Abstract
To ensure that metal recovery processes in electronic waste are truly sustainable from an industrial perspective, studies on the performance of such methodologies are necessary to verify the economic, environmental, social, and technological viabilities. The importance of conducting multicriteria and comparative investigations into the actual performances of methods used in the recovery of these materials is emphasized, considering trade-offs such as high efficiency in metal extraction balanced against intense consumption of energy and chemical reagents. The analytical hierarchy process, multicriteria decision support tool, and the life cycle assessment tool are proposed to be used in combination in this work to assess and contrast the environmental effects of two hydrometallurgical paths for the recuperation of copper in electronic circuit boards (PCBs). The results indicate that the sulfuric acid method had a copper solubilization efficiency of 90.05%, whereas the route employing the combination of ammonium sulfate and ammonia had an estimated copper solubilization efficacy of 49%. It was feasible to calculate the life cycle effects of the hydrometallurgical procedures connected to the copper recovery activities on the PCBs with regard to the LCA. Compared to the acidic leaching pathway, alkaline leaching was responsible for about 71% of the environmental damage discovered in the study, according to the AHP tool.
1. Introduction
An increasing quantity of electronic waste has been produced by the rise in the use of electronic and electrical apparatuses and the speed at which they are replaced as a result of the quick advancements in technology. The correct handling of waste materials that are discharged into the environment is an increasingly pressing issue, given the hazardous or poisonous substances they contain. In fact, this problem has sparked a lot of research over the last few decades on different methods for treating this kind of waste [1].
The hydrometallurgical process is used to extract metals present in WEEE through the use of chemical reagents. This process has been extensively researched, in part because of the fact that it is predictable and manageable. Any hydrometallurgical process needs the following two primary steps: leaching, which involves transforming metals from a solid matrix into an aqueous state, and separation of the desired metals from unwanted components in the solution [2,3].
The principal attribute of hydrometallurgical processing is the existence of the solid/liquid interface. In general, the process involves the leaching of WEEE with a leaching agent, which can be acids, alkalis, thiosulfates, and thiourea, followed by the dissolution of the metals. The process of leaching is dictated by the procedure’s aim, allowing for a combination of leaching agents to achieve selective leaching of the materials of interest [4,5]. With an appropriate choice of reagents and control over parameters such as pH and temperature, the selective extraction of the metals is achievable [6].
Common examples of lixiviation agents include strong acids, such as aqua regia, H2SO4, HNO3, and HCl, which are used in various forms for metal recovery [7]. The recovery of gold and silver also makes use of cyanide, thiosulfate, and thiourea [8]. Among other things, basic and valuable metals are recovered using iodides and chlorides [9].
These lixiviation agents do have certain restrictions though. Despite cyanide’s great efficacy in recovering gold and silver, its extreme toxicity puts both people and the environment at serious risk [10]. The recovery of these same valuable metals may be accomplished with the utilization of thiosulfate and thiourea, and careful observation of the reaction conditions is essential to avoid the creation of undesirable byproducts [11]. Furthermore, because of their corrosivity, the use of acids and halides for metal recovery may raise the price of building supplies [12].
In addition, managing and preserving acids and halides can be difficult and raise process expenses [13]. The limited selectivity of acid lixiviation leads to the leaching of most metals into the solution, which is another problem [14]. As a result, after acid lixiviation, multiple-step separation procedures are needed to extract particular metals, which can raise expenses and cause secondary contamination.
Consequently, finding a leaching reagent that permits the selective recovery of base metals is essential. Because of its ability to selectively leach base metals, alkaline leaching, in particular ammonia leaching, has attracted significant interest in contrast to strong acids [15].
A relevant method for extracting precious metals from a variety of sources is ammonia leaching. Metals like copper (Cu), nickel (Ni), and lithium (Li) form stable compounds with amine. The basic metal used in PCBs, copper, can be released using an ammoniacal or an acidic solution. Prior to treating valuable metals, basic metals are typically extracted through leaching. This makes it possible to enrich the solid residue during the first leaching stage, which contains the precious metals [16,17,18,19,20,21,22].
Regarding copper recovery, various hydrometallurgical approaches have been investigated with the goal of removing copper (Cu) from PCB arrangements, which is thought to be the primary metallic component of these wastes. As a result, sulfuric acid has shown to be an affordable reagent that is frequently utilized in industry to recover metal from ores. It also shows good effectiveness in dissolving copper when hydrogen peroxide is present. As a result, this technology has become more well known in the technical and scientific community and is now thought to be an excellent chemical leaching method for removing copper from used printed circuit boards [23,24].
According to Koyama et al., the ammoniacal system is selective for recovering copper from PCBs, based on previous research [25]. Precious metals create ammonia complexes when they leach with ammonia. Nonetheless, because of the similarities in their physicochemical characteristics, the refining of the nickel, copper, and cobalt in an ammonia-based solution poses a significant level of difficulty [26].
Although the hydrometallurgical route is considered a process with less of an environmental impact when compared to other methods, the process is highly dependent on the physical–mechanical steps. Another challenge inherent to the process is the need to minimize the losses of solvents and chemical reagents during the process [27]. Additionally, other aspects must also be considered when choosing the copper recovery methodology, such as the effects on society and economy. Thus, the determination of the system utilized in the multicriteria decision-making process to extract metals from electronic waste demands tools that assist in the systematization and selection of recovery routes [28].
