Abstract
Ecological floating beds, with their compact footprint and mobility, offer a promising solution for sustainable surface water remediation in rural areas. However, low removal efficiency and instability still limit its application. In this study, iron–carbon-based fillers were integrated into ecological floating beds to investigate their impact and mechanisms in removing pollutants, including carbon, nitrogen, phosphorus, and heavy metals. Results indicate that all five fillers (activated carbon, iron–carbon fillers, sponge iron, activated carbon + iron–carbon fillers, and activated carbon + sponge iron) can completely remove orthophosphate, and the sponge iron filler system can completely remove nitrate. Then, fillers were applied to ecological floating beds, and the iron–carbon microelectrolysis (activated carbon + sponge iron filler)-enhanced ecological floating bed showed superior removal efficiency for pollutants. It achieved 95% removal of NH4+-N, 85% removal of NO3−-N, 75% removal of total phosphorus, 90% removal of chemical oxygen demand, and 90% removal of heavy metals. Typical nitrifying bacteria Nitrospira, denitrifying bacteria Denitratisoma, and a variety of bacterial genera with denitrification functions (e.g., Rhodobacter, Dechloromonas, Sediminibacterium, and Novosphingobium) coexisted in the system, ensuring efficient and robust nitrogen removal performance. These findings will provide support for the sustainable treatment of surface water in rural areas.
1. Introduction
Rural rivers are numerous and widely distributed, with various functions such as flood control and drainage, irrigation water supply, agricultural drainage, and ecological landscaping, making them an important part of the plain river network system [1]. Historically, they have formed close relationships with human life and production, becoming the most critical natural resource and environmental carrier in rural areas, inseparable from the production and life of rural residents. In recent decades, agricultural production has rapidly developed, especially in developing countries, with increasing inputs of chemical fertilizers, pesticides, agricultural films, and other agricultural chemicals per unit area, and the booming development of livestock and poultry farming [2,3]. However, due to ineffective governance and management, the degradation and functional decline of river environments have become severe, significantly affecting the sustainable development of the rural economy and the well-being of the population, thereby impeding the sustainable development of rural economies and societies.
Ecological remediation technology is a widely used surface water restoration method, offering cost-effective and straightforward operation and management when compared with physical and chemical techniques [4,5]. Currently, various ecological restoration methods are available, such as ecological revetments, artificial wetlands, oxidation ponds, and ecological floating beds. Artificial revetments, artificial wetlands, and oxidation ponds typically occupy a significant land area and operate at a relatively slow pace, and artificial revetments especially sometimes require certain construction conditions. Notably, ecological floating beds are an ecological remediation technology that utilizes soilless cultivation, planting vegetation directly in water to absorb nutrients, and can be regarded as a combination of stabilizing ponds and artificial wetlands [6,7]. Ecological floating beds can effectively remove suspended solids and pollutants, adaptable to varying water depths and levels, making them cost-effective for new construction or retrofitting existing pond systems [8,9,10]. Compared with other remediation methods, floating beds are space-efficient and portable, offering promising prospects for sustainable surface water remediation in rural areas [11,12]. However, currently, there are still issues in the application of ecological floating beds, such as low removal efficiency of pollutants and instability, which limit the development of this technology.
In traditional ecological floating beds, microorganisms mainly attach to the surface of plant roots. However, the root system is constrained by surface area, leading to lower efficiency and removal rates of pollutants [13,14]. Therefore, introducing suspended fillers (mainly elastic fillers and fiber fillers) into traditional ecological floating beds to increase the attachment surface area for microorganisms can effectively enhance the removal of pollutants [8,15,16]. For example, Wu et al. incorporated fiber fillers into traditional ecological floating beds, increasing the removal rates of NH4+-N, NO3−-N, total phosphorus (TP), and chemical oxygen demand (COD) by 13.9%, 7.1%, 12.8%, and 14.7%, respectively [15]. Iron–carbon microelectrolysis, also known as internal electrolysis, or the iron–carbon method, involves introducing iron fillers (typically iron–carbon alloys) and carbon fillers (such as activated carbon, coke, graphite, coal, etc.) into contaminated water [17]. The iron–carbon microelectrolysis process relies on the electrochemical reactions occurring within microscopic galvanic cells formed by the electrode materials (iron and carbon) [18]. In recent years, the iron–carbon microelectrolysis technology has gained prominence for its cost-effectiveness, ease of operation, high pollutant removal efficiency, and versatile application in treating various wastewater types, including pharmaceutical, printing, and dyeing, as well as chemical industry wastewater [19,20,21]. However, there is currently limited research on the application of iron–carbon microelectrolysis fillers to enhance the removal of pollutants in traditional ecological floating beds.
Therefore, in this paper, we introduced iron–carbon microelectrolysis fillers into traditional ecological floating beds to enhance their pollutant removal capacity. Firstly, different fillers were combined for batch experiments to explore the differences in pollutant removal efficiency. Subsequently, the differences in pollutant removal efficiency of floating beds with iron–carbon microelectrolysis fillers were investigated. Finally, the filler material and microbial community were detected to elucidate the mechanism behind the differences in pollutant removal efficiency. The results offer valuable insights into the sustainable treatment of surface water in rural regions.
