Biomass Partitioning, Carbon Storage, and Pea (Pisum sativum L.) Crop Production under a Grewia optiva-Based Agroforestry System in the Mid-Hills of the Northwestern Himalayas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Trial Establishment
2.3. Observation Recorded
2.4. Soil Analysis
2.5. Photosynthetic Active Radiation (PAR)
2.6. Bioeconomic Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Tree Density on Growth and Biomass Partitioning
3.2. Carbon Storage and C Sequestration Potential of the G. optiva AFS
3.3. Performance of the Pea Crop
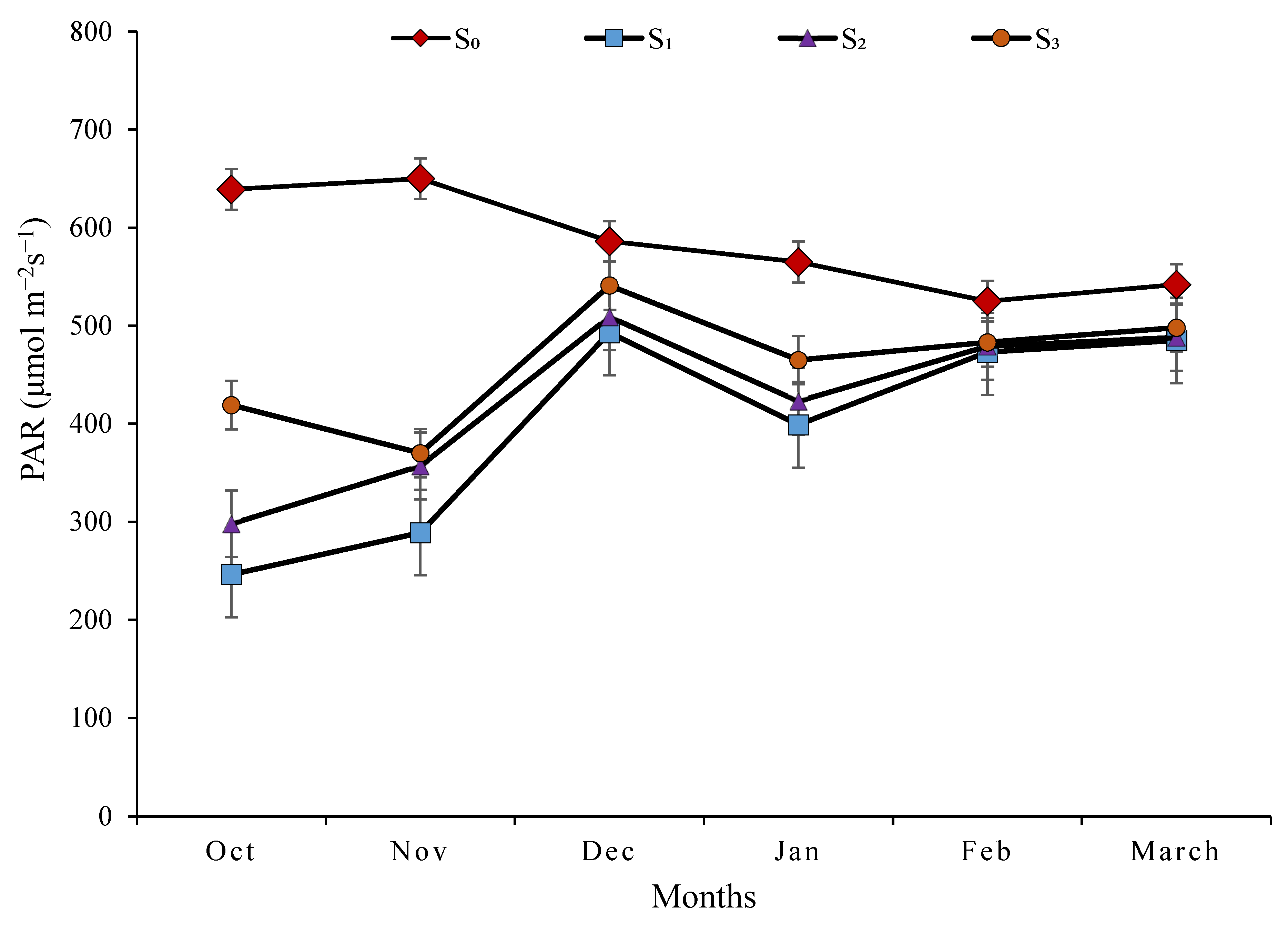
3.4. Photosynthetic Active Radiation (µmol m−2 s−1)
3.5. Soil Physicochemical Properties
3.6. Bioeconomic Appraisal
4. Discussion
4.1. Effect of Tree Density on Growth and Biomass Partitioning
4.2. Carbon Storage and C Sequestration Potential of G. optiva AFSs
4.3. Performance of the Pea Crop
4.4. Photosynthetic Active Radiation (µmol m−2 s−1)
4.5. Soil Physicochemical Properties
4.6. Bioeconomic Appraisal
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Bishnoi, M.; Baloch, M.R.; Singh, G. Tree biomass, resource use and crop productivity in agri-horti-silvicultural Systems in the dry region of Rajasthan, India. Arch. Agron. Soil Sci. 2014, 60, 1031–1049. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. Food and Agricultural Organization of the United Nations. 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 13 March 2022).