Life cycle assessments (LCAs) are used because every system, good, procedure, or activity has an environmental effect starting from when natural resources are extracted to the end of their usefulness, at which point they are released back into the environment as wastes, air emissions, or aqueous effluents. The ability of an LCA to assess the environmental impact of goods and services sets it apart [29].
Apart from the ecological consequences linked to these systems and products, it is equally crucial to assess the following two other factors: economic and social. The economic assessment, commonly referred to as life cycle costs (LCC), encompasses both internal costs (Cin) and external costs (Cex). Internal costs are the ongoing expenses that businesses cannot avoid during the copper manufacturing procedure. Conversely, external costs are the unreported expenses related to reducing the consequences for society, the environment, and human health that are usually not included in conventional cost estimates [30,31].
A methodology known as a societal life cycle assessment (S-LCA) assesses the socio-economic and the social effects of goods and services over the course of their life cycles. The S-LCA focuses specifically on the effects on society. Its goal is to comprehend the effects on individuals and communities of a product’s or service’s manufacture, use, and discard stages. The framework for the S-LCA created by UNEP/SETAC [32] presents the primary indicators considered. These constitute the following: consumer—health and safety (HS) and end-of-life responsibility (ELR); local community—safe and healthy living conditions (SHLC), local employment (LE), and secure living conditions (SLC); society—public commitments to sustainability issues (PCSI), contributions to economic development (CED), and technological development (TD).
Therefore, the selection of the most suitable hydrometallurgy route relies on decision making, which involves defining appropriate parameters, material to be recovered, environmental impacts generated, operating costs, among others. An essential component of the decision-making process is selecting the relevant factors. In the analytic-hierarchy-process (AHP) method, these factors can be organized into a hierarchical structure based on a set of criteria, subcriteria, and alternatives at successive levels [33,34].
Thus, the goal of this study is to provide a methodical process for assessing and contrasting two hydrometallurgical methods for copper extraction in PCB. This process will be guided by the life-cycle-assessment (LCA) approach and the multicriteria decision-making tool, and maximizing the positive environmental effects of metal recovery is the goal.
The relevance of this study lies in developing a mechanism that integrates LCA and AHP tools, allowing for a combination of environmental, social, and economic criteria and subcriteria in evaluating methodologies for recovering metals in electronic waste. This mechanism aims to optimize the benefits of routes under consideration. Furthermore, the study contributes to the stimulation of waste recovery practices. These materials, which can have high economic values in addition to posing risks to the environment and human health when discarded in an unregulated way, can be reinserted into the industrial chain, contributing to sustainable industrial models, such as the circular economy.
2. Materials and Methods
This study used PCBs (printed circuit boards) from computers, commonly referred to as motherboards, to guarantee the samples’ homogeneity. Even if boards are taken from the same kind of electrical equipment, they can display differences in their fundamental makeup. The PCB waste was initially crushed using hammer mill equipment, model EWZ M400/I-200, with the separation of an 8 mm grain diameter. The resulting material was then classified into the following three sizes: larger than 1 mm (+1 mm), between 1 mm and 0.45 mm (−1 mm +0.45 mm), and smaller than 0.45 mm (−0.45 mm). Following granulometry separation, an oscillating table was used to separate the sample based on density. Based on the parameters of this investigation, four product classes—concentrate, mixed, sterile, and organic matter—were produced using this procedure.
To acquire fractions potentially richer in copper, magnetic separation was carried out by hand using a magnetic instrument. As a result, the examined materials were classified as magnetic (M) and nonmagnetic (NM).
For the recovery of existing copper in the concentrate nonmagnetic (CNM) +1 mm product, because of the enrichment of this product with copper, two hydrometallurgy test routes were conducted. In the first route, leaching occurred in an acidic medium, utilizing sulfuric acid as the leaching agent along with the oxidant hydrogen peroxide. The second route involved leaching in a basic medium, using ammonium sulfate and ammonia as a solvent agent when hydrogen peroxide, an oxidant, was present. Both processes were conducted in a closed system under mechanical agitation.
For the tests in the acidic medium, the leaching process took place for a maximum of 6 h. A sample of the CNM +1 mm product weighing approximately 10 g was placed in an Erlenmeyer flask with a capacity of 250 mL, with an overall amount of 100 mL of solution. The tests were performed in accordance with the guidelines listed in Table 1, which included parameters such as agitation speed, stoichiometry of leaching and oxidizing agents, and leaching time and temperature. The parameters were defined with the aim of minimizing the environmental impacts of the system under study, according to [35].

Table 1.
Leaching parameters in acid medium.
The literature contains various parameters, which differ based on the leaching objectives, as noted in [36,37,38]. Regarding the tests in the ammoniacal system, the leaching process occurred over a minimum period of 4 h and a maximum of 12 h. Similar to the acidic medium tests, a sample of the CNM +1 mm product, weighing approximately 10 g, was placed in an Erlenmeyer flask with a capacity of 250 mL, with a total solution volume of 100 mL. Samples were taken over time for analyses of the metals to monitor the dissolution of metals into the solution. Table 2 shows the conditions tested in these experiments. Atomic absorption spectrometry, employing a UNICAM 969 AA spectrometer, was used to identify the metals present in the solutions.

