Effects of Nutrient Accumulation and Microbial Community Changes on Tomato Fusarium Wilt Disease in Greenhouse Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Statistics and Calculation of Tomato Fusarium Wilt Disease Index and Yield
2.3. Determination of Soil Physical and Chemical Properties
2.4. Soil DNA Extraction and Absolute Quantification PCR
2.5. Gene Sample Amplification and Illumina MiSeq Sequencing
2.6. Sequence Processing and Bioinformatic Analyses
2.7. Statistical Analyses
3. Results
3.1. Tomato Fusarium Wilt Incidence Index and Yield in Greenhouses of Different Planting Years
3.2. Differences in Soil Properties between Health and Disease Groups of Tomatoes
3.3. Differences in Soil Microbial Abundance and Alpha Diversity between the Health and Disease Groups
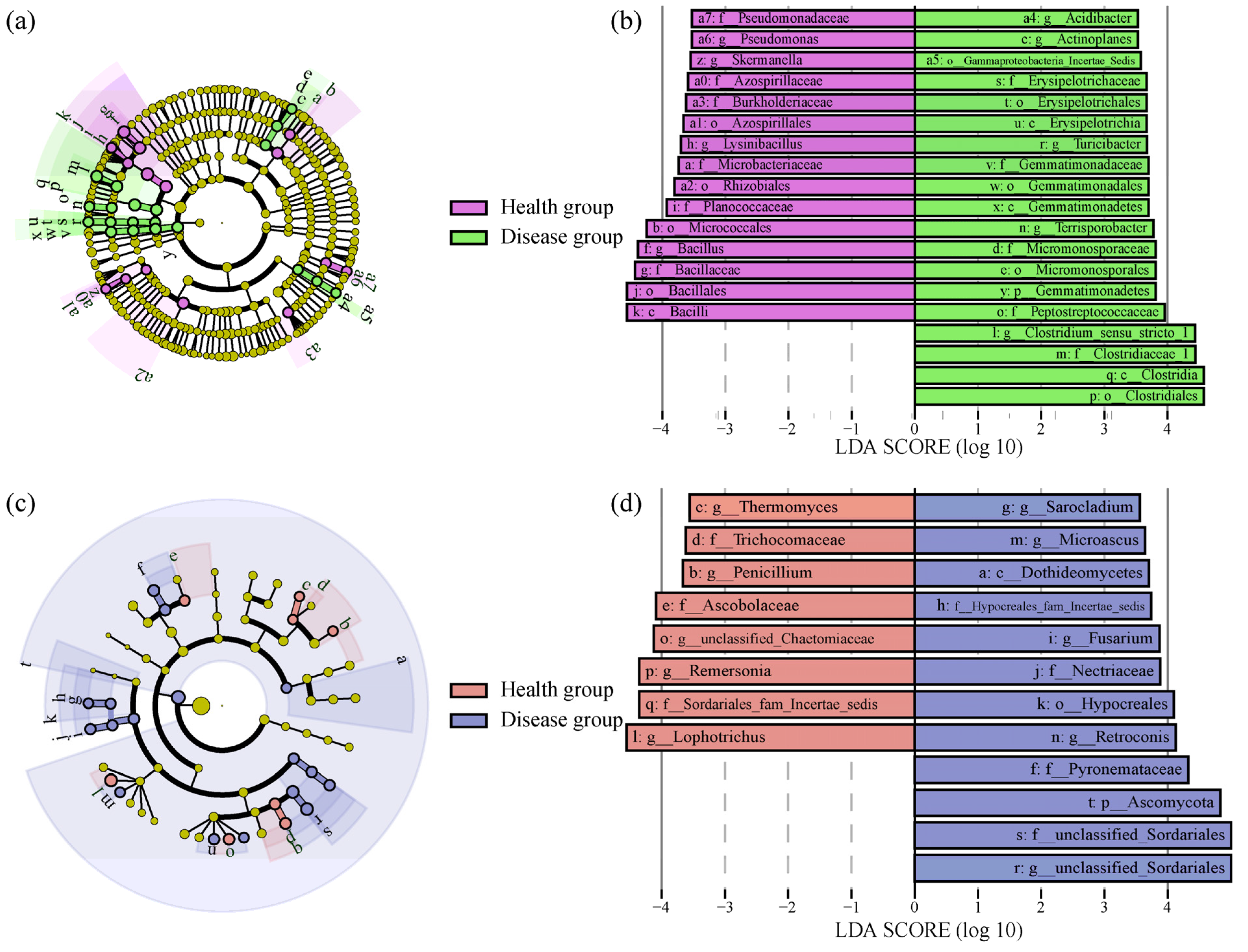
3.4. Differences in Soil Microbial Community Composition between Health and Disease Groups
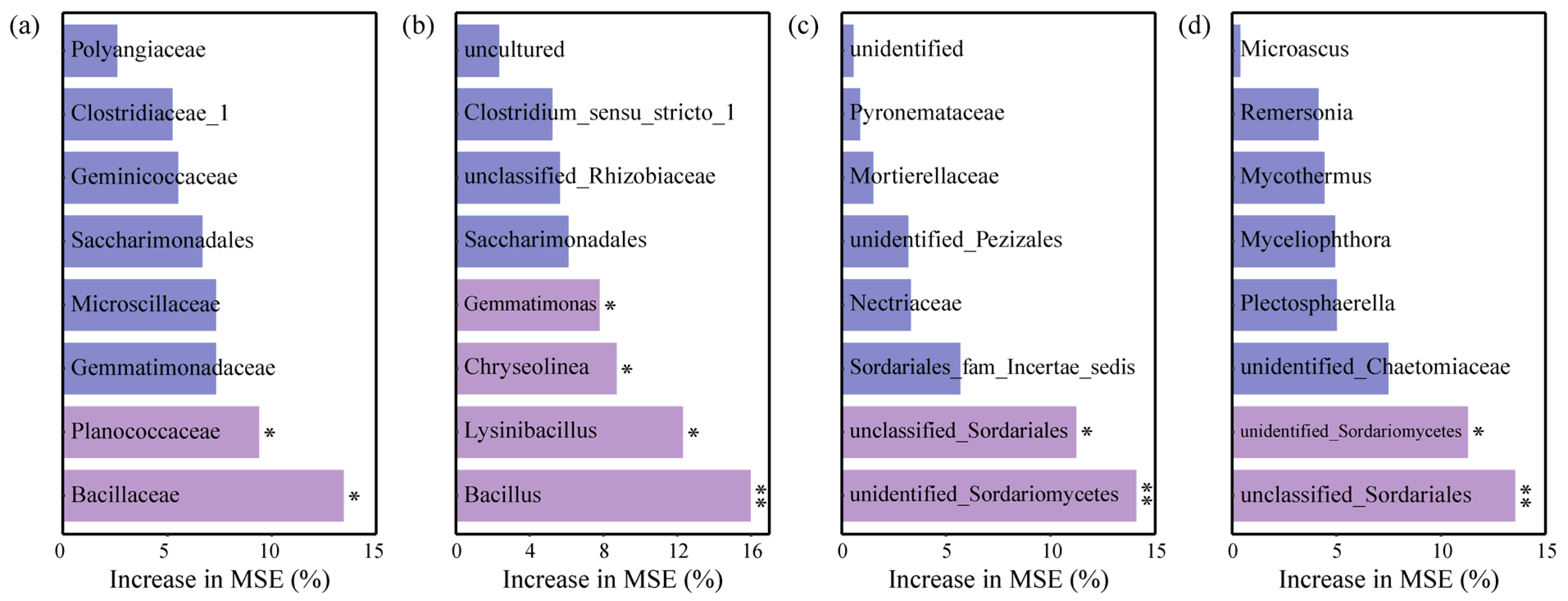
3.5. Effects of Soil Chemical Properties on Microbial Taxa in Greenhouse Tomato Soils
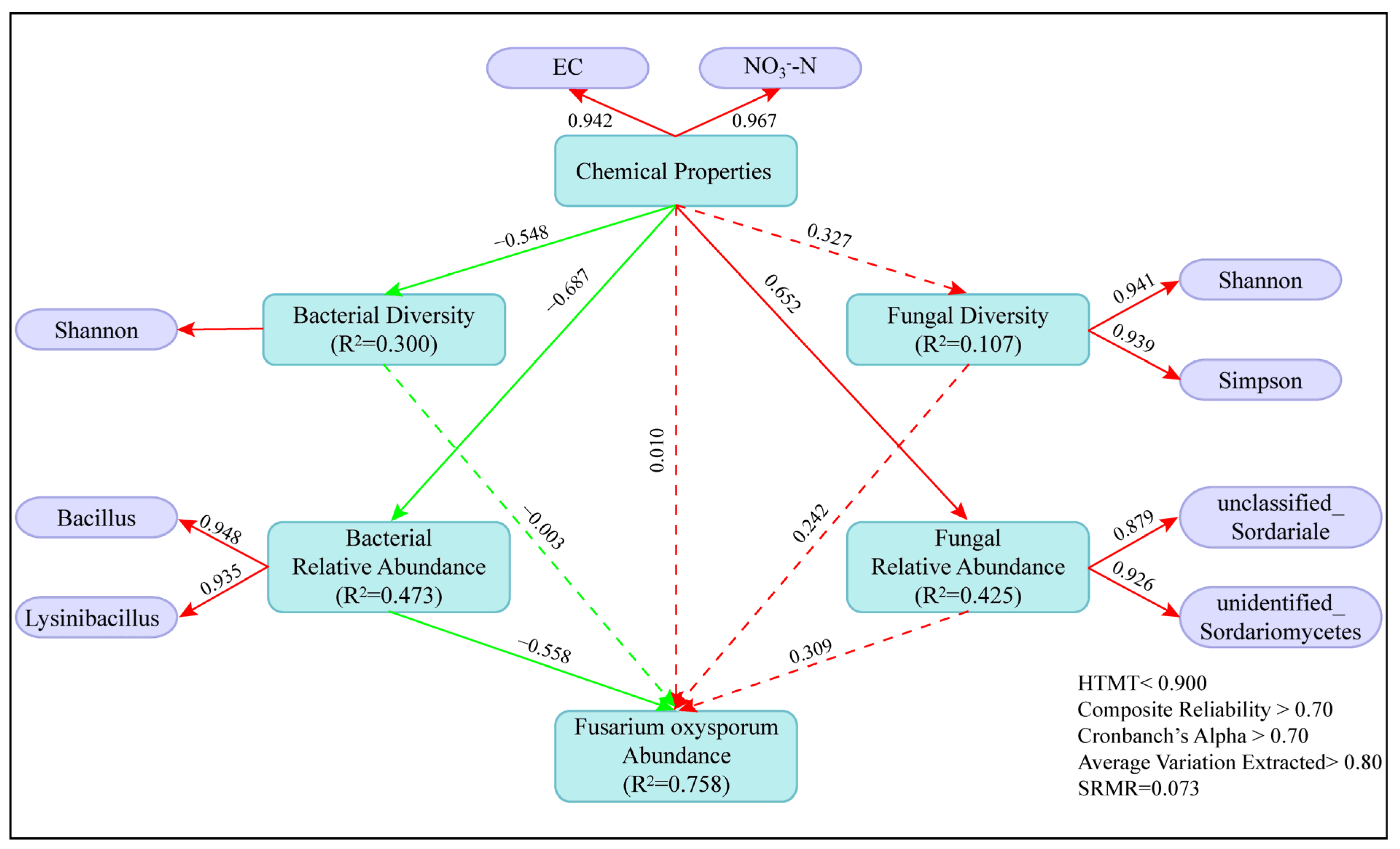
3.6. Direct and Indirect Effects of Soil Factors on the Abundance of Pathogen Fusarium Oxysporum in Greenhouse Tomato Soils
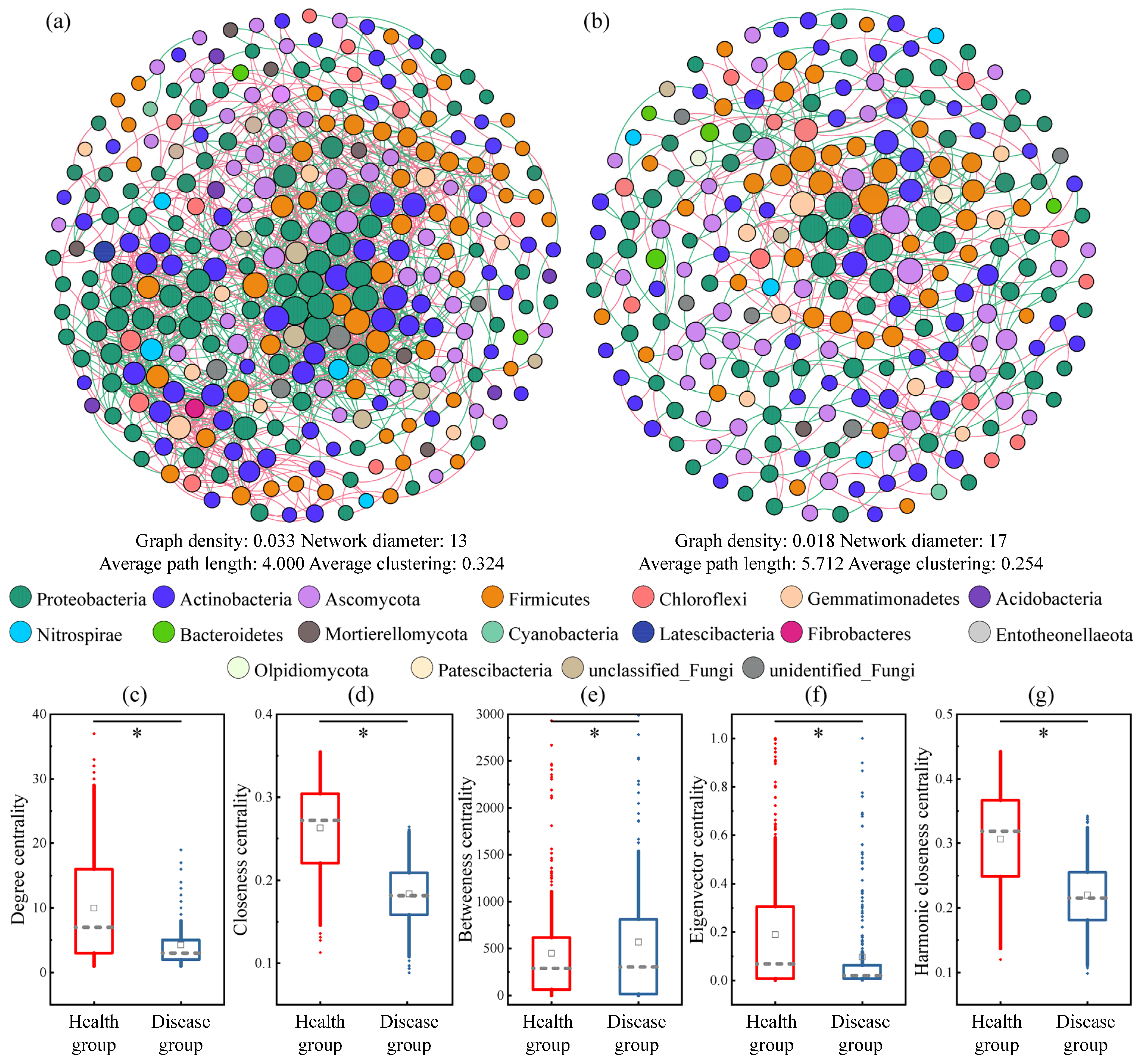
3.7. Differences in Co-Occurrence Networks of Soil Microbial Communities between the Health and Disease Groups of Greenhouse Tomatoes
4. Discussion
4.1. Effect of Soil Nutrient Enrichment on Tomato Fusarium Wilt in Greenhouses
4.2. Relationship between Fusarium Wilt of Greenhouse Tomatoes and the Diversity and Composition of Rhizosphere Soil Microorganisms
4.3. Changes in Bacterial Communities Caused by NO3−–N Accumulation in Greenhouse Soils Are Key to the Increased Fol Abundance
4.4. Nutrification of Greenhouse Soils Alters Microbial Networks and Increases Pathogen Invasion Risk
4.5. Implications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Siddiqui, Z.A. Effects of Bacillus subtilis, Pseudomonas fluorescens and Aspergillus awamori on the Wilt-Leaf Spot Disease Complex of Tomato. Phytoparasitica 2014, 43, 61–75. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 August 2024).
- Scheffer, R.P.; Walker, J.C. The Physiology of Fusarium Wilt of Tomato. Phytopathology 1953, 43, 116–125. [Google Scholar]
- Nofal, A.; El-Rahman, M.; Abdelghany, T.; AbdEl-Mongy, M. Mycoparasitic Nature of Egyptian Trichoderma Isolates and Their Impact on Suppression of Fusarium Wilt of Tomato. Egypt. J. Pest Control 2021, 31, 103. [Google Scholar] [CrossRef]
