Can Ammoniacal Nitrogen from Gold Mining Effluent Be a Promising Alternative for Fertilizing Boreal Forest Stands?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Greenhouse Trials
- N0B0: control, without fertilization;
- N0B4: 12.8 g of anhydrous biochar (corresponding to 4090 kg·ha−1; according to Hart and Luckai [44], this represents the maximum concentration of biochar in the natural boreal forest in North America). The biochar used was chemically composed of C (75.4%), H (3.5%), N (0.9%), S (0.5%), and O (19.7%);
- N2B0: 14.4 mL of 21% ammonium sulfate solution (corresponding to 200 kg N·ha−1);
- N4B0: 28.8 mL of 21% ammonium sulfate solution (corresponding to 400 kg N·ha−1);
- N2B4: 12.8 g of biochar and 14.4 mL of 21% ammonium sulfate solution.

2.2.1. Plant Monitoring
2.2.2. Soil Measurements
2.3. Field Plantation Experiment
2.3.1. Study Area and Experiment Setup
2.3.2. Measurements and Monitoring
2.4. Statistical Analyses
3. Results
3.1. Greenhouse
3.1.1. Growth
3.1.2. Biomass
3.1.3. Soil Properties
3.2. Field Experiment
3.2.1. Growth
3.2.2. Soil Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doucet, R.; Côté, M. Manuel de Foresterie; Éditions MultiMondes: Quebec, QC, Canada, 2009; ISBN 9782895441380. Available online: https://editionsmultimondes.com/livre/manuel-de-foresterie/ (accessed on 20 May 2023).
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef]
- Zhao, P.; Chi, J.; Nilsson, M.B.; Löfvenius, M.O.; Högberg, P.; Jocher, G.; Lim, H.; Mäkelä, A.; Marshall, J.; Ratcliffe, J.; et al. Long-term nitrogen addition raises the annual carbon sink of a boreal forest to a new steady-state. Agric. For. Meteorol. 2022, 324, 109112. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Gearhart, M.M.; Villagarcía, S. Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: A review. Soil Sci. 2011, 176, 327–335. [Google Scholar] [CrossRef]
- Chien, S.H.; Gearhart, M.M.; Collamer, D.J. The effect of different ammonical nitrogen sources on soil acidification. Soil Sci. 2008, 173, 544–551. [Google Scholar] [CrossRef]
- Tamm, C.O.; Aronsson, A.; Popovic, B.; Flower-Ellis, J. Optimum Nutrition and Nitrogen Saturation in Scots Pine Stands; SLU Publication: Uppsala, Sweden, 1999; ISBN 0039-3150. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S. Influence of nitrogen fertilization on abundance and diversity of plants and animals in temperate and boreal forests. Environ. Rev. 2018, 26, 26–42. [Google Scholar] [CrossRef]
- Pukkala, T. Optimal nitrogen fertilization of boreal conifer forest. For. Ecosyst. 2017, 4, 2–11. [Google Scholar] [CrossRef]
- Revey, G.F. Practical methods to control explosive losses and reduce ammonia and nitrate levels in mine water. Min. Eng. 1996, 48, 61–64. [Google Scholar]
- Bailey, B.L.; Smith, L.J.D.; Blowes, D.W.; Ptacek, C.J.; Smith, L.; Sego, D.C. The Diavik Waste Rock Project: Persistence of contaminants from blasting agents in waste rock effluent. Appl. Geochem. 2013, 36, 256–270. [Google Scholar] [CrossRef]
- Moloantoa, K.M.; Khetsha, Z.P.; van Heerden, E.; Castillo, J.C.; Cason, E.D. Nitrate Water Contamination from Industrial Activities and Complete Denitrification as a Remediation Option. Water 2022, 14, 799. [Google Scholar] [CrossRef]
- Jermakka, J.; Wendling, L.; Sohlberg, E.; Heinonen, H.; Vikman, M. Potential Technologies for the Removal and Recovery of Nitrogen Compounds From Mine and Quarry Waters in Subarctic Conditions. Crit. Rev. Environ. Sci. Technol. 2015, 45, 703–748. [Google Scholar] [CrossRef]
