Investigating the Potential of Microbially Induced Carbonate Precipitation Combined with Modified Biochar for Remediation of Lead-Contaminated Loess
Abstract
1. Introduction
2. Testing Materials
2.1. Tested Soil
2.2. Red Mud, Biochar, and Modified Biochar
2.3. Bacteria and Cementation Solutions for MICP Treatment
3. Testing Methods
3.1. Specimen Preparation
3.2. Remediation Process for Lead-Contaminated Loess
3.3. Physicochemical Properties of Soil
3.3.1. Measurement of Soil pH
3.3.2. Measurement of Soil EC
3.4. Measurement of Physical and Mechanical Properties of Soil
3.4.1. Unconfined Compressive Strength (UCS) Test
3.4.2. Permeability Test
3.5. Toxic Leaching Test of Soil
3.6. Microstructure Characteristics of Soil
3.6.1. Zeta Potential Measurement
3.6.2. XRD Tests
3.6.3. SEM Tests
4. Results and Discussion
4.1. Response of Soil pH and EC Values
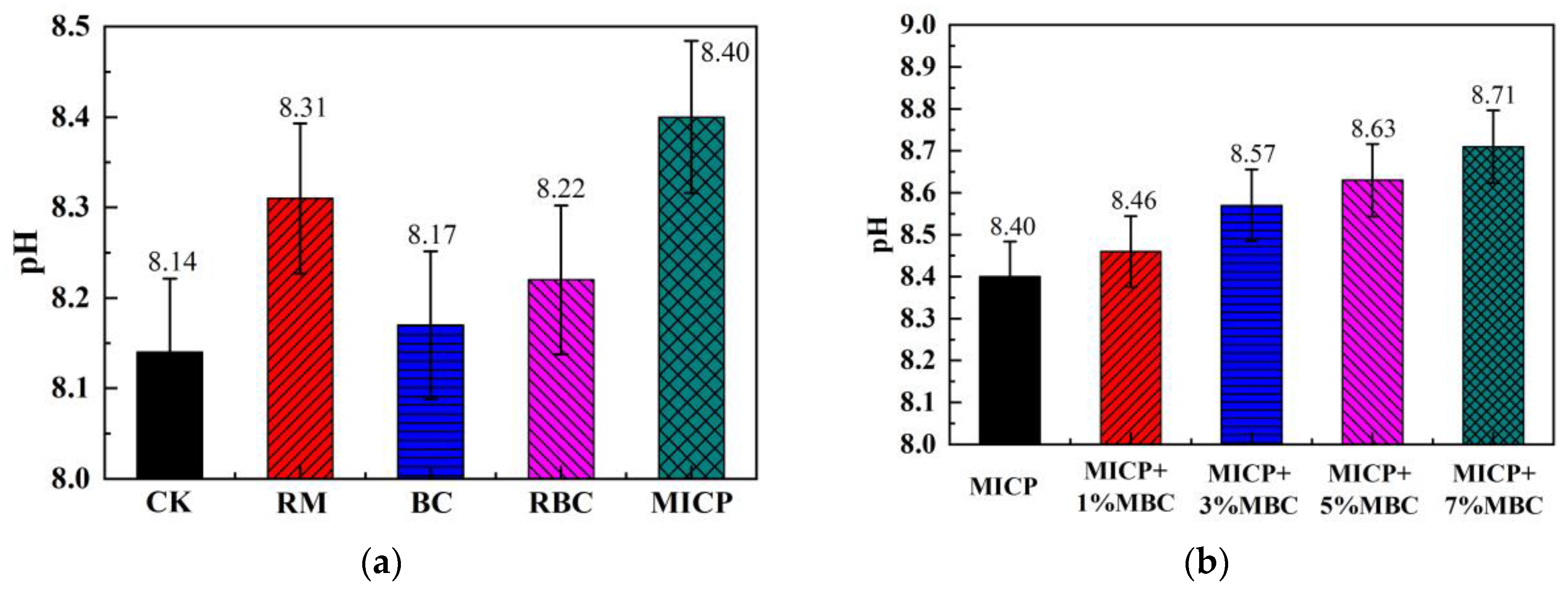
4.1.1. Soil pH
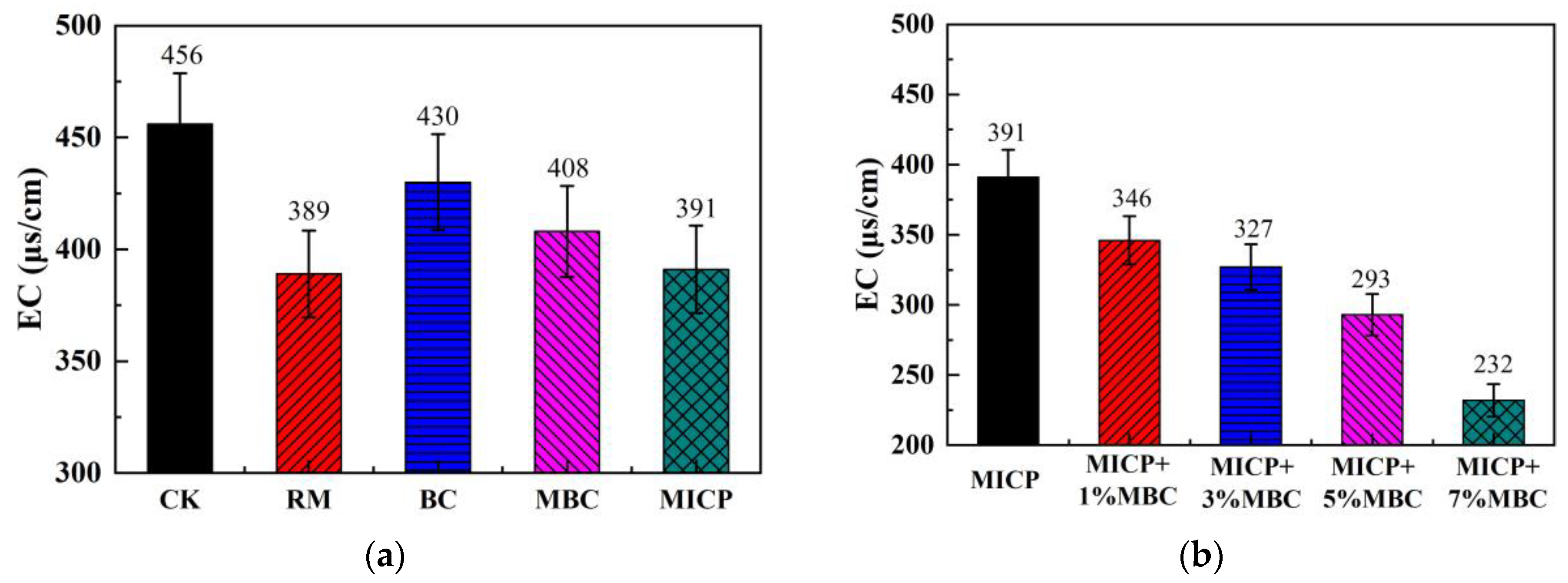
4.1.2. Soil EC
4.2. Response of Physical and Mechanical Properties
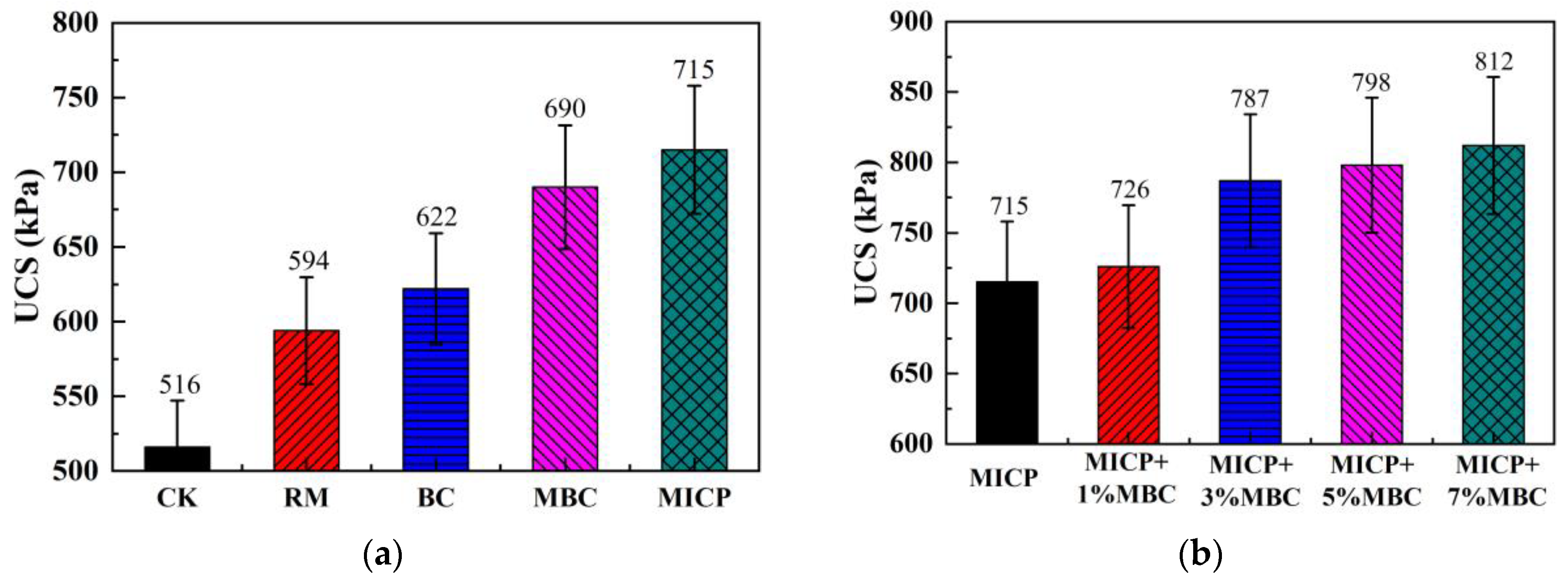
4.2.1. UCS