Table 2.
Leaching parameters in basic medium.
This life cycle assessment study set out to evaluate the life cycle impacts of hydrometallurgical processes by the sulfuric acid route and the ammoniacal route, specifically linked to copper recovery operations in PCBs, adhering to the standards and general principles outlined in NBR ISO 14040 and ISO 14044 (2006) [39,40].
Both preliminary treatment and chemical leaching processes were taken into consideration when setting the whole system frontier for the assessment. A life cycle assessment (LCA) for this group of activities was performed independently, given that all of the procedures that follow share a pretreatment stage, in order to assess their distinct environmental effects. The two sets of processes (Figure 1) that compose the first system boundary under examination are Process I, which entails crushing and sieving process operations, and Process II, which includes separation according to density and separation by magnetic fields.
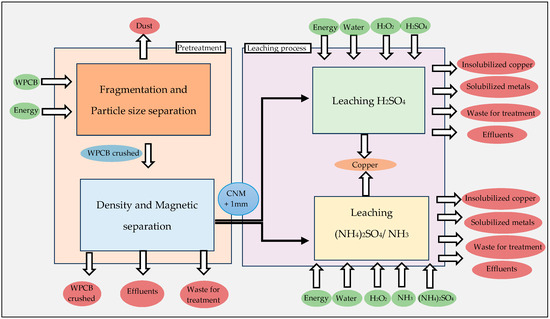
Figure 1.
System boundary for the LCA and considering the pretreatment phase and leaching phase.
Since this is the input for the subsequent leaching procedures, a yield of 1 kg of CNM +1 mm (with a copper content of 78%) was therefore defined as the functional unit. For the leaching processes, the functional unit adopted was 1 kg of copper, that is, obtaining 1 kg of copper after such processes. The ReCiPE midpoint approach of an LCA was used.
Data were gathered, measured, computed, or estimated for determining the inputs and outputs in the life cycle inventory for every stage, as shown in Table 3, which helped with the life cycle impact assessment (LCIA). SimaPRO® 8.0 software assisted in the study and made it easier to retrieve more data from the Ecoinvent 3.0 database.

Table 3.
LCIs of Processes I and II, with 1 kg CNM +1 mm (78% Cu) as the functional unit.
A mass balancing instrument with an emphasis on product environmental performance is Simapro®. The impact on the environment of various goods and services can be analyzed and tracked by users. According to the ISO 14040 and 14044 recommendations, users can model and analyze complicated life cycles. Its databases comprise primary impact assessment techniques, as well as a number of procedures [41].
A set of data and processes were used in the LCI phase to quantify the inputs and the outputs (liquid effluents and solid waste) that are essential for carrying out studies on environmental impacts. The ReCiPE midpoint method was used to conduct an overall evaluation of the life cycle’s effects.
Taking into consideration the effects of the processes on the categories of environmental impacts, the goal of applying the AHP technique may be characterized as showing the hierarchy of the general environmental impacts related to the ways of recovering metals from electronic materials.
The criteria were defined with the aim of achieving the general objective, focusing on industrial sustainability, and were represented by environmental factors extracted from the LCIA environment of the SimaPRO® 8.0 program. The alternatives are represented by the methods of hydrometallurgical metal recovery (leaching in sulfuric acid and leaching in ammonia). In view of this, a hierarchy was developed containing the following elements: goal, criteria, and alternative choices. The decision-tree model’s structure is shown in Figure 2. The following impact categories were taken into consideration: fossil depletion (FD), agriculture land occupation (OA), urban land occupation (OUL), freshwater eutrophication (FE), human toxicity (HT), freshwater ecotoxicity (FEC), terrestrial ecotoxicity (TE), ionizing radiation (IR), climate change (CC), ozone layer depletion (DO), terrestrial acidification (TA), and freshwater eutrophication.
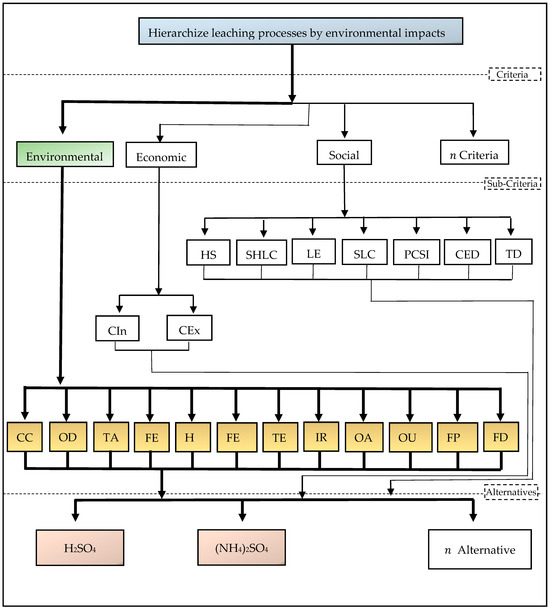
Figure 2.
Decision tree for the proposed model, with goal, criteria, subcriteria, and alternatives.