2. Materials and Methods
2.1. Batch Experiment
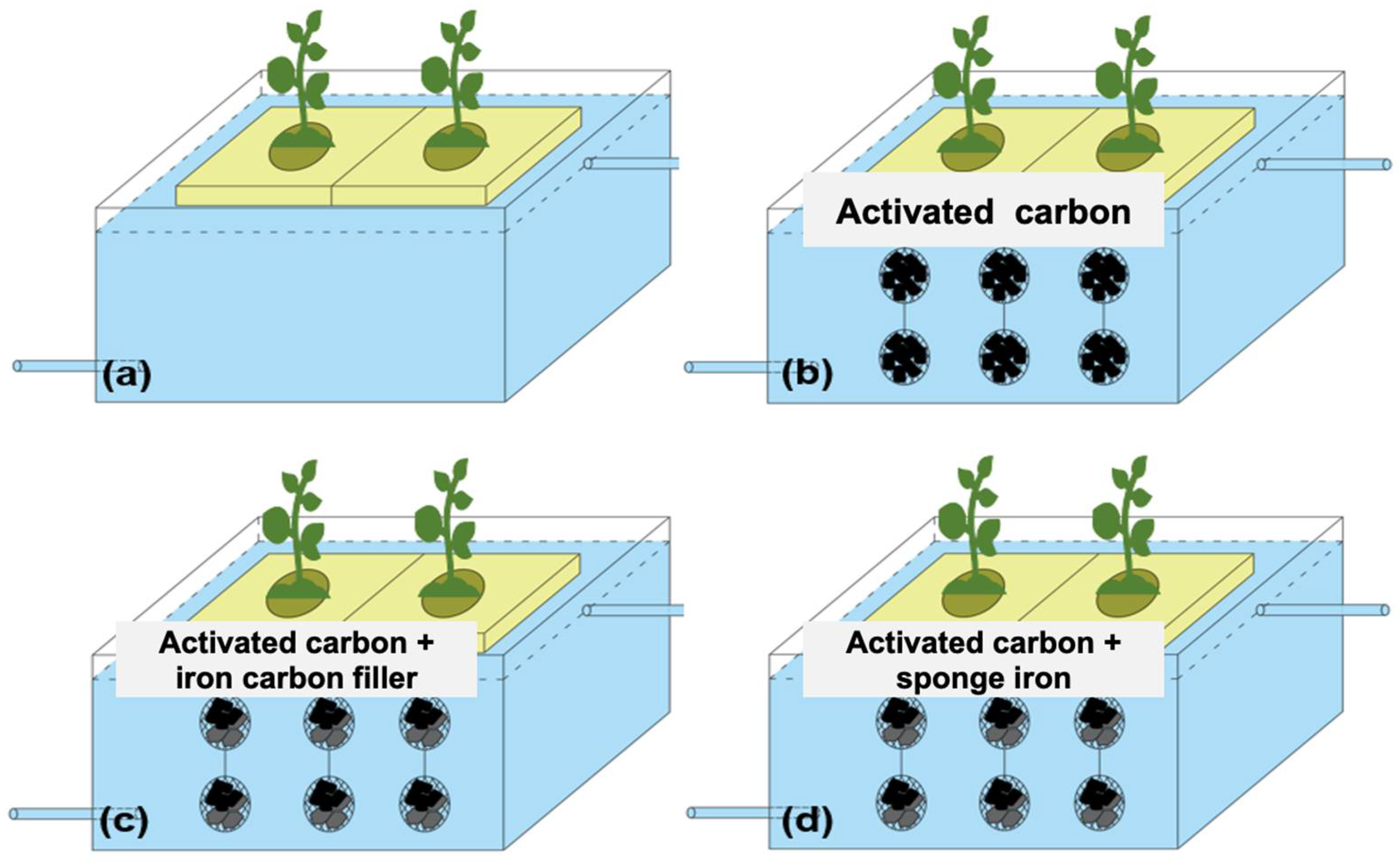
Currently, research on the integration of ecological floating beds with iron–carbon fillers is limited. Therefore, we selected several iron–carbon fillers that are commonly used in other wastewater treatment processes, including activated carbon, iron–carbon fillers, sponge iron, activated carbon combined with iron–carbon fillers, and activated carbon combined with sponge iron. To investigate the impact of fillers on pollutant removal, a series of batch experiments were conducted using five different filler systems. The removal efficiency of NH4+-N, NO3−-N, total phosphorus, and COD in the systems was investigated. The experiment was conducted in 150 mL conical flasks with 5 g iron–carbon fillers, and the experiment was designed with pollutant concentrations of 8 mg/L for NH4+-N, 8 mg/L for NO3−-N, 1.5 mg/L for orthophosphate, and 50 mg/L for COD accruing to practical pollution of surface water. Forty conical flasks were prepared, with each treatment set up in duplicate. In the activated carbon filler group, 5 g of activated carbon was added to each conical bottle; in the iron–carbon filler group, 5 g of iron–carbon filler was added to each conical bottle; in the sponge iron filler group, 5 g of sponge iron was added to each conical bottle; in the activated carbon + iron–carbon filler group, each conical bottle was filled with 5 g of activated carbon and 5 g of iron–carbon filler; and in the activated carbon + sponge iron filler group, each conical bottle was filled with 5 g of activated carbon and 5 g of sponge iron.
The conical flasks were placed in a constant temperature shaker without light at 25 °C, with a rotation speed of 120 r/min. Samples were taken at 0 h, 1 h, 3 h, 8 h, 1 d, 2 d, 3 d, 5 d, 7 d, and 10 d, with a sampling volume of 2.5 mL. Water quality testing indicators included NH4+-N, NO2−-N, NO3−-N, TN, and total Fe.
2.2. Long-Term Operation of Ecological Floating Beds
For long-term operation, the experimental device measures 70 cm × 40 cm × 40 cm in dimensions. The device features an inlet positioned 5 cm above the bottom and an outlet located 5 cm below the top, resulting in a water depth of 35 cm. Two floaters, each measuring 330 mm × 330 mm × 58 mm, float on the device’s surface. These floaters have a central circular hole with a diameter of 160 mm for placing planting pots. The planting pots above the floating bed are planted with yellow flag iris, with a density of around 50 plants per square meter. Each pot is filled with roughly 300 g of zeolite to secure the yellow flag iris and prevent lodging.
Four sets of ecological floating beds, including those with no filler (FC1), active carbon (FC2), active carbon + iron–carbon filler (FC3), and active carbon + sponge iron filler (FC4), were used to treat artificially polluted surface water (Figure 1). Activated carbon is cylindrical, with a diameter of approximately 6 mm; iron–carbon fillers are oval-shaped blocks, ranging in diameter from 10–20 mm; sponge iron is block-shaped, with a size of around 6–10 mm. The fillers are suspended below the float using a specific method that uses hollow plastic balls with an 80 mm diameter to contain the fillers. In the activated carbon floating bed, each plastic ball is filled with 240 g of activated carbon. In the activated carbon + iron–carbon filler floating bed, each plastic ball is filled with 120 g of activated carbon + 120 g of iron–carbon filler. In the activated carbon + sponge iron floating bed, each plastic ball is filled with 120 g of activated carbon + 120 g of sponge iron. The plastic balls containing the fillers are connected with nylon ropes, with 3 plastic balls attached to each nylon rope and 6 nylon ropes suspended beneath each float.
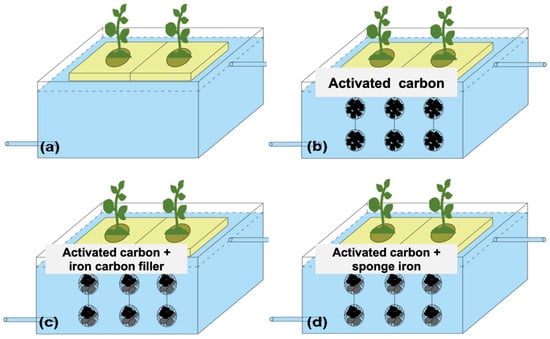
Figure 1.
Four sets of ecological floating beds, including those with no filler (a), activated carbon (b), activated carbon + iron–carbon filler (c), and activated carbon + sponge iron (d).
The entire operation lasted 150 days and can be divided into four stages based on operational performance and strategies. The influent pollutant concentration of the ecological floating beds was set according to the “Environmental Quality Standards for Surface Water” (GB3838-2002) for Class V water bodies (Table 1) [22]. Phase I is the start-up phase, during which it was observed that the floating bed was in an anaerobic state to simulate the dissolved oxygen (DO) concentration in actual river channels. In phase II, intermittent aeration was initiated from 10:00 to 12:00 and 22:00 to 24:00 daily, with an aeration rate of 4 L/min. To explore the removal efficiency of pollutants under the impact of heavy metals, Cu2+, Zn2+, and Cd2+ were added to the water in phase III. In phase IV, the nitrate-nitrogen concentration was increased (from 2 mg/L to 6 mg/L) to investigate the removal efficiency of the ecological floating bed for high concentrations of nitrogen.