- Licker, R.; Johnston, M.; Foley, J.A.; Barford, C.; Kucharik, C.J.; Monfreda, C.; Ramankutty, N. Mind the gap: How do climate and agricultural management explain the ‘yield gap’ of croplands around the world? Glob. Ecol. Biogeogr. 2010, 19, 769–782. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, D.R.; Singh, M.K.; Nigam, R.; Pala, N.A.; Kumar, A.; Verma, K.; Kumar, D.; Thakur, P. Geospatial technology in agroforestry: Status, prospects, and constraints. Environ. Sci. Pollut. Res. 2022, 30, 116459–116487. [Google Scholar] [CrossRef]
- Verma, K.; Sharma, P.; Bhardwaj, D.R.; Kumar, R.; Kumar, N.M.; Singh, A.K. Land and environmental management through agriculture, forestry and other land use (AFOLU) system. In Land and Environmental Management through Forestry; Raj, A., Jhariya, M.K., Banerjee, A., Nema, S., Bargali, K., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 247–271. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, M.; Tiwari, P.; Verma, K. Agroforestry systems: Opportunities and challenges in India. J. Pharmacogn. Phytochem. 2017, 7, 953–957. [Google Scholar]
- Lehmann, L.M.; Smith, J.; Westaway, S.; Pisanelli, A.; Russo, G.; Borek, R.; Sandor, M.; Gliga, A.; Smith, L.; Ghaley, B.B. Productivity and economic evaluation of agroforestry systems for sustainable production of food and nonfood products. Sustainability 2020, 12, 5429. [Google Scholar] [CrossRef]
- Bhardwaj, D.R.; Salve, A.; Kumar, J.; Kumar, A.; Sharma, P.; Kumar, D. Biomass production and carbon storage potential of agroforestry land use systems in high hills of northwestern Himalaya: An approach toward natural based climatic solution. Biomass Convers. Biorefinery 2023, 14, 18079–18092. [Google Scholar] [CrossRef]
- Panwar, P.; Mahalingappa, D.G.; Kaushal, R.; Bhardwaj, D.R.; Chakravarty, S.; Shukla, G.; Thakur, N.S.; Chavan, S.B.; Pal, S.; Nayak, B.G.; et al. Biomass Production and Carbon Sequestration Potential of Different Agroforestry Systems in India: A Critical Review. Forests 2022, 13, 1274. [Google Scholar] [CrossRef]
- Verma, K.; Sharma, P.; Kumar, D.; Vishwakarma, S.P.; Meena, N.K. Strategies sustainable management of agroforestry in climate change mitigation and adaptation. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 2439–2449. [Google Scholar] [CrossRef]
- Dufour, L.; Gosme, M.; Le Bec, J.; Dupraz, C. Does pollarding trees improve the crop yield in a mature alley-cropping agroforestry system? J. Agron. Crop Sci. 2020, 206, 640–649. [Google Scholar] [CrossRef]
- Kumar, J.; Thakur, C.L.; Bhardwaj, D.R.; Kumar, S.; Dutt, B. Effects of integrated nutrient management on performance of bhringraj (eclipta prostrata l.) and soil fertility under the Grewia optiva Drummond. canopy in a mid-hill agroecosystem of north western Himalayas. Agrofor. Syst. 2023, 97, 711–726. [Google Scholar] [CrossRef]
- Thevathasan, N.V.; Gordon, A.M. Ecology of tree intercropping systems in the North temperate region: Experiences from Southern Ontario, Canada. Agrofor. Syst. 2004, 61–62, 257–268. [Google Scholar] [CrossRef]
- Nair, P.; Kumar, B.M.; Nair, V.D. An Introduction to Agroforestry: Four Decades of Scientific Developments; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Navale, M.R.; Bhardwaj, D.R.; Bishist, R.; Thakur, C.L.; Sharma, S.; Sharma, P.; Kumar, D.; Probo, M. Seasonal variations in the nutritive value of fifteen multipurpose fodder tree species: A case study of northwestern Himalayan mid-hills. PLoS ONE 2022, 17, e0276689. [Google Scholar] [CrossRef]
- IGFRI. Vision 2050. 2013. Available online: https://www.igfri.res.in/2013/Vision-2050.pdf (accessed on 26 March 2022).
- Khybri, M.L.; Gupta, R.K.; Ram, S.; Tomar, H.P.S. Crop yields of rice and wheat grown in rotation as intercrops with three tree species in the outer hills of western Himalaya. Agrofor. Syst. 1992, 17, 193–204. [Google Scholar] [CrossRef]
- Francis, C.; Lieblein, G.; Gliessman, S.; Breland, T.A.; Creamer, N.; Harwood, R.; Salomonsson, L.; Helenius, J.; Rickerl, D.; Salvador, R.; et al. Agroecology: The ecology of food systems. J. Sustain. Agric. 2003, 22, 99–118. [Google Scholar] [CrossRef]
- Pantera, A.; Mosquera-Losada, M.R.; Herzog, F.; Den Herder, M. Agroforestry and the environment. Agrofor. Syst. 2021, 95, 767–774. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Migliorini, P.; Wezel, A. Converging and diverging principles and practices of organic agriculture regulations and agroecology. A review. Agron. Sustain. Dev. 2017, 37, 63. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- De Candolle, A. Origin of Cultivated Plants.; Honfer publishing Company: New York, NY, USA, 1886. [Google Scholar]