Within the framework of the AHP, the elements of a layer or hierarchy’s levels are compared in pairs in relation to each of the elements connected in a higher layer of the hierarchy. Specifically, for each criterion, pairwise comparisons are made among the available alternatives. Thus, each alternative, when compared pairwise in relation to a criterion, will receive a value for its performance index according to the metric adopted by the LCA program used.
In this way, information from the LCIA was applied, in the normalized modality, wherein the results of the category indicators were relativized to a reference situation; that is, the impact categories were evaluated in the same dimension, enabling an examination of how each category contributes to the process’s total impact.
From the normalized data, it was possible to obtain an equal judgment matrix by subtracting criterion from criterion , for and building a comparison matrix obeying the following: , where is the normalized impact value of criterion , and is the normalized impact value of criterion . Thus, , or , is defined for each entry of the square matrix of the order . In addition, the adapted scale [42] was used to obtain the final matrix weights of the judgment. Thus, the attributions of the weights adapted according to the AHP were obtained.
To adapt the scale [42], the values obtained in each entry, , were classified according to the amplitude, , and class, . Therefore, the amplitude was calculated using the formula , where corresponds to the highest value, corresponds to the lowest value obtained for , and corresponds to the number of classes being expressed by . Table 4 shows the class intervals considered in this study for the adaptation of the scale [42].

Table 4.
Scale adapted from Saaty (2000) to compare the criteria.
In view of the aforementioned definitions, the weights were defined based on the following relationships:
After the pairwise analysis of the criteria, the weights of each alternative were defined. In this step, the percentage results of the contribution of each leaching route to the assessed impact category were used. Thus, an adaptation of the Saaty scale [41] was employed to define the comparison matrix of each alternative against the evaluated criteria. The process was similar to the construction of the comparison matrix among the criteria, where it was possible to obtain a matrix of equal judgment, performing the subtraction of the impact value of alternative from alternative for each impact category evaluated, , for , building a comparison vector, obeying the following: , where and e . Thus, we define or , for each entry in the vector of order .
Thus, the weights for the comparison matrix among the alternatives were defined by the following relationships:
After structuring the decision tree and the weights associated with the criteria and alternatives, the average local priorities (PMLs) and average global priorities (GPs) were calculated. The PMLs can be calculated by means of the average of the values in the horizontal direction, that is, by the average of the lines of the matrix . The calculation of the global average priorities (GPs), according to [43], requires a combination of the PMLs’ hierarchical structure to define the best alternative. The equation below presents the mathematical structure for obtaining the GPs:
The consistency ratio can be calculated based on the following formula, according to [34]:
It is noteworthy that for the validation of the weights assigned in the construction of the RC weights matrix, the following condition must be respected: .
3. Results and Discussion
The sample contained a copper amount of 78%, a granulometry larger than 1 mm, and nonmagnetic properties as a result of the separation procedures. These operations effectively concentrated metals, particularly copper, into a specific fraction, thereby streamlining the subsequent hydrometallurgical processes.
According to acid leaching tests, it was noticed that the conditions established for test V, as displayed in Figure 3, ensured the greatest extraction rate, where about 90% of the Cu in the sample was solubilized.
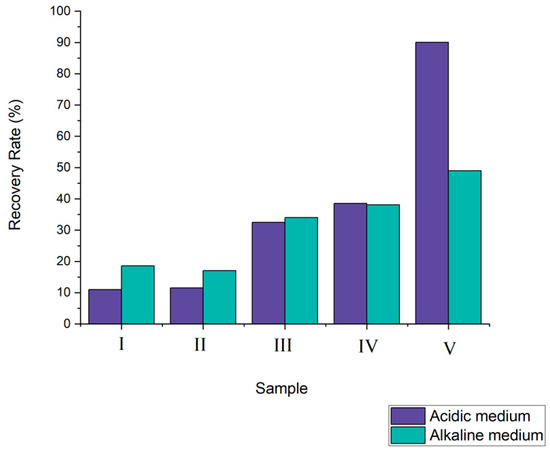
Figure 3.
Copper recovery rate according to the hydrometallurgical route.
It became necessary to analyze the procedure of adding the oxidant (H2O2) to the system, which is performed via regular applications over the test’s runtime. This characteristic is crucial, since by dividing the applications regularly over the course of the test, the resulting dissolution of the copper granules in the solid/liquid system is guaranteed. If all of the oxidant is applied at the beginning of the process, it potentially reacts at the beginning of the test and reduces its action later, with metallic copper particles in a solid physical state still showing when the test is over.
According to [25], the ammoniacal solution exhibits selectivity for the copper recovery from PCBs. Thus, in the ammoniacal system during the leaching process, precious metals form ammonia complexes [44]. However, during the tests, it was noted that the reaction of the copper’s dissolution under the studied parameters is associated with the leaching time. The highest extraction rate (49%) was recorded after 12 h.
In order to evaluate the effects related to obtaining the fraction of concentrated copper, an LCA was applied to the hydrometallurgy process for extracting copper from WPCBs, beginning with the pretreatment stage of these wastes.
Process I, which consists of crushing and sieving activities, was the primary contributor to each of the assessed impact categories when the overall impact values obtained during the initial treatment stage for copper extraction from the WPCBs were analyzed. These operations accounted for more than 90% of the effect values that were recorded in seven of the nine categories that were analyzed. The LCIA data for the pretreatment stage are displayed in Figure 4.
