah, I. Eco-Friendly Solutions to Emerging Contaminants: Unveiling the Potential of Bioremediation in Tackling Microplastic Pollution in Water. Adv. Sustain. Syst. 2024, 2400172. [Google Scholar] [CrossRef]
- Kalak, T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Martinez, V.; Stolar, T.; Karadeniz, B.; Brekalo, I.; Užarević, K. Advancing mechanochemical synthesis by combining milling with different energy sources. Nat. Rev. Chem. 2023, 7, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Tee, K.; Elnahass, M.; Ahmed, R. Assessing the environmental impacts of renewable energy sources: A case study on air pollution and carbon emissions in China. J. Environ. Manag. 2023, 345, 118525. [Google Scholar] [CrossRef]
- Algarni, S.; Tirth, V.; Alqahtani, T.; Alshehery, S.; Kshirsagar, P. Contribution of renewable energy sources to the environmental impacts and economic benefits for sustainable development. Sustain. Energy Technol. Assess. 2023, 56, 103098. [Google Scholar] [CrossRef]
- Ibrahim, O.; Bakare, M.S.; Amosa, T.I.; Otuoze, A.O.; Owonikoko, W.O.; Ali, E.M.; Adesina, L.M.; Ogunbiyi, O. Development of fuzzy logic-based demand-side energy management system for hybrid energy sources. Energy Convers. Manag. X 2023, 18, 100354. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Chu, W.; Fang, S.; Zheng, C.; Xue, M.; Wang, X.; Hu, T.; Guo, W. Harvesting energy from atmospheric water: Grand challenges in continuous electricity generation. Adv. Mater. 2024, 36, 2211165. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; An, C.; Chen, Z. The role of clean energy in achieving decarbonization of electricity generation, transportation, and heating sectors by 2050: A meta-analysis review. Renew. Sustain. Energy Rev. 2023, 182, 113404. [Google Scholar] [CrossRef]
- Shao, B.; Song, Y.; Song, Z.; Wang, Y.; Wang, Y.; Liu, R.; Sun, B. Electricity generation from phase transitions between liquid and gaseous water. Adv. Energy Mater. 2023, 13, 2204091. [Google Scholar] [CrossRef]
- Sonawane, A.V.; Rikame, S.; Sonawane, S.H.; Gaikwad, M.; Bhanvase, B.; Sonawane, S.S.; Mungray, A.K.; Gaikwad, R. A review of microbial fuel cell and its diversification in the development of green energy technology. Chemosphere 2024, 350, 141127. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, S.; Pandey, N.; Bhatnagar, P.; Chadha, G.; Rawat, N.; Joshi, N.C.; Tomar, M.S.; Eyvaz, M.; Gururani, P. A concise review on wastewater treatment through microbial fuel cell: Sustainable and holistic approach. Environ. Sci. Pollut. Res. 2024, 31, 6723–6737. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.M.; Noor, Z.Z.; Mutamim, N.S.A.; Baharuddin, N.H.; Aris, A.; Faizal, A.N.M.; Ibrahim, R.S.; Suhaimin, N.S. A critical review of ceramic microbial fuel cell: Economics, long-term operation, scale-up, performances and challenges. Fuel 2024, 365, 131150. [Google Scholar] [CrossRef]
- Jalili, P.; Ala, A.; Nazari, P.; Jalili, B.; Ganji, D.D. A comprehensive review of microbial fuel cells considering materials, methods, structures, and microorganisms. Heliyon 2024, 10, e25439. [Google Scholar] [CrossRef]
- Rincón-Catalán, N.I.; Cruz-Salomón, A.; Sebastian, P.J.; Pérez-Fabiel, S.; Hernández-Cruz, M.D.C.; Sánchez-Albores, R.M.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E.; Esquinca-Avilés, H.A.; Ríos-Valdovinos, E.I.; et al. Banana Waste-to-Energy Valorization by Microbial Fuel Cell Coupled with Anaerobic Digestion. Processes 2022, 10, 1552. [Google Scholar] [CrossRef]
- Agudelo-Escobar, L.M.; Cabrera, S.E.; Avignone Rossa, C. A bioelectrochemical system for waste degradation and energy recovery from industrial coffee wastewater. Front. Chem. Eng. 2022, 4, 814987. [Google Scholar] [CrossRef]
- Abdallah, Y.K.; Estevez, A.T.; Tantawy, D.E.D.M.; Ibraheem, A.M.; Khalil, N.M. Employing laccase-producing Aspergillus sydowii NYKA 510 as a cathodic biocatalyst in self-sufficient lighting microbial fuel cell. J. Microbiol. Biotechnol. 2019, 29, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Xie, L.; Ishii, Y.; Kawasaki, S.; Yoshida, N. Anode biomass rather than soluble organic matter is fuel for electricity production in microbial fuel cell at longer hydraulic retention time. J. Clean. Prod. 2024, 439, 140544. [Google Scholar] [CrossRef]