- Kumari, A.; Singh, O.N.; Kumar, R. Effect of integrated nutrient management on growth, seed yield and economics of field pea (Pisum sativum L.) and soil fertility changes. J. Food Legum. 2012, 25, 121–124. [Google Scholar]
- Rajput, R.; Khushwah, S. Effect of integrated nutrient management on yield of pea (Pisum sativum). Legume Res. 2005, 28, 231–232. [Google Scholar]
- Bijalwan, A.; Dobriyal, M.J.R. Productivity of wheat (Triticum aestivum) as intercrop in Grewia optiva based traditional agroforestry system along altitudinal gradient and aspect in mid hills of Garhwal Himalaya, India. Am. J. Environ. Prot. 2014, 2, 89–94. [Google Scholar] [CrossRef]
- Zubay, P.; Kunzelmann, J.; Ittzés, A.; Németh Zámboriné, É.; Szabó, K. Allelopathic effects of leachates of Juglans regia L., Populus tremula L. and Juglone on germination of temperate zone cultivated medicinal and aromatic plants. Agrofor. Syst. 2021, 95, 431–442. [Google Scholar] [CrossRef]
- Verma, K.; Prasad, S.K.; Singh, M.K.; Sharma, P. Assessment of agronomic zinc biofortification of alley cropped pearl millet. Bangladesh J. Bot. 2023, 52, 203–209. [Google Scholar] [CrossRef]
- Verma, K.; Prasad, S.; Singh, M.; Sharma, P. Response of alley-cropped pearl millet (Pennisetum Glaucum) to Nitrogen and Zinc schedules under semiarid regions. Indian J. Agron. 2023, 68, 105–109. [Google Scholar] [CrossRef]
- Chaturvedi, O.; Handa, A.; Kaushal, R.; Uthappa, A.; Sarvade, S.; Panwar, P. Biomass production and carbon sequestration through agroforestry. Range Manag. Agrofor. 2016, 37, 116–127. [Google Scholar]
- Verma, T.; Bhardwaj, D.R.; Sharma, U.; Sharma, P.; Kumar, D.; Kumar, A.; Kumar, A. Agroforestry systems in the mid-hills of the northwestern Himalaya: A sustainable pathway to improved soil health and climate resilience. J. Environ. Manag. 2023, 348, 119264. [Google Scholar] [CrossRef]
- Bhutia, P.L.; Thakur, C.L.; Sarvade, S.; Bhardwaj, D.R.; Kaushal, R.; Bhutia, K.G. Varietal performance of pea (Pisum Sativum L.) under peach-based agroforestry system in mid hill conditions of Himachal Pradesh. Indian J. Agrofor. 2015, 17, 14–17. [Google Scholar]
- FSI. Volume Equations for Forests of India, Nepal and Bhutan; FSI: Arlington, VA, USA, 1996. [Google Scholar]
- Chidumayo, E.N. Above-ground woody biomass structure and productivity in a Zambezian woodland. For. Ecol. Manag. 1990, 36, 33–46. [Google Scholar] [CrossRef]
- Smith, D.M. Maximum Moisture Content Method for Determining Specific Gravity of Small Wood Samples; No. Report 2014; U.S. Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- IPCC. Good Practice Guidance for Land Use, Land-Use Change and Forestry; Institute for Global Environmental Strategies (IGES) for the IPCC: Arlington, VA, USA, 2003. [Google Scholar]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Change Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Rogelj, J.; Schaeffer, M.; Meinshausen, M.; Knutti, R.; Alcamo, J.; Riahi, K.; Hare, W. Zero emission targets as long-term global goals for climate protection. Environ. Res. Lett. 2015, 10, 105007. [Google Scholar] [CrossRef]
- Jackson, M. Soil Chemical Analysis Prentice; Hall India Priv. Limited: New Delhi, India, 1967; p. 498. [Google Scholar]
- Walkley, A.; Black, C. An examination of digestion of method for determining soil organic matter and a proposed modification of the chromic and titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in the soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture Circular 19: Washington, DC, USA, 1954; p. 939. [Google Scholar]
- Merwin, H.D.; Peech, M. Exchangeability of soil potassium in sand, silt and clay fractions as influenced by the nature of the complimentary exchangeable cations. Soil Sci. Soc. Am. Proc. 1950, 15, 125–128. [Google Scholar] [CrossRef]
- Gomez, L.; Gomez, A. Statistical Procedures for Agricultural Research; John Wiley and Sons: Singapore, 1984. [Google Scholar]
- Antón-Fernández, C.; Burkhart, H.E.; Strub, M.; Amateis, R.L. Effects of initial spacing on height development of loblolly pine. For. Sci. 2011, 57, 201–211. [Google Scholar] [CrossRef]
- Lanner, R.M. On the insensitivity of height growth to spacing. For. Ecol. Manag. 1985, 13, 143–148. [Google Scholar] [CrossRef]
