Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Precursor
2.3. Reactivity Test
2.4. AAM Synthesis and Strength Measurement
2.5. XRD and FTIR Analyses
2.6. SEM-EDS Measurement
2.7. Leaching of Synthesized AAMs
3. Results and Discussion
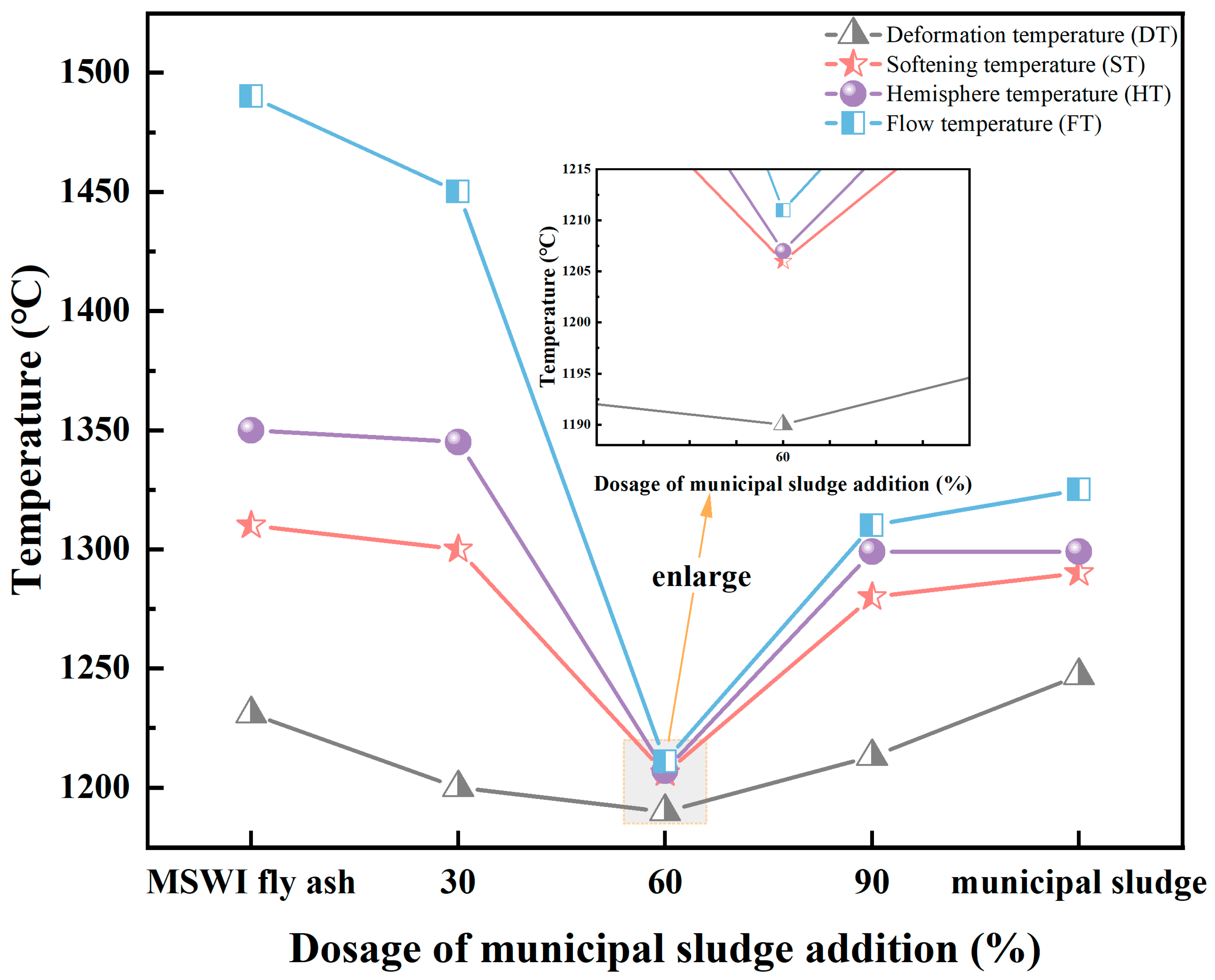
3.1. Physiochemical Property of Precursors
3.2. Reactivities of Precursors
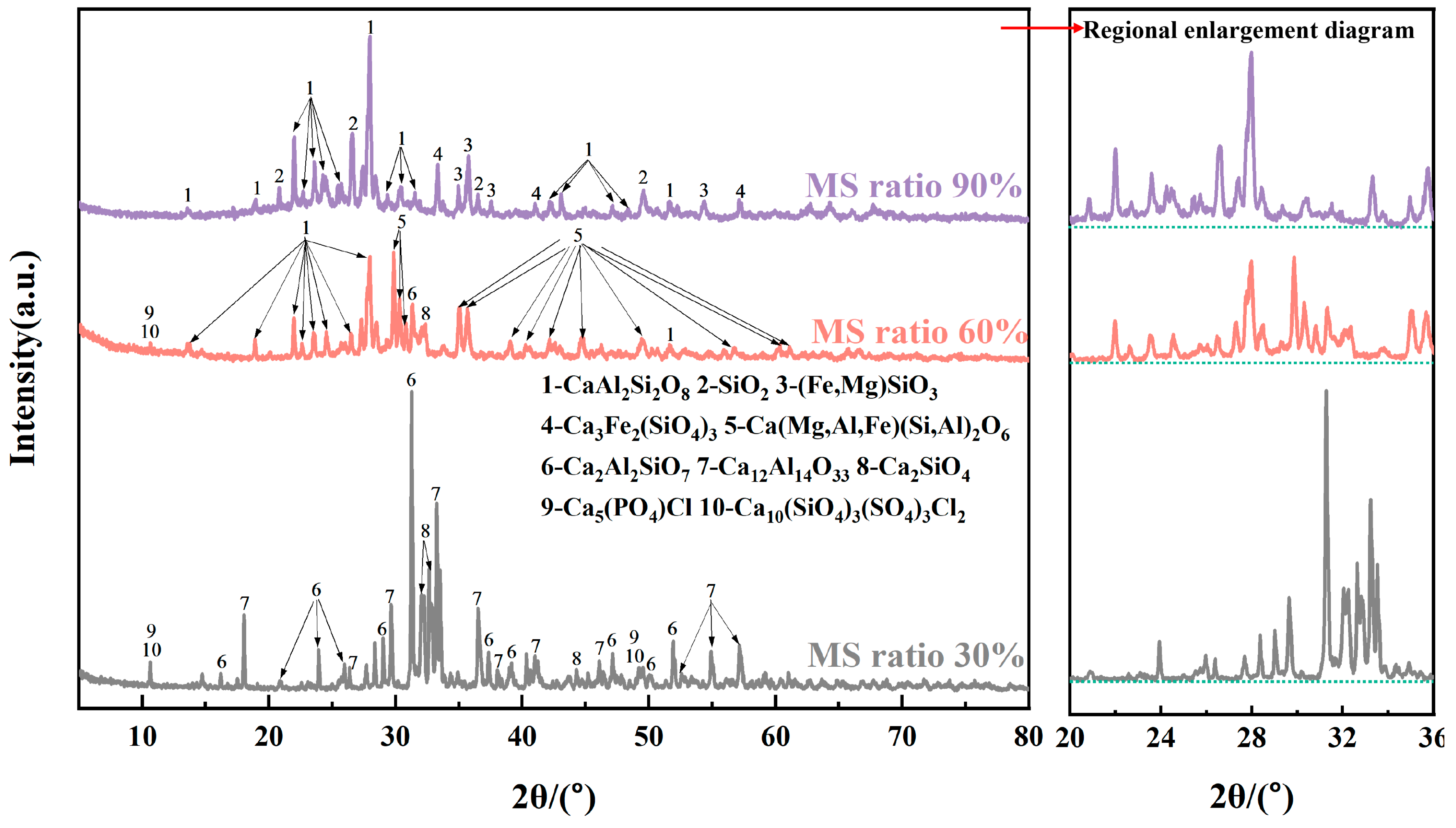
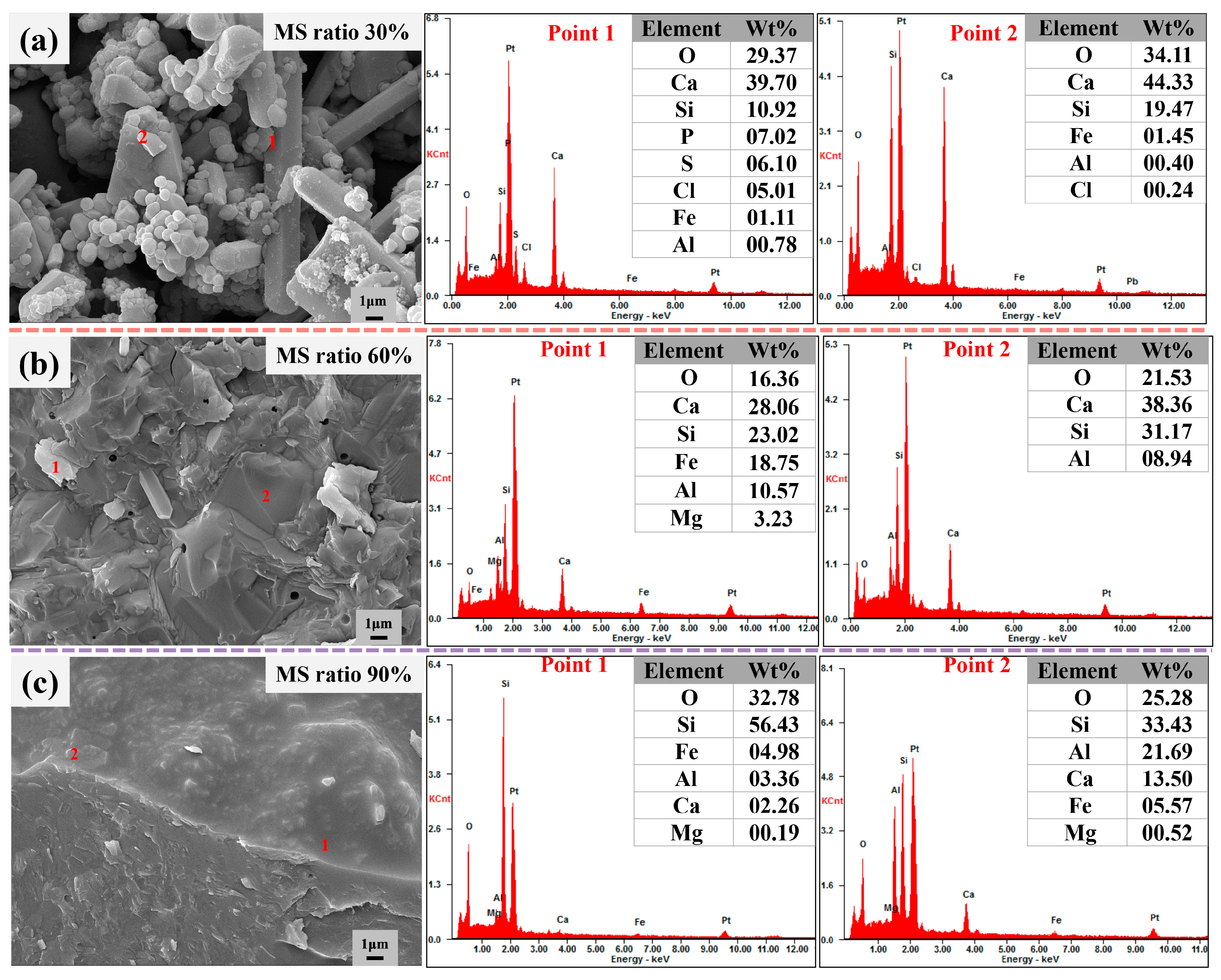
3.3. Alkali Activation Mechanisms
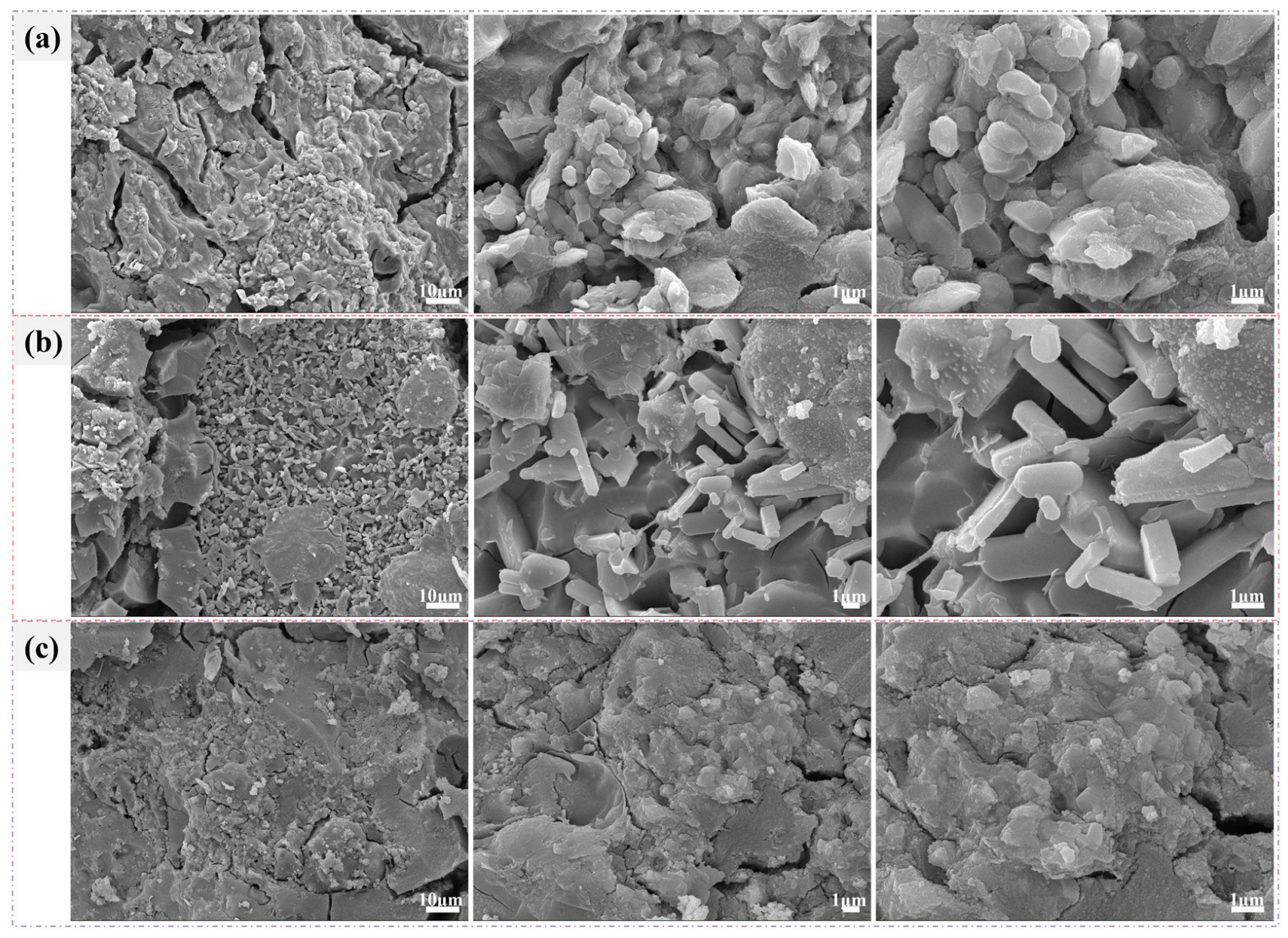
3.4. Mechanical Properties of Synthesized AAM Mortar
3.5. Environmental Compatibility
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, M.; Ge, W.; Xia, Y.; Sun, C.; Lin, X.; Tsang, D.C.W.; Ling, T.-C.; Hu, Y.; Wang, L.; Yan, J. Upcycling MSWI fly ash into green binders via flue gas-enhanced wet carbonation. J. Clean. Prod. 2024, 440, 141013. [Google Scholar] [CrossRef]
- He, H.; Gao, X.; Fei, X. Generation and management of municipal solid waste in top metropolitans of China: A comparison with Singapore. Circ. Econ. 2023, 2, 100041. [Google Scholar] [CrossRef]
- Jiang, Y.; Leng, B.; Xi, J. Assessing the social cost of municipal solid waste management in Beijing: A systematic life cycle analysis. Waste Manag. 2024, 173, 62–74. [Google Scholar] [CrossRef]
- Sun, C.; Ge, W.; Zhang, Y.; Wang, L.; Xia, Y.; Lin, X.; Huang, Q.; Lu, S.; Tsang, D.C.W.; Yan, J. Designing low-carbon cement-free binders for stabilization/solidification of MSWI fly ash. J. Environ. Manag. 2023, 339, 117938. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. China Statistical Yearbook; China Statistics Press: Beijing, China, 2023. [Google Scholar]
- Yuan, Z.; Cai, G.; Gao, L.; Wu, M.; Kong, L.; Bai, J.; Bai, Z.; Li, H.; Li, W. The physical encapsulation and chemical fixation of Zn during thermal treatment process of municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2023, 166, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Siao, H.-J.; Gau, S.-H.; Kuo, J.-H.; Li, M.-G.; Sun, C.-J. Life-Cycle Assessment of Municipal Solid Waste Incineration Fly Ash Recycling as a Feedstock for Brick Manufacturing. Sustainability 2023, 15, 10284. [Google Scholar] [CrossRef]
