Selecting the Most Sustainable Phosphorus Adsorbent for Lake Restoration: Effects on the Photosynthetic Activity of Chlorella sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Species and Culture Medium
2.2. General Characterization of Adsorbents
2.3. Toxicological Tests with Chlorella sp.
2.3.1. Effects of Adsorbent Addition on Physico-Chemical Variables
2.3.2. Effects of the Adsorbents on Algal Growth
2.3.3. Photosynthetic Activity of Chlorella sp.
- F′m: instantaneous maximum fluorescence induced by a saturating light pulse (~5300 μmol photons m−2 s−1 in 0.8 s).
- F′t: current steady-state fluorescence of light-adapted cells induced by an actinic light ~419 W m−2 in light-adapted cells.
- Fm: maximal fluorescence of the dark-adapted sample.
- NPQ was calculated for each sample from the Fm value stored by the software.
- a = initial measurement time
- b = final measurement time
- f(x) = curve describing ΦPSII or NPQ over time for each treatment.
- X = Variable response considered in samples under control (C) and treatments (T).
2.4. Statistical Analyses
3. Results
3.1. Effect of Adsorbents Addition on Physico-Chemical Variables
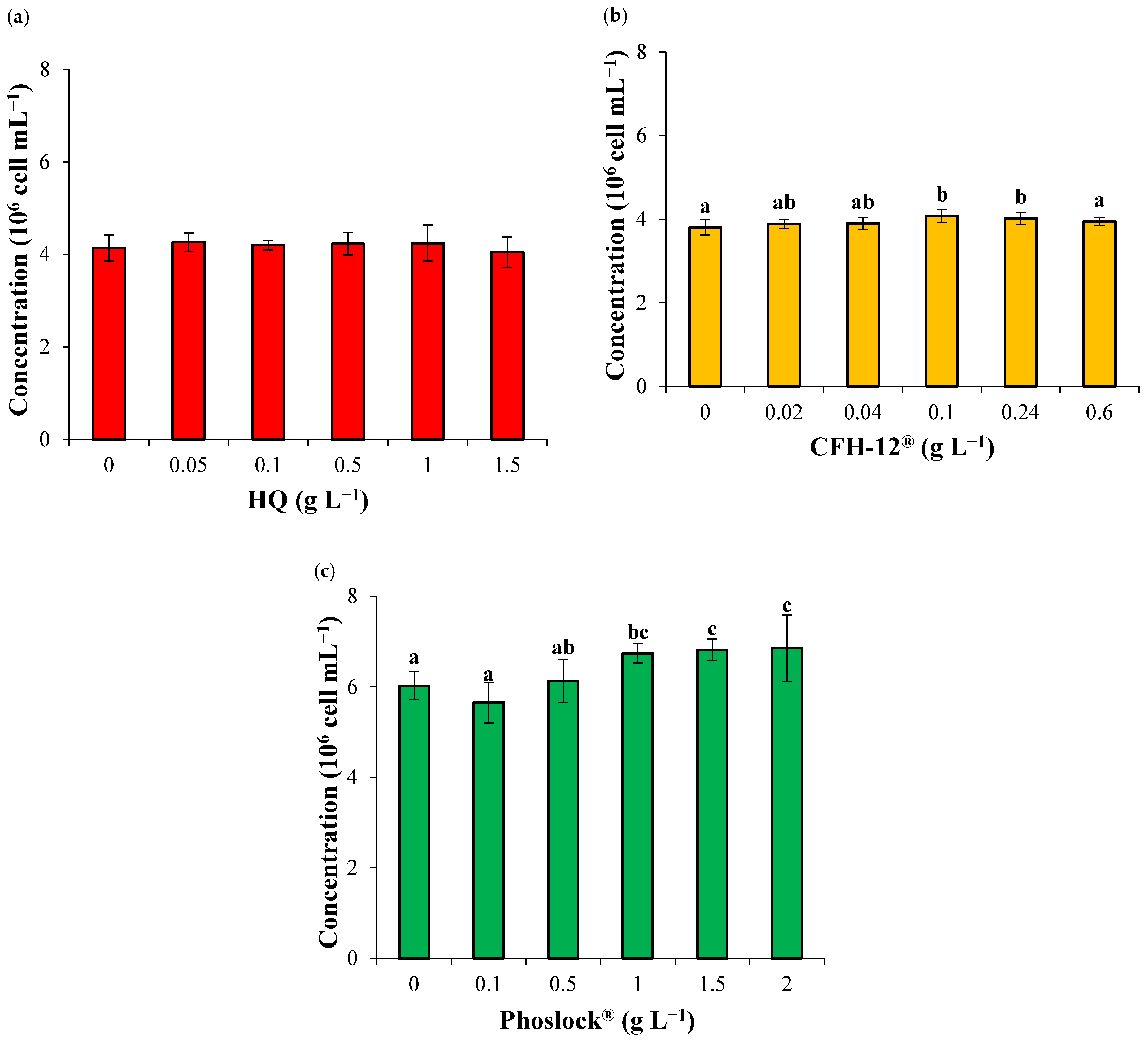
3.2. Effect of Adsorbents Addition on Algal Growth
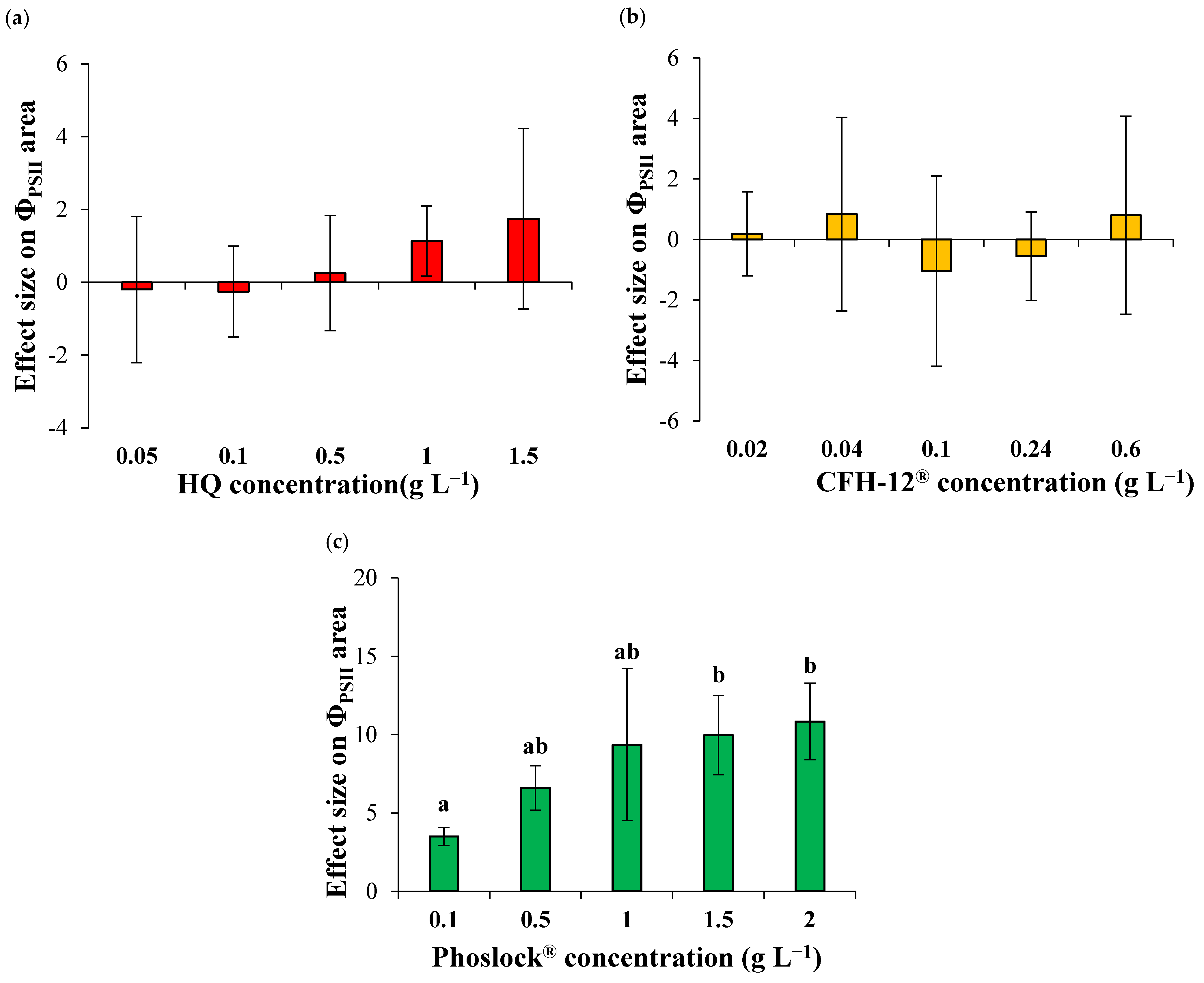
3.3. Effects of the Adsorbents on the Photosynthetic Activity of Chlorella sp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hupfer, M.; Hilt, S. Lake Restoration. In Ecological Engineering; Jørgense, S.E., Fath, B.D., Eds.; Elsevier: Oxford, UK, 2009; Volume 3, pp. 2080–2093. [Google Scholar]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J.; Pitts, K.L. Policy Analysis Policy Analysis Eutrophication of U.S. Freshwaters: Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, J.; Harrison, I.J.; Dudgeon, D.; Clausnitzer, V.; Darwall, W.; Farrell, T.; Savy, C.; Tockner, K.; Tubbs, N. Sustaining freshwater biodiversity in the Anthropocene. In The Global Water System in the Anthropocene: Challenges for Science and Governance; Bahuri, A., Bogardi, J., Leentvaar, J., Marx, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 17, pp. 247–270. [Google Scholar]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. Int. 2003, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Stendera, S.; Adrian, R.; Bonada, N.; Cañedo-Argüelles, M.; Hugueny, B.; Januschke, K.; Pletterbauer, F.; Hering, D. Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: A review. Hydrobiologia 2012, 696, 1–28. [Google Scholar] [CrossRef]
