Effects of Mulching on Soil Properties and Yam Production in Tropical Region
Abstract
:1. Introduction
2. Materials and Methods
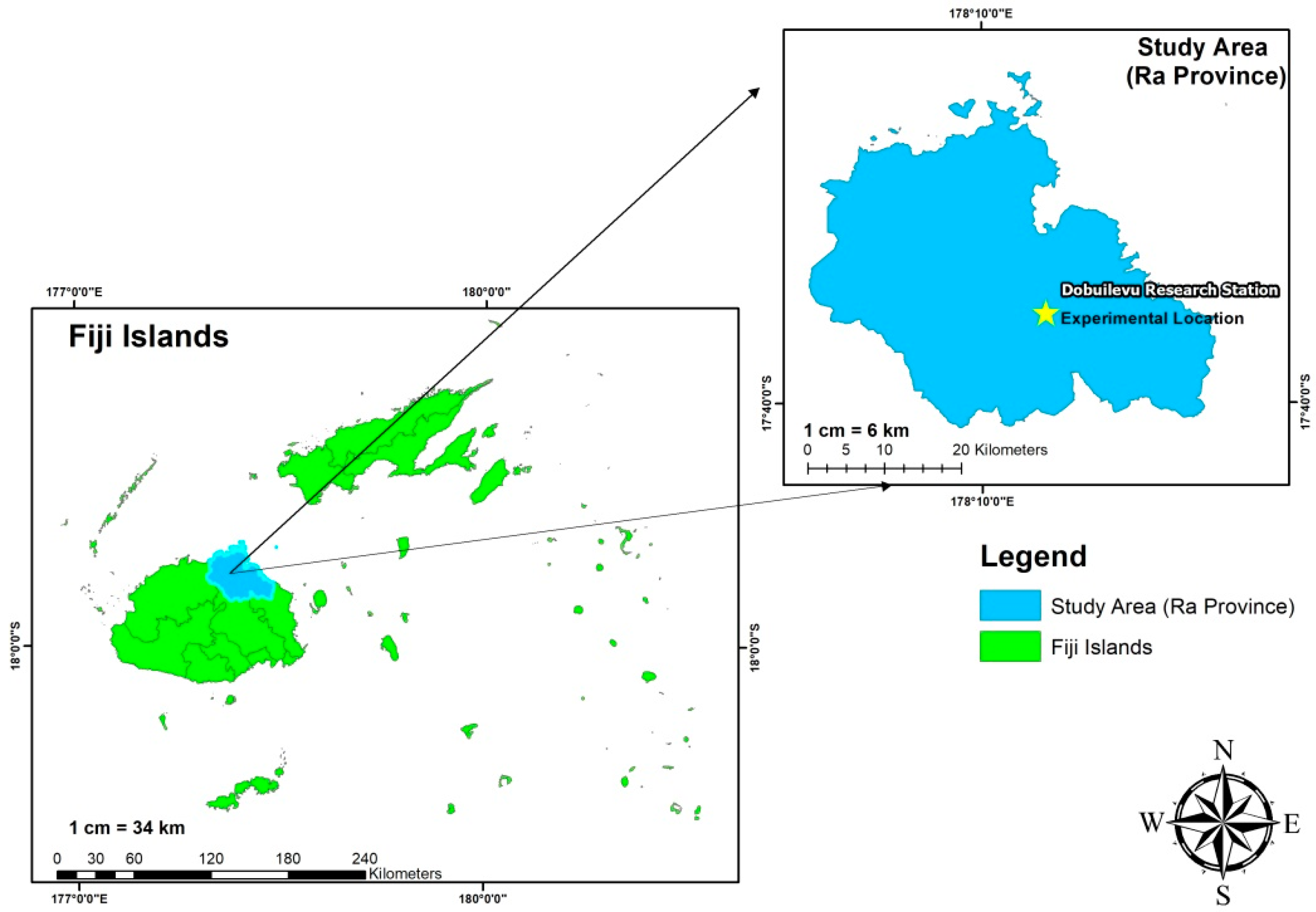
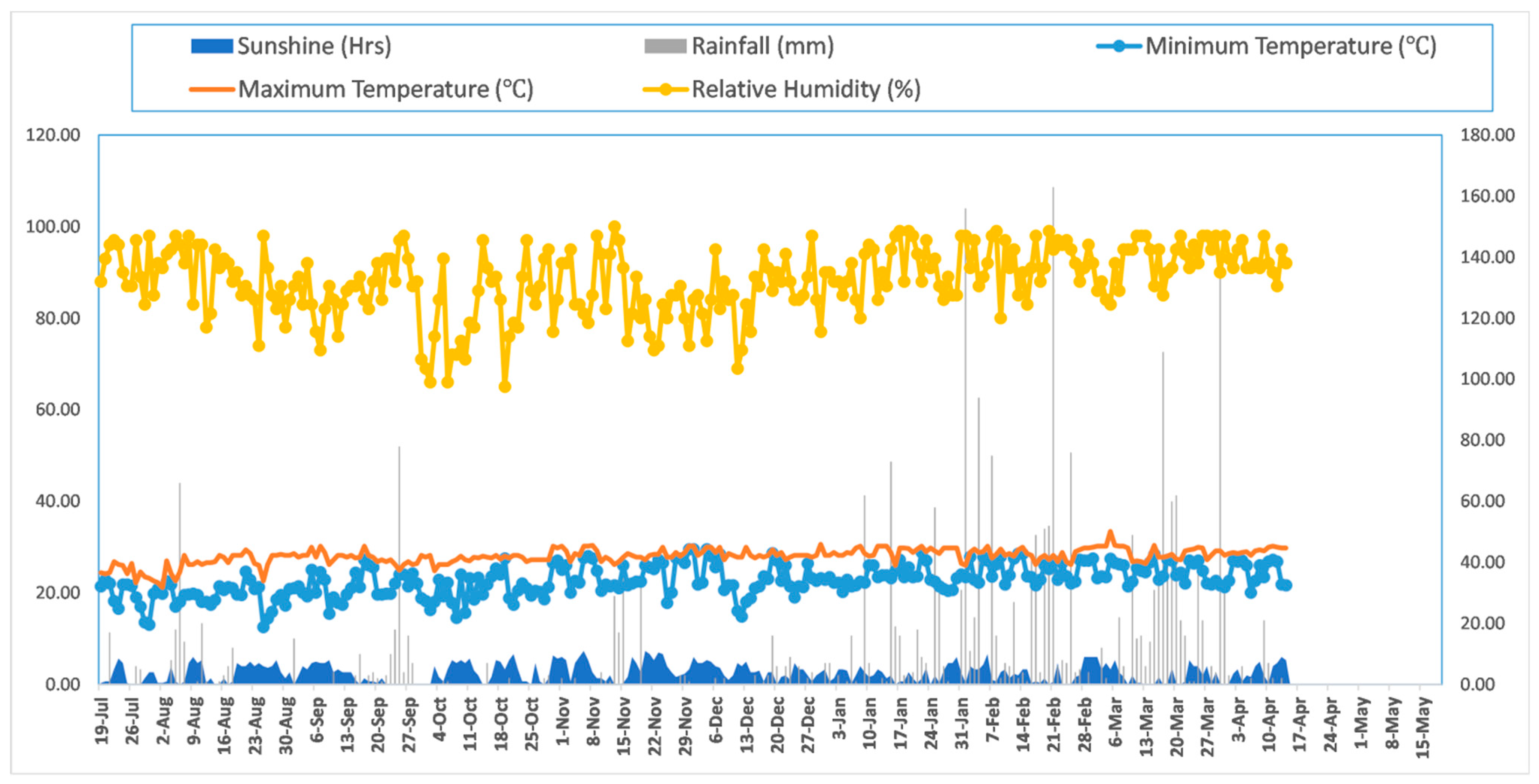
2.1. Experimental Site Description
2.2. Experimental Design and Management
2.3. Soil Physico-Chemical Properties
2.4. Soil and Environment Variable Analysis
2.4.1. Basic Soil Properties
2.4.2. SOC and Essential Soil Nutrients
2.4.3. Soil Micronutrients
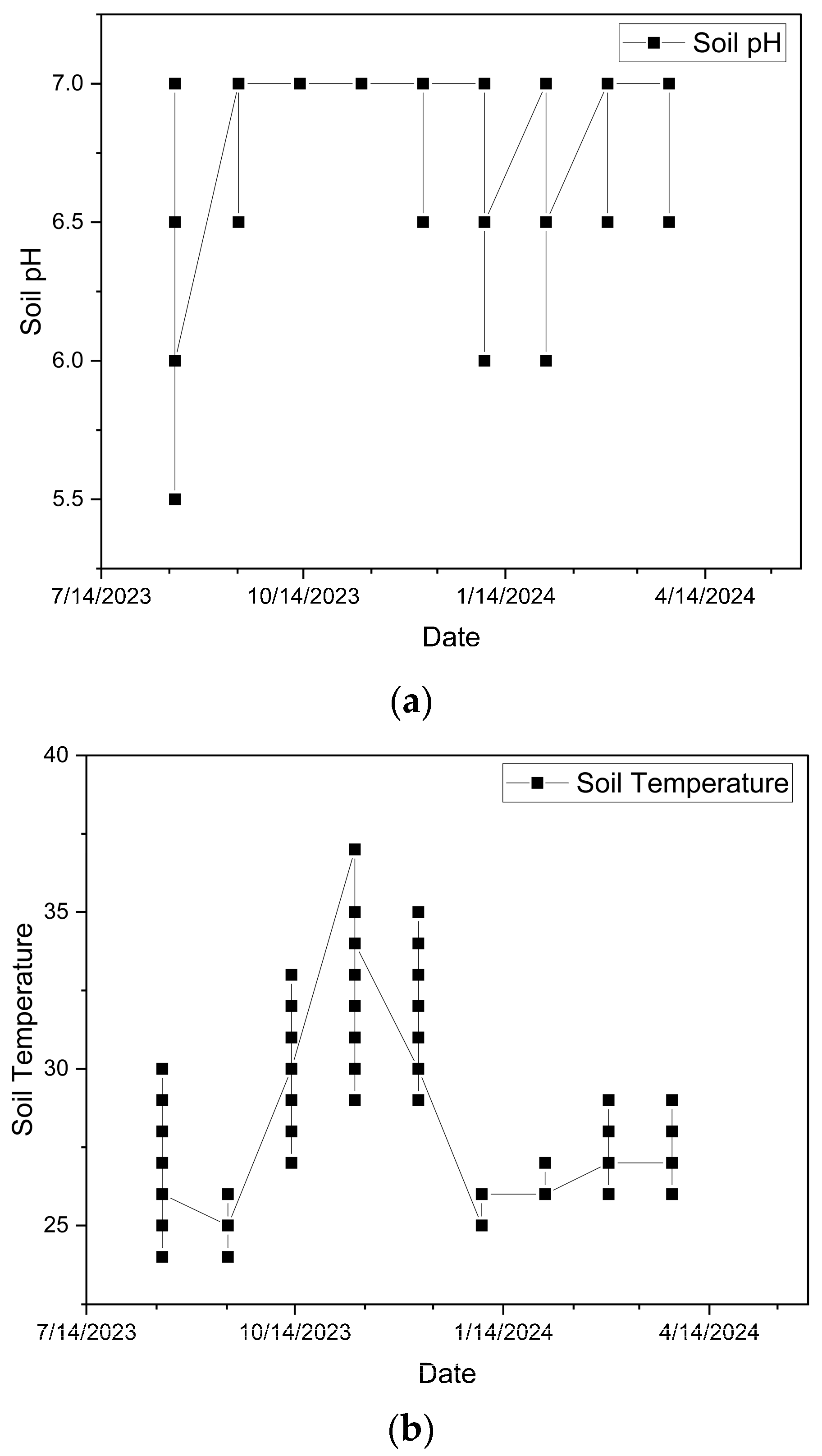
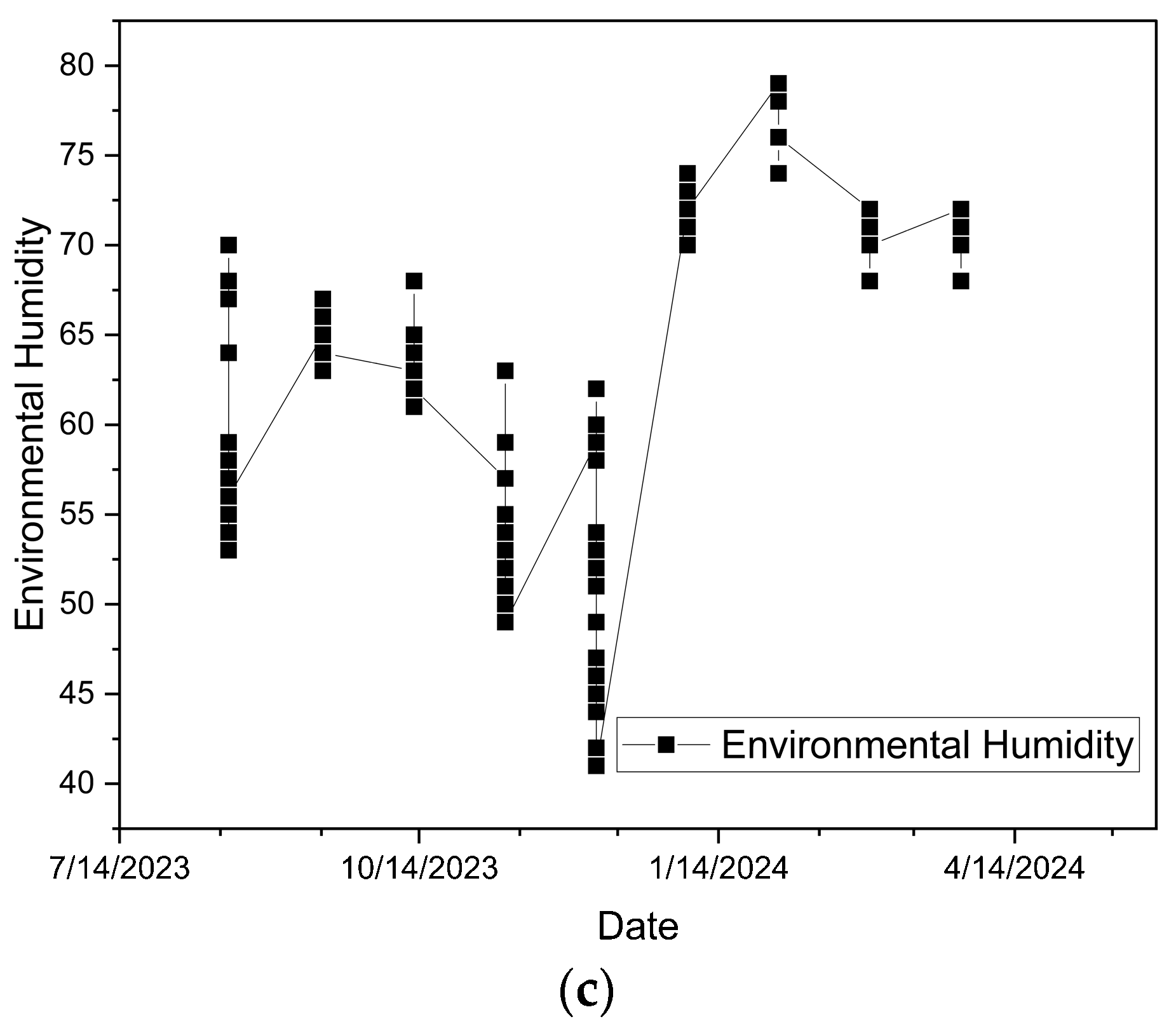
2.4.4. Time Series of Soil pH, Temperature, and Environmental Humidity
2.4.5. Soil Quality Index Calculations
2.5. Crop Growth and Yield Observations
2.6. Statistical Analysis
3. Results and Discussion
3.1. Influence of Mulch Material on Soil Properties
3.2. Influence of Mulch Material on SOC and Essential Soil Nutrients
3.3. Influence of Mulch Material on Soil Micronutrients
3.4. Seasonal Variations in Soil pH, Temperature and Environmental Humidity
3.5. Influence of Mulch Material on Soil Quality
3.6. Influence of Mulch Material on Yam Growth and Production
3.7. Influence of Mulch Material on Economics of Yam Cultivation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owusu Danquah, E.; Danquah, F.O.; Frimpong, F.; Dankwa, K.O.; Weebadde, C.K.; Ennin, S.A.; Opoku, A.Y. Sustainable intensification and climate-smart yam production for improved food security in West Africa: A review. Front. Agron. 2022, 4, 858114. [Google Scholar] [CrossRef]
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Osman, K.T. Poorly Fertile Soils. In Management of Soil Problems; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Fageria, N.K. Role of soil organic matter in maintaining sustainability of cropping systems. Commun. Soil Sci. Plant Anal. 2012, 43, 2063–2113. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Dikilitas, M.; Saxena, A.K. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Powlson, D.S.; Gregory, P.J.; Whalley, W.R.; Quinton, J.N.; Hopkins, D.W.; Whitmore, A.P.; Goulding, K.W. Soil management in relation to sustainable agriculture and ecosystem services. Food Policy 2011, 36, S72–S87. [Google Scholar] [CrossRef]
- Bhardwaj, R.L. Effect of mulching on crop production under rainfed condition: A review. Agric. Rev. 2013, 34, 188–197. [Google Scholar] [CrossRef]
- Ngosong, C.; Okolle, J.N.; Tening, A.S. Mulching: A sustainable option to improve soil health. In Soil Fertility Management for Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2019; pp. 231–249. [Google Scholar] [CrossRef]
- Gurmu, G. Soil organic matter and its role in soil health and crop productivity improvement. For. Ecol. Manag. 2019, 7, 475–483. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Gil, J. Effects of mulching on soil physical properties and runoff under semi-arid conditions in southern Spain. CATENA 2010, 81, 77–85. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Indurthi, S.; Ashoka, P.; Saikanth, D.R.K.; Das, H.; Kumar, V.; Pancholi, R. Application and Impacts of Mulch Installation Techniques on Indian Horticulture: An In-depth Review. Int. J. Plant Soil Sci. 2023, 35, 2135–2147. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Cai, H.; Fan, J.; Chau, H.W.; Malone, R.W.; Zhang, C. Organic amendments improve wheat root growth and yield through regulating soil properties. Agron. J. 2019, 111, 482–495. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion impact on agronomic productivity and environment quality. Crit. Rev. Plant Sci. 1998, 17, 319–464. [Google Scholar] [CrossRef]