- Benomar, L.; DesRochers, A.; Larocque, G.R. The effects of spacing on growth, morphology and biomass production and allocation in two hybrid poplar clones growing in the boreal region of Canada. Trees 2012, 26, 939–949. [Google Scholar] [CrossRef]
- Prasad, J.V.N.S.; Korwar, G.R.; Rao, K.V.; Mandal, U.K.; Rao, G.R.; Srinivas, I.; Venkateswarlu, B.; Rao, S.N.; Kulkarni, H.D. Optimum stand density of Leucaena Leucocephala for wood production in Andhra Pradesh, Southern India. Biomass Bioenergy 2011, 35, 227–235. [Google Scholar] [CrossRef]
- Manasa, P.C.; Salimath, K.S.; Hegde, R.; Amanulla, B.; Kumar, C. Biomass and Carbon stock in forest plantations: Manipulation through spacing. J. Tree Sci. 2016, 35, 71. [Google Scholar] [CrossRef]
- Chotchutima, S.; Kangvansaichol, K.; Tudsri, S.; Sripichitt, P. Effect of spacing on growth, biomass yield and quality of Leucaena leucocephala for renewable energy in Thailand. J. Sustain. Bioenergy Syst. 2013, 03, 48–56. [Google Scholar] [CrossRef]
- Bernardo, A.L.; Reis, M.G.F.; Reis, G.G.; Harrison, R.B.; Firme, D.J. Effect of spacing on growth and biomass distribution in Eucalyptus camaldulensis, E. pellita and E. urophylla plantations in southeastern Brazil. For. Ecol. Manag. 1998, 104, 1–13. [Google Scholar] [CrossRef]
- Hein, S.; Weiskittel, A.R.; Kohnle, U. Effect of wide spacing on tree growth, branch and sapwood properties of young Douglas-fir [Pseudotsuga Menziesii (Mirb.) Franco] in southwestern Germany. Eur. J. For. Res. 2008, 127, 481–493. [Google Scholar] [CrossRef]
- Carter, R.E.; Miller, I.M.; Klinka, K. Relationships between growth form and stand density in immature Douglas-fir. For. Chron. 1986, 62, 440–445. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hein, S. Effect of wide spacing on increment and branch properties of young Norway spruce. Eur. J. For. Res. 2006, 125, 239–248. [Google Scholar] [CrossRef]
- Hegazy, S.; Aref, I.; Al-Mefarrej, H.; Hamad & El-Juhany, L. Effect of spacing on the biomass production and allocation in Conocarpus erectus L. trees grown in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 315–322. [Google Scholar]
- Routray, S.; Bhol, N.; Nayak, R.; Mohapatra, S. Biomass production and carbon sequestraion of mangium (Acacia Mangium Willd.) under varying plant spacing. Indian For. 2018, 144, 527–531. [Google Scholar]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Sharma, R.; Pradhan, L.; Kumari, M.; Bhattacharya, P. Assessment of Carbon Sequestration Potential of Tree Species in Amity University Campus Noida. Environ. Sci. Proc. 2021, 3, 52. [Google Scholar] [CrossRef]
- Peichl, M.; Thevathasan, N.V.; Gordon, A.M.; Huss, J.; Abohassan, R.A. Carbon sequestration potentials in temperate tree-based intercropping systems, southern Ontario, Canada. Agrofor. Syst. 2006, 66, 243–257. [Google Scholar] [CrossRef]
- Rabach, V.O.; Koske, J.; Muna, M.M.; Muriuki, J.; Ngare, I.O. Carbon sequestration in agroforestry systems between conservation agriculture and conventional practice in the Asal area of Machakos County, Kenya. J. Appl. Agric. Sci. Technol. 2020, 4, 118–133. [Google Scholar] [CrossRef]
- Nolte, C.; Tiki-Manga, T.; Badjel-Badjel, S.; Gockowski, J.; Hauser, S.; Weise, S.F. Effects of calliandra planting pattern on biomass production and nutrient accumulation in planted fallows of southern Cameroon. For. Ecol. Manag. 2003, 179, 535–545. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D.; Mohan Kumar, B.; Showalter, J.M. Carbon Sequestration in Agroforestry Systems. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 108, pp. 237–307. [Google Scholar] [CrossRef]
- Fernández-Núñez, E.; Rigueiro-Rodríguez, A.; Mosquera-Losada, M.R. Carbon allocation dynamics one decade after afforestation with Pinus radiata D. Don and Betula alba L. under two stand densities in NW Spain. Ecol. Eng. 2010, 36, 876–890. [Google Scholar] [CrossRef]
- Islam, M.; Dey, A.; Rahman, M. Effect of tree diversity on soil organic carbon content in the homegarden agroforestry system of northeastern Bangladesh. Small-Scale For. 2015, 14, 91–101. [Google Scholar] [CrossRef]
- Oelbermann, M.; Voroney, R.P. Carbon and nitrogen in a temperate agroforestry system: Using stable isotopes as a tool to understand soil dynamics. Ecol. Eng. 2007, 29, 342–349. [Google Scholar] [CrossRef]