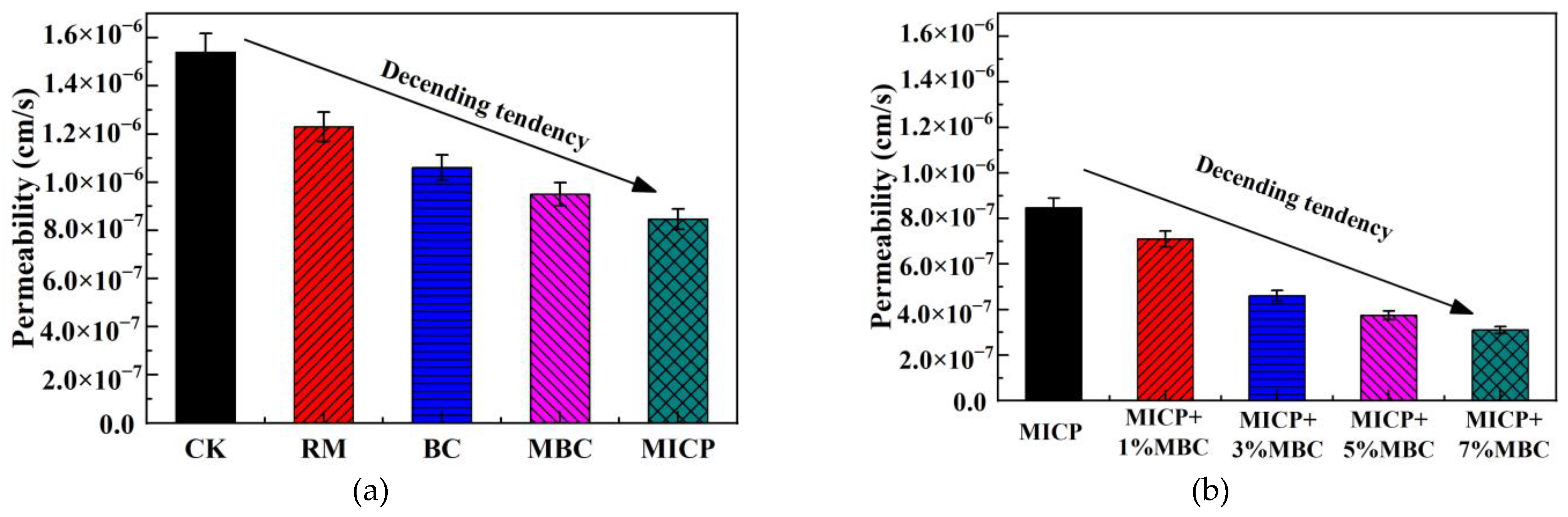
4.2.2. Permeability
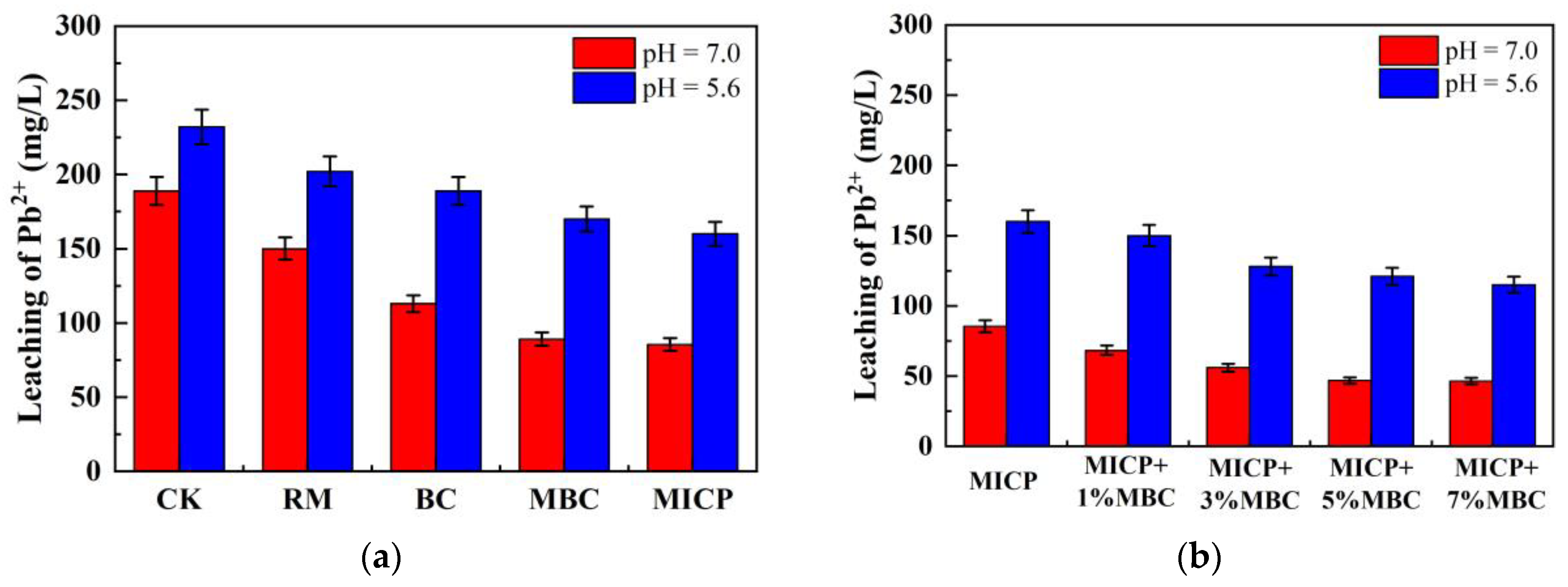
4.3. Response of Lead Ion Leachate Concentration
4.4. Analysis of Microscopic Results
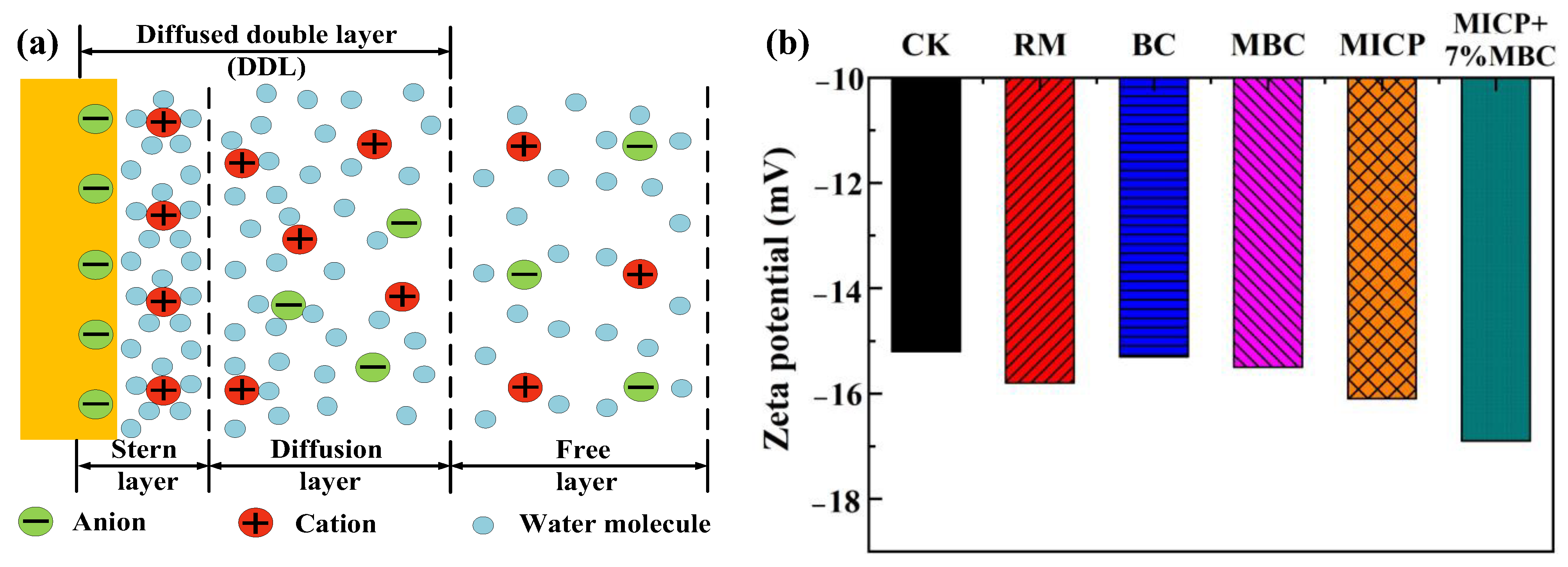
4.4.1. Zeta Potential Test Results
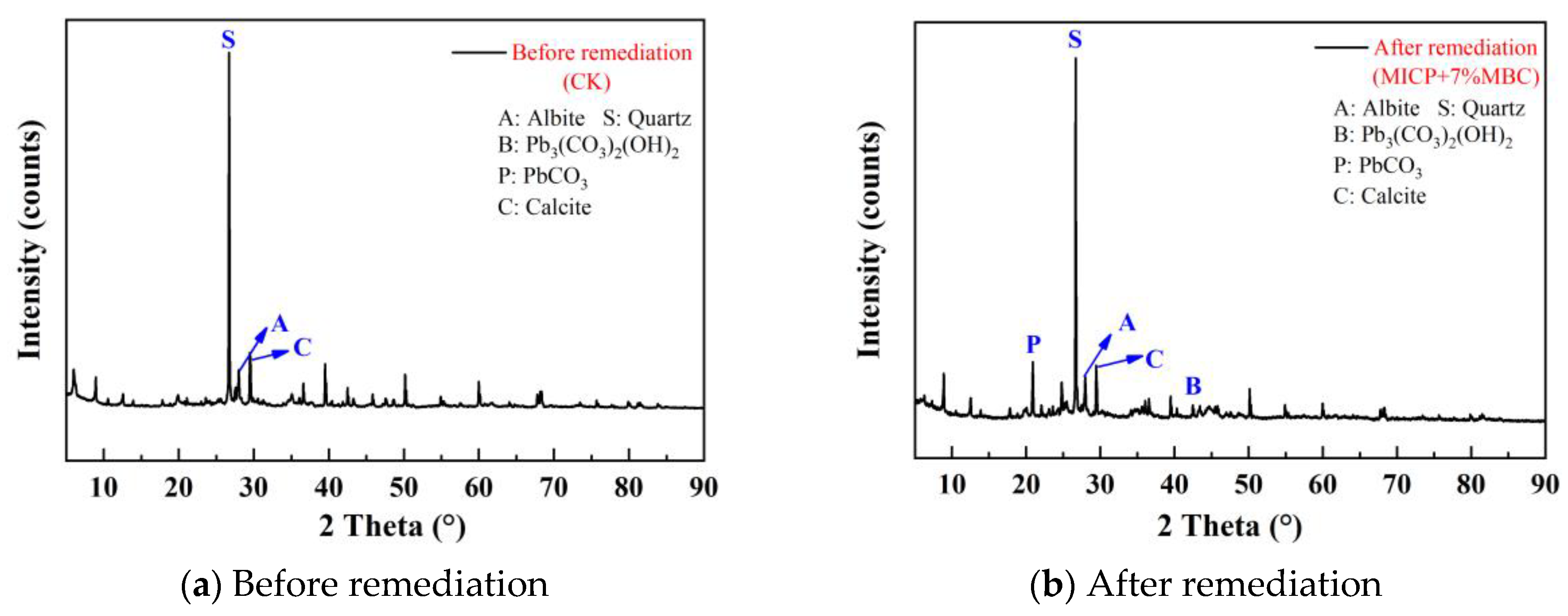
4.4.2. XRD Test Results
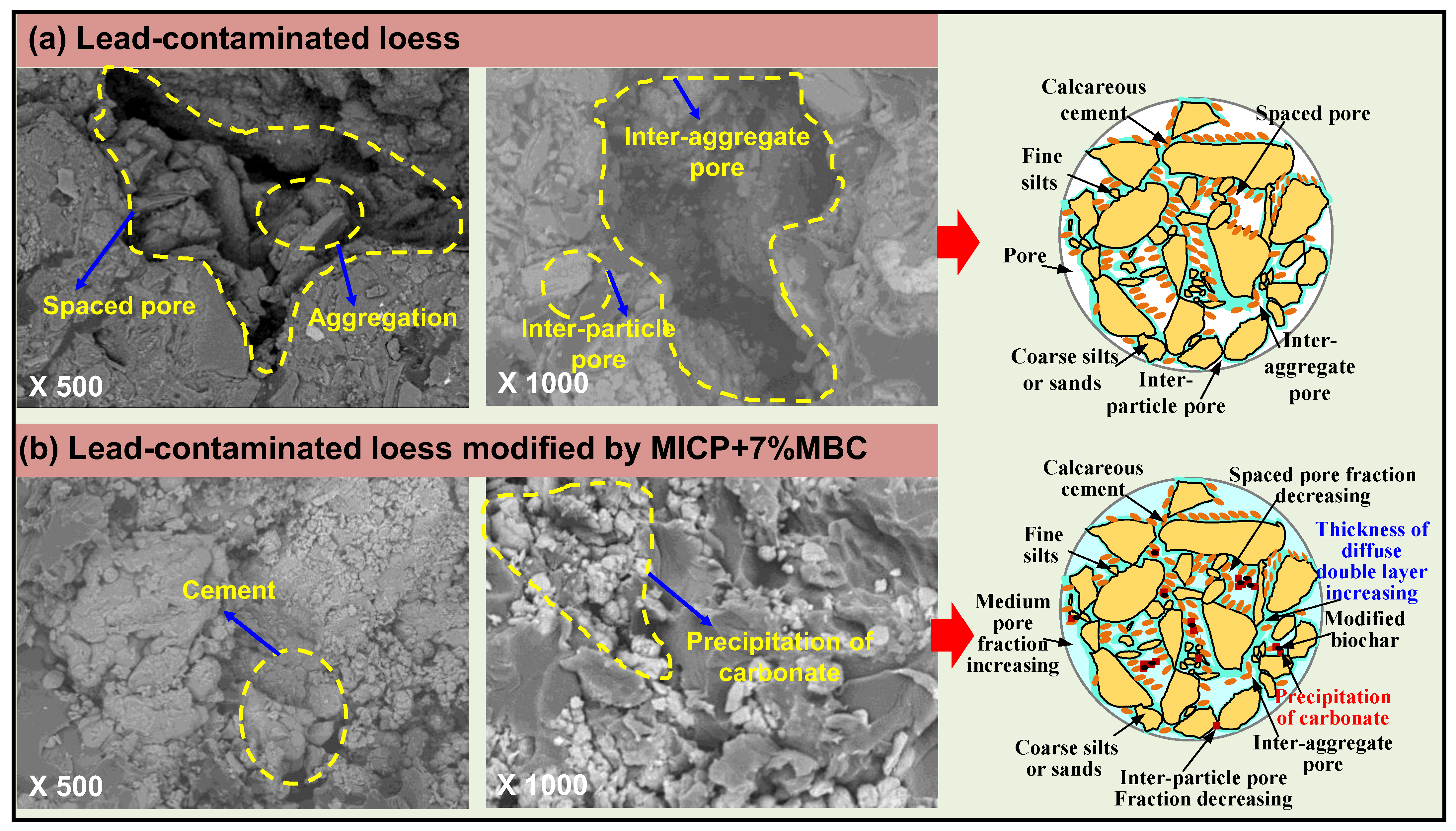
4.4.3. SEM Test Results
4.5. Discussion
- (1)
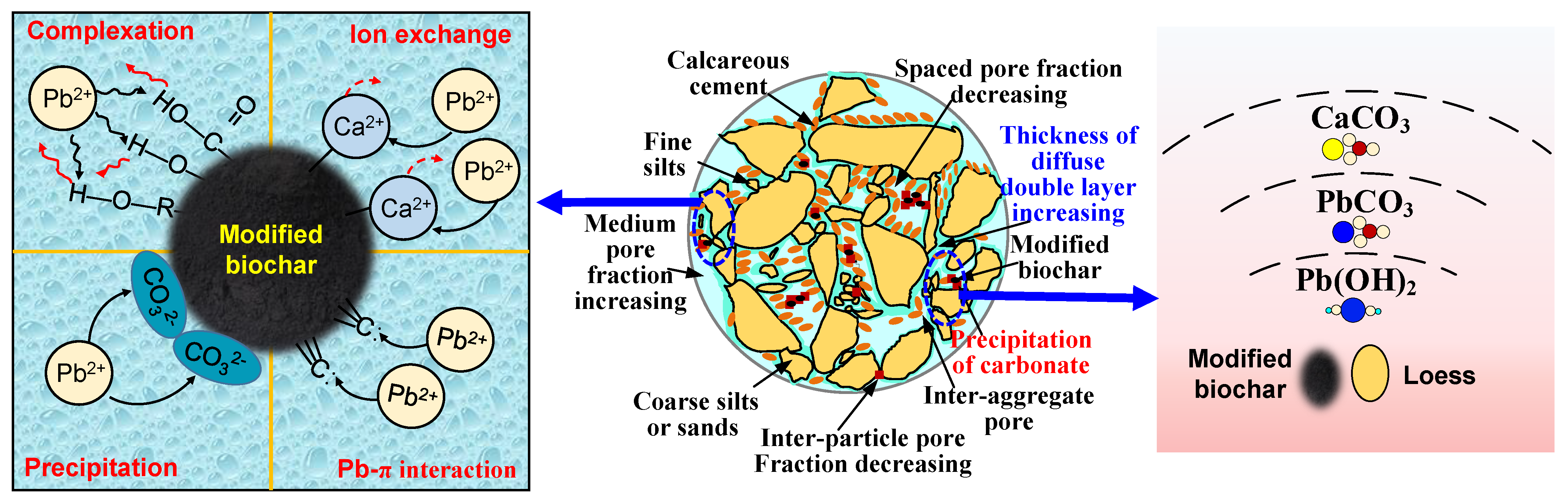
- MBC solidification of Pb2+: The solidification of Pb2⁺ by MBC occurs through several distinct mechanisms. Firstly, the alkaline nature of biochar elevates the pH of the soil, promoting the reaction between Pb2⁺ and hydroxide ions (OH⁻), which leads to the formation of lead hydroxide (Pb(OH)₂), an insoluble compound that significantly reduces the leaching of lead [52]. Secondly, MBC possesses a large specific surface area, enabling it to adsorb substantial amounts of free Pb2⁺. This adsorption capacity effectively immobilizes the lead ions within the soil matrix, further decreasing the leachate concentration of Pb2⁺.