- Dongyang, H.; Hongyun, H.; Facun, J.; Wu, Z.; Changqi, L.; Hao, X.; Lu, D.; Xinye, W. Thermal separation of heavy metals from municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2023, 467, 143344. [Google Scholar]
- Zou, D.; Wang, X.; Wu, C.; Li, T.; Wang, M.; Liu, S.; Wang, Q.; Shimaoka, T. Dechlorination of Municipal Solid Waste Incineration Fly Ash by Leaching with Fermentation Liquid of Food Waste. Sustainability 2020, 12, 4389. [Google Scholar] [CrossRef]
- Clavier, K.A.; Liu, Y.; Intrakamhaeng, V.; Townsend, T.G. Re-evaluating the TCLP’s Role as the Regulatory Driver in the Management of Municipal Solid Waste Incinerator Ash. Environ. Sci. Technol. 2019, 53, 7964–7973. [Google Scholar] [CrossRef]
- Bie, R.; Chen, P.; Song, X.; Ji, X. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 89, 704–712. [Google Scholar] [CrossRef]
- Lindberg, D.; Molin, C.; Hupa, M. Thermal treatment of solid residues from WtE units: A review. Waste Manag. 2015, 37, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Shi, W.; Shi, Y.; Chen, D.; Liu, B.; Chu, C.; Li, D.; Li, Y.; Chen, G. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash. J. Hazard. Mater. 2021, 408, 124809. [Google Scholar] [CrossRef]
- Xu, S.; Hu, H.; Guo, G.; Gong, L.; Liu, H.; Yao, H. Investigation of properties change in the reacted molten salts after molten chlorides cyclic thermal treatment of toxic MSWI fly ash. J. Hazard. Mater. 2022, 421, 126536. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yuan, R.; Gan, M.; Ji, Z.; Sun, Z. Subcritical hydrothermal treatment of municipal solid waste incineration fly ash: A review. Sci. Total Environ. 2023, 865, 160745. [Google Scholar] [CrossRef]