- Luedeling, E.; Kindt, R.; Huth, N.I.; Koenig, K. Agroforestry systems in a changing climate—Challenges in projecting future performance. Curr. Opin. Environ. Sustain. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Smith, D.; Liu, W.; Chen, X.; Yeng, W. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. Afr. J. Biotechnol. 2011, 10, 14392–14398. [Google Scholar] [CrossRef]
- Rizwan, M.; Rauf, A.; Rahmawaty, E.N.A. Physiology Response of Soybean Variety to Various Types of Shading in Agroforestry System. In Proceedings of the 7th International Conference on Multidisciplinary Research, Medan, Indonesia, 5–6 September 2018; SCITEPRESS—Science and Technology Publications: Sumatera Utara, Indonesia, 2018; pp. 225–230. [Google Scholar] [CrossRef]
- Hossain, M.A.; Akamine, H.; Ishimine, Y.; Teruya, R.; Aniya, Y.; Yamawaki, K. Effects of relative light intensity on the growth, yield and curcumin content of turmeric (Curcuma Longa L.) in Okinawa, Japan. Plant Prod. Sci. 2009, 12, 29–36. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Ramesh, K.; Singh, B. Effects of shade regimes and planting geometry on growth, yield and quality of the natural sweetener plant stevia (Stevia Rebaudiana Bertoni) in northwestern Himalaya. Arch. Agron. Soil Sci. 2013, 59, 963–979. [Google Scholar] [CrossRef]
- Hadi, H.; Ghassemi-Golez, K.; Khoei, F.R.; Valizadeh, M.; Shakib, M.R. Response of common bean (Phaseolus vulgaris L.) to different levels of shade. J. Agron. 2006, 5, 595–599. [Google Scholar] [CrossRef]
- Priyadharshini, A.; Singh, V.; Tiwari, D.; Budige, K.; Mahesh, K. Influence of spacing and organic manures on growth, yield and economics of mung bean (Vigna radiata L.). Biol. Forum–An Int. J. 2021, 13, 617–621. [Google Scholar]
- Khalid, M.H.B.; Raza, M.A.; Yu, H.Q.; Sun, F.A.; Zhang, Y.Y.; Lu, F.Z.; Si, L.; Iqbal, N.; Khan, I.; Fu, F.L.; et al. Effect of shade treatments on morphology, photosynthetic and chlorophyll fluorescence characteristics of soybean (Glycine Max L. Merr.). Appl. Ecol. Environ. Res. 2019, 17, 2551–2569. [Google Scholar] [CrossRef]
- Zaki, H.; Mahmoud, A.; Abd El-Ati, Y.; Hammad, A.; Sayed, R. Studies on pea (Pisum sativum L.) growth and productivity under agroforestry system: 2. Yield and seed quality of pea under alley cropping system with two types of trees. J. Basic Appl. Res. 2017, 3, 1–9. [Google Scholar]
- Swamy, S.L.; Mishra, A.; Puri, S. Biomass production and root distribution of Gmelina arborea under an agrisilvicultre system in subhumid tropics of Central India. New For. 2003, 26, 167–186. [Google Scholar] [CrossRef]
- Arenas-Corraliza, M.G.; Rolo, V.; López-Díaz, M.L.; Moreno, G. Wheat and barley can increase grain yield in shade through acclimation of physiological and morphological traits in Mediterranean conditions. Sci. Rep. 2019, 9, 9547. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.Y.; Díaz-Pérez, J.C.; Nambeesan, S.U. Effect of shade levels on plant growth, physiology, and fruit yield in bell pepper (Capsicum annuum L.). Acta Hortic. 2020, 1268, 311–318. [Google Scholar] [CrossRef]
- Paramesh, V.; Kumar, P.; Bhagat, T.; Nath, A.J.; Manohara, K.K.; Das, B.; Desai, B.F.; Jha, P.K.; Prasad, P.V.V. Integrated Nutrient Management Enhances Yield, Improves Soil Quality, and Conserves Energy under the Lowland Rice–Rice Cropping System. Agronomy 2023, 13, 1557. [Google Scholar] [CrossRef]
- Chaudhary, M.; Narwal, R.P. Effect of long-term application of farmyard manure on soil micronutrient status. Arch. Agron. Soil Sci. 2005, 51, 351–359. [Google Scholar] [CrossRef]
- Premsekhar, M.; Rajashree, V. Influence of organic manures on growth, yield and quality of okra. Am.-Eurasian J. Sustain. Agric. 2009, 3, 6–8. [Google Scholar]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different organic manure sources and npk fertilizer on soil chemical properties, growth, yield and quality of okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Gill, B.; Randhawa, R.; Randhawa, G.; Singh, J. Response of turmeric (Curcuma longa L.) to nitrogen in relation to application of farmyard manure and straw mulch. J. Spices Aromat. Crops 1999, 8, 211–214. [Google Scholar]
- Pandey, A.K.; Gopinath, K.; Chattacharya, P.; Hooda, K.; Sushil, S.; Kundu, S.; Selvakumar, G. Effect of source and rate of organic manures on yield attributes, pod yield and economics of organic garden pea (Pisum Sativum Subsp Hortense) in north west Himalaya. Indian J. Agric. Sci. 2006, 76, 230–234. [Google Scholar]