- Massee, G. The “sleepy disease” of tomatoes. Gard. Chron. Ser. 1896, 3, 707–708. [Google Scholar]
- Cai, Z. Scientific and Technological Issues of Nutrient Management under Greenhouse Cultivation in China. Acta Pedol. Sin. 2019, 56, 36–43. [Google Scholar] [CrossRef]
- Worku, M.; Sahela, S. Review on Disease Management Practice of Tomato Wilt Caused Fusarium oxysporum in Case of Ethiopia. J. Plant Pathol. Microbiol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Sun, H.W.; Wei, C.; Xu, W.S.; Yang, J.Z.; Wang, X.G.; Qiu, Y.F. Characteristics of Salt Contents in Soils under Greenhouse Conditions in China. Environ. Sci. Pollut. Res. 2019, 26, 3882–3892. [Google Scholar] [CrossRef]
- Hu, Y.C.; Song, Z.W.; Lu, W.L.; Poschenrieder, C.; Schmidhalter, U. Current Soil Nutrient Status of Intensively Managed Greenhouses. Pedosphere 2012, 22, 825–833. [Google Scholar] [CrossRef]
- Hoffland, E.; Jeger, M.J.; van Beusichem, M.L. Effect of Nitrogen Supply Rate on Disease Resistance in Tomato Depends on the Pathogen. Plant Soil 2000, 218, 239–247. [Google Scholar] [CrossRef]
- Gu, Z.; Wang, M.; Wang, Y.; Zhu, L.; Mur, L.A.J.; Hu, J. Nitrate Stabilizes the Rhizospheric Fungal Community to Suppress Fusarium Wilt Disease in Cucumber. Mol. Plant Microbe Interact. 2020, 33, 590–599. [Google Scholar] [CrossRef]
- Long, D.H.; Lee, F.N.; TeBeest, D.O. Effect of Nitrogen Fertilization on Disease Progress of Rice Blast on Susceptible and Resistant Cultivars. Plant Dis. 2000, 84, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Easterday, C.A.; Kendig, A.E.; Lacroix, C.; Seabloom, E.W.; Borer, E.T. Long-Term Nitrogen Enrichment Mediates the Effects of Nitrogen Supply and Co-Inoculation on a Viral Pathogen. Ecol. Evol. 2022, 12, e8450. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.A.A.; Abo-Elyousr, K.A.M.; Abdel Alal, A.M.K.; Alshareef, N.O. Management of Fusarium Wilt Disease in Tomato by Combinations of Bacillus amyloliquefaciens and Peppermint Oil. Agronomy 2021, 11, 2536. [Google Scholar] [CrossRef]
- Bastakoti, S.; Belbase, S.; Manandhar, S.; Arjyal, C. Trichoderma Species as Biocontrol Agent against Soil Borne Fungal Pathogens. Nepal J. Biotechnol. 2017, 5, 39–45. [Google Scholar] [CrossRef]
- Jiao, X.L.; Zhang, X.S.; Lu, X.H.; Qin, R.; Bi, Y.M.; Gao, W.W. Effects of Maize Rotation on the Physicochemical Properties and Microbial Communities of American Ginseng Cultivated Soil. Sci. Rep. 2019, 9, 8615. [Google Scholar] [CrossRef]