- Pal, M.; Sharma, R.K. Development of wheat straw based catholyte for power generation in microbial fuel cell. Biomass Bioenergy 2020, 138, 105591. [Google Scholar] [CrossRef]
- Sarma, H.; Bhattacharyya, P.N.; Jadhav, D.A.; Pawar, P.; Thakare, M.; Pandit, S.; Mathuriya, A.S.; Prasad, R. Fungal-mediated electrochemical system: Prospects, applications and challenges. Curr. Res. Microb. Sci. 2021, 2, 100041. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ismail, F.; Imran, M. Biodegradation of synthetic plastics by the extracellular lipase of Aspergillus niger. Environ. Adv. 2024, 17, 100563. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A review of the fungi that degrade plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Segundo, R.F.; Magaly, D.L.C.N.; Luis, C.C.; Luis, N.M.; Soto-Deza, N.; Terrones-Rodriguez, N.; Mayra, D.L.C.C. Obtaining Sustainable Electrical Energy from Pepper Waste. Sustainability 2024, 16, 3448. [Google Scholar] [CrossRef]
- Kumari, A.; Pawar, D.; Saini, S.; Kumar, A.; Budhwar, S.; Sharma, S.P. Challenges in Biodegradation of Plastic Waste from Food Packaging. In Advances in Sustainable Food Packaging Technology; Apple Academic Press: Cambridge, MA, USA, 2024; pp. 251–269. [Google Scholar]
- Barnett, S.A. Homo docens. J. Biosoc. Sci. 1973, 5, 393–403. [Google Scholar] [CrossRef]
- Duan, Y.; Yin, Y.; Ni, Z.; Liu, J.; Gui, H.; Wu, D.; Wu, X.; Wang, L. The effective and green biodegradation of polyethylene microplastics by the screening of a strain with its degrading enzymes. Biochem. Eng. J. 2024, 210, 109429. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Li, Y.; Sun, Y.; Zhang, X.; Weng, L.; Ren, T.; Li, Y. Shifting interactions among bacteria, fungi and archaea enhance removal of antibiotics and antibiotic resistance genes in the soil bioelectrochemical remediation. Biotechnol. Biofuels 2019, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.T.; Jain, S.C.; Ghangrekar, M.M.; Mukherjee, C.K. Biofouling inhibition and enhancing performance of microbial fuel cell using silver nano-particles as fungicide and cathode catalyst. Bioresour. Technol. 2016, 220, 183–189. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Jayabalan, T.; Sethupathi, M.; Kim, W.; Muniyasamy, S.; Sengottuvelan, N.; Nainamohamed, S.; Ponnuchamy, K.; Alagarsamy, A. Bioelectricity generation by natural microflora of septic tank wastewater (STWW) and biodegradation of persistent petrogenic pollutants by basidiomycetes fungi: An integrated microbial fuel cell system. J. Hazard. Mater. 2021, 412, 125228. [Google Scholar] [CrossRef]
- Votat, S.; Pontié, M.; Jaspard, E.; Lebrun, L. Crystal Violet (CV) Biodegradation Study in a Dual-Chamber Fungal Microbial Fuel Cell with Trichoderma harzianum. Energies 2024, 17, 247. [Google Scholar] [CrossRef]
- Tiwari, S.; Koreti, D.; Kosre, A.; Mahish, P.K.; Jadhav, S.K.; Chandrawanshi, N.K. Fungal Microbial Fuel Cells, an Opportunity for Energy Sources: Current Perspective and Future Challenges. Energy Crises Chall. Solut. 2021, 250–273. [Google Scholar] [CrossRef]
- Yaakop, A.S.; Ahmad, A.; Hussain, F.; Oh, S.E.; Alshammari, M.B.; Chauhan, R. Domestic Organic Waste: A Potential Source to Produce the Energy via a Single-Chamber Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023, 2425735. [Google Scholar] [CrossRef]
- Pan, P.; Bhattacharyya, N. Bioelectricity production from microbial fuel cell (MFC) using Lysinibacillus xylanilyticus strain nbpp1 as a biocatalyst. Curr. Microbiol. 2023, 80, 252. [Google Scholar] [CrossRef] [PubMed]
- Garbini, G.L.; Barra Caracciolo, A.; Grenni, P. Electroactive bacteria in natural ecosystems and their applications in microbial fuel cells for bioremediation: A review. Microorganisms 2023, 11, 1255. [Google Scholar] [CrossRef]
- Meylani, V.; Surahman, E.; Fudholi, A.; Almalki, W.H.; Ilyas, N.; Sayyed, R.Z. Biodiversity in microbial fuel cells: Review of a promising technology for wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109503. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Wang, J.H.; Shen, Y.; Guo, Z.W.; Yang, Y.; Zhu, D.C.; Peters, R.W.; Mostafa, M.K.; Mahmoud, A.S. Performance of the Dual-Chamber Fungal Fuel Cell in Treating Tannery Wastewater. Appl. Sci. 2023, 13, 10710. [Google Scholar] [CrossRef]
- Kongthale, G.; Sotha, S.; Michu, P.; Madloh, A.; Wetchapan, P.; Chaijak, P. Electricity production and phenol removal of winery wastewater by constructed wetland—Microbial fuel cell integrated with ethanol tolerant yeast. Biointerface Res. Appl. Chem. 2023, 13, 157. [Google Scholar]