Table 1.
Influent pollutant concentration of ecological floating bed.
2.3. Analytical Methods
The concentrations of NH4+-N, NO3−-N, and NO2−-N in the wastewater samples were analyzed with ion chromatography equipment (Thermo ICS5000+, Sunnyvale, CA, USA), and the nitrogen removal efficiency (NRE) was determined. The surface morphology of the granular sludge was examined using a ZEISS Gemini SEM 300 microscope (Carl Zeiss, Oberkochen, Germany). The materials before and after the reaction were analyzed using scanning electron microscope–energy dispersive X-ray spectroscopy (SEM-EDS).
2.4. DNA Extraction and 16S rRNA Sequencing
At the conclusion of the operation, biofilm samples were collected from the surfaces of the fillers for total DNA extraction. The DNA extraction was carried out using the E.Z.N.A.® soil DNA Kit, and the quality and quantity of the extracted DNA were evaluated. For the analysis of the 16S rRNA gene, universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were utilized [23]. PCR conditions involved 27 cycles of 30 s at 95 °C, 30 s at 55 °C, and 45 s at 72 °C, following a previously established protocol [24]. The amplicons were sequenced by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China) on the Illumina MiSeq platform with PE300 chemistry, in accordance with the manufacturer’s guidelines.
3. Results and Discussion
3.1. Short-Term Pollution Removal Performance of Different Filler Systems
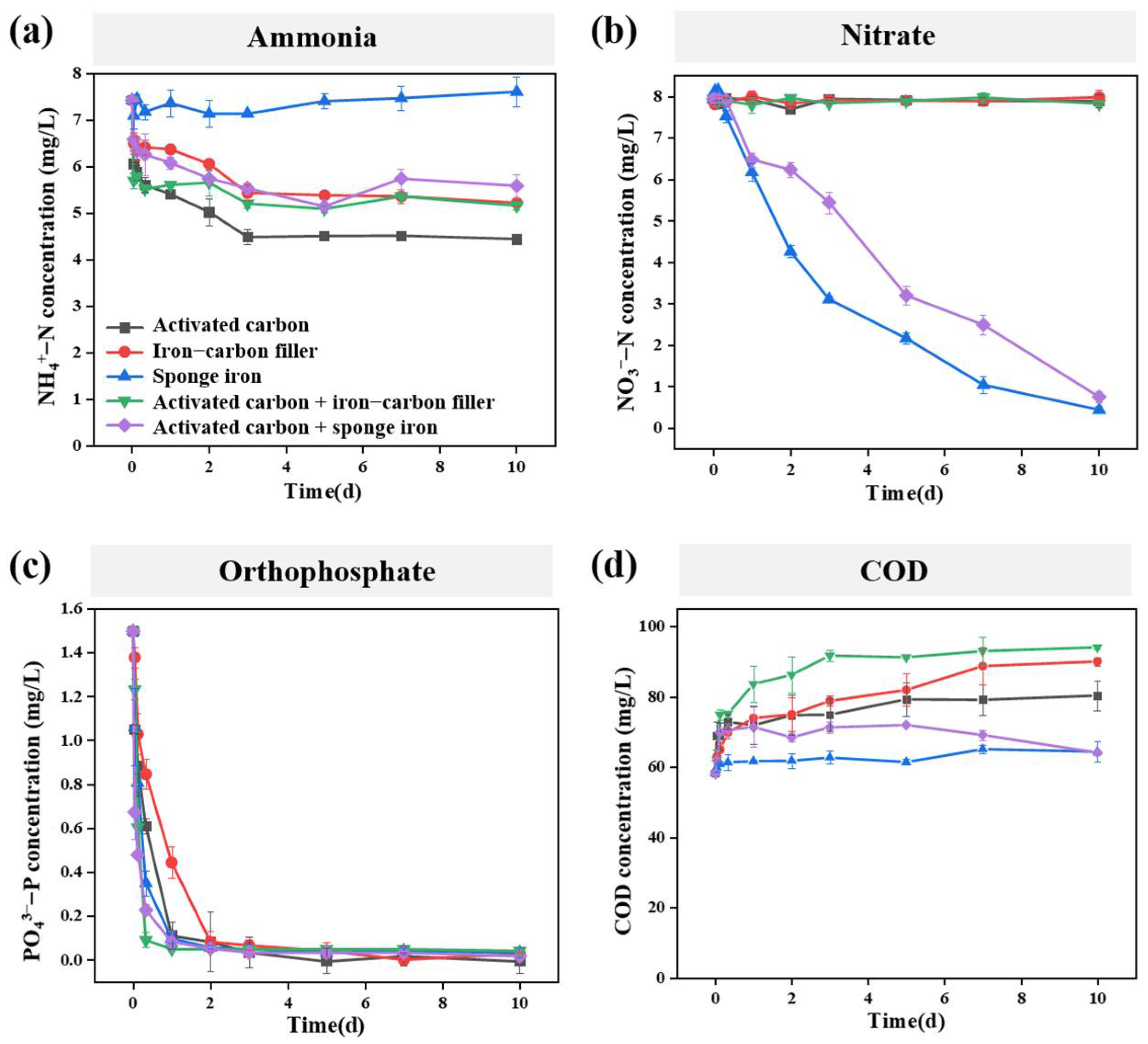
The NH4+-N, NO3−-N, phosphorus, and COD removal efficiency of five different filler systems were first investigated during short-term operation (Figure 2). The results show that the five filler systems can achieve high-efficiency removal of phosphorus, but all have poor removal efficiency for COD. Moreover, only fillers containing sponge iron displayed high removal efficiency for NO3−-N.
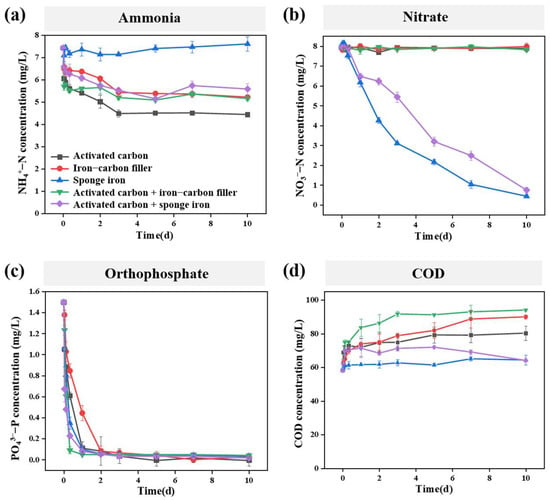
Figure 2.
Short-term removal efficiency of different filler systems: (a) ammonia; (b) nitrate; (c) orthophosphate; (d) Chemical oxygen demand (COD).