- Beckman, C.H. The Nature of Wilt Diseases in Plants; APS Press: St. Paul, MN, USA, 1987; pp. 1–175. [Google Scholar]
- Gosling, P.; Mead, A.; Proctor, M.; Hammond, J.P.; Bending, G.D. Contrasting Arbuscular Mycorrhizal Communities Colonizing Different Host Plants Show a Similar Response to a Soil Phosphorus Concentration Gradient. New Phytol. 2013, 198, 546–556. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsui, M.; Honjo, H.; Becker, J.O.; Fukui, R. Effects of Soil pH on Rhizoctonia Damping-Off of Sugar Beet and Disease Suppression Induced by Soil Amendment with Crop Residues. Plant Soil 2011, 347, 255–268. [Google Scholar] [CrossRef]
- Li, B.B.; Roley, S.S.; Duncan, D.S.; Guo, J.; Quensen, J.F.; Yu, H.Q.; Tiedje, J.M. Long-Term Excess Nitrogen Fertilizer Increases Sensitivity of Soil Microbial Community to Seasonal Change Revealed by Ecological Network and Metagenome Analyses. Soil Biol. Biochem. 2021, 160, 108349. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Santolini, M.; Barabasi, A.L. Predicting Perturbation Patterns from the Topology of Biological Networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified Soil Acidification from Chemical N Fertilization and Prevention by Manure in an 18-Year Field Experiment in the Red Soil of Southern China. J. Soil. Sediment. 2014, 15, 260–270. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007. [Google Scholar]
- Scherf, J.M.; Milling, A.; Allen, C. Moderate Temperature Fluctuations Rapidly Reduce the Viability of Ralstonia solanacearum Race 3, Biovar 2, in Infected Geranium, Tomato, and Potato Plants. Appl. Environ. Microbiol. 2010, 76, 7061–7067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mei, Z.; Zhang, X.; Xue, C.; Zhang, C.; Ma, T.; Zhang, S. Suppression of Fusarium Wilt of Cucumber by Ammonia Gas Fumigation via Reduction of Fusarium Population in the Field. Sci. Rep. 2017, 7, 43103. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Analytical Methods of Soil Agrochemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Dennis, K.L.; Wang, Y.W.; Blatner, N.R.; Wang, S.Y.; Saadalla, A.; Trudeau, E.; Roers, A.; Weaver, C.T.; Lee, J.J.; Gilbert, J.A.; et al. Adenomatous Polyps Are Driven by Microbe-Instigated Focal Inflammation and Are Controlled by IL-10-Producing T Cells. Cancer Res. 2013, 73, 5905–5913. [Google Scholar] [CrossRef] [PubMed]