- (2)
- Influence of MBC on MICP solidification of Pb2+: In the microbially induced calcite precipitation (MICP) process, the effectiveness of solidifying Pb2⁺ is often hindered by the lack of sufficient nucleation sites for free urease enzymes. This can result in the independent formation of calcium carbonate (CaCO₃) within pore spaces, which prevents the full integration of CaCO₃ with soil particles, thus limiting the solidification efficiency of metal ions [53]. MBC, due to its highly porous structure and strong adsorption capacity, introduces additional binding sites by adsorbing both the enzymes and the substrates needed for enzymatic reactions. This adsorption enhances the catalytic decomposition of urea by urease, allowing for more efficient CaCO₃ formation. Moreover, certain metal ions are adsorbed within the pores of MBC, where mineralization reactions occur, leading to localized high concentrations of metal ions. This environment promotes the timely solidification of metal ions through the MICP mineralization process. Other metal ions are either adsorbed within the porous network of MBC or are integrated into CaCO₃ precipitates formed during mineralization [53,54]. These multiple adsorption and mineralization pathways synergistically contribute to the effective immobilization and solidification of metal ions, resulting in successful remediation of Pb2⁺ contamination.
5. Conclusions
- (1)
- Compared to traditional RM and BC methods, MBC releases more hydroxide and carbonate ions, resulting in a higher pH, lower EC, and reduced leachate concentration. In comparison, MICP introduces even more carbonate ions through biomineralization, leading to further increases in pH and reductions in EC. Additionally, the large amounts of carbonate precipitates produced by MICP increase the density of lead-contaminated loess, enhancing UCS and lowering permeability, while also reducing leachate concentration.
- (2)
- The combination of MICP and MBC in lead-contaminated loess remediation leads to higher pH levels. Carbonate precipitates, along with MBC, fill loess pores, improving UCS and reducing permeability. As the MBC proportion increases, the soil becomes denser, enhancing its bearing capacity while further reducing permeability. This combined method effectively adsorbs and immobilizes lead ions, significantly decreasing their concentration in the leachate and achieving successful remediation.
- (3)
- The combined use of MICP and MBC elevates soil pH, creating an alkaline environment. This enhances the negative charge of MBC, promotes the hydrolysis of acidic functional groups on its surface, and increases cation-exchange site density. These changes facilitate ion exchange and complexation reactions between MBC and lead ions. Additionally, the increased negative charge in lead-contaminated loess promotes the further adsorption of lead ions, reducing their mobility and lowering the concentration of soluble lead ions in the loess.
- (4)
- The present study primarily focuses on the synergistic remediation of microbially induced calcite precipitation (MICP) and microbial biochar (MBC) in lead-contaminated loess. Consequently, the results are applicable solely to the behavior observed during the designated period of MICP remediation. The effects of remediation age and potential enhancements through the integration of other technologies have not been addressed in this work, which may limit the applicability of the findings to contaminated sites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adimalla, N. Heavy metals pollution assessment and its associated human health risk evaluation of urban soils from Indian cities: A review. Environ. Geochem. Health 2020, 42, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Ma, Y. Research progress of methods for determining sampling numbers of soil heavy metals survey. Trans. Chin. Soc. Agric. Eng. 2019, 35, 235–245. [Google Scholar]
- Odika, P.O.; Anike, O.L.; Onwuemesi, A.G.; Odika, N.F.; Ejeckam, R.B. Assessment of Environmental Geochemistry of Lead-Zinc Mining at Ishiagu Area, Lower Benue Trough, Southeastern Nigeria. Earth Sci. Res. 2020, 9, 1–31. [Google Scholar]
- Chen, Y.L.; Zuo, M.B.; Yang, D.; He, Y.Q.; Wang, H.M.; Liu, X.X.; Zhao, M.J.; Xu, L.L.; Ji, J.; Liu, Y.; et al. Synergistically effect of heavy metal resistant bacteria and plants on remediation of soil heavy metal pollution. Water Air Soil Pollut. 2024, 235, 296. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Q.W.; Liu, J.Y.; Pei, S.W.; Yang, Z.; Chen, R.T.; Ma, L.; Niu, J.P.; Tian, T. Bacterial-fungal interactions and response to heavy metal contamination of soil in agricultural areas. Front. Microbiol. 2024, 15, 1395154. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Guo, J.; Zhang, S. Feasibility of Microbially Induced Carbonate Precipitation to enhance the internal stability of loess under Zn-contaminated seepage. Buildings 2024, 14, 1230. [Google Scholar] [CrossRef]
- Słaba, M.; Długo’nski, J. Efficient Zn2+ and Pb2+ uptake by filamentous fungus Paecilomyces marquandii with engagement of metal hydrocarbonates precipitation. Int. Biodeterior. Biodegrad. 2011, 65, 954–960. [Google Scholar] [CrossRef]
- Lian, Q.; Yao, L.; Ahmad, Z.U.; Konggidinata, M.I.; Zappi, M.E.; Gang, D.D. Modeling mass transfer for adsorptive removal of Pb(II) onto phosphate modified ordered mesoporous carbon (OMC). J. Contam. Hydrol. 2020, 228, 103562. [Google Scholar] [CrossRef]
- Kalinitchenko, V.P.; Glinushkin, A.P.; Minkina, T.M.; Mandzhieva, S.S.; Sushkova, S.S.; Sushkova, V.A.; Il’ina, L.P.; Makarenkov, D.A. Chemical soil-biological engineering theoretical foundations, technical means, and technology for safe intrasoil waste recycling and long-term higher soil productivity. ACS Omega 2020, 5, 17553–17564. [Google Scholar] [CrossRef]
- Chen, G.N.; Yao, S.Y.; Wang, Y.; Li, Y.C.; Ke, H.; Chen, Y.M. Measurement of contaminant adsorption on soils using cycling modified column tests. Chemosphere 2022, 294, 133822. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Wu, B.R.; Chai, X.L. In situ remediation technology for heavy metal contaminated sediment: A review. Int. J. Environ. Res. Public Health 2022, 19, 16767. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Zhou, Q.; Zhao, J.K.; Wu, H.X.; Li, X.Y.; Liu, Y.B.; Le, Z.G. Performance and mechanism of siderite/zero-valent nickel composite material for removing Se(IV) from solution. J. Solid State Chem. 2024, 335, 124708. [Google Scholar] [CrossRef]