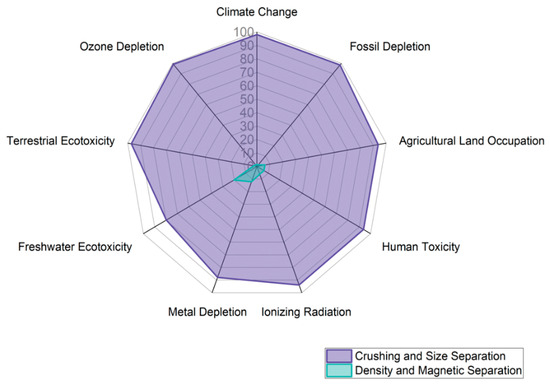
Figure 4.
Evaluation of the pretreatment Life Cycle Impact.
An in-depth examination of Process I revealed that the energy matrix employed at this point had a major effect on its related impacts. This is because, in comparison to the operations in Process II, the equipment used in Process I consumes more energy.
Conversely, the density separation process, conducted on an oscillating table, required water, which resulted in the generation of effluents as byproducts. Additionally, about 20% of the overall impact in the freshwater ecotoxicity impact category was attributed to Process II, which was aided by the disposal of garbage in subterranean deposits. The crushing operation is essential because it releases metals, particularly copper, from PCBs, including those embedded in the inner layers. However, a sizable amount of the materials that are ground during the process are polymeric or ceramic, which reduces the quality and quantity of the metals recovered in the subsequent steps.
The aim of this analysis was to conduct a comparative assessment of two hydrometallurgical methods for recovering copper from electronic circuit board waste using the leaching process life cycle assessment. Chemical leaching was conducted in an alkaline medium (NH3/(NH4)2SO4) and an acid medium (H2SO4). In addition to obtaining information on the associated impacts, according to the assessment categories, the elaboration of this LCA provided input data for the proposition of a decision-making model, based on multicriteria analysis, through standardized impact information and features.
It is noteworthy that the tests were developed at the European level, specifically in Portugal, thus using information from the Ecoinvent 3.0 database referring to European characterizations. Table 5 presents the complementary LCI data for the LCIA modeling. It is noteworthy that the functional unit (FU) established for the assessment of the leaching operations occurred in favor of the recovery of 1 kg of copper; that is, the entire process was dimensioned in order to obtain an output product of 1 kg of copper.

Table 5.
ICV of the sulfuric acid leaching process and the ammonia/ammonium sulfate leaching process.
An analysis of the comparisons was carried out to assess the performance of each tested route according to each impact category defined as of interest for this study. Figure 5 presents a comparison of the environmental performances among the leaching scenarios studied. The main impact categories affected by the acid leaching were urban land occupation, agricultural land occupation, and freshwater ecotoxicity. The leaching in ammonia’s greatest impacts were recorded in the categories freshwater eutrophication, ionizing radiation, fossil depletion, climate change, and ozone depletion. Table 6 and Table 7 present the results extracted using the software in the characterization and normalization modalities, respectively.

Figure 5.
Life cycle impact assessment for the leaching scenarios.

Table 6.
AICV comparative characterization between the acid and alkaline leaching processes.

Table 7.
AICV normalized comparative analysis between the acid and alkaline leaching processes.
According to the definition of the scale, adapted from Saaty [42], and the obtained normalized data on the impacts associated with each criterion, it became possible to fill in the comparison matrix for the selected criteria. It is noteworthy that the basic procedure for filling in the comparison matrix initially consisted of subtracting criterion from criterion for and building a comparison matrix obeying the following , where ci is the normalized impact value of criterion , and is the normalized impact value of criterion , considering the arrangement shown in Table 8.

Table 8.
Comparison matrix of the criteria, .
Supplementary Materials Table S1 presents the values obtained after the process of subtracting between the criteria pair by pair. After obtaining the values, the proposed scale, adapted from [43], was used to define the weights associated with each entry. Negative values were consulted with the adapted scale according to their value in the module and characterized by presenting . Subsequently, the following relationships were used for the assignment of the weights:
Supplementary Materials Table S2 presents the assignment of weights for each criterion on an equal basis and obeying the attributes defined in the adapted scale. It should be noted that the obtained matrix presents the values of its lower triangular region inversely to those of the upper triangular region. For this reason, the comparison matrix (Supplementary Materials Table S1) only has valuation in its upper triangular area.
After obtaining the criteria comparison matrix with the respective assigned weights, it was possible to generate a normalized criteria comparison matrix, which consisted in dividing each element, , by adding together each matrix column’s value, as reported in Supplementary Materials Table S3.
The calculation of the PMLs corresponded to the arithmetic means of the values arranged in each row of the normalized criteria matrix (Supplementary Materials Table S3). Thus, it was observed that the fossil depletion and human toxicity criteria presented greater related environmental effects in contrast to the other criteria, assuming 34% and 21% of the global impacts of the processes, respectively.
To assess the consistency of the process of assigning weights to the criteria, the AHP methodology recommends calculating the consistency ratio (CR) based on the matrix of the assigned weights. Thus, CR was calculated by the following equation:
The value of was obtained by multiplying the vectors (Supplementary Materials Table S2) Σ column and normalized eigenvector. Thus, the values referring to the consistency ratio for assigning weights in the criteria matrix are shown in Table 9.