- Zuttah, Y. Destruction de L’ammoniaque dans les Effluents Miniers. Master’s Thesis, Université Laval, Québec, QC, Canada, 1999. [Google Scholar]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Robert, É.; Braghiroli, F.L. Development of a Biochar-Based Substrate Added with Nitrogen from a Mining Effluent for the Production of Picea mariana Seedlings. Clean Technol. 2022, 4, 770–784. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönen, E. Forest fertilization research in Finland: A literature review. Scand. J. For. Res. 2001, 16, 514–535. [Google Scholar] [CrossRef]
- Hof, A.R.; Girona, M.M.; Fortin, M.-J.; Tremblay, J.A. Editorial: Using Landscape Simulation Models to Help Balance Conflicting Goals in Changing Forests. Front. Ecol. Evol. 2021, 9, 795736. [Google Scholar] [CrossRef]
- Girona, M.M.; Aakala, T.; Aquilué, N.; Bélisle, A.-C.; Chaste, E.; Danneyrolles, V.; Díaz-Yáñez, O.; D’Orangeville, L.; Grosbois, G.; Hester, A.; et al. Challenges for the Sustainable Management of the Boreal Forest Under Climate Change. In Boreal Forests in the Face of Climate Change; Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023; Volume 74, pp. 773–837. ISBN 978-3-031-15987-9. [Google Scholar]
- Achim, A.; Moreau, G.; Coops, N.C.; Axelson, J.N.; Barrette, J.; Bédard, S.; Byrne, K.E.; Caspersen, J.; Dick, A.R.; D’Orangeville, L.; et al. The changing culture of silviculture. For. Int. J. For. Res. 2022, 95, 143–152. [Google Scholar] [CrossRef]
- Girona, M.M.; Morin, H.; Gauthier, S.; Bergeron, Y. (Eds.) Boreal Forests in the Face of Climate Change; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023; Volume 74, ISBN 978-3-031-15987-9. [Google Scholar]
- Paré, D.; Rochon, P.; Brais, S. Assessing the geochemical balance of managed boreal forests. Ecol. Indic. 2002, 1, 293–311. [Google Scholar] [CrossRef]
- Barrette, M.; Leblanc, M.; Thiffault, N.; Paquette, A.; Lavoie, L.; Bélanger, L.; Bujold, F.; Côté, L.; Lamoureux, J.; Schneider, R.; et al. Issues and solutions for intensive plantation silviculture. For. Chron. 2014, 90, 748–762. [Google Scholar] [CrossRef]
- Messier, C.; Tittler, R.; Kneeshaw, D.D.; Gélinas, N.; Paquette, A.; Berninger, K.; Rheault, H.; Meek, P.; Beaulieu, N. TRIAD zoning in Quebec: Experiences and results after 5 years. For. Chron. 2009, 85, 885–896. [Google Scholar] [CrossRef]
- Maynard, D.G.; Paré, D.; Thiffault, E.; Lafleur, B.; Hogg, K.E.; Kishchuk, B. How do natural disturbances and human activities affect soils and tree nutrition and growth in the Canadian boreal forest?1. Environ. Rev. 2014, 22, 161–178. [Google Scholar] [CrossRef]
- Raymond, P.; Löf, M.; Comeau, P.; Rytter, L.; Girona, M.M.; Puettmann, K.J. Silviculture of Mixed-Species and Structurally Complex Boreal Stands. In Boreal Forests in the Face of Climate Change; Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y., Eds.; Advances in Global Change Research; Springer International Publishing: Cham, Switzerland, 2023; Volume 74, pp. 403–416. ISBN 978-3-031-15987-9. [Google Scholar]
- Gabira, M.M.; da Silva, R.B.G.; Bortolheiro, F.P.d.A.P.; Mateus, C.d.M.D.; Villas Boas, R.L.; Rossi, S.; Girona, M.M.; da Silva, M.R. Composted sewage sludge as an alternative substrate for forest seedlings production. IForest 2021, 14, 569–575. [Google Scholar] [CrossRef]
- Lévesque, V.; Oelbermann, M.; Ziadi, N. Biochar in temperate soils: Opportunities and challenges. Can. J. Soil Sci. 2020, 102, 1–26. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: A review and meta-analysis of tree growth responses. New For. 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Sackett, T.E.; Basiliko, N.; Noyce, G.L.; Winsborough, C.; Schurman, J.; Ikeda, C.; Thomas, S.C. Soil and greenhouse gas responses to biochar additions in a temperate hardwood forest. GCB Bioenergy 2015, 7, 1062–1074. [Google Scholar] [CrossRef]
- Pluchon, N.; Vincent, A.G.; Gundale, M.J.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. The impact of charcoal and soil mixtures on decomposition and soil microbial communities in boreal forest. Appl. Soil Ecol. 2016, 99, 40–50. [Google Scholar] [CrossRef]