- Ansari, A.A.; Gill, S.S.; Khan, F.A. Eutrophication: Threat to Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Singh Gill, S., Lanza, G.R., Rast, W., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 143–170. [Google Scholar]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Zhou, J.; Leavitt, P.R.; Zhang, Y.; Qin, B. Anthropogenic eutrophication of shallow lakes: Is it occasional? Water Res. 2022, 221, 118728. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of Sediment and Internal Loading of Phosphorus in Shallow Lakes Martin. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Nikolai, S.J.; Dzialowski, A.R. Effects of Internal Phosphorus Loading on Nutrient Limitation in a Eutrophic Reservoir. Limnologica 2014, 49, 33–41. [Google Scholar] [CrossRef]
- Spears, B.M.; Mackay, E.B.; Yasseri, S.; Gunn, I.D.M.; Waters, K.E.; Andrews, C.; Cole, S.; De Ville, M.; Kelly, A.; Meis, S.; et al. A Meta-Analysis of Water Quality and Aquatic Macrophyte Responses in 18 Lakes Treated with Lanthanum Modified Bentonite (Phoslock®). Water Res. 2016, 97, 111–121. [Google Scholar] [CrossRef]
- Cooke, D.G.; Welch, E.B.; Peterson, S.A.; Nicholas, S.A. Restoration and Management of Lakes and Reservoirs, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005; 658p. [Google Scholar]
- Bakker, E.S.; Van Donk, E.; Immers, A.K. Lake Restoration by In-Lake Iron Addition: A Synopsis of Iron Impact on Aquatic Organisms and Shallow Lake Ecosystems. Aquat. Ecol. 2016, 50, 121–135. [Google Scholar] [CrossRef]
- Kuster, A.C.; Kuster, A.T.; Huser, B.J. A Comparison of Aluminum Dosing Methods for Reducing Sediment Phosphorus Release in Lakes. J. Environ. Manag. 2020, 261, 110195. [Google Scholar] [CrossRef]
- de Vicente, I.; Jensen, H.S.; Andersen, F. Factors Affecting Phosphate Adsorption to Aluminum in Lake Water: Implications for Lake Restoration. Sci. Total Environ. 2008, 389, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pacioglu, O.; Cornut, J.; Gessner, M.O.; Kasprzak, P. Prevalence of Indirect Toxicity Effects of Aluminium Flakes on a Shredder-Fungal-Leaf Decomposition System. Freshw. Biol. 2016, 61, 2013–2025. [Google Scholar] [CrossRef]