Specifically, the NH4+-N removal efficiency of ammonia nitrogen in the five filler systems of activated carbon, iron–carbon filler, sponge iron, activated carbon + iron–carbon filler, and activated carbon + sponge iron group were 40.2%, 29.7%, −2.4%, 30.5%, and 24.8%, respectively (Figure 2a). However, in terms of NO3−-N, the removal rates in the five filler systems of activated carbon, iron–carbon filler, sponge iron, activated carbon + iron–carbon filler, and activated carbon + sponge iron group were 0.6%, −0.5%, 94.4%, 1.5%, and 90.5%, respectively (Figure 2b). We speculated that in the sponge iron system, the reduced NO3−-N was converted to NH4+-N (resulting in a negative value of NH4+-N removal efficiency), while in the activated carbon + sponge iron system, the presence of activated carbon led to the generated NH4+-N being adsorbed by the activated carbon. It is worth noting that in the activated carbon + sponge iron system, the reduction rate of nitrate was smaller than that in the sponge iron system, which may be related to the pH of the solution. The reduction in nitrate by zero-valent iron is an acid-driven reaction that consumes H+ [1]. Thus, in the activated carbon + sponge iron system, the substances released by activated carbon increase the pH of the system, leading to a slower reaction rate.
Under the condition of an influent orthophosphate concentration of 1.50 mg/L and an operation time of 10 days, the removal rates of orthophosphate in the five filler systems—activated carbon, iron–carbon filler, sponge iron, activated carbon + iron–carbon filler, and activated carbon + sponge iron—can reach 100%, 98.0%, 97.6%, 97.1%, and 98.7%, respectively (Figure 2c). This suggested that all five filler systems can effectively achieve the complete removal of orthophosphate. Studies have indicated that activated carbon and iron–carbon fillers all have good adsorption effects on phosphorus [25,26]. However, in the case of sponge iron, the primary mechanism for phosphorus removal involves the corrosion of iron to generate iron ions, which subsequently react with orthophosphate ions to form precipitates [27].
Further, COD is defined as the amount of oxygen equivalents needed to oxidize organic substances in water, serving as a key indicator of organic pollution in water. In this study, the five filler systems exhibited no significant treatment effect on COD, and in certain instances, the effluent COD concentration even slightly exceeded that of the influent due to the release of organic carbon. (Figure 2d).
3.2. Long-Term Removal Performance of Iron–Carbon Microelectrolysis Ecological Floating Bed
According to the results regarding the impact of fillers on pollution, three types of fillers were utilized in ecological floating beds to treat artificially polluted surface water. These included beds without fillers (FC1), beds with activated carbon (FC2), beds with a combination of activated carbon and iron–carbon filler (FC3), and beds with a combination of activated carbon and sponge iron filler (FC4) (Figure 1). The results confirm that the iron–carbon microelectrolysis filler, particularly the activated carbon + iron–carbon filler, can greatly improve the stable and efficient removal of pollutants from surface water by ecological floating beds (Figure 3).
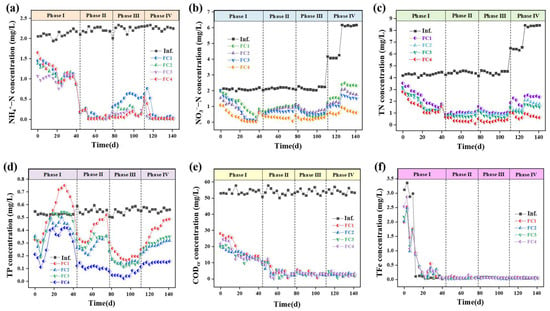
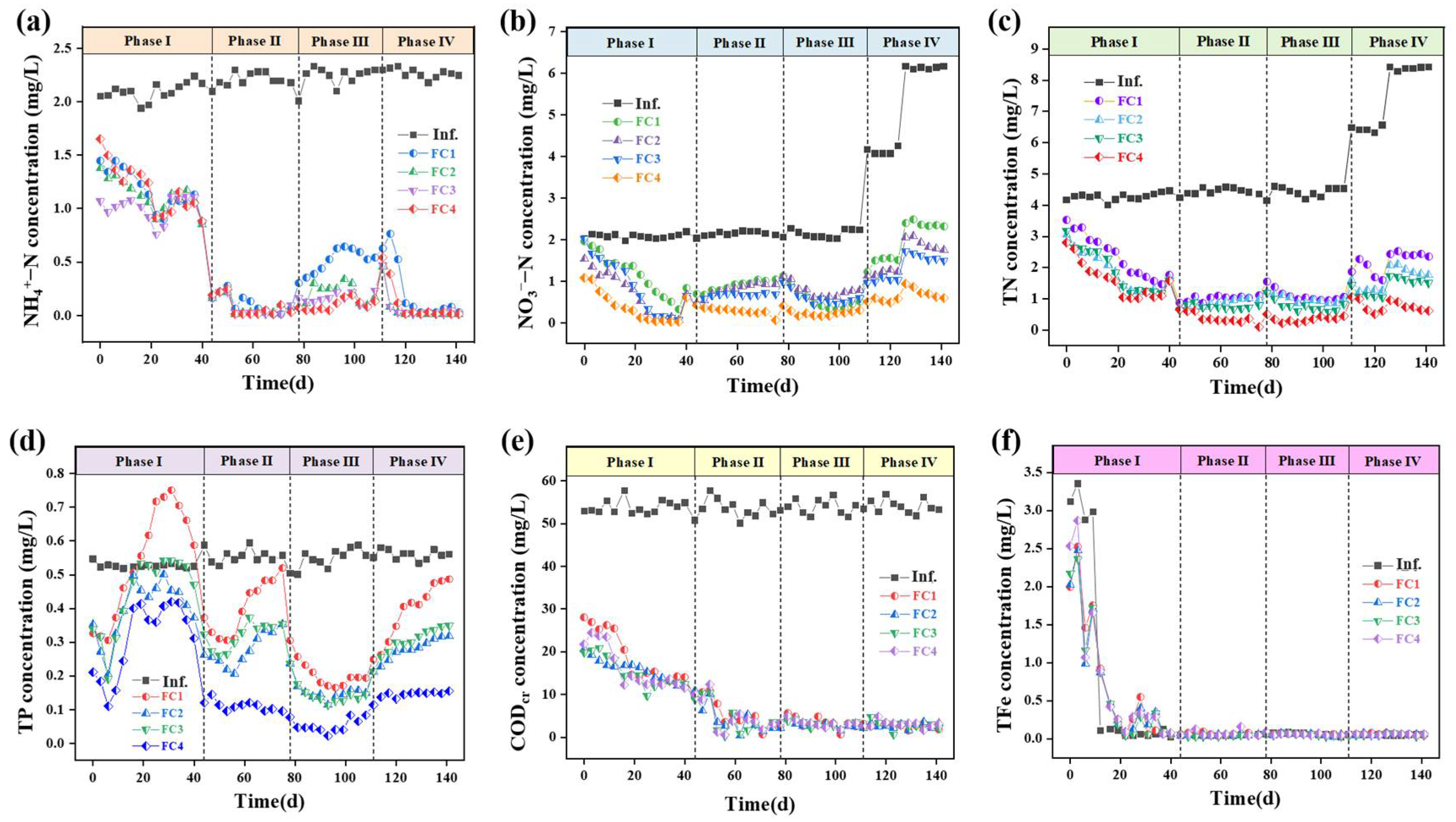
Figure 3.
Long-term removal efficiency of ecological floating beds: (a) ammonia; (b) nitrate; (c) Total nitrogen (TN); (d) TP; (e) COD; (f) total Fe.