- Manter, D.K.; Vivanco, J.M. Use of the ITS Primers, ITS1F and ITS4, to Characterize Fungal Abundance and Diversity in Mixed-Template Samples by qPCR and Length Heterogeneity Analysis. J. Microbiol. Methods 2007, 71, 7–14. [Google Scholar] [CrossRef]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector Gene Screening Allows Unambiguous Identification of Fusarium oxysporum f. sp. lycopersici Races and Discrimination from Other Formae Speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef]
- Walker, N.J. Real-Time and Quantitative PCR: Applications to Mechanism-Based Toxicology. J. Biochem. Mol. Toxicol. 2001, 15, 121–127. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Feng, K.; Peng, X.; Zhang, Z.; Gu, S.; He, Q.; Shen, W.; Wang, Z.; Wang, D.; Hu, Q.; Li, Y.; et al. iNAP: An Integrated Network Analysis Pipeline for Microbiome Studies. iMeta 2022, 1, e13. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Gallardo, A.; Covelo, F.; Prado-Comesaña, A.; Ochoa, V.; Maestre, F.T.; Schweitzer, J. Differences in Thallus Chemistry Are Related to Species-Specific Effects of Biocrust-Forming Lichens on Soil Nutrients and Microbial Communities. Funct. Ecol. 2015, 29, 1087–1098. [Google Scholar] [CrossRef]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM), 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2022. [Google Scholar]
- Bertrand, F.; Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Partial Least Squares Path Modeling (PLS-PM), R Package Version 0.5.1. 2024. Available online: https://cran.r-project.org/web/packages/plspm/index.html (accessed on 25 August 2024). [CrossRef]
- Du, L.Y.; Pan, D.W.; Zhang, H.L.; Liang, C.H.; Xu, Z.Q. Effects of Different Fertilization Treatments on Potassium Content in Soils under Vegetable Protection Cultivation. Chin. J. Soil Sci. 2006, 37, 945–949. [Google Scholar] [CrossRef]
- Elrys, A.S.; Desoky, E.M.; Abo El-Maati, M.F.; Elnahal, A.S.; Abdo, A.I.; Raza, S.; Zhou, J. Can Secondary Metabolites Extracted from Moringa Seeds Suppress Ammonia Oxidizers to Increase Nitrogen Use Efficiency and Reduce Nitrate Contamination in Potato Tubers? Ecotoxicol. Environ. Saf. 2019, 185, 109689. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Hue, N.V.; Adams, F.; Evans, C.E. Sulfate Retention by an Acid BE Horizon of an Ultisol. Soil Sci. Soc. Am. J. 1985, 49, 1196–1200. [Google Scholar] [CrossRef]
- Andrade Foronda, D.; Colinet, G. Prediction of Soil Salinity/Sodicity and Salt-Affected Soil Classes from Soluble Salt Ions Using Machine Learning Algorithms. Soil Syst. 2023, 7, 47. [Google Scholar] [CrossRef]