- Cattanio, J.H.; Kuehne, R.; Vlek, P.L. Organic material decomposition and nutrient dynamics in a mulch system enriched with leguminous trees in the Amazon. Rev. Bras. Ciênc. Solo 2008, 32, 1073–1086. [Google Scholar] [CrossRef]
- Patil Shirish, S.; Kelkar Tushar, S.; Bhalerao Satish, A. Mulching: A soil and water conservation practice. Res. J. Agric. For. Sci. 2013, 2320, 6063. [Google Scholar]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2020, 148, 107866. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ojeniyi, S.O. Evaluation of tomato growth and soil properties under methods of seedbed preparation in an Alfisol in the rainforest zone of Southwest Nigeria. Soil Tillage Res. 2002, 64, 155–161. [Google Scholar] [CrossRef]
- Seglah, P.A.; Wang, Y.; Wang, H.; Bi, Y.; Zhou, K.; Wang, Y.; Feng, X. Crop straw utilization and field burning in Northern region of Ghana. J. Clean. Prod. 2020, 261, 121191. [Google Scholar] [CrossRef]
- Anikwe, M.A.N.; Mbah, C.N.; Ezeaku, P.I.; Onyia, V.N. Tillage and plastic mulch effects on soil properties and growth and yield of cocoyam (Colocasia esculenta) on an ultisol in southeastern Nigeria. Soil Tillage Res. 2007, 93, 264–272. [Google Scholar] [CrossRef]
- Agbede, T.M.; Adekiya, A.O.; Ogeh, J.S. Effects of Chromolaena and Tithonia mulches on soil properties, leaf nutrient composition, growth and yam yield. West Afr. J. Appl. Ecol. 2013, 21, 15–30. [Google Scholar]
- Gas, B.; Yo, O. Effect of Planting and Mulching Materials on Growth and Yield of White Yam (Dioscorea rotundata) in Ikorodu, Lagos State, Nigeria. J. Exp. Agric. Int. 2024, 46, 110–116. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 2006, 95, 115–125. [Google Scholar] [CrossRef]
- Nedunchezhiyan, M.; Sahoo, B.; Pati, K.; Chauhan, V.B.S.; Bansode, V.; Kumar, J.S.; Munshi, R. Polypropylene fabric ground cover effects on weed control and profit in elephant foot yam cultivation. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1100–1111. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.D.; Alves, P.R.L.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 199–224. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 417–435. [Google Scholar] [CrossRef]
- Grossman, R.B.; Reinsch, T.G. 2.1 Bulk density and linear extensibility. In Methods of Soil Analysis: Part 4 Physical Methods; American Society of Agronomy: Madison, WI, USA, 2002; Volume 5, pp. 201–228. [Google Scholar] [CrossRef]
- Gardner, W.H. Water content. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986; Volume 5, pp. 493–544. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen availability indexes. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 1324–1345. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9, pp. 159–165. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of soil quality index using three methods. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Sinkevičienė, A.; Jodaugienė, D.; Pupalienė, R.; Urbonienė, M. The influence of organic mulches on soil properties and crop yield. Agron. Res. 2009, 7, 485–491. [Google Scholar]
- Miller, R.O.; Gavlak, R.; Horneck, D. Soil, Plant and Water Reference Methods for the Western Region; Colorado State University: Fort Collins, CO, USA, 2013; p. 155. [Google Scholar]
- Naramabuye, F.X.; Haynes, R.J. Effect of organic amendments on soil pH and Al solubility and use of laboratory indices to predict their liming effect. Soil Sci. 2006, 171, 754–763. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Impact of mulches on landscape plants and the environment—A review. J. Environ. Hortic. 2007, 25, 239–249. [Google Scholar] [CrossRef]