- Hung, P.C.; Chen, Q.H.; Chang, M.B. Pyrolysis of MWI fly ash—Effect on dioxin-like congeners. Chemosphere 2013, 92, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Chen, C.; Shi, X.; Wu, S.; Jia, Y.; Du, B.; Liu, J. Recovery of metals from municipal solid waste incineration fly ash and red mud via a co-reduction process. Resour. Conserv. Recycl. 2020, 154, 104600. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Z.; Xu, C.; Huang, X. A new efficient paradigm of energy and resource recovery from sewage: AnMBR treating chemically pre-precipitated concentrate in sidestream. Chem. Eng. J. 2023, 478, 147309. [Google Scholar] [CrossRef]
- Ma, D.; Cheng, S.; Zhang, Y.; Ullah, F.; Ji, G.; Li, A. Relation between hydrophilic/hydrophobic characteristics of sludge extracellular polymeric substances and sludge moisture-holding capacity in hot-pressing drying. Sci. Total Environ. 2024, 916, 170233. [Google Scholar] [CrossRef]
- Un, C. Enhancing Sewage Sludge Treatment with Hydrothermal Processing: A Case Study of Adana City. Sustainability 2024, 16, 4174. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Chang, Y. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Heavy metals, volatile organic compounds and combustion characteristics of hydrochar. Chem. Eng. J. 2016, 297, 1–10. [Google Scholar] [CrossRef]
- Elmi, A.; Al-Khaldy, A.a.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Detho, A.; Kadir, A.A.; Ahmad, S. Utilization of wastewater treatment sludge in the production of fired clay bricks: An approach towards sustainable development. Results Eng. 2024, 21, 101708. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Fořt, J.; Klapiszewska, I.; Thomas, M.; Klapiszewski, Ł.; Černý, R. A literature review of the latest trends and perspectives regarding alkali-activated materials in terms of sustainable development. J. Mater. Res. Technol. 2023, 25, 5394–5425. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Tositti, L.; Masi, G.; Morozzi, P.; Zappi, A.; Chiara Bignozzi, M. Cleaner, sustainable, and safer: Green potential of alkali-activated materials in current building industry, radiological good practice, and a few tips. Constr. Build. Mater. 2023, 409, 133879. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Zhang, S. Dispersion, properties, and mechanisms of nanotechnology-modified alkali-activated materials: A review. Renew. Sustain. Energy Rev. 2024, 192, 114215. [Google Scholar] [CrossRef]