- Cucci, G.; Lacolla, G.; Mastro, M.A.; Caranfa, G. Leaching Effect of Rainfall on Soil under Four-Year Saline Water Irrigation. Soil Water Res. 2016, 11, 181–189. [Google Scholar] [CrossRef]
- Minhas, P.S.; Ramos, T.B.; Ben-Gal, A.; Pereira, L.S. Coping with Salinity in Irrigated Agriculture: Crop Evapotranspiration and Water Management Issues. Agric. Water Manag. 2020, 227, 105832. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: Columbus, OH, USA, 2016. [Google Scholar]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Rapid Method for Coextraction of DNA and RNA from Natural Environments for Analysis of Ribosomal DNA- and rRNA-Based Microbial Community Composition. Appl. Environ. Microbiol. 2000, 66, 5488–5491. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Lou, Y.; Xu, M.; He, X.; Duan, Y.; Li, L. Soil Nitrate Distribution, N2O Emission, and Crop Performance after the Application of N Fertilizers to Greenhouse Vegetables. Soil Use Manag. 2012, 28, 299–306. [Google Scholar] [CrossRef]
- Sun, J.; Zou, L.; Li, W.; Yang, J.; Wang, Y.; Xia, Q.; Peng, M. Rhizosphere Soil Properties and Banana Fusarium Wilt Suppression Influenced by Combined Chemical and Organic Fertilizations. Agric. Ecosyst. Environ. 2018, 254, 60–68. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. An Integrated Insight into the Relationship between Soil Microbial Community and Tobacco Bacterial Wilt Disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Zhang, J.; Cai, Z. Highly Connected Taxa Located in the Microbial Network Are Prevalent in the Rhizosphere Soil of Healthy Plant. Biol. Fertil. Soils 2019, 55, 299–312. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Dong, W.; Zhang, Y.; Zhang, G.; Sun, Z.; Yang, L. Vermicompost Can Suppress Fusarium oxysporum f. sp. lycopersici via Generation of Beneficial Bacteria in a Long-Term Tomato Monoculture Soil. Plant Soil 2019, 440, 491–505. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil Health through Soil Disease Suppression: Which Strategy from Descriptors to Indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M. Effects of Selected Root Exudate Components on Soil Bacterial Communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, Toxicity and Microbial Transformation in Soil—A Review. Ann. Microbiol. 2008, 58, 351–357. Available online: https://annalsmicrobiology.biomedcentral.com/articles/10.1007/BF03175528 (accessed on 24 August 2024). [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Li, R.; Kowalchuk, G.A.; Shen, Q. Bio-Fertilizer Application Induces Soil Suppressiveness against Fusarium Wilt Disease by Reshaping the Soil Microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Jamal, Q.M.S.; Ahmad, V. Lysinibacilli: A Biological Factories Intended for Bio-Insecticidal, Bio-Control, and Bioremediation Activities. J. Fungi 2022, 8, 1288. [Google Scholar] [CrossRef] [PubMed]