- Demo, A.H.; Asefa Bogale, G. Enhancing crop yield and conserving soil moisture through mulching practices in dryland agriculture. Front. Agron. 2024, 6, 1361697. [Google Scholar] [CrossRef]
- Burt, C.M.; Mutziger, A.J.; Allen, R.G.; Howell, T.A. Evaporation research: Review and interpretation. J. Irrig. Drain. Eng. 2005, 131, 37–58. [Google Scholar] [CrossRef]
- Pan, R.; Martinez, A.; Brito, T.; Seidel, E. Processes of soil infiltration and water retention and strategies to increase their capacity. J. Exp. Agric. Int. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Awe, G.O.; Reichert, J.M.; Timm, L.C.; Wendroth, O.O. Temporal processes of soil water status in a sugarcane field under residue management. Plant Soil 2015, 387, 395–411. [Google Scholar] [CrossRef]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Nitha, K.; Tamilmani, D. Performance of Tomato Under Best Management Practices. In Engineering Interventions in Sustainable Trickle Irrigation; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 183–260. [Google Scholar]
- Zahed, Z.; Mufti, S.; Kumar, S.S.; Wani, O.A.; Mushtaq, F.; Rasool, R.; Hossain, A. Organic and inorganic mulches combination improves the productivity, quality and profitability of rainfed potato in the temperate himalayan region. Gesunde Pflanz. 2022, 74, 1109–1122. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.; Ghazzawy, H.S. Mulching as a sustainable water and soil saving practice in agriculture: A review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The increase of soil organic matter reduces global warming, myth or reality? Science 2021, 3, 18. [Google Scholar] [CrossRef]
- Lal, R. Soil organic matter and water retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Liu, C.; Ding, Y.; Liu, L.; Tian, Y.; Zhao, Z. Mulching practices alter soil microbial functional diversity and benefit to soil quality in orchards on the Loess Plateau. J. Environ. Manag. 2020, 271, 110985. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Use of plastic mulch in agriculture and strategies to mitigate the associated environmental concerns. Adv. Agron. 2020, 164, 231–287. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, X.; Chen, K.; Li, Z.; Guo, S.; Shangguan, Y.; Qin, Y. Long-term straw mulch effects on crop yields and soil organic carbon fractions at different depths under a no-till system on the Chengdu Plain, China. J. Soils Sediments 2019, 19, 2143–2152. [Google Scholar] [CrossRef]
- Phukongchai, W.; Kaewpradit, W.; Rasche, F. Inoculation of cellulolytic and ligninolytic microorganisms accelerates decomposition of high C/N and cellulose rich sugarcane straw in tropical sandy soils. Appl. Soil Ecol. 2022, 172, 104355. [Google Scholar] [CrossRef]
- Dahiya, R.; Malik, R.S.; Jhorar, B.S. Effect of sugarcane trash and enriched sugarcane trash mulches on ratoon cane yield and soil properties. J. Indian Soc. Soil Sci. 2003, 51, 504–508. [Google Scholar]
- Cao, Y.; He, Z.; Zhu, T.; Zhao, F. Organic-C quality as a key driver of microbial nitrogen immobilization in soil: A meta-analysis. Geoderma 2021, 383, 114784. [Google Scholar] [CrossRef]
- Sulman, B.N.; Brzostek, E.R.; Medici, C.; Shevliakova, E.; Menge, D.N.; Phillips, R.P. Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol. Lett. 2017, 20, 1043–1053. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Trumbore, S.E. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Y.; Xi, B.; Wei, Z.; Li, X.; Cao, Z. Changes in phosphorus fractions during organic wastes composting from different sources. Bioresour. Technol. 2015, 189, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Jodaugienė, D.; Pupalienė, R.; Sinkevičienė, A.; Marcinkevičienė, A.; Žebrauskaitė, K.; Baltaduonytė, M.; Čepulienė, R. The influence of organic mulches on soil biological properties. Zemdirb.-Agric. 2010, 97, 33–40. [Google Scholar]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of microbial communities in the formation and decomposition of soil organic matter. In Soil Microbiology and Sustainable Crop Production; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–118. [Google Scholar] [CrossRef]