3.2.1. Nitrogen Removal
Comparatively, the FC4 achieved the best total nitrogen removal performance, with an average total nitrogen removal rate above 90%. For NH4+-N removal, after aeration (phase II), all four floating beds showed excellent removal efficiency (>95%) (Figure 3a). There are two possible reasons for this phenomenon: (i) the fillers themselves may adsorb NH4+-N; (ii) the fillers can act as a substrate for microbial growth, resulting in significantly higher microbial quantities compared with floating beds without fillers [28]. Notably, the addition of activated carbon + iron–carbon filler effectively enhanced the floating beds’ resistance to heavy metal impacts, with the FC4 (90%) demonstrating the highest NH4+-N removal efficiency than other groups in phase III. But in phase IV, without the addition of heavy metals, the removal of NH4+-N by the four floating beds all increased rapidly, with an average removal efficiency of over 90%, and there was no significant difference among them.
The FC4 system also demonstrated advantages in NO3−-N removal (>85%), particularly under high NO3−-N influent concentration (Figure 3b). This may be due to the reaction between NO3−-N and zero-valent iron in addition to denitrification. Also, the improved performance of the activated carbon floating bed (FC2) and the activated carbon + iron–carbon filler floating bed (FC3) compared with the control floating bed (FC1) can be attributed to the increased biomass resulting from the addition of fillers [29]. Additionally, the activated carbon or iron–carbon filler releases organic compounds, which may enhance denitrification. In addition, we also conducted tests on the accumulation of NO2−-N and Fe in the effluent of the floating bed. The results indicate that either nitrification or denitrification processes in the floating bed are relatively complete, and during stable operation, there is minimal accumulation of NO2−-N and Fe in the effluent (Figure 3c,f).
3.2.2. Phosphorus Removal
Similarly, the FC4 also demonstrated a good removal efficiency for phosphorus TP. After stable operation, the average removal efficiency can be around 75% (Figure 3d). Specifically, in phase I, as the floating bed gradually transitioned to anaerobic conditions during operation, the effluent TP initially decreased briefly before gradually rising, indicating the occurrence of anaerobic phosphorus release [30]. Then, in phase II, the TP concentration decreased significantly after aeration. However, at the end of this stage, except for FC4, the TP concentrations of the other three groups of floating beds began to rise again, indicating that the aerobic phosphorus absorption had reached a bottleneck. The FC4 floating bed, consisting of activated carbon and sponge iron, effectively removes TP with an 80% average removal rate and an effluent concentration of 0.11 mg/L. Also, aeration speeds up the corrosion of sponge iron, increasing the release of iron ions, which contributes to the bed’s efficiency (Figure 3f) [31]. Even if aerobic phosphorus absorption reaches a bottleneck, the precipitation of iron ions with phosphate ions can still precipitate most of the phosphate ions, achieving a good TP removal performance. During phase III, the TP concentrations in all four groups of floating beds decreased rapidly when heavy metals were added, indicating a notable enhancement in removal rates attributed to the precipitation of heavy metals with phosphate ions. Subsequently, in phase IV, while the TP removal efficiency decreased with higher influent NO3−-N concentrations, the performance of FC4 remained relatively stable.
3.2.3. COD and Heavy Metal Removal
In phase I, the removal efficiency of COD by the four floating beds gradually increased (Figure 3e and Table 2). After aeration, the operation of the four floating beds stabilized, and subsequently, the average removal efficiency was above 90% in phase II, phase III, and phase IV. In phase III, in order to investigate the COD removal efficiency of pollutants under the impact of heavy metals, a certain concentration of heavy metals was added to the influent. As shown in Table 1, the four floating beds have high removal efficiency for the three heavy metals, with removal efficiency approximately exceeding 90% (except Cd in FC1), indicating that the floating beds can withstand a certain concentration of heavy metal impact. It was hypothesized that some of the heavy metals could be removed by forming precipitates with phosphate, adsorbed by plant roots, or utilized by microorganisms [32].

Table 2.
Average inlet and outlet concentration and average removal rate of heavy metals in floating beds.
Therefore, compared with fiber fillers, the addition of iron–carbon fillers significantly enhanced the removal of nitrogen, phosphorus, organic matter, and heavy metals [15].
3.3. Characterization of the Filling Materials
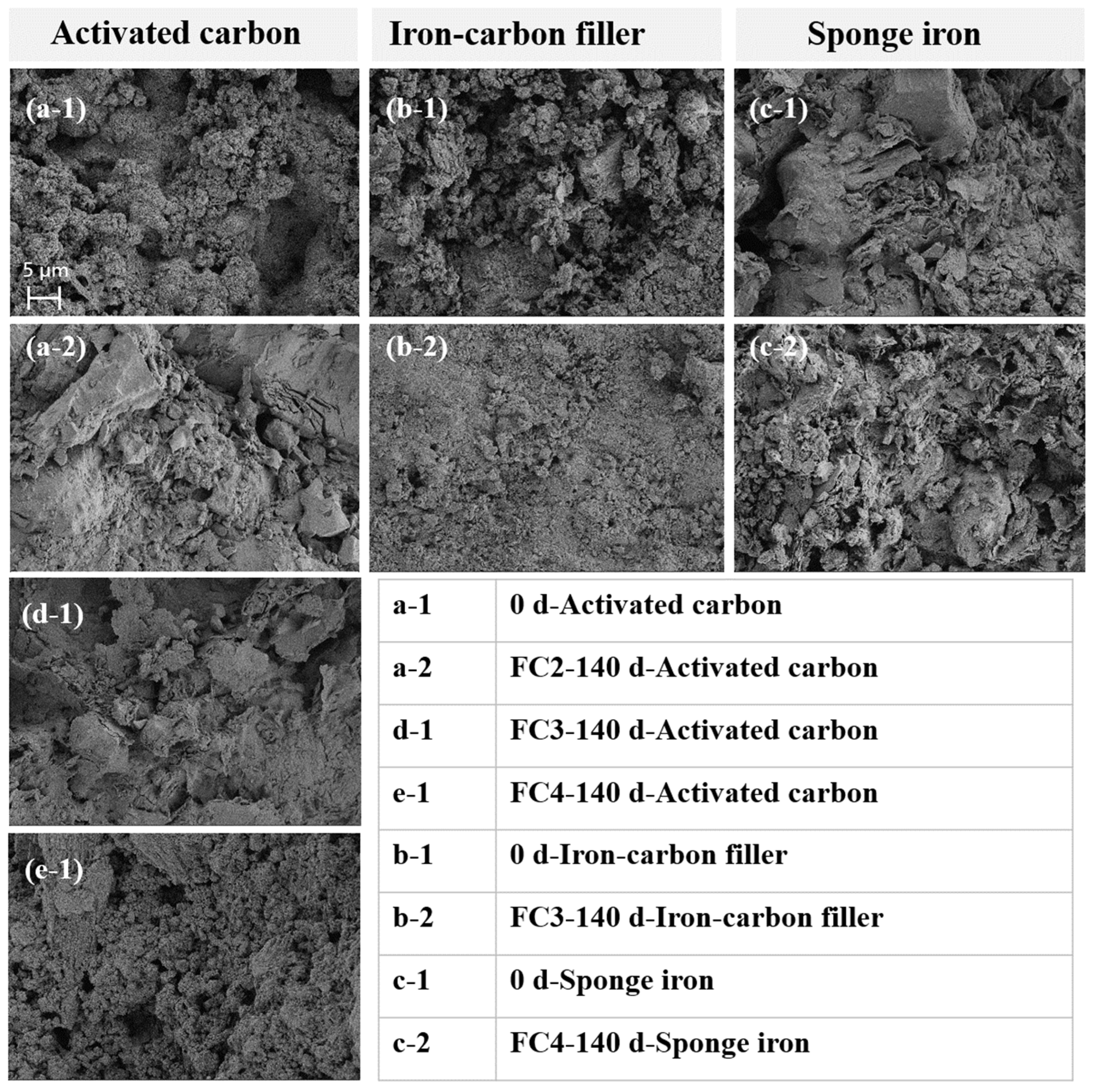
In order to investigate the surface morphology and elemental distribution changes in the selected materials after the operation of the ecological floating beds, the filling materials were analyzed using SEM-EDS (Figure 4). The activated carbon used in this experiment features a layered porous structure. After the operation, the morphology of the activated carbon in both the activated carbon floating bed and the activated carbon + iron–carbon filling floating bed remained unchanged, suggesting that there was no microbial aggregation on the surface. This may be attributed to the experiment being conducted under natural conditions with low nutrient levels in the influent, leading to slow microbial growth [33]. Moreover, intermittent aeration was initiated from the second stage onwards, potentially causing air bubbles to disperse the microbes on the activated carbon surface.