- Palviainen, M.; Aaltonen, H.; Laurén, A.; Köster, K.; Berninger, F.; Ojala, A.; Pumpanen, J. Biochar amendment increases tree growth in nutrient-poor, young Scots pine stands in Finland. For. Ecol. Manag. 2020, 474, 118362. [Google Scholar] [CrossRef]
- Grau-Andrés, R.; Pingree, M.R.A.; Öquist, M.G.; Wardle, D.A.; Nilsson, M.C.; Gundale, M.J. Biochar increases tree biomass in a managed boreal forest, but does not alter N2O, CH4, and CO2 emissions. GCB Bioenergy 2021, 13, 1329–1342. [Google Scholar] [CrossRef]
- Deng, Z.; van Linden, N.; Guillen, E.; Spanjers, H.; van Lier, J.B. Recovery and applications of ammoniacal nitrogen from nitrogen-loaded residual streams: A review. J. Environ. Manag. 2021, 295, 113096. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.L.; Bouafif, H.; Hamza, N.; Bouslimi, B.; Neculita, C.M.; Koubaa, A. The influence of pilot-scale pyro-gasification and activation conditions on porosity development in activated biochars. Biomass Bioenergy 2018, 118, 105–114. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Oudad, M.A.; Kumar, P.; Chaali, M.; Brar, S.K.; Ramirez, A.A. Optimized ammonium sulphate recovery by stripping-scrubbing sequence system from compost leachate at mesophilic temperatures. Case Stud. Chem. Environ. Eng. 2022, 5, 100198. [Google Scholar] [CrossRef]
- Boivin, J.R.; Salifu, K.F.; Timmer, V.R. Late-season fertilization of Picea mariana seedlings: Intensive loading and outplanting response on greenhouse bioassays. Ann. For. Sci. 2004, 61, 737–745. [Google Scholar] [CrossRef]
- Hart, S.A.; Luckai, N.J. Charcoal carbon pool in North American boreal forests. Ecosphere 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Pluchon, N.; Gundale, M.J.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Stimulation of boreal tree seedling growth by wood-derived charcoal: Effects of charcoal properties, seedling species and soil fertility. Funct. Ecol. 2014, 28, 766–775. [Google Scholar] [CrossRef]
- White, J.W.; Mastalerz, J.W. Soil moisture as related to container capacity. Proc. Am. Soc. Hortic. Sci. 1996, 121, 758–765. [Google Scholar]
- Salifu, K.F.; Timmer, V.R. Nitrogen Retranslocation Response of Young Picea mariana to Nitrogen-15 Supply. Soil Sci. Soc. Am. J. 2003, 67, 309–317. [Google Scholar] [CrossRef]
- Timmer, V.R.; Teng, Y. Pretransplant fertilization of containerized Picea mariana seedlings: Calibration and bioassay growth response. Can. J. For. Res. 2004, 34, 2089–2098. [Google Scholar] [CrossRef]
- Sharifi, M.; Lynch, D.H.; Zebarth, B.J.; Zheng, Z.; Martin, R.C. Evaluation of nitrogen supply rate measured by in situ placement of Plant Root SimulatorTM probes as a predictor of nitrogen supply from soil and organic amendments in potato crop. Am. J. Potato Res. 2009, 86, 356–366. [Google Scholar] [CrossRef]
- Centre d’expertise en analyse environnementale du Québec. Détermination de L’AZOTE TOTAL KJELDAHL et du Phosphore Total: Digestion Acide—Méthode Colorimétrique Automatisée; Québec, Canada, 2014; pp. 1–16. Available online: http://www.ceaeq.gouv.qc.ca/methodes/pdf/MA300NTPT20.pdf (accessed on 20 May 2023).
- Centre d’expertise en analyse environnementale du Québec. Détermination des Métaux: Méthode par Spectrométrie de Masse à Source Ionisante au Plasma d’Argon; Quebec, Canada, 2023; Available online: https://www.ceaeq.gouv.qc.ca/methodes/pdf/methode-analyse-200-metaux.pdf (accessed on 20 May 2023).
- Béland, M.; Bergeron, Y. Height growth of jack pine ( Pinus hanksiana ) in relation to site types in boreal forests of Abitibi, Quebec. Can. J. For. Res. 1996, 26, 2170–2179. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 7 June 2022).