- Kleeberg, A.; Herzog, C.; Hupfer, M. Redox Sensitivity of Iron in Phosphorus Binding Does Not Impede Lake Restoration. Water Res. 2013, 47, 1491–1502. [Google Scholar] [CrossRef]
- Reitzel, K.; Jensen, H.S.; Egemose, S. PH Dependent Dissolution of Sediment Aluminum in Six Danish Lakes Treated with Aluminum. Water Res. 2013, 47, 1409–1420. [Google Scholar] [CrossRef]
- Egemose, S.; Wauer, G.; Kleeberg, A. Resuspension Behaviour of Aluminium Treated Lake Sediments: Effects of Ageing and pH. Hydrobiologia 2009, 636, 203–217. [Google Scholar] [CrossRef]
- Egemose, S.; Reitzel, K.; Andersen, F.Ø.; Flindt, M.R. Chemical Lake Restoration Products: Sediment Stability and Phosphorus Dynamics. Environ. Sci. Technol. 2010, 44, 985–991. [Google Scholar] [CrossRef]
- Robb, M.; Greenop, B.; Goss, Z.; Douglas, G.; Adeney, J. Application of PhoslockTM, an Innovative Phosphorus Binding Clay, to Two Western Australian Waterways: Preliminary Findings. Hydrobiologia 2003, 494, 237–243. [Google Scholar] [CrossRef]
- Meis, S.; Spears, B.M.; Maberly, S.C.; O’Malley, M.B.; Perkins, R.G. Sediment Amendment with Phoslock® in Clatto Reservoir (Dundee, UK): Investigating Changes in Sediment Elemental Composition and Phosphorus Fractionation. J. Environ. Manag. 2012, 93, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Spears, B.M.; Meis, S.; Anderson, A.; Kellou, M. Comparison of Phosphorus (P) Removal Properties of Materials Proposed for the Control of Sediment p Release in UK Lakes. Sci. Total Environ. 2013, 442, 103–110. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Effects of Lanthanum and Lanthanum-Modified Clay on Growth, Survival and Reproduction of Daphnia Magna. Water Res. 2010, 44, 309–319. [Google Scholar] [CrossRef]
- Lürling, M.; Waajen, G.; Van Oosterhout, F. Humic Substances Interfere with Phosphate Removal by Lanthanum Modified Clay in Controlling Eutrophication. Water Res. 2014, 54, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Funes, A.; Saar, K.; Reitzel, K.; Jensen, H.S. Evaluation of Dried Amorphous Ferric Hydroxide CFH-12® as Agent for Binding Bioavailable Phosphorus in Lake Sediments. Sci. Total Environ. 2018, 628–629, 990–996. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, I.; Merino-Martos, A.; Cruz-Pizarro, L.; de Vicente, J. On the Use of Magnetic Nano and Microparticles for Lake Restoration. J. Hazard. Mater. 