- Du, Y.J.; Zhou, Q.; Zhao, J.K.; Wu, H.X.; Li, X.Y.; Liu, Y.B.; Le, Z.G. Adsorptive removal of selenium(IV) from aqueous solution by ferrous hydroxide complex-zero nickel composites. Solvent Extr. Ion Exch. 2023, 41, 680–696. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, X.X.; Zhang, C.Y.; Hu, W.J.H.; Liu, Y.C.; Fu, X.Z.; Yao, J.; Sun, W. Adsorption and selective mechanism of Pb2+ and Cd2+ on the surface of calcined modified attapulgite. Seperation Purif. Technol. 2025, 353, 128377. [Google Scholar]
- Jiang, N.J.; Liu, R.; Du, Y.J.; Bi, Y.Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Unconfined compressive strength of MICP and EICP treated sands subjected to cycles of wetting-drying, freezing-thawing and elevated temperature: Experimental and EPR modelling. J. Rock Mech. Geotech. Eng. 2023, 5, 1226–1247. [Google Scholar] [CrossRef]
- Xu, F.L.; Wang, D.X. Review on soil solidification and heavy metal stabilization by microbial-induced carbonateprecipitation (MICP) technology. Geomicrobiol. J. 2023, 40, 503–518. [Google Scholar] [CrossRef]
- Ji, G.S.; Huan, C.C.; Zeng, Y.; Lyu, Q.Y.; Du, Y.L.; Liu, Y.; Xu, L.S.; He, Y.; Tian, X.P.; Yan, Z.Y. Microbiologically induced calcite precipitation (MICP) in situ remediated heavy metal contamination in sludge nutrient soil. J. Hazard. Mater. 2024, 473, 134600. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.W.; Mishra, S.; Zhang, W.; Zhang, Y.L.; Zhang, Q.R.; Yu, Z.G. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- He, Z.F.; Xu, Y.T.; Wang, W.Y.; Yang, X.L.; Jin, Z.Z.; Zhang, D.Y.; Pan, X.L. Synergistic mechanism and application of microbially induced carbonate precipitation (MICP) and inorganic additives for passivation of heavy metals in copper-nickel tailings. Chemosphere 2023, 311, 136981. [Google Scholar] [CrossRef]
- Li, C.; Tian, L.; Dong, C.H.; Zhang, Y.F.; Wang, Y.X. Experimental study on zinc-lead composite contaminated soil solidified/stabilized by MICP technology combined with porous silicon adsorption materials. Rock Soil Mech. 2022, 43, 307–316. [Google Scholar]
- Chen, M.J.; Cao, D.; Li, B.W.; Pang, H.; Zheng, C.L. Sodium citrate increases the aggregation capacity of calcium ions during microbial mineralization to accelerate the formation of calcium carbonate. Environ. Res. 2023, 224, 115479. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Jeong, S.W. Changes in the health of metal-contaminated soil before and after stabilization and solidification. Environ. Pollut. 2023, 331, 121929. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, K.; Wang, H.S.; Shi, B.; Tang, C.S. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2022, 9, 135–148. [Google Scholar] [CrossRef]
- Meng, Z.W.; Wu, J.W.; Huang, S.; Xin, L.; Zhao, Q. Competitive adsorption behaviors and mechanisms of Cd, Ni, and Cu by biochar when coexisting with microplastics under single, binary, and ternary systems. Sci. Total Environ. 2024, 913, 169524. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.B.; Tao, X.D.; Wang, H.; Li, W.K.; Ding, X.; Chu, H.Q. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.L.; Wang, L.; Liu, R.Q.; Sun, W.; He, D.D. Efficient removal of Pb(II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, L.Y.; Dapaah, M.F.; Niu, Q.J.; Cheng, L. Bio-remediation of heavy metal-contaminated soil by microbial-induced carbonate precipitation (MICP)—A critical review. Sustainability 2023, 15, 7622. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, Z.Z.; Lyu, Q.Y.; Wang, X.X.; Du, Y.L.; Huan, C.C.; Liu, Y.; Yan, Z.Y. Mechanism of microbiologically induced calcite precipitation for cadmium mineralization. Sci. Total Environ. 2022, 852, 158465. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Jia, X.X.; Shao, M.A. Loess thickness variations across the loess plateau of China. Surv. Geophys. 2018, 39, 715–727. [Google Scholar] [CrossRef]
- Xu, P.P.; Zhang, Q.Y.; Qian, H.; Li, M.N.; Yang, F.X. An investigation into the relationship between saturated permeability and microstructure of remolded loess: A case study from Chinese Loess Plateau. Geoderma 2021, 382, 114774. [Google Scholar] [CrossRef]
- Xu, P.P.; Qian, H.; Li, W.Q.; Ren, W.H.; Yang, F.X.; Wang, L.B. New insights into the seepage behavior of heavy metal-contaminated loess and its underlying geochemical mechanism. J. Hydrol. 2023, 620 Part B, 129476. [Google Scholar] [CrossRef]
- Hou, X.K.; Qi, S.W.; Li, T.L.; Guo, S.F.; Wang, Y.; Li, Y.; Zhang, L.X. Microstructure and soil-water retention behavior of compacted and intact silt loess. Eng. Geol. 2020, 277, 105814. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Ministry of Water Resources of the People’s Republic of China, Standard for Geotechnical Test Methods. China Planning Press: Beijing, China, 2019.
- D2487; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 2011.
- Li, R.B.; Zhang, T.G.; Liu, Y.; Lv, G.Z.; Xie, L.Q. Calcification-carbonation method for red mud processing. J. Hazard. Mater. 2016, 316, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.T.; Xu, X.; Niu, L.; Wang, Z.; Huang, X.G.; Zheng, C.L. Study on the mechanism and stability of microbially induced struvite precipitation (MISP) for enhanced immobilization of uranium. J. Clean. Prod. 2024, 449, 141536. [Google Scholar] [CrossRef]
- Feng, D.L.; Yu, Y.; Wang, J.; Fang, C.X.; Liang, S.H. Experimental study on shear and disintegration resistance of MICP-treated residual granite soil. Environ. Earth Sci. 2024, 83, 179. [Google Scholar] [CrossRef]
- Song, H.W.; Li, D.; Qiu, H.; Yu, Z.G.; Kumar, A.; Yan, X.X.; Hu, F.Y.; Wang, B.Y.; An, J. Microbial-induced carbonate precipitation effectively prevents Pb2+migration through the soil profile: Lab experiment and model simulation. Sci. Total Environ. 2024, 927, 172268. [Google Scholar] [CrossRef]
- GB 36600-2018; Ministry of Water Resources of the People’s Republic of China, Soil Environmental Quality/Risk Control Standard for Soil Contamination of Development Land. China Planning Press: Beijing, China, 2018.