- Ailar, H.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valorization 2016, 8, 225–233. [Google Scholar]
- Wang, K.-t.; Du, L.-q.; Lv, X.-s.; He, Y.; Cui, X.-m. Preparation of drying powder inorganic polymer cement based on alkali-activated slag technology. Powder Technol. 2017, 312, 204–209. [Google Scholar] [CrossRef]
- Kravchenko, E.; Lazorenko, G.; Jiang, X.; Leng, Z. Alkali-activated materials made of construction and demolition waste as precursors: A review. Sustain. Mater. Technol. 2024, 39, e00829. [Google Scholar] [CrossRef]
- Mahutjane, T.C.; Tchadjié, L.N.; Sithole, T.N. The feasibility of utilizing sewage sludge as a source of aluminosilicate to synthesise geopolymer cement. J. Mater. Res. Technol. 2023, 25, 3314–3323. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, L.; Hu, C.; Gong, J.; Xu, T.; Li, J.; Yang, L.; Bai, J.; Zhong, S. Co-disposal of MSWI fly ash and electrolytic manganese residue based on geopolymeric system. Waste Manag. 2018, 82, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Rao, F.; Leon-Patino, C.A.; Song, S. Effects of aluminum on the expansion and microstructure of alkali-activated MSWI fly ash-based pastes. Chemosphere 2020, 240, 124986. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- de Godoy, L.G.G.; Rohden, A.B.; Garcez, M.R.; da Costa, E.B.; Da Dalt, S.; de Oliveira Andrade, J.J. Valorization of water treatment sludge waste by application as supplementary cementitious material. Constr. Build. Mater. 2019, 223, 939–950. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Wen, Z.; Yan, D.; Liu, M.; Huang, Q.; Zhu, Z. Removal of dioxins from municipal solid waste incineration fly ash by low-temperature thermal treatment: Laboratory simulation of degradation and ash discharge stages. Waste Manag. 2023, 168, 45–53. [Google Scholar] [CrossRef]