- Chalise, D.; Kumar, L.; Sharma, R.; Kristiansen, P. Assessing the impacts of tillage and mulch on soil erosion and corn yield. Agronomy 2020, 10, 63. [Google Scholar] [CrossRef]
- der Merwe, V.; Prins, J.D. The Effects of Organic and Inorganic Mulches on the Yield and Fruit Quality of ‘Cripps’ Pink’apple Trees. Doctoral Dissertation, Stellenbosch University, Stellenbosch, South Africa, 2012. [Google Scholar]
- Prem, M.; Ranjan, P.; Seth, N.; Patle, G.T. Mulching techniques to conserve the soil water and advance the crop production—A Review. Curr. World Environ. 2020, 15, 10–30. [Google Scholar] [CrossRef]
- Dahiya, R.; Malik, R.S.; Jhorar, B.S.; Dahiya, J.B. Organic mulch decomposition kinetics in semiarid environment at bare and crop field conditions. Arid Land Res. Manag. 2001, 15, 49–60. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, A.; Chen, H.; Luo, S.; Bu, L.; Chen, X.; Li, S. Response of nitrogen use efficiency and soil nitrate dynamics to soil mulching in dryland maize (Zea mays L.) fields. Nutr. Cycl. Agroecosyst. 2015, 101, 271–283. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. Sustain. Agric. 2011, 2, 761–786. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.D.; Neilsen, G.H.; Forge, T.A.; Neilsen, D.; Losso, I.; Jones, M.D.; Fentabil, M.M. Irrigation practices, nutrient applications, and mulches affect soil nutrient dynamics in a young Merlot (Vitis vinifera L.) vineyard. Can. J. Soil Sci. 2016, 96, 23–36. [Google Scholar] [CrossRef]
- Ashiono, F.A. Effects of Sawdust and Cow Manure Mixtures on Growth Characteristics of Blue Gum (Eucalyptus saligna) Seedlings in South Kinangop Forest, Kenya. Doctoral Dissertation, Karatina University, Karatina, Kenya, 2020. [Google Scholar]
- Djigal, D.; Saj, S.; Rabary, B.; Blanchart, E.; Villenave, C. Mulch type affects soil biological functioning and crop yield of conservation agriculture systems in a long-term experiment in Madagascar. Soil Tillage Res. 2012, 118, 11–21. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems; CABI Publishing: Wallingford, UK, 2001. [Google Scholar]
- Ikhajiagbe, B.; Anoliefo, G.O.; Okoh, H.U.; Owenaezee, I. Impact of organic mulching on the enhanced natural attenuation of a petroleum hydrocarbon polluted soil. NISEB J. 2019, 13, 32–42. [Google Scholar]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Dubey, S.K.; Kumar, D.; Toor, A.S.; Walia, S.S.; Randhawa, M.K.; Shivey, Y.S. Enhanced Organic Carbon Triggers Transformations of Macronutrients, Micronutrients, and Secondary Plant Nutrients and Their Dynamics in the Soil under Different Cropping Systems-A Review. J. Soil Sci. Plant Nutr. 2024, 1–21. [Google Scholar] [CrossRef]
- Du, C.; Li, L.; Effah, Z. Effects of straw mulching and reduced tillage on crop production and environment: A review. Water 2022, 14, 2471. [Google Scholar] [CrossRef]
- Murray, H.; Pinchin, T.A.; Macfie, S.M. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J. Soils Sediments 2011, 11, 815–829. [Google Scholar] [CrossRef]