- Andriukonis, E.; Celiesiute-Germaniene, R.; Ramanavicius, S.; Viter, R.; Ramanavicius, A. From microorganism-based amperometric biosensors towards microbial fuel cells. Sensors 2021, 21, 2442. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Boddula, R.; Pothu, R. Microbial fuel cells: Technologically advanced devices and approach for sustainable/renewable energy development. Energy Convers. Manag. X 2022, 13, 100160. [Google Scholar] [CrossRef]
- Rafaqat, S. Development of Fungal Biocathode Based Microbial Fuel Cell and Laccase-Based Biosensor for Biodegradation and Detection of Chlorpyrifos Organophosphate. Ph.D. Thesis, Quaid I Azam University Islamabad, Islamabad, Pakistan, 2022. [Google Scholar]
- Žalnėravičius, R.; Paškevičius, A.; Samukaitė-Bubnienė, U.; Ramanavičius, S.; Vilkienė, M.; Mockevičienė, I.; Ramanavičius, A. Microbial fuel cell based on nitrogen-fixing Rhizobium anhuiense bacteria. Biosensors 2022, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Karim, A.; Mishra, P.; Dubowski, J.J.; Yousuf, A.; Sarmin, S.; Khan, M.M.R. Microbial synergistic interactions enhanced power generation in co-culture driven microbial fuel cell. Sci. Total Environ. 2020, 738, 140138. [Google Scholar] [CrossRef]
- Ballestas, E.R.; Bortoluzzi, E.C.; Minervino, A.H.H.; Palma, H.H.; Neckel, A.; Ramos, C.G.; Moreno-Ríos, A.L. Power generation potential of plant microbial fuel cells as a renewable energy source. Renew. Energy 2024, 221, 119799. [Google Scholar] [CrossRef]
- Bhatt, P.; Paudel, P.; Regmi, D.; Soni, S.; Dhungana, P.; Joshi, J. Degradation of potato peels using amylase-and pectinase-producing fungal strain in an electrochemical cell and by-product analysis. Int. J. Sustain. Energy 2024, 43, 2345735. [Google Scholar] [CrossRef]
- Nasrabadi, A.E.; Ramavandi, B.; Bonyadi, Z. Recent progress in biodegradation of microplastics by Aspergillus sp. in aquatic environments. Colloid Interface Sci. Commun. 2023, 57, 100754. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Pracejus, B.; Novo, L.A. Bioremediation of copper using indigenous fungi Aspergillus species isolated from an abandoned copper mine soil. Chemosphere 2023, 314, 137688. [Google Scholar] [CrossRef]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145. [Google Scholar] [CrossRef]
- Raaman, N.; Rajitha, N.; Jayshree, A.; Jegadeesh, R. Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around Chennai. J. Acad. Ind. Res. 2012, 1, 313–316. [Google Scholar]
- Mathur, G.; Mathur, A.; Prasad, R. Colonization and degradation of thermally oxidized high-density polyethylene by Aspergillus niger (ITCC No. 6052) isolated from plastic waste dumpsite. Bioremediation J. 2011, 15, 69–76. [Google Scholar] [CrossRef]
- El-Dash, H.A.; Yousef, N.E.; Aboelazm, A.A.; Awan, Z.A.; Yahya, G.; El-Ganiny, A.M. Optimizing eco-friendly degradation of polyvinyl chloride (PVC) plastic using environmental strains of Malassezia species and Aspergillus fumigatus. Int. J. Mol. Sci. 2023, 24, 15452. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Flores, S.; De La Cruz-Noriega, M.; Otiniano, N.M.; Cabanillas-Chirinos, L. Sustainable Use of the Fungus Aspergillus sp. to Simultaneously Generate Electricity and Reduce Plastic through Microbial Fuel Cells. Sustainability 2024, 16, 7413. https://doi.org/10.3390/su16177413
Rojas-Flores S, De La Cruz-Noriega M, Otiniano NM, Cabanillas-Chirinos L. Sustainable Use of the Fungus Aspergillus sp. to Simultaneously Generate Electricity and Reduce Plastic through Microbial Fuel Cells. Sustainability. 2024; 16(17):7413. https://doi.org/10.3390/su16177413
Chicago/Turabian StyleRojas-Flores, Segundo, Magaly De La Cruz-Noriega, Nélida Milly Otiniano, and Luis Cabanillas-Chirinos. 2024. "Sustainable Use of the Fungus Aspergillus sp. to Simultaneously Generate Electricity and Reduce Plastic through Microbial Fuel Cells" Sustainability 16, no. 17: 7413. https://doi.org/10.3390/su16177413
APA StyleRojas-Flores, S., De La Cruz-Noriega, M., Otiniano, N. M., & Cabanillas-Chirinos, L. (2024). Sustainable Use of the Fungus Aspergillus sp. to Simultaneously Generate Electricity and Reduce Plastic through Microbial Fuel Cells. Sustainability, 16(17), 7413. https://doi.org/10.3390/su16177413