- Gan, M.; Xing, J.; Tang, Q.; Ji, Z.; Fan, X.; Zheng, H.; Sun, Z.; Chen, X. Basic Research on Co-treatment of Municipal Solid Waste Incineration Fly Ash and Municipal Sludge for Energy-Saving Melting. ACS Omega 2022, 7, 45153–45164. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, Y.; Yu, M.; Cheng, H.; Li, Z.; Xu, W. Mineral phase evolution and heavy metals migration during the hydrothermal treatment of municipal solid waste incineration fly ash. Fuel 2024, 357, 129790. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, N.; Li, X.; Xi, Y.; Shen, W.; Wu, P. Triggered heavy metals and chlorine simultaneous removal from hazardous waste incineration fly ash. Process Saf. Saf. Environ. Environ. Prot. Prot. 2023, 175, 796–805. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, M.; Zhou, Y.; Wang, T.; Fang, Y.; Sui, L.; Gong, G. Chloride binding mechanism and free chloride reduction method of alkali-activated slag/fly ash mixed with seawater. Constr. Constr. Build. Build. Mater. Mater. 2023, 409, 134079. [Google Scholar] [CrossRef]
- Ascensão, G.; Marchi, M.; Segata, M.; Faleschini, F.; Pontikes, Y. Reaction kinetics and structural analysis of alkali activated Fe–Si–Ca rich materials. J. Clean. Prod. 2020, 246, 119065. [Google Scholar] [CrossRef]
- Fan, M.X.; Chen, F.X.; Zhang, X.Y.; Wang, R.K.; Yu, R. Effect of Ca/Si ratio on the characteristics of alkali-activated ultra-high performance concrete (A-UHPC): From hydration kinetics to microscopic structure development. Constr. Build. Mater. 2023, 394, 132158. [Google Scholar] [CrossRef]
- Chen, B.; Perumal, P.; Aghabeyk, F.; Adediran, A.; Illikainen, M.; Ye, G. Advances in using municipal solid waste incineration (MSWI) bottom ash as precursor for alkali-activated materials: A critical review. Resour. Conserv. Recycl. 2024, 204, 107516. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Li, W.; Nie, Y.; Wang, F.; Bian, R.; Wang, H.; Wang, Y.N.; Gong, Z.; Lu, J.; et al. Solidification/stabilization pre-treatment coupled with landfill disposal of heavy metals in MSWI fly ash in China: A systematic review. J. Hazard. Mater. 2024, 478, 135479. [Google Scholar] [CrossRef]