2010, 181, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Merino-Martos, A.; de Vicente, J.; Cruz-Pizarro, L.; de Vicente, I. Setting up High Gradient Magnetic Separation for Combating Eutrophication of Inland Waters. J. Hazard. Mater. 2011, 186, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Funes, A.; del Arco, A.; Álvarez-Manzaneda, I.; de Vicente, J.; de Vicente, I. A Microcosm Experiment to Determine the Consequences of Magnetic Microparticles Application on Water Quality and Sediment Phosphorus Pools. Sci. Total Environ. 2017, 579, 245–253. [Google Scholar] [CrossRef]
- Funes, A.; Martínez, F.J.; Álvarez-Manzaneda, I.; Conde-Porcuna, J.M.; De Vicente, J.; Guerrero, F.; De Vicente, I. Determining Major Factors Controlling Phosphorus Removal by Promising Adsorbents Used for Lake Restoration: A Linear Mixed Model Approach. Water Res. 2018, 141, 377–386. [Google Scholar] [CrossRef]
- Álvarez-Manzaneda, I.; Laza, N.; Navarro, F.B.; Suárez-Rey, E.M.; Segura, M.L.; de Vicente, I. Assessing the Viability of Recovered Phosphorus from Eutrophicated Aquatic Ecosystems as a Liquid Fertilizer. J. Environ. Manag. 2021, 285, 112156. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, Harmful Algae and Biodiversity—Challenging Paradigms in a World of Complex Nutrient Changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Nesme, T.; Metson, G.S.; Bennett, E.M. Global Phosphorus Flows through Agricultural Trade. Glob. Environ. Chang. 2018, 50, 133–141. [Google Scholar] [CrossRef]
- Gilbert, N. Environment: The Disappearing Nutrient. Nature 2009, 461, 716–718. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A.L. Towards Global Phosphorus Security: A Systems Framework for Phosphorus Recovery and Reuse Options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J. Phosphorus: A Limiting Nutrient for Humanity? Curr. Opin. Biotechnol. 2012, 23, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Manzaneda, I.; Guerrero, F.; Cruz-Pizarro, L.; Rendón, M.; de Vicente, I. Magnetic Particles as New Adsorbents for the Reduction of Phosphate Inputs from a Wastewater Treatment Plant to a Mediterranean Ramsar Wetland (Southern Spain). Chemosphere 2021, 270, 128640. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Manzaneda, I.; Baun, A.; Cruz-Pizarro, L.; De Vicente, I. Ecotoxicity Screening of Novel Phosphorus Adsorbents Used for Lake Restoration. Chemosphere 2019, 222, 469–478. [Google Scholar] [CrossRef] [PubMed]