- Lei, X.G. The types of loess pores in China and their relationship with collapsibility. Sci. Sin. Ser. B-Chem. Biol. Agric. Med. Earth Sci. 1988, 31, 1398–1410. [Google Scholar]
- Li, M.D.; Wen, K.J.; Li, Y.; Zhu, L.P. Impact of oxygen availability on microbially induced calcite precipitation (MICP) treatment. Geomicrobiol. J. 2018, 35, 15–22. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tang, J.; Li, K. Removal behavior of heavy metals from aqueous solutions via microbially induced carbonate precipitation driven by acclimatized Sporosarcina pasteurii. Appl. Sci. 2022, 12, 9958. [Google Scholar] [CrossRef]
- Lin, H.; Muhannad, S.; Derick, B.G.; Kavazanjian, J.R. Mechanical behavior of sands treated by microbially induced carbonate precipitation. J. Geotech. Geoenviron. Eng. 2016, 142, 015066. [Google Scholar] [CrossRef]
- Safaa, M.E. A critical review of microbially induced carbonate precipitation for soil stabilization: The global experiences and future prospective. Pedosphere 2023, 5, 717–730. [Google Scholar]
- Su, F.; Yang, Y.Y.; Qi, Y.; Zhang, H.N. Combining microbially induced calcite precipitation (MICP) with zeolite: A new technique to reduce ammonia emission and enhance soil treatment ability of MICP technology. J. Environ. Chem. Eng. 2022, 10, 107770. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jiang, N.J.; Saracho, A.C.; Doygun, O.; Du, Y.J.; Han, X.L. Compressibility characteristics of bio-cemented calcareous sand treated through the bio-stimulation approach. J. Rock Mech. Geotech. Eng. 2023, 2, 510–522. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, S.K.; Li, Z.Y.; Garg, A. Exploring the application of the MICP technique for the suppression of erosion in granite residual soil in Shantou using a rainfall erosion simulator. Acta Geotech. 2023, 18, 3273–3285. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Konstantinou, C.; Soga, K.; Biscontion, G.; Kabla, A.J. Use of microfluidic experiments to optimize MICP treatment protocols for effective strength enhancement of MICP-treated sandy soils. Acta Geotech. 2022, 17, 3817–3838. [Google Scholar] [CrossRef]
- Su, Y.H.; Luo, B.; Luo, Z.D.; Xu, F.; Huang, H.; Long, Z.W.; Shen, C.P. Mechanical characteristics and solidification mechanism of slag/fly ash-based geopolymer and cement solidified organic clay: A comparative study. Journal of Building Engineering 2023, 71, 106498. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Zhao, Y.; Pan, W.; Qu, J.; Yang, T.; Huang, X.; Liu, X.; Xu, N. Numerical simulation of forming MICP horizontal seepage reducing body in confined aquifer for deep excavation. Appl. Sci. 2023, 13, 601. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, Q.; Chen, X.J.; Zhang, L.M.; Zhou, C.; Wan, L. Experimental study on influence of microbial mineralization on permeability of clay. Adv. Sci. Technol. Water Resour. 2024, 44, 29–36. (In Chinese) [Google Scholar]
- Wen, K.; Li, Y.; Liu, S.; Bu, C.; Li, L. Development of an improved immersing method to enhance microbial induced calcite precipitation treated sandy soil through multiple treatments in low cementation media concentration. Geotech. Geol. Eng. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Yu, X.P.; Xiao, H.B.; Li, Z.Y.; Qian, J.F.; Luo, S.P.; Su, H.Y. Experimental study on microstructure of unsaturated expansive soil improved by MICP method. Appl. Sci. 2022, 12, 342. [Google Scholar] [CrossRef]