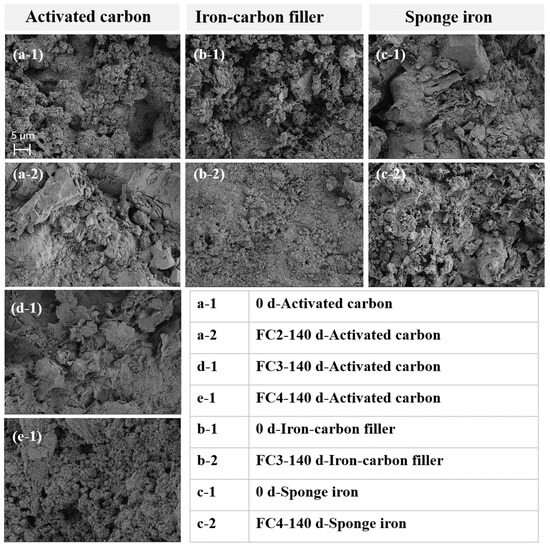
Figure 4.
Characterization of the filling materials, including activated carbon, iron–carbon filler, and sponge iron.
Moreover, the surface of the activated carbon in the activated carbon + sponge iron floating bed was extensively coated with iron rust, indicating a notable iron–carbon microelectrolysis effect (Figure 4). Both the activated carbon and the iron–carbon filler possessed a layered porous structure, with the only noticeable difference being their macroscopic shapes—cylindrical for activated carbon and elliptical for the iron–carbon filler. As a result, there was minimal alteration in the surface morphology of the iron–carbon filler before and after the reaction. Notably, no microbial aggregation or biofilm formation was observed on the surface of the iron–carbon filler throughout the experiment, underscoring its effectiveness in the treatment process.
3.4. Microbial Diversity in Iron–Carbon Microelectrolysis Floating Bed
From Table 3, it can be observed that the ACE index and Chao index of the floating beds with added fillers were greater than those without fillers, indicating that the addition of fillers increased the total number of species. However, after heavy metals were added to the influent in phase III, the ACE index and Chao index of all four floating beds decreased, indicating a significant inhibitory effect of heavy metals on microorganisms. The Shannon index and Simpson index are used to characterize the microbial diversity in samples, with microbial diversity considering both the number of species and the evenness of species distribution [34]. In this study, the microbial diversity of the floating beds with added fillers was higher than those without fillers.

Table 3.
Alpha diversity index of the microbial community.
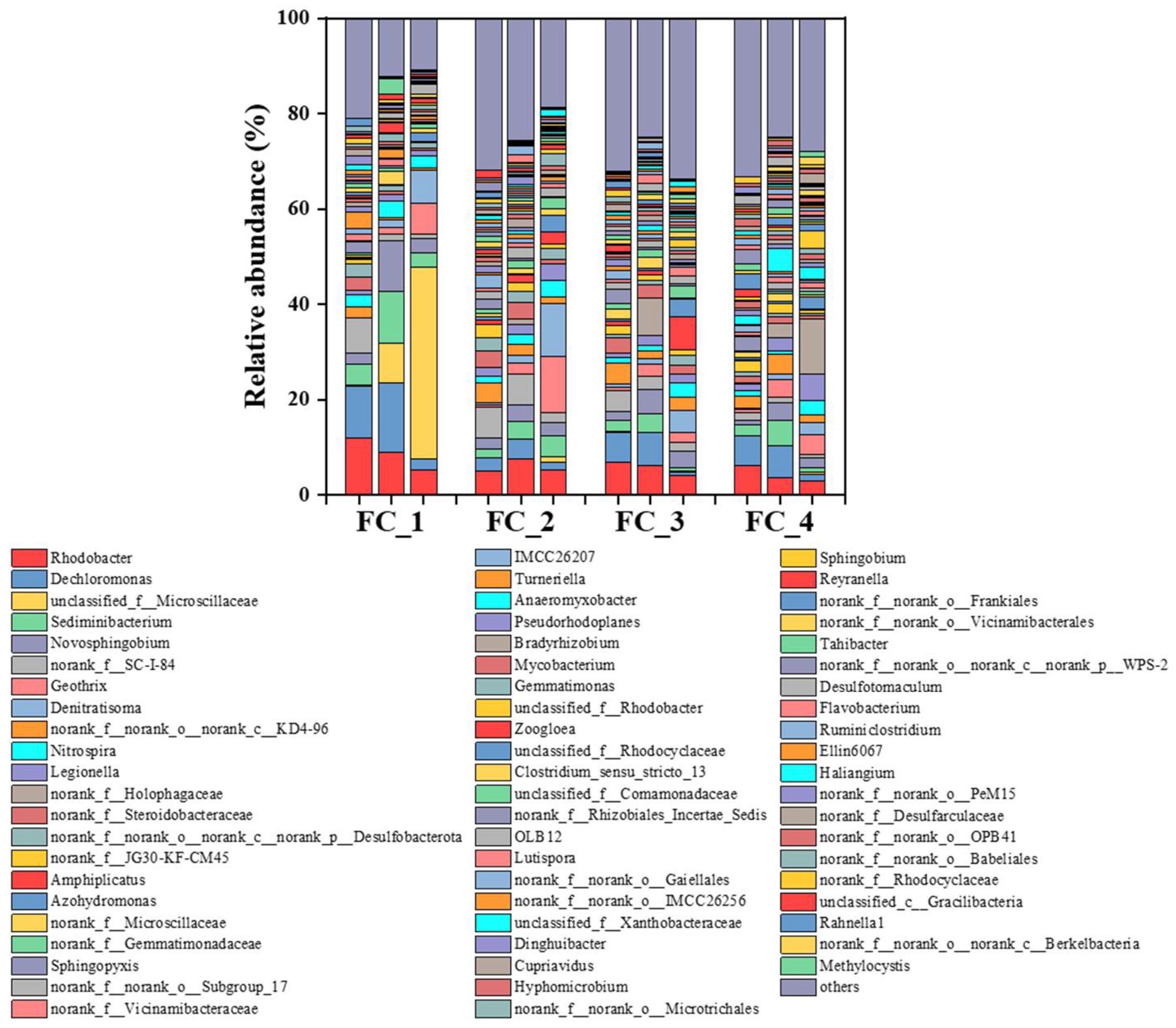
In the biofilm samples collected at the end of phases II, III, and IV of the four floating beds, a total of 54 bacterial phyla were identified, with 16 phyla having an abundance greater than 1%. Among them, the Proteobacteria phylum dominated in all four floating beds. Additionally, the Bacteroidota, Acidobacteriota, Actinobacteriota, Firmicutes, and Nitrospirota phyla were detected in all four floating beds, which was consistent with the research [35]. At the genus level, the typical nitrifying bacteria genus Nitrospira was detected in all four floating beds, with relative abundances ranging from 2.5% to 3.5%, 1.4% to 3.5%, 1.1% to 3.0%, and 0.7% to 3.0%, respectively, in FC1, FC2, FC3, and FC4 (Figure 5). The denitrifying bacteria genus Denitratisoma was also detected, with relative abundances ranging from 0.1% to 7.0%, 0.5% to 11.1%, 0.6% to 4.5%, and 0.3% to 2.6%, respectively (Figure 5). Additionally, the relative abundances of Denitratisoma gradually increased, especially when the nitrate-nitrogen concentration increased in the influent