- Carvalho, F.P. Mining industry and sustainable development: Time for change. Food Energy Secur. 2017, 6, 61–77. [Google Scholar] [CrossRef]
- Högberg, P.; Fan, H.; Quist, M.; Binkley, D.; Tamm, C.O. Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob. Chang. Biol. 2006, 12, 489–499. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, F.; Hobbie, E.A.; Zhu, W.; Li, S.; Gurmesa, G.A.; Wang, A.; Fang, X.; Zhu, J.; Gundersen, P.; et al. Effects of nitrogen deposition on carbon allocation between wood and leaves in temperate forests. Plants People Planet 2022, 5, 267–280. [Google Scholar] [CrossRef]
- Henneb, M.; Thiffault, N.; Valeria, O. Regional climate, edaphic conditions and establishment substrates interact to influence initial growth of black spruce and jack pine planted in the boreal forest. Forests 2020, 11, 139. [Google Scholar] [CrossRef]
- Hamilton, W.N.; Krause, H.H. Relationship between jack pine growth and site variables in New Brunswick plantations. Can. J. For. Res. 1985, 15, 922–926. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Biochar enhances the retention capacity of nitrogen fertilizer and affects the diversity of nitrifying functional microbial communities in karst soil of southwest China. Ecotoxicol. Environ. Saf. 2021, 226, 112819. [Google Scholar] [CrossRef]
- Peng, J.; Han, X.; Li, N.; Chen, K.; Yang, J.; Zhan, X.; Luo, P.; Liu, N. Combined application of biochar with fertilizer promotes nitrogen uptake in maize by increasing nitrogen retention in soil. Biochar 2021, 3, 367–379. [Google Scholar] [CrossRef]
- Morris, D.M.; Reid, D.E.B.; Kwiaton, M.; Hunt, S.L.; Gordon, A.M. Comparing growth patterns of jack pine and black spruce in mixed natural stands and plantations. Ecoscience 2014, 21, 1–10. [Google Scholar] [CrossRef]
- Subedi, A.; Marchand, P.; Bergeron, Y.; Morin, H.; Girona, M.M. Climatic conditions modulate the effect of spruce budworm outbreaks on black spruce growth. Agric. For. Meteorol. 2023, 339, 109548. [Google Scholar] [CrossRef]