- van Oosterhout, F.; Lürling, M. The Effect of Phosphorus Binding Clay (Phoslock®) in Mitigating Cyanobacterial Nuisance: A Laboratory Study on the Effects on Water Quality Variables and Plankton. Hydrobiologia 2013, 710, 265–277. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, C.; Liu, J.; Weng, Y.; Li, H.; Zhang, D. Assessing the Impacts of Phosphorus Inactive Clay on Phosphorus Release Control and Phytoplankton Community Structure in Eutrophic Lakes. Environ. Pollut. 2016, 219, 620–630. [Google Scholar] [CrossRef]
- Kang, L.; Mucci, M.; Lürling, M. Compounds to Mitigate Cyanobacterial Blooms Affect Growth and Toxicity of Microcystis Aeruginosa. Harmful Algae 2022, 118, 102311. [Google Scholar] [CrossRef]
- Álvarez-Manzaneda, I.; de Vicente, I. Assessment of Toxic Effects of Magnetic Particles Used for Lake Restoration on Chlorella sp. and on Brachionus calyciflorus. Chemosphere 2017, 187, 347–356. [Google Scholar] [CrossRef]
- Solovchenko, A.; Baulina, O.; Ptushenko, O.; Gorelova, O. Ultrastructural Patterns of Photoacclimation and Photodamage to Photosynthetic Algae Cell under Environmental Stress. Physiol. Plant. 2019, 166, 251–263. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Israel, A.; Neori, A.; Martínez, B.; Malta, E.J.; Put, A.; Inken, S.; Marquardt, R.; Abdala, R.; Korbee, N. Effect of Nutrient Supply on Photosynthesis and Pigmentation to Short-Term Stress (UV Radiation) in Gracilaria Conferta (Rhodophyta). Mar. Pollut. Bull. 2010, 60, 1768–1778. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of Photosynthesis in Nature. Annu. Rev. Plant Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Li, R.; Yao, H.; Lu, Z.; Yang, X. Photosynthetic Toxicity and Oxidative Damage Induced by Nano-Fe3O4 on Chlorella vulgaris in Aquatic Environment. Open J. Ecol. 2012, 02, 21–28. [Google Scholar] [CrossRef]
- Silva, A.; Figueiredo, S.A.; Sales, M.G.; Delerue-Matos, C. Ecotoxicity Tests Using the Green Algae Chlorella vulgaris—A Useful Tool in Hazardous Effluents Management. J. Hazard. Mater. 2009, 167, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bold, H.C. The Morphology of Chlamydomonoas Chlamydogama. Bull. Torrey Bot. Club 1949, 76, 101–108. [Google Scholar] [CrossRef]
- ISO 8692:2012; Water Quality-Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. ISO: Geneva, Switzerland, 2012.