- Beckler, J.S.; Jones, M.E.; Taillefert, M. The origin, composition, and reactivity of dissolved iron (III) complexes in coastal organic-and iron-rich sediments. Geochim. Cosmochim. Acta 2015, 152, 72–88. [Google Scholar] [CrossRef]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Pan, H.J.; Zhang, K.Y.; Sun, W.; Wang, X.J.; Gao, H. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Raza, Q.U.A.; Bashir, M.A.; Rehim, A.; Sial, M.U.; Ali Raza, H.M.; Atif, H.M.; Geng, Y. Sugarcane industrial byproducts as challenges to environmental safety and their remedies: A review. Water 2021, 13, 3495. [Google Scholar] [CrossRef]
- Dania, S.O.; Ayegbe, A.O.; Amenkhienan, B.E. Effect of different rates of sawdust-piggery compost on soil properties and yield of maize in nutrient depleted soil. World J. Adv. Eng. Technol. Sci. 2021, 3, 016–022. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Kaur, M.; Singh, P.; Hossain, A. Effect of addition of organic manures on basmati yield, nutrient content and soil fertility status in north-western India. Heliyon 2023, 9, e14514. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Mai, T.; Uktta, M.; Sekine, M.; Higuchi, T. Distributions of iron, manganese, copper and zinc in various composts and amended soils. Environ. Technol. 2003, 24, 1517–1525. [Google Scholar] [CrossRef]
- Paul, E.A. Dynamics of organic matter in soils. Plant Soil 1984, 76, 275–285. [Google Scholar] [CrossRef]
- Yang, Y.J.; Dungan, R.S.; Ibekwe, A.M.; Valenzuela-Solano, C.; Crohn, D.M.; Crowley, D.E. Effect of organic mulches on soil bacterial communities one year after application. Biol. Fertil. Soils 2003, 38, 273–281. [Google Scholar] [CrossRef]
- Hitchmough, J. Establishment of Planted Nursery Stock. In Plant User Handbook: A Guide to Effective Specifying; Blackwell Science Ltd.: Chichester, UK, 2003; pp. 95–112. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Mandal, A.; Singh, R.; Dhaliwal, M.K. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Education: London, UK, 1996. [Google Scholar]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. Methods Assess. Soil Qual. 1997, 49, 169–185. [Google Scholar] [CrossRef]
- Lal, R. Soil health and climate change: An overview. In Soil Health and Climate Change; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–24. [Google Scholar] [CrossRef]
- Region-ASHS, S. American Society for Horticultural Science. HortScience 2022, 57, S2. [Google Scholar]
- Durán, J.; Morse, J.L.; Groffman, P.M.; Campbell, J.L.; Christenson, L.M.; Driscoll, C.T.; Templer, P.H. Winter climate change affects growing-season soil microbial biomass and activity in northern hardwood forests. Glob. Chang. Biol. 2014, 20, 3568–3577. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Olson, R.A. Effect of acid rain on soils. Crit. Rev. Environ. Sci. Technol. 1985, 15, 65–110. [Google Scholar] [CrossRef]
- Reddy, A.A. The soil health card Scheme in India: Lessons learned and challenges for replication in other developing countries. J. Nat. Resour. Policy Res. 2019, 9, 124–156. [Google Scholar] [CrossRef]
- Pramanik, P.; Bhattacharya, P.; Chakrabarti, B.; Ghosh, T. Improved Soil Environment Under Conservation Agriculture. In Sustainable Management of Soil and Environment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 169–192. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, J.; Zhang, Y.; Wu, J.; Zhang, J.; Pan, X.; He, F. Effects of tillage and mulching measures on soil moisture and temperature, photosynthetic characteristics and yield of winter wheat. Agric. Water Manag. 2018, 201, 299–308. [Google Scholar] [CrossRef]