- Newton, P.F.; Amponsah, I.G. Systematic review of short-term growth responses of semi-mature black spruce and jack pine stands to nitrogen-based fertilization treatments. For. Ecol. Manag. 2006, 237, 1–14. [Google Scholar] [CrossRef]
- Sheedy, G. La Fertilisation des Plantations. Résultats de Dix ans Pour 34 Plantations; résineuses ministère des fôrets du Québec, Direction de la recherche, Note de recherche n° 52 forestière; Gouvernement du Québec: Québec, QC, Canada, 1993; Volume 18. [Google Scholar]
- Houle, D.; Moore, J.D.; Ouimet, R.; Marty, C. Tree species partition N uptake by soil depth in boreal forests. Ecology 2014, 95, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Brockley, R.P.; Simpson, D.G. Effects of Intensive Fertilization on the Foliar Nutrition and Growth of Young Lodgepole Pine and Spruce Forests in the Interior of British Columbia (E.P. 886.13); B.C. Ministry of Forests: Victoria, BC, Canada, 2004. [Google Scholar]
- Templer, P.H.; Mack, M.C.; Chapin, F.S.; Christenson, L.M.; Compton, J.E.; Crook, H.D.; Currie, W.S.; Curtis, C.J.; Dail, D.B.; D’Antonio, C.M.; et al. Sinks for nitrogen inputs in terrestrial ecosystems: A meta-analysis of 15N tracer field studies. Ecology 2012, 93, 1816–1829. [Google Scholar] [CrossRef]
- Quoreshi, M.; Timmer, V.R. Growth, nutrient dynamics, and ectomycorrhizal development of container-grown Picea mariana seedlings in response to exponential nutrient loading. Can. J. For. Res. 2000, 30, 191–201. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Lupi, C. Role of Soil Nitrogen for the Conifers of the Boreal Forest: A Critical Review. Int. J. Plant Soil Sci. 2013, 2, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Houle, D. Forest floor gross and net nitrogen mineralization in three forest types in Quebec, Canada. Soil Biol. Biochem. 2006, 38, 2135–2143. [Google Scholar] [CrossRef]