- Lyngsie, G.; Penn, C.J.; Hansen, H.C.B.; Borggaard, O.K. Phosphate Sorption by Three Potential Fi Lter Materials as Assessed by Isothermal Titration Calorimetry. J. Environ. Manag. 2014, 143, 26–33. [Google Scholar] [CrossRef]
- Reitzel, K.; Andersen, F.T.; Egemose, S.; Jensen, H.S. Phosphate Adsorption by Lanthanum Modified Bentonite Clay in Fresh and Brackish Water. Water Res. 2013, 47, 2787–2796. [Google Scholar] [CrossRef]
- Funes, A.; Álvarez-Manzaneda, I.; Arco, A.d.; de Vicente, J.; de Vicente, I. Evaluating the Effect of CFH-12® and Phoslock® on Phosphorus Dynamics during Anoxia and Resuspension in Shallow Eutrophic Lakes. Environ. Pollut. 2021, 269, 116093. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Visnapuu, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Gibbs, M.M. A Simple Method for the Rapid Determination of Iron in Natural Waters. Water Res. 1979, 13, 295–297. [Google Scholar] [CrossRef]
- Juneau, P.; El Berdey, A.; Popovic, R. PAM Fluorometry in the Determination of the Sensitivity of Chlorella vulgaris, Selenastrum capricornutum, and Chlamydomonas reinhardtii to Copper. Arch. Environ. Contam. Toxicol. 2002, 42, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2020, 51, 659–668. [Google Scholar] [CrossRef]
- González-Olalla, J.M.; Medina-Sánchez, J.M.; Cabrerizo, M.J.; Villar-Argáiz, M.; Sánchez-Castillo, P.M.; Carrillo, P. Contrasting Effect of Saharan Dust and UVR on Autotrophic Picoplankton in Nearshore versus Offshore Waters of Mediterranean Sea. J. Geophys. Res. Biogeosci. 2017, 122, 2085–2103. [Google Scholar] [CrossRef]
- Yamada-Ferraz, T.M.; Sueitt, A.P.E.; Oliveira, A.F.; Botta, C.M.; Fadini, P.S.; Nascimento, M.R.; Faria, B.M.; Mozeto, A.A. Assessment of Phoslock® application in a tropical eutrophic reservoir: An integrated evaluation from laboratory to field experiments. Environ. Technol. Innov. 2015, 4, 194–205. [Google Scholar] [CrossRef]
- Cheloni, G.; Slaveykova, V. Combined Effects of Trace Metals and Light on Photosynthetic Microorganisms in Aquatic Environment. Environments 2018, 5, 81. [Google Scholar] [CrossRef]
- Corcoll, N.; Bonet, B.; Leira, M.; Montuelle, B.; Tlili, A.; Guasch, H. Light history influences the response of fluvial biofilms to Zn exposure. J. Phycol. 2012, 48, 1411–1423. [Google Scholar] [CrossRef]
- Xu, K.; Li, Z.-K.; Qiu, B.-S.; Juneau, P. Different responses to high light stress of toxic and non-toxic Microcystis aeruginosa acclimated under two light intensities and zinc concentrations. Toxicol. Environ. Chem. 2013, 95, 1145–1156. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Serôdio, J.; Lavaud, J. A model for describing the light response of the nonphotochemical quenching of chlorophyll fluorescence. Photosynth. Res. 2011, 108, 61–76. [Google Scholar] [CrossRef]
- Hjorth, R.; Skjolding, L.M.; Sørensen, S.N.; Baun, A. Regulatory adequacy of aquatic ecotoxicity testing of nanomaterials. NanoImpact 2017, 8, 28–37. [Google Scholar] [CrossRef]
- Reynolds, C.S. Eutrophication and the Management of Planktonic Algae: What Vollenweider Couldn’t Tell Us. In Eutrophication: Research and Application to Water Supply; Sutcliffe, D.W., Jones, G., Eds.; Freshwater Biological Association: Ambleside, UK; International Water Supply Association, Queen Anne’s Gate: London, UK, 1992; pp. 4–29. [Google Scholar]
- Reynolds, C.S. Metabolic Sensitivities of Lacustrine Ecosystems to anthropogenic forcing. Aquat. Sci. 1999, 61, 183–205. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajisani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of Optimal Condition for Chlorella vulgaris Microalgae Growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar] [CrossRef]