- Pinamonti, F. Compost mulch effects on soil fertility, nutritional status and performance of grapevine. Nutr. Cycl. Agroecosyst. 1998, 51, 239–248. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Interactions between light and growing season temperatures on, growth and development and gas exchange of Semillon (Vitis vinifera L.) vines grown in an irrigated vineyard. Plant Physiol. Biochem. 2012, 54, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Miles, C.; Gerdeman, B.; LaHue, D.G.; DeVetter, L. Plastic mulch use in perennial fruit cropping systems—A review. Sci. Hortic. 2021, 281, 109975. [Google Scholar] [CrossRef]
- Eruola, A. Response of yam varieties to soil moisture regime in Southwestern Nigeria. Ital. J. Agrometeorol. 2021, 2, 3–14. [Google Scholar] [CrossRef]
- Olasantan, F.O. Effect of time of mulching on soil temperature and moisture regime and emergence, growth and yield of white yam in western Nigeria. Soil Tillage Res. 1999, 50, 215–221. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Holland, E.; Rebetzke, G.J.; Guo, Y.; Zheng, B. Dynamic quantification of canopy structure to characterize early plant vigour in wheat genotypes. J. Exp. Bot. 2016, 67, 4523–4534. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.L.; Rechcigl, J.E. Organic mulches, wood products, and composts as soil amendments and conditioners. In Handbook of Soil Conditioners; CRC Press: Boca Raton, FL, USA, 2020; pp. 43–95. [Google Scholar]
- Sastre, B.; Álvarez, B.; Antón, O.; Pérez, M.Á.; Marques, M.J.; Bienes, R.; García-Díaz, A. Groundcovers in olive groves in semiarid climates: Are they always beneficial? Water 2020, 12, 2230. [Google Scholar] [CrossRef]
- Karthika, K.S.; Rashmi, I.; Parvathi, M.S. Biological functions, uptake and transport of essential nutrients in relation to plant growth. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–49. [Google Scholar] [CrossRef]
- Acharya, M.; Ghimire, S.; Gautam, N. Evaluating the impact of mulching and fertilizer combinations in maximizing cucumber (Cucumis sativus L.) growth and production. Technol. Hortic. 2024, 4, e012. [Google Scholar] [CrossRef]
- Waheed, A.; Li, C.; Muhammad, M.; Ahmad, M.; Khan, K.A.; Ghramh, H.A.; Zhang, D. Sustainable potato growth under straw mulching practices. Sustainability 2023, 15, 10442. [Google Scholar] [CrossRef]
- Filipović, A.; Perčin, A.; Hadžiabulić, A.; Mandić, A. Transformation of organic matter and impact on the ecosystem. In Agroforestry for Carbon and Ecosystem Management; Academic Press: Cambridge, MA, USA, 2024; pp. 311–329. [Google Scholar] [CrossRef]
- Jabran, K.; Chauhan, B.S. Weed control using ground cover systems. In Non-Chemical Weed Control; Academic Press: Cambridge, MA, USA, 2018; pp. 61–71. [Google Scholar] [CrossRef]
- Shah, S.T.; Ullah, I.; Basit, A.; Sajid, M.; Arif, M.; Mohamad, H.I. Mulching is a mechanism to reduce environmental stresses in plants. In Mulching in Agroecosystems: Plants, Soil & Environment; Springer Nature: Singapore, 2022; pp. 353–376. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 75. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, J.; Dai, H.; Wang, D.; Li, D. Effect of ridge–furrow and plastic-mulching planting patterns on yield formation and water movement of potato in a semi-arid area. Agric. Water Manag. 2014, 131, 87–94. [Google Scholar] [CrossRef]
- Davis, A.J.; Strik, B.C. Long-term organic production systems in northern highbush blueberry: Placing weed mat over existing organic mulches and changing to nitrogen-only fertilizer sources increased yield. HortScience 2021, 56, 897–908. [Google Scholar] [CrossRef]
- Westbrook, A.S.; Bhaskar, V.; DiTommaso, A. Weed control and community composition in living mulch systems. Weed Res. 2022, 62, 12–23. [Google Scholar] [CrossRef]
- Bhaskar, V.; Bellinder, R.R.; DiTommaso, A.; Walter, M.F. Living mulch performance in a tropical cotton system and impact on yield and weed control. Agriculture 2018, 8, 19. [Google Scholar] [CrossRef]