- Marty, C.; Piquette, J.; Dussault-Chouinard, É.; Morin, H.; Thiffault, N.; Houle, D.; Bradley, R.L.; Ouimet, R.; Simpson, M.J.; Paré, M.C. Canopy Nitrogen Addition and Soil Warming Affect Conifer Seedlings’ Phenology but Have Limited Impact on Growth and Soil N Mineralization in Boreal Forests of Eastern Canada. Front. For. Glob. Chang. 2020, 3, 581363. [Google Scholar] [CrossRef]
- Lam, K.L.; Zlatanović, L.; van der Hoek, J.P. Life cycle assessment of nutrient recycling from wastewater: A critical review. Water Res. 2020, 173, 115519. [Google Scholar] [CrossRef] [PubMed]
- Kyttä, V.; Helenius, J.; Tuomisto, H.L. Carbon footprint and energy use of recycled fertilizers in arable farming. J. Clean. Prod. 2021, 287, 125063. [Google Scholar] [CrossRef]
- González-Cencerrado, A.; Ranz, J.P.; López-Franco Jiménez, M.T.; Gajardo, B.R. Assessing the environmental benefit of a new fertilizer based on activated biochar applied to cereal crops. Sci. Total Environ. 2020, 711, 134668. [Google Scholar] [CrossRef]
- Basosi, R.; Spinelli, D.; Fierro, A.; Jez, S. Mineral nitrogen fertilizers: Environmental impact of production and use. In Fertilizers: Components, Uses in Agriculture and Environmental Impacts; NOVA Science Publishers: Hauppauge, NY, USA, 2014; pp. 3–43. [Google Scholar]

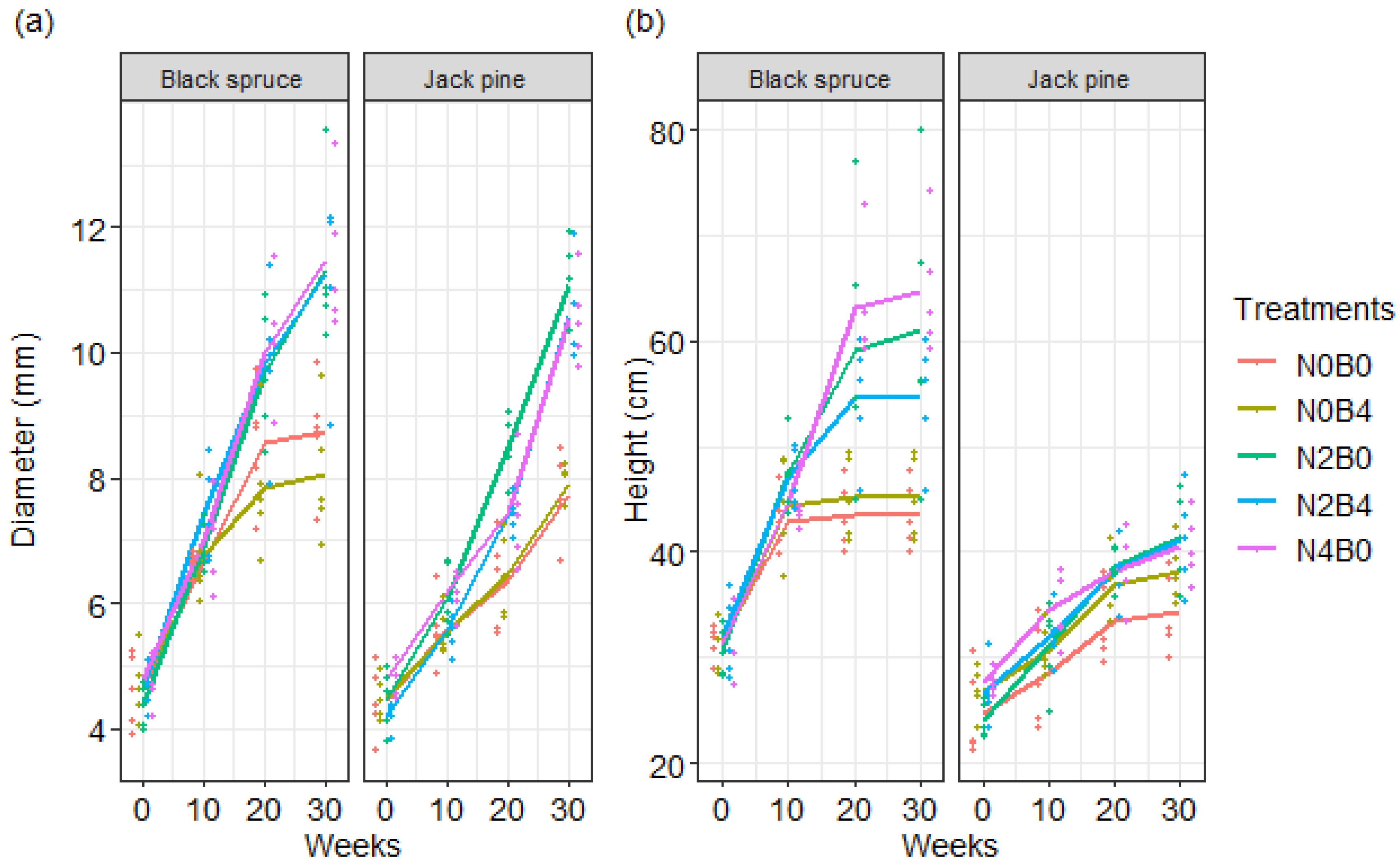
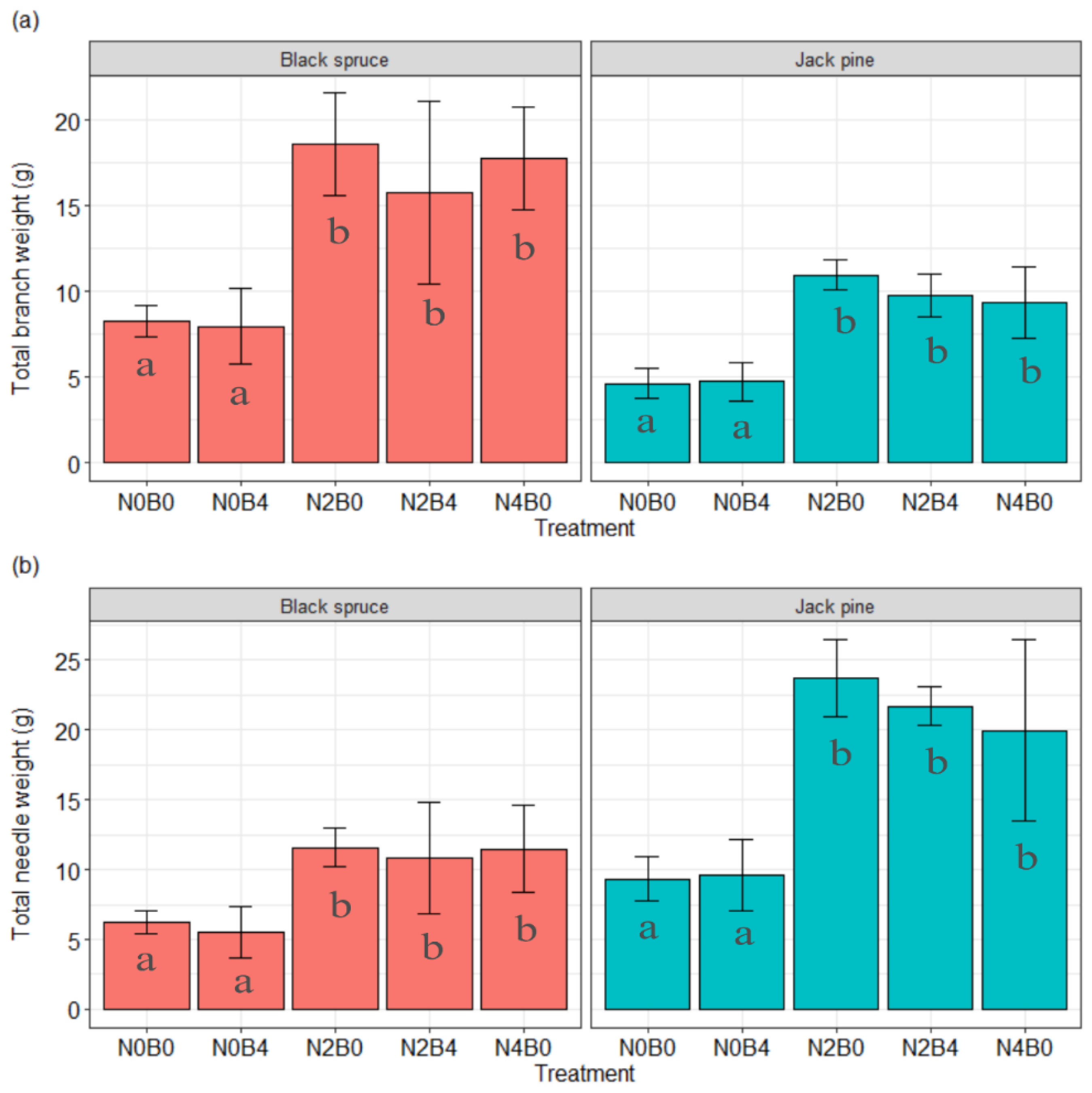
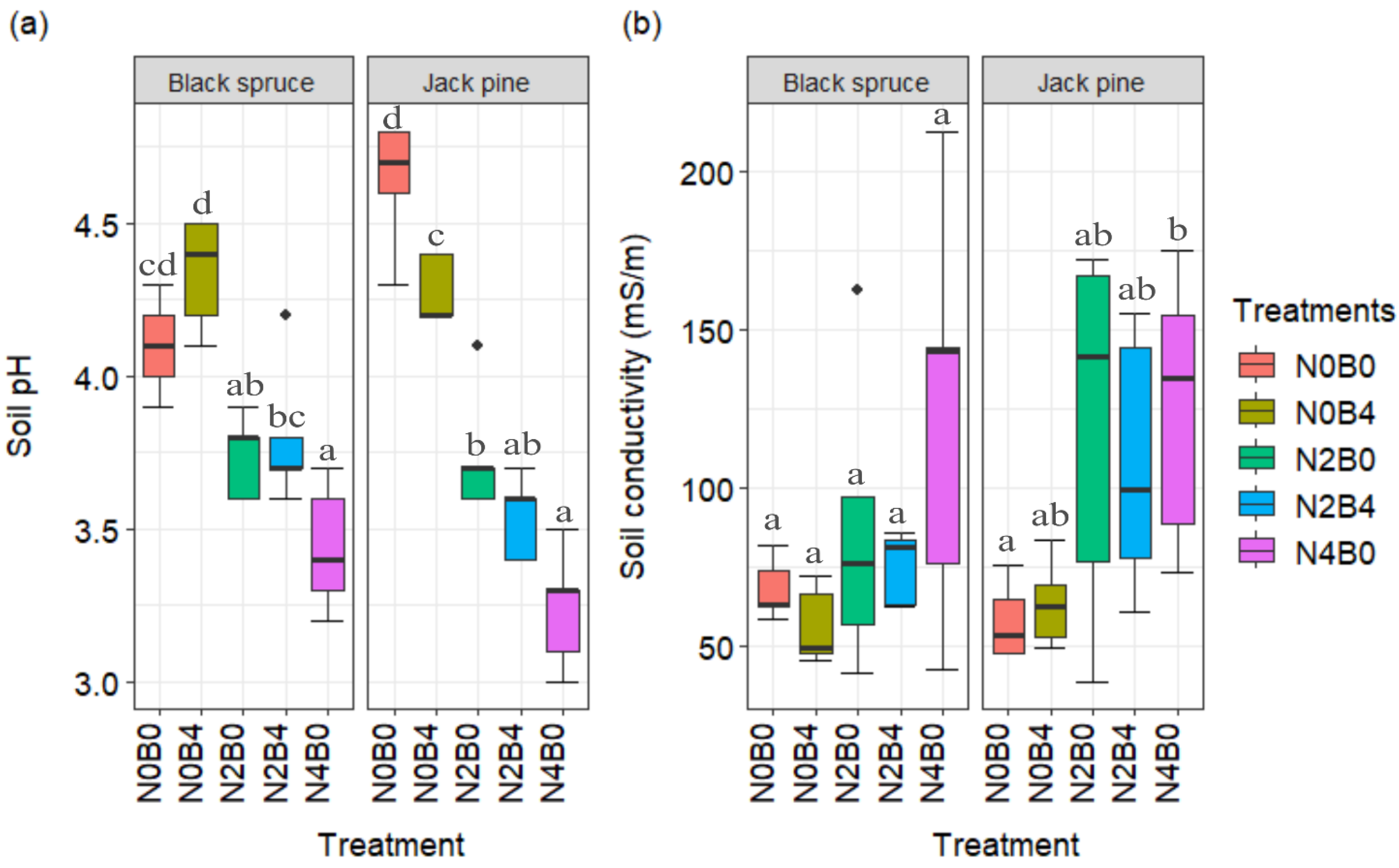


| Species | Week | Treatment | Diameter (mm) | Height (cm) | Biomass (g) | Change (%) Compared to Control in | |||
|---|---|---|---|---|---|---|---|---|---|
| Needle | Branch | Diameter | Height | Biomass | |||||
| Black spruce | 0 | N0B0 | 4.62 | 31.5 | - | - | - | - | - |
| 10 | N0B0 | 6.66 | 42.98 | - | - | - | - | - | |
| 10 | N0B4 | 6.75 | 44.40 | - | - | 1.35 | 3.30 | - | |
| 10 | N2B0 | 6.91 | 47.28 | - | - | 3.75 | 10.00 | - | |
| 10 | N2B4 | 7.43 | 46.92 | - | - | 11.56 | 9.17 | - | |
| 10 | N4B0 | 7.01 | 44.52 | - | - | 5.26 | 3.58 | - | |
| 20 | N0B0 | 8.56 | 43.50 | - | - | - | - | - | |
| 20 | N0B4 | 7.84 | 45.20 | - | - | −8.41 | 3.91 | - | |