- Ren, J.; Hu, L.; Dong, Z.; Tang, L.; Xing, F.; Liu, J. Effect of silica fume on the mechanical property and hydration characteristic of alkali-activated municipal solid waste incinerator (MSWI) fly ash. J. Clean. Prod. 2021, 295, 126317. [Google Scholar] [CrossRef]
- Liu, J.; Xie, G.; Wang, Z.; Zeng, C.; Fan, X.; Li, Z.; Ren, J.; Xing, F.; Zhang, W. Manufacture of alkali-activated cementitious materials using municipal solid waste incineration (MSWI) ash: Immobilization of heavy metals in MSWI fly ash by MSWI bottom ash. Constr. Build. Mater. 2023, 392, 131848. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Lothenbach, B.; De Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs. Mater. Struct. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Li, L.; Ren, Q.; Li, S.; Lu, Q. Effect of Phosphorus on the Behavior of Potassium during the Co-combustion of Wheat Straw with Municipal Sewage Sludge. Energy Fuels 2013, 27, 5923–5930. [Google Scholar] [CrossRef]
- Long, Y.; Song, Y.; Yang, Y.; Huang, H.; Fang, H.; Shen, D.; Geng, H.; Ruan, J.; Gu, F. Co-vitrification of hazardous waste incineration fly ash and hazardous waste sludge based on CaO–SiO2–Al2O3 system. J. Environ. Manag. 2023, 338, 117776. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Fotio, D.; Rüscher, C.H.; Kamseu, E.; Djobo, J.N.Y.; Bignozzi, M.C.; Leonelli, C. The effects of synthesized calcium phosphate compounds on the mechanical and microstructural properties of metakaolin-based geopolymer cements. Constr. Build. Mater. 2018, 163, 776–792. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S. Time-resolved and spatially-resolved infrared spectroscopic observation of seeded nucleation controlling geopolymer gel formation. J. Colloid Interface Sci. 2011, 357, 384–392. [Google Scholar] [CrossRef]
- Díaz, A.G.; Bueno, S.; Villarejo, L.P.; Eliche-Quesada, D. Improved strength of alkali activated materials based on construction and demolition waste with addition of rice husk ash. Constr. Build. Mater. 2024, 413, 134823. [Google Scholar] [CrossRef]