- Saket, S.; Singh, S.; Namdeo, K.; Parihar, S. Effect of organic and inorganic fertilizers on yield, quality and nutrients uptake of lentil. Ann. Plant Soil Res. 2014, 16, 238–241. [Google Scholar]
- Gonmei, L.; Umrao, R.; Ramchandra, V. Effect of different types of organic manure on the growth and yield of pea (Pisum sativum) under Jatropha curcas based agroforestry system. Int. J. Multidiscip. Res. Dev. 2018, 5, 157–159. [Google Scholar]
- Aher, S.B.; Lakaria, B.L.; Kaleshananda, S.; Singh, A.B.; Ramana, S.; Ramesh, K.; Thakur, J.K. Effect of organic farming practices on soil and performance of soybean (Glycine max) under semiarid tropical conditions in Central India. J. Appl. Nat. Sci. 2015, 7, 67–71. [Google Scholar] [CrossRef]
- Solanki, R.; Bhalu, V.; Jadav, K. Organic farming approach for sustaining productivity of rainfed groundnut. Ann. Agric. Res. 2006, 27, 236–239. [Google Scholar]
- Zalate, P.; Padmani, D. Effect of organic manure and biofertilizers on yield, harvest index, shelling percentage and quality of kharif groundnut (Arachis hypogeae L.). Int. J. Agric. Sci. 2009, 5, 417–419. [Google Scholar]
- Babu, S.; Singh, R.; Avasthe, R.K.; Yadav, G.S.; Das, A.; Singh, V.K.; Mohapatra, K.P.; Rathore, S.S.; Chandra, P.; Kumar, A. Impact of land configuration and organic nutrient management on productivity, quality and soil properties under baby maize in eastern Himalayas. Sci. Rep. 2020, 10, 16129. [Google Scholar] [CrossRef]
- Thongney, P.L.; Khare, N.; Rout, S.; Debbarma, R. Effect of different level of vermicompost and fym organic manure on growth and yield of cucumber intercropped with citrus based agroforestry system. Adv. Bioresearch 2020, 11, 11–20. [Google Scholar]
- Patil, H.; Udmale, K. Response of different organic inputs on growth and yield of soybean on Inceptisol. Int. J. Recent Sci. Res. 2016, 7, 14116–14120. [Google Scholar]
- Gopinath, K.; Mina, B. Effect of organic manures on agronomic and economic performance of garden pea (Pisum sativum) and on soil properties. Indian J. Agric. Sci. 2011, 81, 236–239. [Google Scholar]
- Garima; Bhardwaj, D.R.; Thakur, C.L.; Kaushal, R.; Sharma, P.; Kumar, D.; Kumari, Y. Bamboo-based agroforestry system effects on soil fertility: Ginger performance in the bamboo subcanopy in the Himalayas (India). Agron. J. 2021, 113, 2832–2845. [Google Scholar] [CrossRef]
- Sharma, U.; Bhardwaj, D.R.; Sharma, S.; Sankhyan, N.; Thakur, C.L.; Rana, N.; Sharma, S. Assessment of the efficacy of various mulch materials on improving the growth and yield of ginger (Zingiber officinale) under bamboo-based agroforestry system in NW-Himalaya. Agrofor. Syst. 2022, 96, 925–940. [Google Scholar] [CrossRef]
- Kaushal, R.; Verma, K.; Chaturvedi, O.; Alam, N. Leaf litter decomposition and nutrient dynamics in four multipurpose tree species. Range Manag. Agrofor. 2012, 33, 20–27. [Google Scholar]
- Bisht, N.; Sah, V.K.; Satyawali, K.; Tiwari, S. Comparison of wheat yield and soil properties under open and poplar based agroforestry system. J. Appl. Nat. Sci. 2017, 9, 1540–1543. [Google Scholar] [CrossRef]
- Kar, S.; Pant, K.; Chandel, A.; Roshanzada, S. Trend of soil parameters under different spacings of grewia based agroforestry system in the mid hill zones of Himachal Pradesh. Int. J. Chem. Stud. 2019, 7, 1904–1907. [Google Scholar]
- Zhao, Z.; Zhang, C.; Li, F.; Gao, S.; Zhang, J. Effect of compost and inorganic fertilizer on organic carbon and activities of carbon cycle enzymes in aggregates of an intensively cultivated Vertisol. PLoS ONE 2020, 15, e0229644. [Google Scholar] [CrossRef]
- Albiach, R.; Canet, R.; Pomares, F.; Ingelmo, F. Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Bioresour. Technol. 2000, 75, 43–48. [Google Scholar] [CrossRef]
- Ghosh, S.; Wilson, B.; Ghoshal, S.; Senapati, N.; Mandal, B. Organic amendments influence soil quality and carbon sequestration in the indo-gangetic plains of India. Agric. Ecosyst. Environ. 2012, 156, 134–141. [Google Scholar] [CrossRef]
- Singh, I.; Awasthi, O.; Meena, S. Influence of tree plantation on soil physico-chemical properties in arid region. Indian J. Agrofor. 2010, 12, 42–47. [Google Scholar]
- Edmeades, D.C. The long-term effects of manures and fertilizers on soil productivity and quality: A review. Nutr. Cycl. Agroecosystems 2003, 66, 165–180. [Google Scholar] [CrossRef]