| 20 | N2B0 | 9.69 | 59.16 | - | - | 13.20 | 36.00 | - | |
| 20 | N2B4 | 9.83 | 54.66 | - | - | 14.84 | 25.66 | - | |
| 20 | N4B0 | 9.99 | 63.08 | - | - | 16.71 | 45.01 | - | |
| 30 | N0B0 | 8.73 | 43.58 | 6.23 | 8.26 | - | - | - | |
| 30 | N0B4 | 8.04 | 45.26 | 5.56 | 7.95 | −7.90 | 3.85 | −10.80 | |
| 30 | N2B0 | 11.32 | 61.02 | 11.60 | 18.57 | 29.67 | 40.02 | 86.06 | |
| 30 | N2B4 | 11.25 | 54.68 | 10.81 | 15.77 | 28.87 | 25.47 | 73.39 | |
| 30 | N4B0 | 11.49 | 64.76 | 11.48 | 17.78 | 31.62 | 48.60 | 84.12 | |
| Jack pine | 0 | N0B0 | 4.45 | 24.74 | - | - | - | - | - |
| 10 | N0B0 | 5.59 | 28.46 | - | - | - | - | - | |
| 10 | N0B4 | 5.55 | 30.80 | - | - | −0.72 | 8.22 | - | |
| 10 | N2B0 | 6.08 | 31.16 | - | - | 8.77 | 9.49 | - | |
| 10 | N2B4 | 5.59 | 32.04 | - | - | 0.00 | 12.58 | - | |
| 10 | N4B0 | 6.17 | 34.44 | - | - | 10.38 | 21.01 | - | |
| 20 | N0B0 | 6.35 | 33.46 | - | - | - | - | - | |
| 20 | N0B4 | 6.47 | 36.94 | - | - | 1.89 | 10.40 | - | |
| 20 | N2B0 | 8.49 | 38.56 | - | - | 33.70 | 15.24 | - | |
| 20 | N2B4 | 7.42 | 38.10 | - | - | 16.85 | 13.87 | - | |
| 20 | N4B0 | 7.43 | 38.26 | - | - | 17.01 | 14.35 | - | |
| 30 | N0B0 | 7.72 | 34.36 | 9.32 | 4.64 | - | - | - | |
| 30 | N0B4 | 7.90 | 38.10 | 9.62 | 4.75 | 2.33 | 10.88 | 3.21 | |
| 30 | N2B0 | 11.08 | 41.40 | 23.69 | 10.97 | 43.52 | 20.49 | 154.19 | |
| 30 | N2B4 | 10.59 | 41.20 | 21.68 | 9.76 | 37.18 | 19.91 | 132.58 | |
| 30 | N4B0 | 10.54 | 40.44 | 19.98 | 9.35 | 36.53 | 17.69 | 114.37 | |
| Soil Ions | Treatment | |||
|---|---|---|---|---|
| N0B0 | N2B0 | N4B0 | ||
| Elemental composition (mg/m2 of membrane/two weeks) | Total N | 3.04 | 645.53 | 887.84 |
| NO3− | 3.03 | 373.97 | 331.58 | |
| NH4+ | 0.01 | 271.55 | 556.26 | |
| Ca2+ | 481.37 | 857.67 | 908.62 | |
| Mg2+ | 167.98 | 301.52 | 318.58 | |
| K+ | 71.15 | 43.89 | 41.37 | |
| PO43− | 0.49 | 0.44 | 0.19 | |
| Fe2+ | 12.32 | 11.63 | 6.41 | |
| Mn2+ | 3.03 | 25.87 | 22.86 | |
| Cu+ | 1.07 | 1.1 | 0.66 | |