during Phase IV. The iron-reducing bacterium Geothrix genus was also detected in the four floating bed systems, with relative abundances increased from 0.1% to 6.4%, 0.4% to 11.8%, 0.8% to 2.7%, and 0.8% to 4.2%. Meanwhile, the genera Rhodobacter, Dechloromonas, Sediminibacterium, and Novosphingobium demonstrated relatively high abundances. Rhodobacter and Sediminibacterium have been reported for their aerobic denitrification function, Dechloromonas has been proven to be a denitrifying polyphosphate-accumulating bacterium, and Novosphingobium acts as an oligotrophic aerobic denitrifying bacterium [36]. These denitrifying functional microorganisms in the ecological floating beds worked together to ensure the efficiency of total nitrogen and carbon removal.
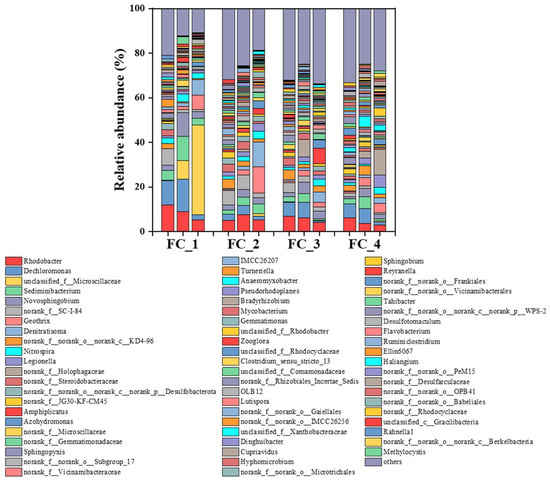
Figure 5.
The microbial community variation at the end of phase II, phase III, and phase IV in ecological floating beds (genus level).
4. Conclusions
In this study, iron–carbon-based fillers were integrated into ecological floating beds to investigate their impact and mechanisms in removing pollutants including carbon, nitrogen, phosphorus, and heavy metals, aiming to provide support for the efficient and sustainable treatment of surface water in rural areas. The main research conclusions are as follows:
- (i)
- All five fillers (activated carbon, iron–carbon fillers, sponge iron, activated carbon + iron–carbon fillers, and activated carbon + sponge iron) can completely remove orthophosphate, and the sponge iron filler and activated carbon + sponge iron filler system can completely remove nitrate nitrogen.
- (ii)
- Fillers are applied to an ecological floating bed, with influent concentrations of 2 mg/L of NH4+-N, 2~6 mg/L of NO3−-N, 0.5 mg/L of total phosphorus, 50 mg/L of COD, 2 mg/L of Cu2+, 4 mg/L of Zn2+, and 0.1 mg/L of Cd2+. The iron–carbon microelectrolysis (activated carbon + sponge iron filler) enhanced ecological floating bed showed superior removal efficiency for various pollutants. It achieved 95% removal of NH4+-N, 85% removal of NO3−-N, 75% removal of total phosphorus, 90% removal of COD, and 90% removal of heavy metals.
- (iii)
- Typical nitrifying bacteria of the Nitrospira, denitrifying bacteria Denitratisoma, and a variety of bacterial genera with denitrification functions (e.g., Rhodobacter, Dechloromonas, Sediminibacterium, and Novosphingobium) coexisted in the system, ensuring efficient and robust nitrogen removal performance.
Therefore, the iron–carbon microelectrolysis ecological floating bed offers advantages such as a compact design, cost-effectiveness in construction and operation, high efficiency in pollutant removal, and fast treatment rates. It shows great potential for application in economically underdeveloped rural areas, providing an affordable solution for sustainable treatment of surface water without harming the local ecosystem.