- Almeida, R.F.; Queiroz, I.D.S.; Mikhael, J.E.R.; Oliveira, R.C.; Borges, E.N. Enriched animal manure as a source of phosphorus in sustainable agriculture. Int. J. Recycl. Org. Waste Agric. 2019, 8, 203–210. [Google Scholar] [CrossRef]
- Biratu, G.K.; Elias, E.; Ntawuruhunga, P. Soil fertility status of cassava fields treated by integrated application of manure and NPK fertilizer in Zambia. Environ. Syst. Res. 2019, 8, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, P.; Gulati, A. Viable agroforestry models and their economics in Yamunanagar district of Haryana and Haridwar district of Uttaranchal. Indian For. 2004, 130, 131–146. [Google Scholar]
- Singh, D.; Kumar, R.; Walia, S.S.; Brar, A.S.; Singh, R. Productivity and economics of turmeric (Curcuma longa L.) in responseto nitrogen applied through different sources in conjunction with biofertilizer consortium. J. Appl. Nat. Sci. 2017, 9, 497–501. [Google Scholar]
- Chandana, P.; Madhavi Lata, A.; Aariff Khan, M.A.; Krishna, A. Climate change smart option and doubling farmer’s income through melia dubia based agri-silviculture system. Curr. Sci. 2020, 118, 444. [Google Scholar] [CrossRef]





| Sr. No. | Parameters | Spacing (m2) | ||
|---|---|---|---|---|
| S1 (10 m × 1 m) | S2 (10 m × 2 m) | S3 (10 m × 3 m) | ||
| Mean | Mean | Mean | ||
| 1. | Height (m) | 4.67 ± 0.14 | 4.33 ± 0.17 | 3.60 ± 0.33 |
| 2. | Collar diameter (cm) | 19.21 ± 1.30 | 20.44 ± 1.41 | 22.88 ± 1.51 |
| 3. | Crown spread (m) | 3.51 ± 0.12 | 3.70 ± 0.23 | 3.73 ± 0.28 |
| 4. | No. of primary branches tree−1 | 2.27 ± 0.36 | 2.87 ± 0.36 | 3.27 ± 0.37 |
| 5. | Stem biomass (Mg ha−1) | 145.54 ± 22.56 | 83.47 ± 10.08 | 71.98 ± 10.48 |
| 6. | Leaf biomass (Mg ha−1) | 3.05 ± 0.13 | 1.79 ± 0.07 | 1.52 ± 0.12 |
| 7. | Branch biomass (Mg ha−1) | 12.18 ± 0.32 | 6.60 ± 0.28 | 5.08 ± 0.22 |
| 8. | Above-ground biomass (Mg ha−1) | 160.78 ± 22.71 | 91.86 ± 9.97 | 78.58 ± 10.62 |
| 9. | Below-ground biomass (Mg ha−1) | 51.45 ± 7.27 | 29.40 ± 3.19 | 25.15 ± 3.40 |
| 10. | Total biomass (Mg ha−1) | 212.23 ± 29.98 | 121.26 ± 13.16 | 103.73 ± 14.02 |
| 11. | Total biomass carbon stock (Mg ha−1) | 106.11 ± 14.99 | 60.63 ± 6.58 | 51.86 ± 7.01 |
| Spacing | Component | Rate of C Sequestration in Biomass (Mg ha−1 yr−1) | Total (A + B + C) | Carbon Sequestration Rate (Biomass + Soil) (Mg ha−1 yr−1) | ||
|---|---|---|---|---|---|---|
| Crop (A) | Tree (B) | Soil (C) | ||||
| S0 (Open) | 0.94 ± 0.21 | - | 31.15 ± 5.66 | 0.94 | 32.09 | 0.94 |
| S1 (10 m × 1 m) | 1.04 ± 0.20 | 106.11 ± 27.14 | 31.27 ± 4.19 | 6.63 | 138.42 | 6.64 |
| S2 (10 m × 2 m) | 1.06 ± 0.25 | 60.63 ± 25.49 | 32.55 ± 4.99 | 3.79 | 94.24 | 3.88 |
| S3 (10 m × 3 m) | 1.20 ± 0.42 | 51.86 ± 58.04 | 32.25 ± 3.46 | 3.25 | 85.31 | 3.32 |
| Treatment | Plant Height (cm) | Number of Branches | Days to 50% Flowering | Pod Length (cm) | Pod Diameter (mm) | Pod Weight (g) | 100-Seed Weight (g) |
|---|---|---|---|---|---|---|---|
| Tree Spacing | |||||||
| S0 (Open) | 44.61 b ± 4.47 | 2.83 b ± 0.41 | 96.33 b ± 3.03 | 9.57 ± 0.22 | 10.57 ± 0.26 | 5.48 b + 0.34 | 38.85 ± 1.63 |
| S1 (10 m × 1 m) | 47.03 b ± 4.49 | 2.91 b ± 0.31 | 98.72 b ± 1.14 | 9.64 ± 0.19 | 10.59 ± 0.19 | 5.73 b ± 0.34 | 39.24 ± 2.66 |
| S2 (10 m × 2 m) | 54.06 a± 4.17 | 3.89 a ±0.47 | 100.33 ab ± 2.37 | 9.66 ± 0.25 | 10.73 ± 0.25 | 5.94 ab ± 0.38 | 39.65 ± 2.03 |
| S3 (10 m × 3 m) | 55.08 a ± 2.47 | 3.90 a ± 0.52 | 103.17 a ± 3.32 | 9.79 ± 0.16 | 10.89 ± 0.37 | 6.29 a ± 0.15 | 40.68 ± 2.17 |
| CD0.05 | 5.43 | 0.45 | 4.39 | NS | NS | 0.53 | NS |
| Fertilizer Treatment | |||||||
| T0 (Control) | 48.23 b ± 4.69 | 3.15 ± 0.56 | 99.92 ± 2.30 | 9.37 c ± 0.12 | 10.32 b ± 0.03 | 5.54 b ± 0.55 | 38.18 ± 0.97 |
| T1 (FYM) | 55.29 a ± 1.73 | 3.57 ± 0.86 | 99.75 ± 1.62 | 9.63 ab ± 0.09 | 10.78 a ± 0.43 | 5.94 ab ± 0.42 | 40.38 ± 1.55 |
| T2 (VC) | 51.33 ab ± 8.43 | 3.28 ± 0.68 | 100.33 ± 6.02 | 9.68 ab ± 0.21 | 10.64 a ± 0.09 | 5.78 b ± 0.38 | 39.49 ± 1.33 |
| T3 (Jeevamrut) | 48.29 b ± 5.12 | 3.27 ± 0.95 | 100.33 ± 3.37 | 9.79 ab ± 0.14 | 10.63 ab ± 0.22 | 5.64 b ± 0.31 | 38.23 ± 1.49 |
| T4 (FYM + VC) | 49.02 b ± 6.73 | 3.42 ± 0.77 | 99.25 ± 3.03 | 9.92 a ± 0.06 | 11.01 a ± 0.16 | 6.32 a ± 0.21 | 41.11 ± 1.39 |
| T5(RDF) | 49.04 b ± 6.96 | 3.62 ± 0.35 | 98.25 ± 5.06 | 9.61 bc ± 0.15 | 10.79 a ± 0.15 | 5.93 ab ± 0.33 | 40.26 ± 3.96 |
| CD0.05 | 4.86 | NS | NS | 0.29 | 0.38 | 0.43 | NS |
| Treatment | Number of Grains Pod−1 | Number of Pods Plant−1 | Green Pod Yield Plant−1 (g) | Shelling Percentage (%) | Yield Per Hectare (t ha−1) | TSS (°Brix) |