- Xing, W.; Zhou, F.; Zhu, R.; Wang, X.D.; Chen, T.Z. Performance and mechanism of Zn-contaminated soil through Microbe-Induced Calcium Carbonate Precipitation. Buildings 2023, 13, 1974. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.Q.; Lu, C.W.; Fang, H.; Huang, Y.X.; Chen, L.X.; Song, X.Y. Feasible utilization of waste limestone as a calcium source for microbially induced carbonate precipitation (MICP). Fermentation 2023, 9, 307. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.; Deng, W.; Wang, R.; Sun, X.; Lu, Z. Biocementation of pyrite tailings using microbially induced calcite carbonate precipitation. Molecules 2022, 27, 3608. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Chuan, X.; Yu, L. An indoor experimental study in the remediation of cadmium-contaminated red caly by immobilized microbial technology. J. Chang. River Sci. Res. Inst. 2024, 41, 106–113. (In Chinese) [Google Scholar] [CrossRef]
- Hadi, Z.S.; Saeed, K.A. Effect of microbial-induced calcite precipitation (MICP) on the strength of soil contaminated with lead nitrate. J. Mech. Behav. Mater. 2022, 31, 143–149. [Google Scholar] [CrossRef]
- Zha, F.; Wang, H.; Kang, B.; Liu, C.; Xu, L.; Tan, X. Improving the strength and leaching characteristics of Pb-contaminated silt through MICP. Crystals 2021, 11, 1303. [Google Scholar] [CrossRef]

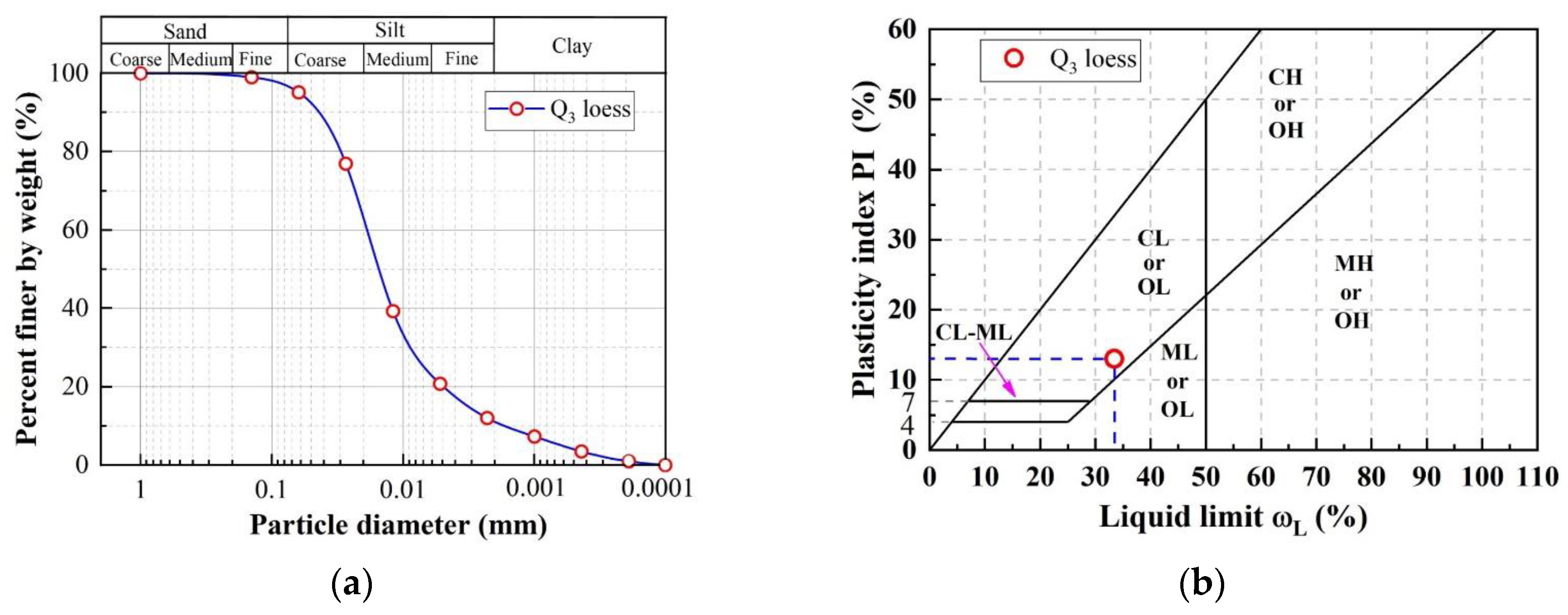
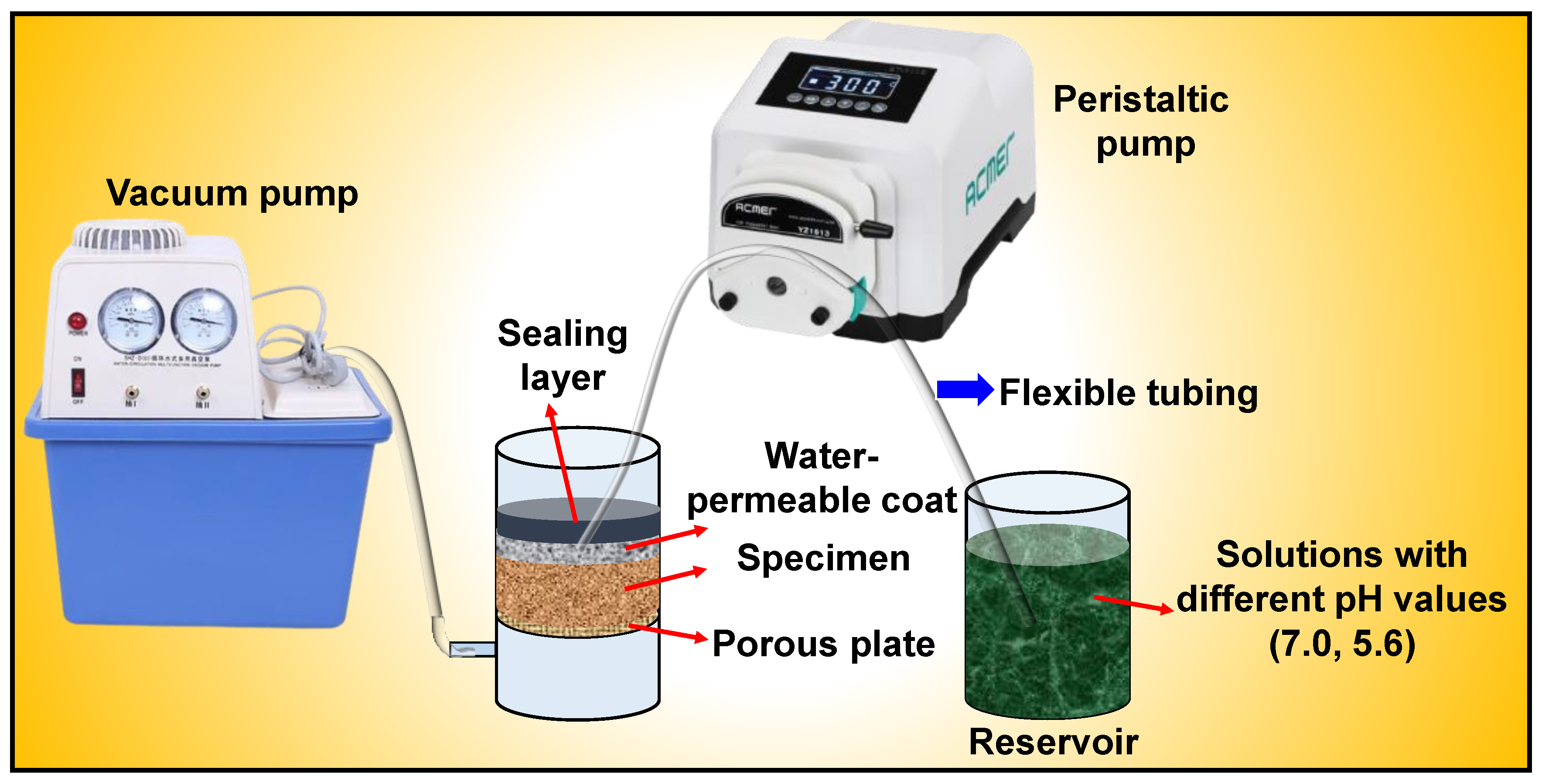

| Physical Index. | Data |
|---|---|
| Fines (%) | 91.18 |
| Sand (%) | 8.82 |
| Gravel (%) | 0 |
| Specific gravity, Gs | 2.72 |
| Void ratio, e | 0.88 |
| Dry density, ρdmax/(g/cm3) | 1.78 |
| Initial water content, ѡn/% | 16.4 |
| The Atterberg limit | |
| Liquid limit, ωL/% | 33.42 |
| Plastic limit, ωP/% | 20.43 |
| Soil classification | CL |
| Chemical Element | Loess (%) | Red Mud (%) |
|---|---|---|
| SiO2 | 55.08 | 31.14 |
| CaO | 14.47 | 18.31 |
| Al2O3 | 13.58 | 24.32 |
| Fe2O3 | 7.73 | 9.41 |
| MgO | 2.63 | 1.25 |
| K2O | 3.36 | 0.64 |
| Na2O | 1.41 | 9.60 |
| SO3 | - | 0.18 |
| Category | Materials | Incorporation Ratio (%) | Ration of MBC (%) | Treatment Time (d) | Influencing Factors |
|---|---|---|---|---|---|
| CK | CK | 3 | / | 30 | Material type |
| RM | RM | 3 | / | 30 | |
| BC | BC | 3 | / | 30 | |
| MBC | MBC | 3 | / | 30 | |
| MICP | MICP | 3 | / | 30 | |
| MICP + 1%MBC | MICP | 3 | 1 | 30 | Ration of MBC |
| MICP + 3%MBC | MICP | 3 | 3 | 30 | |
| MICP + 5%MBC | MICP | 3 | 5 | 30 | |
| MICP + 7%MBC | MICP | 3 | 7 | 30 |
| Contaminant | Initial Concentration (mg/kg) | Treatment Time (d) | Soil Type | UCS (kPa) | Leaching Concentration (mg/L) | References |
|---|---|---|---|---|---|---|
| Zn | 1000 | 10 | XinJiang soil | 625 | 105 | [55] |
| / | / | 1 | Sand | 730 | / | [56] |
| Mn | 836.62 | 45 | Pyrite tailings Sand | 587.86 | 13.68 | [57] |
| Cd | 1000 | 21 | Red clay | 229.7 | 0.002 | [58] |
| Pb | 400 | 28 | Sand | 512 | / | [59] |
| Pb | 100 | 14 | / | 630 | 0.5 | [60] |
| Pb | 3000 | 30 | Loess | 812 | 46.4 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Guo, J.; Zhang, S. Investigating the Potential of Microbially Induced Carbonate Precipitation Combined with Modified Biochar for Remediation of Lead-Contaminated Loess. Sustainability 2024, 16, 7550. https://doi.org/10.3390/su16177550
He P, Guo J, Zhang S. Investigating the Potential of Microbially Induced Carbonate Precipitation Combined with Modified Biochar for Remediation of Lead-Contaminated Loess. Sustainability. 2024; 16(17):7550. https://doi.org/10.3390/su16177550
Chicago/Turabian StyleHe, Pengli, Jinjun Guo, and Shixu Zhang. 2024. "Investigating the Potential of Microbially Induced Carbonate Precipitation Combined with Modified Biochar for Remediation of Lead-Contaminated Loess" Sustainability 16, no. 17: 7550. https://doi.org/10.3390/su16177550
APA StyleHe, P., Guo, J., & Zhang, S. (2024). Investigating the Potential of Microbially Induced Carbonate Precipitation Combined with Modified Biochar for Remediation of Lead-Contaminated Loess. Sustainability, 16(17), 7550. https://doi.org/10.3390/su16177550