Author Contributions
Conceptualization, H.W.; investigation, T.W. and W.W.; writing—review and editing, H.W. and Y.Y.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (42107246).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahn, S.C.; Oh, S.Y.; Cha, D.K. Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J. Hazard. Mater. 2008, 156, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Alan Bojanic, H. The rapid agricultural development of Brazil in the last 20 years. EuroChoices 2017, 16, 5–10. [Google Scholar] [CrossRef]
- Reardon, T.; Echeverria, R.; Berdegué, J.; Minten, B.; Liverpool-Tasie, S.; Tschirley, D.; Zilberman, D. Rapid transformation of food systems in developing regions: Highlighting the role of agricultural research & innovations. Agric. Syst. 2019, 172, 47–59. [Google Scholar]
- Karki, B.K.; Lamichhane, K.; Joshi, L.; Raj, K.C.; Sah, M.K.; Pathak, M.; Karki, K.R. Risk assessment of heavy metals in the major surface water system of Nepal with potential remediation technologies. Environ. Chall. 2024, 14, 100865. [Google Scholar] [CrossRef]
- Md Anawar, H.; Chowdhury, R. Remediation of polluted river water by biological, chemical, ecological and engineering processes. Sustainability 2020, 12, 7017. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S. Ecological floating bed (EFB) for decontamination of polluted water bodies: Design, mechanism and performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, D.; Qiu, Y.; Li, D.; Yan, C.; Li, J.; Li, D.; Liu, G.; Feng, Y. Performance and mechanism of pilot-scale carbon fibers enhanced ecological floating beds for urban tail water treatment in optimized ecological floating beds water surface coverage. Bioresour. Technol. 2024, 393, 130095. [Google Scholar] [CrossRef] [PubMed]
- Kumwimba, M.N.; Li, X.; Huang, J.; Muyembe, D.K.; Dzakpasu, M.; Sanganyado, E. Performance of various fillers in ecological floating beds planted with Myriophyllum aquaticum treating municipal wastewater. Sci. Total Environ. 2022, 842, 156827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Song, H.L.; Li, W.; Lu, X.W.; Nishimura, O. An integrated ecological floating-bed employing plant, freshwater clam and biofilm carrier for purification of eutrophic water. Ecol. Eng. 2010, 36, 382–390. [Google Scholar] [CrossRef]
- Lyu, J.C.; Lin, G.H.; Fan, Z.Y.; Lin, W.X.; Dai, Z. Suitable plant combinations for ecological floating beds in eutrophic subtropical coastal wetlands under different salinities: Experimental evidences. Int. J. Environ. Sci. Technol. 2020, 17, 4505–4516. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S.; Upadhyay, S. Assessing the impact of vegetation coverage ratio in a floating water treatment bed of Pistia stratiotes. SN Appl. Sci. 2021, 3, 120. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Ketola, T. Biomass accumulations and nutrient uptake of plants cultivated on artificial floating beds in China’s rural area. Ecol. Eng. 2011, 37, 1460–1466. [Google Scholar] [CrossRef]
- ShahShahid, M.J.; Al-Surhanee, A.A.; Kouadri, F.; Ali, S.; Nawaz, N.; Afzal, M.; Rizwan, M.; Ali, B.; Soliman, M.H. Role of microorganisms in the remediation of wastewater in floating treatment wetlands: A review. Sustainability 2020, 12, 5559. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhang, M.; He, S. Simultaneous improving nitrogen removal and decreasing greenhouse gas emission with biofilm carriers addition in ecological floating bed. Bioresour. Technol. 2019, 292, 121944. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hu, Y.; Li, S.; Peng, S.; Zhao, H. Microbial mechanisms of using enhanced ecological floating beds for eutrophic water improvement. Bioresour. Technol. 2016, 211, 451–456. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; He, Y.; Cheng, Q.; Wu, Q.; Liu, Z.; Li, Y.; Yang, Z.; Tan, Y.; Yuan, Y. Enhanced nitrogen and phosphorus removal by a novel ecological floating bed integrated with three-dimensional biofilm electrode system. J. Environ. Manag. 2023, 348, 119346. [Google Scholar] [CrossRef]
- Do, T.H.; Vu, D.N.; Ngan, T.T.K. Internal Micro-electrolysis Using Fe/C Material for Pre-Treatment of Concentrated Coking Wastewater. Pol. J. Chem. Technol. 2021, 23, 41–46. [Google Scholar]
- Zheng, X.; Zhou, C.; Wu, F.; Xu, H.; Zhao, Z.; Han, Z.; Zhang, H.; Yang, S. Enhanced removal of organic, nutrients, and PFCs in the iron-carbon micro-electrolysis constructed wetlands: Mechanism and iron cycle. Chem. Eng. J. 2023, 457, 141174. [Google Scholar] [CrossRef]
- Defilippi, C.; Mukadam, M.O.A.; Nicolae, S.A.; Lees, M.R.; Giordano, C. Iron Carbide@ Carbon nanocomposites: A tool box of functional materials. Materials 2019, 12, 323. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Pazos, M.; Sanromán, M.A.; Rosales, E. Heterogeneous electro-Fenton system using Fe-MOF as catalyst and electrocatalyst for degradation of pharmaceuticals. Chemosphere 2023, 340, 139942. [Google Scholar] [CrossRef]
- Zhao, E.; Fan, J.; Zhang, H.; Wu, S.; Yu, Y.; Yang, Y.; Mei, S. Iron-carbon composite as flow electrode active material for highly efficient electrosorption of uranium from radioactive wastewater. Sep. Purif. Technol. 2025, 354, 128405. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standards for Surface Water. State Environmental Protection Administration of China: Beijing, China, 2002.
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Y.; Zhou, M.; Liu, J.; Li, X.; Wang, Y. Metagenomics insight into the long-term effect of ferrous ions on the mainstream anammox system. Environ. Res. 2023, 238, 117243. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Braun, J.C.; Borba, C.E.; Godinho, M.; Perondi, D.; Schontag, J.M.; Wenzel, B.M. Phosphorus adsorption in Fe-loaded activated carbon: Two-site monolayer equilibrium model and phenomenological kinetic description. Chem. Eng. J. 2019, 361, 751–763. [Google Scholar] [CrossRef]
- Li, J.; Zeng, W.; Liu, H.; Wu, Y.; Miao, H. Performances and mechanisms of simultaneous nitrate and phosphate removal in sponge iron biofilter. Bioresour. Technol. 2021, 337, 125390. [Google Scholar] [CrossRef]
- Huong, D.T.; Nguyen, V.T.; Ha, X.L.; Nguyen Thi, H.L.; Duong, T.T.; Nguyen, D.C.; Nguyen Thi, H.T. Enhanced degradation of phenolic compounds in coal gasification wastewater by methods of microelectrolysis Fe-C and anaerobic-anoxic—Oxic moving bed biofilm reactor (A2O-MBBR). Processes 2020, 8, 1258. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Saleha, S.; Maulina, F.P.; Idroes, R. Polyurethane film prepared from ball-milled algal polyol particle and activated carbon filler for NH3–N removal. Heliyon 2020, 6, E04590. [Google Scholar]
- Amarawansha, G.; Kumaragamage, D.; Flaten, D.; Zvomuya, F.; Tenuta, M. Predicting phosphorus release from anaerobic, alkaline, flooded soils. J. Environ. Qual. 2016, 45, 1452–1459. [Google Scholar] [CrossRef]
- Zafarani, H.R.; Bahrololoom, M.E.; Javidi, M.; Shariat, M.H.; Tashkhourian, J. Removal of chromate ion from aqueous solutions by sponge iron. Desalin. Water Treat. 2014, 52, 7154–7162. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Salgar-Chaparro, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Nutrient level determines biofilm characteristics and subsequent impact on microbial corrosion and biocide effectiveness. Appl. Environ. Microbiol. 2020, 86, e02885-19. [Google Scholar] [CrossRef] [PubMed]
- Nagendra, H. Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 2002, 22, 175–186. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, Y.; Yu, Y.; Ji, Y.; Li, H.; Liao, M.; Li, D.; Liang, D.; Liu, G.; Feng, Y. Long-term operation of cathode-enhanced ecological floating bed coupled with microbial electrochemical system for urban surface water remediation: From lab-scale research to engineering application. Water Res. 2023, 237, 119967. [Google Scholar] [CrossRef]
- Coates, J.D.; Chakraborty, R.; Lack, J.G.; O’Connor, S.M.; Cole, K.A.; Bender, K.S.; Achenbach, L.A. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 2001, 411, 1039–1043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).