- Badar, S.N.; Mohammad, M.; Emdadi, Z.; Yaakob, Z. Algae and their growth requirements for bioenergy: A review. Biofuels 2018, 12, 307–325. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Limits to growth. In Wastewater Treatment with Algae; Wong, Y.S., Tam, N.F.Y., Eds.; Springer: Heidelberg/Berlin, Germany, 1998; pp. 203–226. [Google Scholar]
- Organization for Economic Cooperation and Development. Guideline for Testing of Chemicals; No.201. Alga Growth Inhibition Test; Organization for Economic Cooperation and Development: Paris, France, 1984. [Google Scholar]
- Rachlin, J.W.; Grosso, A. The Effects of pH on the Growth of Chlorella vulgaris and Its Interactions with Cadmium Toxicity. Arch. Environ. Contam. Toxicol. 1991, 20, 505–508. [Google Scholar] [CrossRef]
- Rozman, K.K.; Doull, J. Dose and Time as Variables of Toxicity. Toxicology 2000, 144, 169–178. [Google Scholar] [CrossRef]
- Nyholm, N. Response Variable in Algal Growth Inhibition Tests—Biomass or Growth Rate? Water Res. 1985, 19, 273–279. [Google Scholar] [CrossRef]
- Connell, D.W.; Yu, Q.J.; Verma, V. Influence of Exposure Time on Toxicity—An Overview. Toxicology 2016, 355–356, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of Nanosized TiO2. Review of in Vivo Data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lin, K.-C.; Yang, D.-T. Comparison of the Relative Toxicity Relationships Based on Batch and Continuous Algal Toxicity Tests. Chemosphere 1997, 35, 1959–1965. [Google Scholar] [CrossRef]
- Millington, L.A.; Goulding, K.H.; Adams, N. The Influence of Growth Medium Composition on the Toxicity of Chemicals to Algae. Water Res. 1988, 22, 1593–1597. [Google Scholar] [CrossRef]
- Ouyang, H.L.; Kong, X.Z.; He, W.; Qin, N.; He, Q.S.; Wang, Y.; Wang, R.; Xu, F.L. Effects of Five Heavy Metals at Sub-Lethal Concentrations on the Growth and Photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 2012, 57, 3363–3370. [Google Scholar] [CrossRef]
- Xiao, A.; Wang, C.; Chen, J.; Guo, R.; Yan, Z.; Chen, J. Carbon and Metal Quantum Dots Toxicity on the Microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2016, 133, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Cheng, J.; Hu, X.L.; Wang, L.; Li, D.; Gao, K. Biological Effects of TiO2 and CeO2 Nanoparticles on the Growth, Photosynthetic Activity, and Cellular Components of a Marine Diatom Phaeodactylum tricornutum. Sci. Total Environ. 2017, 575, 87–96. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, I.; Serrano, L.; Amores, V.; Clavero, V.; Cruz-Pizarro, L. Sediment Phosphate Fractionation and Interstitial Water Phosphate Concentration in Two Coastal Lagoons (Albuferas de Adra, SE Spain). Hydrobiologia 2003, 492, 95–105. [Google Scholar] [CrossRef]



| Adsorbent | Composition | Maximum P Adsorption Capacity (mg g−1) | Size | References |
|---|---|---|---|---|
| HQ | Fe (98); C (0.9); N (0.9); O (0.5) 1 (manufacturer) | 18.83 | 805 ± 10 nm | [27] |
| CFH-12® | O (59); Fe (28); C (9); S (2); Mg (0.5) and Ca (0.3) 2 | 15.1 | 0.85–2 mm (93%); <0.85 nm (6%) | [26,30,52] |
| Phoslock® | O (66); C (6); Si (19); Al (5); La (1); Na (1); Mg (1); Ca (1) and Fe (1) 2 | 13.6 | 2–4 × 1–3 mm (manufacturer) | [30,52] |
| pH | DO (%) | ||||
|---|---|---|---|---|---|
| Treatment (g L−1) | Initial | Final | Initial | Final | |
| HQ | Control | 7.47 ± 0.02 | 7.52 ± 0.01 | 100.00 ± 0.00 | 96.00 ± 1.04 |
| 0.05 | 7.49 ± 0.06 | 7.55 ± 0.04 | 100.00 ± 0.00 | 94.75 ± 1.05 | |