- Volpiano, C.G.; Sant’Anna, F.H.; Ambrosini, A.; de Sao Jose, J.F.B.; Beneduzi, A.; Whitman, W.B.; de Souza, E.M.; Lisboa, B.B.; Vargas, L.K.; Passaglia, L.M.P. Genomic Metrics Applied to Rhizobiales (Hyphomicrobiales): Species Reclassification, Identification of Unauthentic Genomes and False Type Strains. Front. Microbiol. 2021, 12, 614957. [Google Scholar] [CrossRef]
- Bonilla, N.; Gutiérrez-Barranquero, J.; Vicente, A.; Cazorla, F. Enhancing Soil Quality and Plant Health Through Suppressive Organic Amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Qu, X.; Luo, Z.; Lu, S.; Kuzyakov, Y.; Alharbi, H.A.; Yuan, J.; Niu, G. Microbial Communities and Functions in the Rhizosphere of Disease-Resistant and Susceptible Camellia spp. Front. Microbiol. 2021, 12, 732905. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hamonts, K.; Anderson, I.C.; Singh, B.K. Keystone Microbial Taxa Regulate the Invasion of a Fungal Pathogen in Agro-Ecosystems. Soil Biol. Biochem. 2017, 111, 10–14. [Google Scholar] [CrossRef]
- Stein, L.Y.; Klotz, M.G. The Nitrogen Cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhang, Y.; Zhou, B.; Wang, Q.; Gu, J.; Liu, G.; Qin, Z.; Li, Z. Changes in Antibiotic Resistance Genes and Mobile Genetic Elements during Cattle Manure Composting after Inoculation with Bacillus subtilis. Bioresour. Technol. 2019, 292, 122011. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Y.Y.; Yan, W.; Cao, L.L.; Xiao, Y.; Ye, Y.H. Chaetomium globosum CDW7, a Potential Biological Control Strain and Its Antifungal Metabolites. FEMS Microbiol. Lett. 2017, 364, fnw287. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.O.; Bigirimana, V.D.P.; Hua, G.K.H.; Oni, F.E.; Bertier, L.; Onwughalu, J.; Oyetunji, O.E.; Ogunbayo, A.; Van De Velde, M.; Nyamangyoku, O.I.; et al. Fusarium and Sarocladium Species Associated with Rice Sheath Rot Disease in Sub-Saharan Africa. Diversity 2023, 15, 1090. [Google Scholar] [CrossRef]