Table 9.
RC matrix of weights assigned to the criteria.
According to the values obtained, it was possible to verify through the that the variance among the assigned weights was relatively low, since the further the value of is from the n order of the matrix, the greater the variance. In addition, the RI value corresponded to 0.028, respecting the condition and ensuring the consistency of the calculations.
For the definition of the matrix to compare the alternatives, the process was similar to the construction of the matrix to compare the criteria, where it was possible to obtain a matrix of equal judgment, performing the subtraction of the impact value of alternative from alternative according to each impact category evaluated, , for and building a comparison vector obeying the following: , where and ; considering the structures, Table 10 and Table 11 present the comparison matrices between each alternative in view of the associated impact criteria.

Table 10.
Matrix of the alternative comparisons, .

Table 11.
Matrix of the alternative comparisons according to each criterion.
After obtaining these data, weights were assigned to each alternative according to the proposed scale, adapted from Saaty [42] (Table 4). It is worth mentioning that for the consultation using the scale adapted from Saaty [42], negative values were analyzed according to their modules. Subsequently, the weights for the comparison matrix among the alternatives were defined by the following relationships:
Supplementary Materials Table S4 presents the weights assigned to each of the alternatives in the comparative process. It should be noted that the matrices obtained, as well as the results of the calculation of the comparison matrix for the criteria, present values for their lower triangular regions that are inverse to those of the upper triangular region.
After the normalization process, which consisted in dividing each element, , by the sum of its respective matrix column, it was possible to calculate the matrix of the average local priorities. Table 12 shows that the majority of the leaching route based on the ammoniacal system had the highest PMLs, contributing more than 75% of the impacts associated with seven of the twelve criteria studied.

Table 12.
PMLs for each alternative according to each criterion.
To determine the global priorities (GPs), a multiplication process was carried out between the PMLs obtained through the criteria (Table 12) and the PMLs obtained through the alternatives (Supplementary Materials Table S3). The relationship for calculating the GPs is established in Figure 6, in matrix form.
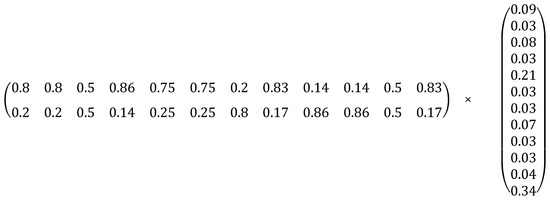
Figure 6.
Matrix structure for calculating growth rates of the PGs.
After the matrix calculation, the GP’s values were obtained (Table 13), which present the results of the analysis of the hierarchical process in question. It should be noted that the scope of this analysis consisted of evaluating two methodologies for recovering metals from waste, given their environmental impacts. It should be emphasized that environmental impact, in this context, was considered as an indirect attribute, meaning that the higher the value associated with an alternative, the worse its environmental performance becomes. According to the GPs, the copper recovery alternative based on the ammoniacal system exhibited 71.25% of the associated environmental impacts, whereas the route based on the acidic medium showed 28.75%.

Table 13.
PGs for each alternative.
4. Conclusions
According to the results obtained in the execution of the tests, it was possible to confirm the importance of the residue preparation phases in the leaching process. This aspect contributed to the enrichment of the fraction of the metal of interest, in addition to reducing the consumption of reagents in the leaching process, as well as to the mitigation of the general environmental impacts of the systems studied. The following highlights stand out:
- While materials are being recovered from electronic waste, density separation greatly improves the effectiveness of both the physical separation process and the pretreatment phase, particularly in the extraction and recovery of metals like copper;
- The crushing and separation processes by granulometry contributed the greatest environmental impact associated with the pretreatment phase;
- Based on the parameters adopted for the studied hydrometallurgical processes, it is concluded that copper recovery by sulfuric acid leaching exhibits greater efficiency within a shorter runtime;
- It is also inferred that hydrogen peroxide’s addition in a fractional manner throughout the process contributes to increasing the efficiency of the acid leaching;
- The hierarchical process analysis provided insights into the environmental performances of the two copper recovery methodologies;
- The proposal to utilize the multicriteria decision-making method in conjunction with a life cycle assessment represents a structured mechanism that contributes to business decision making and the formulation of public environmental policies;
- Since WPCBs are heterogeneous wastes with a wide variety of metals in their compositions, future studies are recommended on the development of environmental impact assessments associated with the purification stage, which involves significant associated costs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16188002/s1. Table S1: Comparison matrix of the criteria, Table S2: Matrix of weights assigned to each criterion; Table S3: Normalized matrix of the criteria and PMLs; Table S4: Matrix of the weights assigned to each alternative for each criterion.
Author Contributions
Conceptualization, J.D., A.G.P.d.S., J.N.F.d.H. and S.C.P.; Methodology, J.D. and A.G.P.d.S.; Validation, J.D., A.G.P.d.S., J.N.F.d.H. and S.C.P.; Investigation, J.D., A.G.P.d.S., J.N.F.d.H., S.C.P. and G.M.d.M.J.; Writing—original draft preparation, J.D., A.G.P.d.S., J.N.F.d.H. and S.C.P.; Writing—review and editing, J.D.; Supervision, A.G.P.d.S., J.N.F.d.H. and S.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). The authors thank the international cooperation program, capes/PDSE/88881.190561/2018-0. The authors would like to acknowledge the financial support of the Espírito Santo Foundation for Innovation and Research—FAPES.