| 0.1 | 7.63 ± 0.05 | 7.70 ± 0.05 | 100.00 ± 0.00 | 93.60 ± 0.34 | |
| 0.5 | 8.26 ± 0.07 | 8.33 ± 0.09 | 98.30 ± 0.92 | 93.70 ± 0.64 | |
| 1 | 8.30 ± 0.11 | 8.30 ± 0.04 | 92.08 ± 1.98 | 91.63 ± 0.23 | |
| 1.5 | 8.44 ± 0.04 | 8.28 ± 0.04 | 88.90 ± 1.41 | 91.63 ± 0.40 | |
| CFH-12® | Control | 7.42 ± 0.04 | 7.27 ± 0.29 | 93.75 ± 5.54 | 91.33 ± 0.21 |
| 0.02 | 7.41 ± 0.03 | 7.44 ± 0.01 | 86.88 ± 0.57 | 91.23 ± 0.37 | |
| 0.04 | 7.39 ± 0.02 | 7.44 ± 0.04 | 87.50 ± 0.85 | 90.38 ± 0.26 | |
| 0.1 | 7.44 ± 0.05 | 7.43 ± 0.02 | 85.40 ± 6.66 | 90.38 ± 0.57 | |
| 0.24 | 7.44 ± 0.05 | 7.43 ± 0.01 | 86.80 ± 5.10 | 88.13 ± 1.18 | |
| 0.6 | 7.36 ± 0.03 | 7.40 ± 0.02 | 80.07 ± 7.14 | 87.27 ± 0.12 | |
| Phoslock® | Control | 7.83 ± 0.10 | 7.70 ± 0.16 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 0.1 | 7.85 ± 0.04 | 7.82 ± 0.07 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 0.5 | 7.92 ± 0.01 | 7.92 ± 0.06 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 1 | 7.94 ± 0.04 | 7.95 ± 0.10 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 1.5 | 7.95 ± 0.02 | 7.98 ± 0.09 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| 2 | 7.93 ± 0.05 | 8.16 ± 0.10 | 100.00 ± 0.00 | 100.00 ± 0.00 | |
| Treatment (g L−1) | Turbidity (NTU) | DIP (mg L−1) | Tot-Fedis (mg L−1) | |
|---|---|---|---|---|
| HQ | Control | 28.23 ± 2.00 | 145.77 ± 8.49 | n.d. |
| 0.05 | 57.34 ± 7.15 | 142.81 ± 8.28 | n.d. | |
| 0.1 | 69.50 ± 6.45 | 137.64 ± 7.83 | 0.01 ± 0.02 | |
| 0.5 | 254.00 ± 15.71 | 100.12 ± 4.00 | 18.67 ± 0.60 | |
| 1 | 223 ± 15.72 | 88.96 ± 1.54 | 19.81 ± 1.50 | |
| 1.5 | 157.75 ± 24.10 | 72.86 ± 4.38 | 15.97 ± 3.48 | |
| CFH-12® | Control | 32.80 ± 15.66 | 63.57 ± 1.53 | n.d. |
| 0.02 | 42.37 ± 0.85 | 67.22 ± 0.99 | n.d. | |
| 0.04 | 46.74 ± 3.44 | 65.58 ± 3.73 | n.d. | |
| 0.1 | 64.50 ± 3.11 | 64.72 ± 1.34 | n.d. | |
| 0.24 | 98.75 ± 4.27 | 62.50 ± 4.71 | n.d. | |
| 0.6 | 261.00 ± 32.91 | 58.95 ± 2.27 | n.d. | |
| Phoslock® | Control | 121.25 ± 10.60 | 71.65 ± 1.72 | - |
| 0.1 | 106.50 ± 12.45 | 56.63 ± 0.56 | - | |
| 0.5 | 137.75 ± 29.33 | 54.08 ± 0.90 | - | |
| 1 | 204.75 ± 8.77 | 50.68 ± 1.59 | - | |
| 1.5 | 264.50 ± 9.11 | 44.68 ± 5.86 | - | |
| 2 | 366.50 ± 8.96 | 42.84 ± 2.55 | - | |
| HQ | CFH-12® | Phoslock® | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| pH a,b | 5 | 292.07 | *** | 5 | 0.59 | ns | 5 | 8.40 | *** |
| DO a,b | 5 | 101.83 | *** | 5 | 2.90 | * | - | - | - |
| Turbidity a,b | 5 | 12.36 | *** | 5 | 11.87 | *** | 5 | 178.01 | *** |
| DIP b | 5 | 182.99 | *** | 5 | 1.39 | ns | 5 | 6.69 | ** |
| Tot-Fedis a,b | 5 | 10.76 | ** | - | - | - | - | - | - |
| Algal concentration b | 5 | 0.68 | ns | 5 | 3.87 | ** | 5 | 10.53 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Manzaneda, I.; Castaño-Hidalgo, Á.; de Vicente, I. Selecting the Most Sustainable Phosphorus Adsorbent for Lake Restoration: Effects on the Photosynthetic Activity of Chlorella sp. Sustainability 2024, 16, 8305. https://doi.org/10.3390/su16198305
Álvarez-Manzaneda I, Castaño-Hidalgo Á, de Vicente I. Selecting the Most Sustainable Phosphorus Adsorbent for Lake Restoration: Effects on the Photosynthetic Activity of Chlorella sp. Sustainability. 2024; 16(19):8305. https://doi.org/10.3390/su16198305
Chicago/Turabian StyleÁlvarez-Manzaneda, Inmaculada, Álvaro Castaño-Hidalgo, and Inmaculada de Vicente. 2024. "Selecting the Most Sustainable Phosphorus Adsorbent for Lake Restoration: Effects on the Photosynthetic Activity of Chlorella sp." Sustainability 16, no. 19: 8305. https://doi.org/10.3390/su16198305