| S. No | Soil Properties | Value | Rating |
|---|---|---|---|
| 1 | Soil pH | 5.77 | Slightly acidic |
| 2 | EC (mS cm−1) | 0.05 | Very low salinity |
| 3 | BD (g cm−3) | 1.09 | Optimal |
| 4 | Temperature (°C) | 27.33 | Warm |
| 5 | SOC (%) | 2.27 | High |
| 6 | SAN (%) | 0.08 | Low |
| 7 | SAP (mg kg−1) | 12.33 | Medium |
| 8 | SEK (me/100 g) | 0.40 | Low |
| 9 | SECa (me/100 g) | 18.52 | High |
| 10 | SEMg (me/100 g) | 11.30 | High |
| 11 | SENa (me/100 g) | 0.04 | Very low |
| 12 | SEFe (mg/kg) | 30.62 | High |
| 13 | SEMn (mg/kg) | 45.20 | Very high |
| 14 | SECu (mg/kg) | 6.55 | High |
| 15 | SEZn (mg/kg) | 1.50 | Medium |
| Treatments | Soil pH | EC (mS/cm) | Moisture (%) | Temperature (°C) |
|---|---|---|---|---|
| BPM | 5.73 ± 0.09 b | 0.05 ± 0.003 ab | 10.20 ± 0.96 ab | 28.80 ± 0.44 a |
| WMM | 5.83 ± 0.12 ab | 0.05 ± 0.010 b | 10.10 ± 1.07 ab | 28.50 ± 0.29 ab |
| SSM | 5.60 ± 0.06 b | 0.07 ± 0.003 a | 9.50 ± 1.57 b | 28.50 ± 0.01 ab |
| OCM | 6.37 ± 0.38 a | 0.07 ± 0.012 a | 12.50 ± 0.32 ab | 28.80 ± 0.17 a |
| CLM | 5.83 ± 0.07 ab | 0.04 ± 0.007 b | 12.70 ± 0.21 ab | 28.70 ± 0.17 ab |
| JGM | 5.60 ± 0.01 b | 0.05 ± 0.003 ab | 10.80 ± 2.02 ab | 28.30 ± 0.17 ab |
| SDM | 5.63 ± 0.23 b | 0.03 ± 0.003 b | 12.90 ± 0.29 ab | 28.20 ± 0.17 ab |
| CON | 5.87 ± 0.15 ab | 0.04 ± 0.003 b | 13.50 ± 0.45 a | 28.00 ± 0.01 b |
| CD (p ≤ 0.05) | 1.69 | 0.06 | 10.12 | 2.12 |
| SE (d) | 0.26 | 0.01 | 1.58 | 0.33 |
| Treatments | SOC (%) | SAN (%) | SAP (mg/kg) | SEK (me/100 g) | SECa (me/100 g) | SEMg (me/100 g) | SENa (me/100 g) |
|---|---|---|---|---|---|---|---|
| BPM | 2.63 ± 0.03 ab | 0.19 ± 0.02 b | 12.30 ± 2.03 b | 0.61 ± 0.10 ab | 26.10 ± 6.04 a | 14.70 ± 0.47 a | 0.08 ± 0.03 a |
| WMM | 2.60 ± 0.01 ab | 0.21 ± 0.01 ab | 17.70 ± 0.33 b | 1.07 ± 0.27 a | 20.70 ± 1.42 a | 14.10 ± 0.75 a | 0.05 ± 0.02 a |
| SSM | 2.60 ± 0.15 ab | 0.29 ± 0.06 a | 18.70 ± 6.69 b | 0.80 ± 0.10 ab | 21.80 ± 0.77 a | 14.60 ± 0.31 a | 0.10 ± 0.02 a |
| OCM | 2.90 ± 0.31 a | 0.20 ± 0.02 ab | 38.00 ± 16.30 a | 0.63 ± 0.12 ab | 27.30 ± 4.77 a | 14.60 ± 0.70 a | 0.06 ± 0.02 a |
| CLM | 2.47 ± 0.18 ab | 0.21 ± 0.01 ab | 11.00 ± 1.00 b | 0.50 ± 0.10 b | 21.10 ± 0.93 a | 14.60 ± 0.60 a | 0.11 ± 0.04 a |
| JGM | 2.50 ± 0.21 ab | 0.18 ± 0.01 b | 10.70 ± 3.28 b | 0.90 ± 0.20 ab | 17.40 ± 0.74 a | 13.80 ± 0.60 a | 0.10 ± 0.03 a |
| SDM | 2.53 ± 0.09 ab | 0.22 ± 0.01 ab | 11.30 ± 2.19 b | 0.46 ± 0.11 b | 20.70 ± 0.46 a | 13.60 ± 1.63 a | 0.10 ± 0.02 a |
| CON | 2.37 ± 0.18 b | 0.24 ± 0.02 ab | 7.67 ± 2.91 b | 0.46 ± 0.08 b | 21.70 ± 2.18 a | 15.20 ± 0.12 a | 0.12 ± 0.01 a |
| CD (p ≤ 0.05) | 1.23 | 0.23 | 57.37 | 1.32 | 27.84 | 5.02 | 0.22 |
| SE (d) | 0.19 | 0.04 | 8.98 | 0.21 | 4.36 | 0.79 | 0.03 |
| Treatments | SEFe (mg/kg) | SEMn (mg/kg) | SECu (mg/kg) | SEZn (mg/kg) |
|---|---|---|---|---|
| BPM | 81.70 ± 3.53 a | 76.70 ± 4.91 a | 9.00 ± 0.01 a | 2.00 ± 0.01 b |
| WMM | 70.70 ± 1.20 ab | 70.30 ± 4.91 a | 8.33 ± 0.33 a | 2.00 ± 0.01 b |
| SSM | 76.70 ± 10.70 a | 79.30 ± 4.67 a | 7.67 ± 0.67 a | 2.00 ± 0.58 b |
| OCM | 52.00 ± 2.52 b | 51.00 ± 6.11 b | 7.67 ± 0.33 a | 4.13 ± 1.95 a |
| CLM | 62.70 ± 8.99 ab | 65.00 ± 2.00 ab | 8.33 ± 0.67 a | 1.67 ± 0.33 b |
| JGM | 60.00 ± 9.50 ab | 67.00 ± 6.00 ab | 7.67 ± 0.88 a | 1.67 ± 0.33 b |
| SDM | 82.00 ± 1.00 a | 78.00 ± 9.07 a | 9.00 ± 0.01 a | 2.00 ± 0.01 b |
| CON | 64.00 ± 9.29 ab | 69.70 ± 7.88 a | 7.67 ± 0.88 a | 1.67 ± 0.33 b |
| CD (p ≤ 0.05) | 59.47 | 47.22 | 4.28 | 6.35 |
| SE (d) | 9.31 | 7.39 | 0.67 | 0.99 |
| Treatments | PCA-Based SQI | Regression-Based SQI | Linear Scoring-Based SQI |