Data Availability Statement
The data that support the findings of this study are available upon request from corresponding author Josinaldo Dias.
Acknowledgments
This study represents the continuation of research entitled “Environmental and Technological Assessment of Metal Extraction and Concentration Operations in Electronic Waste”, published in the journal Sustainability, under DOI: https://doi.org/10.3390/su151713175.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ismail, H.; Hanafiah, M.M. An overview of LCA application in WEEE management: Current practices, progress and challenges. J. Clean. Prod. 2019, 232, 79–93. [Google Scholar] [CrossRef]
- Cui, j.; Zhang, l. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Tunsu, C.; Petranikova, M.; Ekberg, C.; Retegan, T. A hydrometallurgical process for the recovery of rare earth elements from fluorescent lamp waste fractions. Sep. Purif. Technol. 2016, 161, 172–186. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C.G. Literature review of hydrometallurgical recycling of printed circuit boards (PCBs). J. Adv. Chem. Eng. 2016, 6, 142–153. [Google Scholar] [CrossRef]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Udayakumar, S.; Abd Razak, M.I.B.; Ismail, S. Recovering valuable metals from Waste Printed Circuit Boards (WPCB): A short review. Mater. Today Proc. 2022, 66, 3062–3070. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Sahin, M.; Akcil, A.; Erust, C.; Altynbek, S.; Gahan, C.S.; Tuncuk, A. A Potential alternative for precious metal recovery from e-waste: Iodine leaching. Separ. Sci. Technol. 2015, 50, 2587–2595. [Google Scholar] [CrossRef]
- Panda, R.; Jadhao, P.; Kishore, K.; Narayan, S. Eco-friendly recovery of metals from waste mobile printed circuit boards using low temperature roasting. J. Hazard. Mater. 2020, 395, 122642. [Google Scholar] [CrossRef]
- Chauhan, G.; Jadhao, P.R.; Pant, K.K.; Nigam, K.D.P. Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: Challenges & opportunities—A review. J. Environ. Chem. Eng. 2018, 6, 1288–1304. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Yan, F.; Zhang, Z.; Shen, X.; Zhang, Z. Recycling of spent lithium-ion batteries: Selective ammonia leaching of valuable metals and simultaneous synthesis of high-purity manganese carbonate. Waste Manag. 2020, 114, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium- ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, J.; Wanaldi, R.; Zhou, X.; Wang, H.; Zhou, C.; Yang, J. A promising selective recovery process of valuable metals from spent lithium-ion batteries via reduction roasting and ammonia leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef]
- Choubey, P.K.; Panda, R.; Jha, M.K.; Lee, J.-C.; Pathak, D. Recovery of copper and recycling of acid from the leach liquor of discarded Printed Circuit Boards (PCBs). Sep. Purif. Technol. 2015, 156, 269–275. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, W.; Li, J.; Wang, X.; Liu, L.; Wang, J. A critical review on the recycling of copper and precious metals from waste printed circuit boards using hydrometallurgy. Front. Environ. Sci. Eng. 2017, 11, 8. [Google Scholar] [CrossRef]
- Longle, H.; Jeong, J.; Lee, J.-C.; Pandey, B.D.; Yoo, J.-M.; Huyunh, T.H. Hydrometallurgical process for copper recovery from waste printed circuit boards (PCBs). Miner. Process. Extr. Metall. Rev. 2011, 32, 90–104. [Google Scholar] [CrossRef]
- Ajiboye, A.E.; Olasehinde, F.E.; Adebayo, O.A.; Ajayi, O.J.; Ghosh, M.K.; Basu, S. Extraction of copper and zinc from waste printed circuit boards. Recycling 2019, 4, 36. [Google Scholar] [CrossRef]
- de Andrade, L.M.; de Carvalho, M.A.; Caldas, M.P.K.; Espinosa, D.T.J. Recovery of Copper and Silver of Printed Circuit Boards from Obsolete Computers by One-Step Acid Leaching. Detritus 2021, 14, 86. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver and copper from waste printed circuit boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Trinh, H.B.; Kim, S.; Lee, J. Selective Copper Recovery by Acid Leaching from Printed Circuit Board Waste Sludge. Metals 2020, 10, 293. [Google Scholar] [CrossRef]
- Dutta, D.; Panda, R.; Kumari, A.; Goel, S.; Jha, M.K. Sustainable recycling process for metals recovery from used printed circuit boards (PCBs). Sustain. Mater. Technol. 2018, 17, e00066. [Google Scholar] [CrossRef]
- Nobahar, A.; Melka, A.B.; Pusta, A.; Lourenço, J.P.; Carlier, J.D.; Costa, M.C. A New Application of Solvent Extraction to Separate Copper from Extreme Acid Mine Drainage Producing Solutions for Electrochemical and Biological Recovery Processes. Mine Water Environ. 2022, 41, 387–401. [Google Scholar] [CrossRef]