- Xu, R.; Wang, H.; Yang, R.; Kong, F.; Hong, T. The potential of copper slag as a precursor for partially substituting blast furnace slag to prepare alkali-activated materials. J. Clean. Prod. 2024, 434, 140283. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Liu, Z. A comparative study on characteristics and leaching toxicity of fluidized bed and grate furnace MSWI fly ash. J. Environ. Manag. 2022, 305, 114345. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.-S.; Zhan, B.-J.; Sharma, U.; Poon, C.S. Compressive strength and microstructural properties of dry-mixed geopolymer pastes synthesized from GGBS and sewage sludge ash. Constr. Build. Mater. 2018, 182, 597–607. [Google Scholar] [CrossRef]
- Londono-Zuluaga, D.; Tobón, J.I.; Aranda, M.A.G.; Santacruz, I.; De la Torre, A.G. Clinkering and hydration of belite-alite-ye’elimite cement. Cem. Concr. Compos. 2017, 80, 333–341. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different admixtures and additives on the properties of alkali-activated fly ash. Mater. Des. 2014, 53, 1005–1025. [Google Scholar] [CrossRef]
- Garanayak, L. Behavior of alkali activated fly ash slag paste at room temperature. Mater. Today Proc. 2021, 43, 1865–1873. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A.; van der Sloot, H.A. pH dependent leaching characterization of major and trace elements from fly ash and metakaolin geopolymers. Cem. Concr. Res. 2019, 125, 105889. [Google Scholar] [CrossRef]
- Hartwich, P.; Vollpracht, A. Influence of leachate composition on the leaching behaviour of concrete. Cem. Concr. Res. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. The role of sulfide in the immobilization of Cr(VI) in fly ash geopolymers. Cem. Concr. Res. 2008, 38, 681–688. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009. [Google Scholar]
- Engelsen, C.J.; van der Sloot, H.A.; Wibetoe, G.; Justnes, H.; Lund, W.; Stoltenberg-Hansson, E. Leaching characterisation and geochemical modelling of minor and trace elements released from recycled concrete aggregates. Cem. Concr. Res. 2010, 40, 1639–1649. [Google Scholar] [CrossRef]








| Material | CaO | SiO2 | Al2O3 | MgO | K2O | Na2O | P2O5 | SO3 | Fe2O3 | TiO2 | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSWI-FA | 58.44 | 2.77 | 0.89 | 1.22 | 2.45 | 2.54 | 0.31 | 7.08 | 1.18 | 0.20 | 15.31 |