- Naeem, M.; Manzoor, S.; Abid, M.U.; Tareen, M.B.K.; Asad, M.; Mushtaq, S.; Ehsan, N.; Amna, D.; Xu, B.J.; Hazafa, A. Fungal Proteases as Emerging Biocatalysts to Meet the Current Challenges and Recent Developments in Biomedical Therapies: An Updated Review. J. Fungi 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, V.; Dubey, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Industrial Applications of Fungal Lipases: A Review. Front. Microbiol. 2023, 14, 1142536. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, D.; Wang, D.; Zhi, Y.; Zhou, P. Heterologous Expression and Biochemical Characterization of Assimilatory Nitrate and Nitrite Reductase Reveals Adaption and Potential of Bacillus megaterium NCT-2 in Secondary Salinization Soil. Int. J. Biol. Macromol. 2017, 101, 1019–1028. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of Maize Root Growth by High Nitrate Supply Is Correlated with Reduced IAA Levels in Roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef]
- Dodds, K.L.; Collins-Thompson, D.L. Nitrite Tolerance and Nitrite Reduction in Lactic Acid Bacteria Associated with Cured Meat Products. Int. J. Food Microbiol. 1984, 1, 163–170. [Google Scholar] [CrossRef]
- Gomez-Gil, L.; Camara Almiron, J.; Rodriguez Carrillo, P.L.; Olivares Medina, C.N.; Bravo Ruiz, G.; Romo Rodriguez, P.; Corrales Escobosa, A.R.; Gutierrez Corona, F.; Roncero, M.I. Nitrate Assimilation Pathway (NAP): Role of Structural (nit) and Transporter (ntr1) Genes in Fusarium oxysporum f. sp. lycopersici Growth and Pathogenicity. Curr. Genet. 2018, 64, 493–507. [Google Scholar] [CrossRef]
- Uchimura, H.; Enjoji, H.; Seki, T.; Taguchi, A.; Takaya, N.; Shoun, H. Nitrate Reductase-Formate Dehydrogenase Couple Involved in the Fungal Denitrification by Fusarium oxysporum. J. Biochem. 2002, 131, 579–586. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The Ecology of the Microbiome: Networks, Competition, and Stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, H.; Wang, L.; Xu, M.; Wan, Y.; Chou, M.; Shi, P.; Wei, G. Multifunctionality and Microbial Communities in Agricultural Soils Regulate the Dynamics of a Soil-Borne Pathogen. Plant Soil 2021, 461, 309–322. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil Networks Become More Connected and Take up More Carbon as Nature Restoration Progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.H.; Liu, H.W.; Liu, T.; Zhao, M.L.; Yang, S.D.; Niu, G.Q.; Hale, L.; Singh, B.K.; Kowalchuk, G.A.; et al. Tapping the Rhizosphere Metabolites for the Prebiotic Control of Soil-Borne Bacterial Wilt Disease. Nat. Commun. 2023, 14, 4497. [Google Scholar] [CrossRef]


| Chemical Characteristics | Health Group | Disease Group |