|---|---|---|---|
| BPM | 0.370 | 0.625 | 0.379 |
| WMM | 0.125 | 0.875 | 0.130 |
| SSM | 0.000 | 1.000 | 0.000 |
| OCM | 0.500 | 0.500 | 0.503 |
| CLM | 1.000 | 0.000 | 1.000 |
| JGM | 0.500 | 0.500 | 0.498 |
| SDM | 0.750 | 0.249 | 0.748 |
| CON | 0.500 | 0.500 | 0.501 |
| Treatments | Vine Length Plant−1 (m) | Stem Diameter Plant−1 (mm) | Total Number of Tubers Plot−1 | Average Tuber Weight Plant−1 (kg) | Tuber Weight Plot−1 (kg) | Tuber Yield (kg ha−1) |
|---|---|---|---|---|---|---|
| BPM | 3.02 ± 0.16 ab | 5.64 ± 0.34 ab | 16.33 ± 1.20 ab | 0.61 ± 0.19 b | 3.76 ± 1.01 bc | 4703.33 ± 1257.55 bc |
| WMM | 3.39 ± 0.11 a | 5.47 ± 0.20 ab | 18.00 ± 4.04 ab | 1.19 ± 0.25 a | 9.51 ± 1.94 a | 11,894.17 ± 2419.80 a |
| SSM | 2.79 ± 0.24 abc | 5.44 ± 0.39 ab | 17.00 ± 0.58 ab | 0.54 ± 0.16 b | 4.90 ± 1.63 bc | 6125.00 ± 2039.80 bc |
| OCM | 2.64 ± 0.32 bcd | 6.17 ± 0.13 a | 20.33 ± 3.18 ab | 0.59 ± 0.14 b | 5.06 ± 1.33 bc | 6328.33 ± 1663.58 bc |
| CLM | 2.25 ± 0.07 cd | 5.44 ± 0.07 ab | 14.00 ± 3.21 ab | 0.12 ± 0.02 b | 1.11 ± 0.42 c | 1380.83 ± 528.00 c |
| JGM | 2.00 ± 0.19 d | 5.00 ± 0.10 b | 25.33 ± 3.71 a | 0.59 ± 0.15 b | 5.61 ± 0.90 b | 7013.33 ± 1121.93 b |
| SDM | 2.00 ± 0.21 bcd | 5.03 ± 0.41 b | 12.00 ± 1.73 b | 0.37 ± 0.06 b | 2.37 ± 0.55 bc | 2965.00 ± 689.51 bc |
| CON | 1.99 ± 0.16 d | 5.25 ± 0.14 b | 17.00 ± 8.50 ab | 0.22 ± 0.10 b | 1.94 ± 0.89 bc | 2426.67 ± 1107.19 bc |
| CD (p ≤ 0.05) | 1.87 | 2.37 | 33.89 | 1.41 | 11.45 | 14,311.58 |
| SE (d) | 0.29 | 0.37 | 5.31 | 0.22 | 1.79 | 2240.137 |
| Treatments | Total Cost of Cultivation (FJD ha−1) | Total Yield (kg ha−1) | Gross Returns (FJD ha−1) | Net Returns (FJD ha−1) | Cost-Benefit Ratio (BCR) | Internal Rate of Return (IRR) | Payback Period (Years) | Return on Investment (ROI) | Break-Even Yield (kg ha−1) | Break-Even Price (FJD kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| BPM | $21,907.63 | 4703.33 | $28,219.98 | $6312.35 | 1.29 | 28.81% | 3.47 | 28.81% | 3651.27 | $4.66 |
| WMM | $35,857.63 | 11,894.17 | $71,365.02 | $35,507.39 | 1.99 | 99.00% | 1.99 | 99.00% | 5976.27 | $3.01 |
| SSM | $27,650.59 | 6125.00 | $36,750.00 | $9099.41 | 1.33 | 32.90% | 3.04 | 32.90% | 4608.43 | $4.52 |
| OCM | $27,650.59 | 6328.33 | $37,969.98 | $10,319.39 | 1.37 | 37.30% | 2.68 | 37.30% | 4608.43 | $4.37 |
| CLM | $17,750.83 | 1380.83 | $8284.98 | −$9465.85 | 0.47 | −53.32% | N/A | −53.32% | 2958.47 | $12.86 |
| JGM | $27,650.59 | 7013.33 | $42,079.98 | $14,429.39 | 1.52 | 52.17% | 2.18 | 52.17% | 3775.10 | $3.94 |
| SDM | $32,650.59 | 2965.00 | $17,790.00 | −$14,860.59 | 0.54 | −45.53% | N/A | −45.53% | 5441.77 | $11.01 |
| CON | $20,840.55 | 2426.67 | $14,560.02 | −$6280.53 | 0.70 | −30.14% | N/A | −30.14% | 3473.43 | $8.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.S.; Wani, O.A.; Prasad, B.; Banuve, A.; Mua, P.; Sharma, A.C.; Prasad, S.; Malik, A.R.; El-Hendawy, S.; Mattar, M.A. Effects of Mulching on Soil Properties and Yam Production in Tropical Region. Sustainability 2024, 16, 7787. https://doi.org/10.3390/su16177787
Kumar SS, Wani OA, Prasad B, Banuve A, Mua P, Sharma AC, Prasad S, Malik AR, El-Hendawy S, Mattar MA. Effects of Mulching on Soil Properties and Yam Production in Tropical Region. Sustainability. 2024; 16(17):7787. https://doi.org/10.3390/su16177787
Chicago/Turabian StyleKumar, Shamal Shasang, Owais Ali Wani, Binesh Prasad, Amena Banuve, Penaia Mua, Ami Chand Sharma, Shalendra Prasad, Abdul Raouf Malik, Salah El-Hendawy, and Mohamed A. Mattar. 2024. "Effects of Mulching on Soil Properties and Yam Production in Tropical Region" Sustainability 16, no. 17: 7787. https://doi.org/10.3390/su16177787