| MS | 3.03 | 49.76 | 15.47 | 1.20 | 2.27 | 0.61 | 5.78 | 0.13 | 12.84 | 1.02 | 0.01 |
| Sample ID | Molar Ratio | ||
|---|---|---|---|
| CaO/SiO2 | (Na2O+CaO)/SiO2 | SiO2/Al2O3 | |
| AAM-30%MS | 1.95 | 1.06 | 7.44 |
| AAM-60%MS | 0.73 | 0.42 | 6.55 |
| AAM-90%MS | 0.18 | 0.14 | 6.21 |
| Sample ID | Compressive Strength in MPa | Strength Activity Index | ||||
|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |
| control | 38.8 | 45.7 | 50.0 | / | / | / |
| OPC-MS-0.3 | 27.5 | 34.7 | 40.0 | 0.71 | 0.76 | 0.80 |
| OPC-MS-0.6 | 30.7 | 40.2 | 48.5 | 0.79 | 0.88 | 0.97 |
| OPC-MS-0.9 | 31.2 | 42.2 | 54.1 | 0.80 | 0.92 | 1.08 |
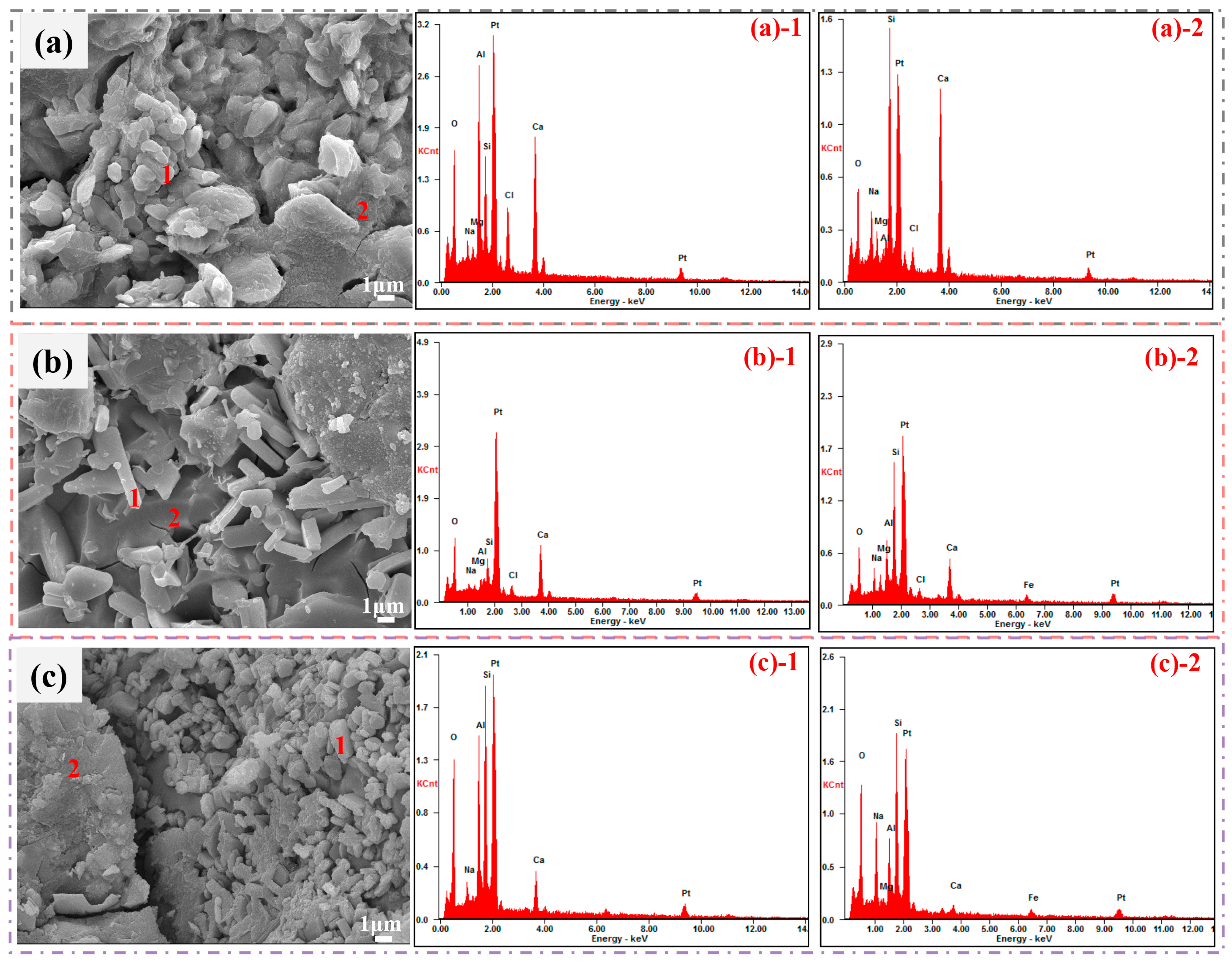
| Point | O | Ca | Si | Al | Na | Mg | Fe | Cl |
|---|---|---|---|---|---|---|---|---|
| (a)-1 | 29.46 | 31.27 | 9.57 | 17.16 | 2.01 | 1.00 | - | 9.53 |
| (a)-2 | 23.71 | 42.33 | 22.11 | 0.43 | 5.66 | 2.83 | - | 2.93 |
| (b)-1 | 43.76 | 37.02 | 8.81 | 1.98 | 3.03 | 1.16 | - | 4.24 |
| (b)-2 | 21.70 | 19.86 | 26.30 | 9.90 | 5.40 | 2.85 | 10.21 | 1.91 |
| (c)-1 | 34.56 | 11.90 | 31.06 | 19.44 | 3.04 | - | - | - |
| (c)-2 | 30.30 | 3.39 | 30.96 | 9.44 | 13.88 | 0.62 | 8.85 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Li, X.; Gan, M.; Ji, Z.; Fan, X.; Xing, J. Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material. Sustainability 2024, 16, 7912. https://doi.org/10.3390/su16187912
Sun Z, Li X, Gan M, Ji Z, Fan X, Xing J. Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material. Sustainability. 2024; 16(18):7912. https://doi.org/10.3390/su16187912
Chicago/Turabian StyleSun, Zengqing, Xiaoyu Li, Min Gan, Zhiyun Ji, Xiaohui Fan, and Jinxin Xing. 2024. "Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material" Sustainability 16, no. 18: 7912. https://doi.org/10.3390/su16187912
APA StyleSun, Z., Li, X., Gan, M., Ji, Z., Fan, X., & Xing, J. (2024). Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material. Sustainability, 16(18), 7912. https://doi.org/10.3390/su16187912