|---|---|---|
| pH | 7.29 ± 0.22 a | 6.75 ± 0.28 b |
| EC (μs cm−1) | 255.60 ± 134.85 a | 458.57 ± 381.34 a |
| TN (g kg−1) | 2.08 ± 0.51 b | 3.14 ± 0.80 a |
| NH4+–N (mg kg−1) | 5.53 ± 7.00 a | 7.10 ± 16.67 a |
| NO3−–N (mg kg−1) | 94.90 ± 36.65 b | 237.87 ± 99.31 a |
| AN (mg kg−1) | 202.86 ± 63.80 b | 332.22 ± 124.12 a |
| AP (mg kg−1) | 551.88 ± 218.04 b | 737.29 ± 154.12 a |
| AK (mg kg−1) | 625.73 ± 233.93 a | 804.34 ± 233.18 a |
| Microbial Community Characteristics | Health Group | Disease Group | ||
|---|---|---|---|---|
| Absolute abundance | 16S rRNA (109 copies g−1) | 7.780 ± 5.281 a | 7.204 ± 4.038 a | |
| ITS (108 copies g−1) | 5.197 ± 2.357 a | 7.276 ± 2.832 a | ||
| Fol (104 copies g−1) | 10.003 ± 2.697 b | 16.175 ± 4.091 a | ||
| Alpha diversity | Bacterial | Chao1 | 3750.40 ± 112.80 a | 3355.59 ± 745.47 a |
| Pielou_e | 0.8808 ± 0.0095 a | 0.8596 ± 0.013 b | ||
| Shannon | 10.26 ± 0.21 a | 9.91 ± 0.20 b | ||
| Simpson | 0.9969 ± 0.0009 a | 0.9956 ± 0.0013 b | ||
| Fungal | Chao1 | 403.83 ± 86.09 a | 394.29 ± 90.05 a | |
| Pielou_e | 0.5747 ± 0.0357 a | 0.5950 ± 0.0339 a | ||
| Shannon | 4.96 ± 0.41 a | 5.10 ± 0.43 a | ||
| Simpson | 0.9086 ± 0.0294 a | 0.9244 ± 0.0201 a | ||
| Group | Bacterial–Bacterial (%) | Fungal–Fungal (%) | Bacterial–Fungal (%) | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Health | 58.47 | 41.53 | 65.52 | 34.48 | 9.60 | 90.40 |
| Disease | 60.17 | 39.83 | 56.76 | 43.24 | 31.97 | 68.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Han, W.; Tan, B.; Wu, Y.; Li, S.; Yi, Y. Effects of Nutrient Accumulation and Microbial Community Changes on Tomato Fusarium Wilt Disease in Greenhouse Soil. Sustainability 2024, 16, 7756. https://doi.org/10.3390/su16177756
Yang L, Han W, Tan B, Wu Y, Li S, Yi Y. Effects of Nutrient Accumulation and Microbial Community Changes on Tomato Fusarium Wilt Disease in Greenhouse Soil. Sustainability. 2024; 16(17):7756. https://doi.org/10.3390/su16177756
Chicago/Turabian StyleYang, Lu, Wei Han, Boyuan Tan, Yue Wu, Song Li, and Yanli Yi. 2024. "Effects of Nutrient Accumulation and Microbial Community Changes on Tomato Fusarium Wilt Disease in Greenhouse Soil" Sustainability 16, no. 17: 7756. https://doi.org/10.3390/su16177756
APA StyleYang, L., Han, W., Tan, B., Wu, Y., Li, S., & Yi, Y. (2024). Effects of Nutrient Accumulation and Microbial Community Changes on Tomato Fusarium Wilt Disease in Greenhouse Soil. Sustainability, 16(17), 7756. https://doi.org/10.3390/su16177756