| Zn2+ | 0.8 | 1.53 | 1.34 | |
| BO33− | 0.03 | 0.03 | 0.01 | |
| S2− | 279.23 | 718.14 | 1048.48 | |
| Pb2+ | 0.1 | 0.02 | 0.03 | |
| Al3+ | 14.9 | 40.34 | 30.75 | |
| Cd2+ | 0 | 0.01 | 0.01 | |
| Treatment | Soil Horizon | Ca2+ | K+ | Mg2+ | PO43− | S2− | pH | C | H | N |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/m2 of Membrane/2 Weeks) | (%) | |||||||||
| N0 | Organic | 2378.33 | 1300.50 | 740.33 | 313.83 | 0.20 | 3.43 | 26.21 | 2.58 | 3.16 |
| Mineral | 2538.67 | 1574.33 | 937.50 | 121.15 | 0.05 | 3.48 | 4.97 | 0.40 | 2.99 | |
| N1.5 | Organic | 2535.83 | 1055.50 | 1103.83 | 337.70 | 0.17 | 2.93 | 27.46 | 2.92 | 0.84 |
| Mineral | 2314.60 | 377.00 | 655.60 | 154.20 | 0.16 | 2.98 | 2.82 | 0.39 | 0.19 | |
| N3 | Organic | 2819.40 | 1906.00 | 437.00 | 404.20 | 0.71 | 3.10 | 44.25 | 4.79 | 1.40 |
| Mineral | 1510.25 | 234.25 | 2267.25 | 74.48 | 0.09 | 3.58 | 2.29 | 0.34 | 0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subedi, A.; Robert, É.; Braghiroli, F.L.; Girona, M.M. Can Ammoniacal Nitrogen from Gold Mining Effluent Be a Promising Alternative for Fertilizing Boreal Forest Stands? Sustainability 2024, 16, 7683. https://doi.org/10.3390/su16177683
Subedi A, Robert É, Braghiroli FL, Girona MM. Can Ammoniacal Nitrogen from Gold Mining Effluent Be a Promising Alternative for Fertilizing Boreal Forest Stands? Sustainability. 2024; 16(17):7683. https://doi.org/10.3390/su16177683
Chicago/Turabian StyleSubedi, Anoj, Émilie Robert, Flavia Lega Braghiroli, and Miguel Montoro Girona. 2024. "Can Ammoniacal Nitrogen from Gold Mining Effluent Be a Promising Alternative for Fertilizing Boreal Forest Stands?" Sustainability 16, no. 17: 7683. https://doi.org/10.3390/su16177683
APA StyleSubedi, A., Robert, É., Braghiroli, F. L., & Girona, M. M. (2024). Can Ammoniacal Nitrogen from Gold Mining Effluent Be a Promising Alternative for Fertilizing Boreal Forest Stands? Sustainability, 16(17), 7683. https://doi.org/10.3390/su16177683