- Koyama, K.; Tanaka, M.; Lee, J. Copper leaching behavior from waste printed circuit board in ammoniacal alkaline solution. Mater. Trans. 2006, 47, 1788–1792. [Google Scholar] [CrossRef]
- Duan, H.; Hou, K.; Li, J.; Zhu, X. Examining the technology acceptance for dismantling of waste printed circuit boards in light of recycling and environmental concerns. J. Environ. Manag. 2011, 92, 392–399. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Selective recovery of nickel from obsolete mobile phone PCBs. Hydrometallurgy 2022, 210, 105843. [Google Scholar] [CrossRef]
- Campos-Guzmán, V.; García-Cáscales, M.S.; Espinosa, N.; Urbina, A. Life Cycle Analysis with Multi-Criteria Decision Making: A review of approaches for the sustainability evaluation of renewable energy technologies. Renew. Sustain. Energy Reviews 2019, 104, 343–366. [Google Scholar] [CrossRef]
- Dias, J.; Xavier, G.; Azevedo, A.; Alexandre, J.; Colorado, H.; Vieira, C.M. Eco-friendly ceramic bricks: A comparative study of life cycle impact methods. Environ. Sci. Pollut. Res. 2022, 29, 76202–76215. [Google Scholar] [CrossRef]
- Wang, C.B.; Zhang, L.X.; Zhou, P.; Chang, Y.; Zhou, D.Q.; Pang, M.Y.; Yin, H. Assessing the environmental externalities for biomass- and coal-fired electricity generation in China: A supply chain perspective. J. Environ. Manag. 2019, 246, 758–767. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Z.; Yang, S.; Liu, Z.; Liu, Z.; Liu, Y.; Yin, H. Life cycle assessment and cost analysis for copper hydrometallurgy industry in China. J. Environ. Manag. 2022, 309, 114689. [Google Scholar] [CrossRef] [PubMed]
- UNEP/SETAC. Methodological Sheets of Sub-Categories in Social Life Cycle Assessment (S-LCA). 2021. Available online: https://www.lifecycleinitiative.org/library/methodological-sheets-for-subcategories-in-social-life-cycle-assessment-s-lca-2021/ (accessed on 11 July 2024).
- Saaty, T.L. How to make a decision: The Analytic Hierarchy Process. Eur. J. Oper. Res. 1990, 48, 9–26. [Google Scholar] [CrossRef]
- Taha, A.H. Operational Research, 8th ed.; Pearson: New York, NY, USA, 2013; p. 363. [Google Scholar]
- Silvas, F.P.C.; Jimenez, M.M.; Caldas, M.P.K.; Moraes, V.T.D.; Espinosa, D.C.R.; Tenorio, J.A.S. Printed circuit board recycling: Physical processing and copper extraction by selective leaching. Waste Manag. 2015, 46, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Liu, Y.; Wang, D.; Cao, H.; Zhu, W.; Yang, R.; Liu, Z. Pressure leaching of selenium and tellurium from scrap copper anode slimes in sulfuric acid-oxygen media. J. Clean. Prod. 2021, 278, 123989. [Google Scholar] [CrossRef]
- Das, D.; Mukherjee, S.; Chaudhuri, M.G. Studies on leaching characteristics of electronic waste for metal recovery using inorganic and organic acids and base. Waste. Manag. Res. 2021, 39, 242–249. [Google Scholar] [CrossRef]
- Duran, J.A.G.; Arroyo, Z.G.; Castro, F.I.G.; Owen, P.Q.; Cadena, L.E.S.; Gómez, M.V.A. Evaluation of the effect of physical and chemical factors in the recovery of Cu, Pb and Fe from waste PCB through acid leaching. Heliyon 2023, 9, e21348. [Google Scholar] [CrossRef]
- NBR 14.040; Environmental Management—Life Cycle Assessment—Principles and Structure. Association Brazilian of Technical Standards: Rio de Janeiro, Brazil, 2006; pp. 1–7.
- NBR 14.044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. Association Brazilian of Technical Standards: Rio de Janeiro, Brazil, 2006; pp. 1–6.
- Silva, D.A.L.; Nunes, A.O.; Piekarski, C.M.; Da Silva Moris, V.A.; De Souza, L.S.M.; Rodrigues, T.O. Why using different Life Cycle Assessment software tools can generate different results for the same product system? A cause–effect analysis of the problem. Sustain. Prod. Consum. 2019, 20, 304–315. [Google Scholar] [CrossRef]
- Decision Making for Leaders, 3rd ed.; RWS Publications: Pittsburgh, PA, USA, 2000; p. 314.
- Costa, H.G. Introdução ao método de análise hierárquica-Análise Multicritério no Auxílio à Decisão. Biblioteca da Escola de Engenharia e Instituto de Computação da UFF. Niterói. 2002. Available online: http://www.din.uem.br/sbpo/sbpo2004/pdf/arq0279.pdf (accessed on 11 July 2024).
- Duan, H.; Wang, Z.; Yuan, X.; Wang, S.; Guo, H.; Yang, X. A novel sandwich supported liquid membrane system for simultaneous separation of copper, nickel and cobalt in ammoniacal solution. Sep. Purif. Technol. 2017, 173, 323–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).