|---|---|---|---|---|---|---|
| Tree Spacing | ||||||
| S0 (Open) | 7.45 b ± 0.18 | 17.17 a ± 2.85 | 69.67 c ± 15.6 | 46.48 ± 2.91 | 4.51 c ± 0.77 | 16.59 a ± 0.85 |
| S1 (10 m × 1 m) | 7.63 b ± 0.19 | 16.01 a ± 2.12 | 75.03 bc ± 9.52 | 47.02 ± 3.49 | 4.73 bc ± 0.67 | 17.24 a ± 1.26 |
| S2 (10 m × 2 m) | 7.86 ab ± 0.20 | 18.24 a ± 3.77 | 87.61 ab ± 20.75 | 48.02 ± 3.47 | 5.56 ab ± 0.77 | 17.28 a ± 0.56 |
| S3 (10 m × 3 m) | 8.10 a ± 0.33 | 21.53 ± 2.79 | 90.91 a ± 16.33 | 49.03 ± 4.5 | 5.72 a ± 0.74 | 18.08 ± 1.05 |
| CD0.05 | 0.43 | 2.93 | 15.64 | NS | 0.92 | 0.73 |
| Fertilizer Treatment | ||||||
| T0 (Control) | 7.47 c ± 0.24 | 15.40 c ± 3.07 | 65.92 c ± 2.98 | 44.24 c ± 1.70 | 4.47 b ± 1.13 | 16.23 b ± 0.61 |
| T1 (FYM) | 7.94 ab ± 0.46 | 22.17 a ± 3.91 | 102.32 a ± 20.45 | 49.35 ab ± 2.55 | 6.04 a ± 0.46 | 17.48 a ± 0.42 |
| T2 (VC) | 7.76 abc ± 0.18 | 17.93 abc ± 2.26 | 78.35 bc ± 14.04 | 46.75 bc ± 2.69 | 5.21 ab ± 1.09 | 17.77 a ± 1.41 |
| T3 (Jeevamrut) | 7.60 c ± 0.33 | 17.55 bc ± 1.20 | 72.52 bc ± 11.26 | 46.56 bc ± 2.22 | 4.60 b ± 0.37 | 18.02 a ± 0.83 |
| T4 (FYM + VC) | 7.96 a ± 0.37 | 19.93 abc ± 2.91 | 90.20 abc ± 13.29 | 50.73 ab ± 4.05 | 5.25 ab ± 0.73 | 17.30 ab ± 1.16 |
| T5(RDF) | 7.81 abc ± 0.20 | 16.45 bc ± 3.45 | 75.53 bc ± 15.34 | 48.21 ab ± 4.78 | 5.23 ab ± 0.58 | 16.98 ab ± 1.07 |
| CD0.05 | 0.28 | 4.40 | 18.98 | 3.88 | 0.85 | 1.15 |
| Treatment | pH | EC (dS m−1) | Bulk Density (gm cm−3) | Organic Carbon (%) | SOC (Mg ha−1) | Available N (kg ha−1) | Available P (kg ha−1) | Available K (kg ha−1) |
|---|---|---|---|---|---|---|---|---|
| Tree Spacing (A) | ||||||||
| S0 (Open) | 7.20 (+) 0.30 | 0.170 (+) 0.02 | 1.26 (−) 0.01 | 1.65 (+) 0.55 | 31.15 (+) 10.19 | 303.11 (+) 34.81 | 51.29 b (+) 20.79 | 198.99 b (+) 8.09 |
| S1 (10 m × 1 m) | 7.16 (+) 0.34 | 0.174 (+) 0.024 | 1.25 (−) 0.006 | 1.67 (+) 0.57 | 31.27 (+) 10.48 | 333.24 a (+) 24.94 | 59.74 ab (+) 24.64 | 202.91 b (+) 10.11 |
| S2 (10 m × 2 m) | 7.12 (+) 0.23 | 0.175 (+) 0.015 | 1.23 (−) 0.005 | 1.76 (+) 0.62 | 32.55(+) 11.35 | 334.30 a (+) 52.70 | 65.75 a (+) 25.05 | 239.34 ab (+) 42.44 |
| S3 (10 m × 3 m) | 7.08 (+) 0.35 | 0.207 (+) 0.050 | 1.22 (−) 0.024 | 1.77 (+) 0.57 | 32.25 (+) 9.93 | 348.82 a (+) 58.02 | 69.61 a(+) 23.31 | 253.03 a (+) 52.13 |
| CD0.05 | NS | NS | NS | NS | NS | 29.93 | 12.64 | 41.99 |
| Fertilizer Treatment (B) | ||||||||
| T0 (Control) | 7.13 (+) 0.29 | 0.153 (−) 0.002 | 1.27 a (+) 0.01 | 1.56 (+) 0.43 | 29.64 (+)8.33 | 298.30 (+) 11.05 | 51.14 c(+) 12.99 | 186.82 c (−) 8.56 |
| T1 (FYM) | 7.11 (+) 0.28 | 0.211 (+) 0.057 | 1.21 b (−) 0.05 | 1.90 (+) 0.76 | 34.32 (+) 13.0 | 347.82 a (+) 60.57 | 72.67 a(+) 34.52 | 252.43 a (+) 57.06 |
| T2 (VC) | 7.14 (+) 0.30 | 0.172 (+) 0.018 | 1.24 ab (−) 0.008 | 1.73 (+) 0.59 | 32.09 (+) 10.77 | 341.63 a (+) 54.38 | 62.90 b (+) 24.75 | 200.18 bc (+) 4.80 |
| T3 (Jeevamrut) | 7.20 (+) 0.36 | 0.164 (+) 0.010 | 1.26 a (+) 0.005 | 1.64 (+) 0.51 | 30.77 (+) 9.45 | 327.84 a (+) 40.59 | 53.47 c (+) 15.32 | 197.32 b (+) 1.94 |
| T4 (FYM+VC) | 7.11(+) 0.27 | 0.189 (+) 0.035 | 1.24 ab (−) 0.01 | 1.74 (+) 0.60 | 32.17 (+) 10.85 | 333.13 a (+) 45.88 | 65.28 ab (+) 27.13 | 266.05 a (+) 70.68 |
| T5(RDF) | 7.16 (+) 0.32 | 0.199 (+) 0.045 | 1.23 b (−) 0.02 | 1.72 (+) 0.59 | 31.83 (+) 10.51 | 330.48 a (+) 43.23 | 64.13 b (+) 25.98 | 238.63 ab (+) 43.25 |
| CD0.05 | NS | NS | 0.03 | NS | NS | 25.56 | 7.9 | 46.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keprate, A.; Bhardwaj, D.R.; Sharma, P.; Kumar, D.; Rana, R.K. Biomass Partitioning, Carbon Storage, and Pea (Pisum sativum L.) Crop Production under a Grewia optiva-Based Agroforestry System in the Mid-Hills of the Northwestern Himalayas. Sustainability 2024, 16, 7438. https://doi.org/10.3390/su16177438
Keprate A, Bhardwaj DR, Sharma P, Kumar D, Rana RK. Biomass Partitioning, Carbon Storage, and Pea (Pisum sativum L.) Crop Production under a Grewia optiva-Based Agroforestry System in the Mid-Hills of the Northwestern Himalayas. Sustainability. 2024; 16(17):7438. https://doi.org/10.3390/su16177438
Chicago/Turabian StyleKeprate, Alisha, Daulat Ram Bhardwaj, Prashant Sharma, Dhirender Kumar, and Rajesh Kumar Rana. 2024. "Biomass Partitioning, Carbon Storage, and Pea (Pisum sativum L.) Crop Production under a Grewia optiva-Based Agroforestry System in the Mid-Hills of the Northwestern Himalayas" Sustainability 16, no. 17: 7438. https://doi.org/10.3390/su16177438








