Abstract
A century after the first scientific research on the chemical structures of pyrethrins was published (in 1923), this paper aims to provide an exhaustive review of the historical research pathways and relative turning points that led to the discovery and mass production of pyrethroids, which have become among the most commercially successful insecticides. These compounds, which are not specific to any particular pest, are used globally and offer cost-effective advantages against a broad spectrum of pests in both agricultural and non-agricultural situations. They are utilized in the context of both harvest and post-harvest applications, as well as in the implementation of public health programs and veterinary applications. Currently, the research for new pyrethroids has essentially reached a standstill due to the increasingly widespread occurrence of insecticide resistance in pests. Nevertheless, several research paths remain open regarding these pesticides. This paper represents the current state of knowledge regarding pyrethroids, exposing both their advantages and disadvantages. Moreover, further investigation, at the molecular level, on their mode of action (MoA) could be very useful to improve their specificity. The results of this review may stimulate additional research for the development of novel pyrethroids having enhanced efficacy, low cost and reduced environmental impact.
1. Introduction
Among the most common insecticides there are natural pyrethrins and synthetic pyrethroids. They are extensively used in both indoor and outdoor applications. In the domestic sphere, they are used in the treatment of parasitic infestations, whereas in agriculture, they are used to control pests, particularly on horticultural crops, corn, and cereals. Additionally, they are used in social preventive medicine to combat, as strongly recommended by the WHO (World Health Organization), mosquito-borne diseases, such as malaria and the Zika virus [1].
It has been estimated that the global market for pyrethrins and pyrethroids in 2015 was approximately USD 4.7 billion, with an annual growth trend of approximately USD 300 million per year [2]. The global market for synthetic pyrethroids alone reached a value of more than USD 3.7 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 4.6 percent during the period 2024–2032, reaching a value of USD 5.6 billion by 2032 [3,4]. Pyrethrins and pyrethroids account for over one-third of the global market share of insecticides [5].
In the scientific literature, the generic term “pyrethroids” is used to refer to both “natural pyrethrins” and “synthetic pyrethroids”; this last subcategory also includes the “pyrethroid-like compounds”. Despite sharing a common MoA, these compounds have disparate application costs, environmental persistence, and ecological risks [6].
In comparison to conventional insecticides, such as organophosphates and organochlorines, pyrethroids possess several notable advantages. These include high insecticidal activity even at low doses, rapid paralysis (knockdown effect), low toxicity to birds and mammals, and low environmental persistence [7]. Their insecticidal and acaricidal effects are achieved through contact, whether direct or indirect). However, they have broad-spectrum activity and, thus, they are toxic to non-target organisms, including useful insects (e.g., pollinators), fish, and amphibians.
Pyrethrins are a mixture of six compounds extracted from the flower heads of Tanacetum cinerariifolium (chrysanthemum). These substances have demonstrated insecticidal properties for centuries and were first employed as insect deterrents [8]. The utilization of pyrethrum-based insecticides became pervasive in the 1800s when the dried flower powder, which is commonly known as “Persian powder” or “Dalmatian dust”, was transported to Europe via trade routes [9,10].
The first identification of the active insecticidal constituents of the pyrethrins took place during the early years of the 20th century. A series of investigations have demonstrated that the insecticidal activity resides in six esters. The active principles act on the nervous systems of insects, causing paralysis and death by contact. They were first used in powders and fumigants to control a broad range of pests. The efficiency of action with rather low-level toxicity against humans and animals made these compounds highly popular for both agricultural and domestic pest control [8,10,11,12]. The characterization of the elements in pyrethrins resulted in the development of synthetic pyrethroids.
Historically, several specific factors have contributed to the rapid decline of natural pyrethrum, including seasonal production variability, labor-intensive harvesting, differences in the concentration of the active compound in pyrethrum powder, very poor environmental persistence, importation issues, and higher production costs [13]. These factors have collectively favored and stimulated the research and the production of synthetic pyrethroids.
Based on the period of their discovery, their effectiveness of action, and other chemical-physical characteristics, pyrethroids can be grouped into several generational classes, which are further subdivided into different paragraphs below. Despite the synthesis of hundreds of pyrethroid molecules, only a few of them have been successfully commercialized and approved for use [11].
A century after the first scientific paper on the chemical constituents of pyrethrins was published (in 1923), this review aims to provide a comprehensive account of the history of pyrethroids and the associated challenges encountered during the lengthy and arduous quest for an “ideal insecticide” to combine efficacy, specificity of action, cost-effectiveness and environmental sustainability. This ideal insecticide would possess high toxicity and selectivity while being harmless to the ecosystem, higher animals, and humans. Over the decades, esteemed organic chemists, botanists, and biologists from around the globe have engaged in a spirited and intellectually stimulating exchange of ideas regarding this intriguing challenge. Nevertheless, the final word has yet to be spoken, as while it is true that pyrethroids still present the lowest environmental impact among other different categories of pesticides, it is also true that their negative effects on non-target organisms and their cross-resistance warrant careful evaluation.
This research aims to provide a comprehensive overview of the historical evolution and status of the most significant pyrethroids. For each pyrethroid, the efficacy, eco-compatibility, and cost-effectiveness will be evaluated, and the advantages and disadvantages will be discussed. Additionally, the limitations of current knowledge will be highlighted, including the incomplete understanding of the common chemical structure that determines their MoA.
2. The Pyrethrum-Derived Natural Compounds
Pyrethrins are a mixture of six neurotoxic botanical substances. They are natural pesticides extracted from pyrethrum, obtained from the dried flower heads of some plants of the Compositae family (Tanacetum cinerariifolium, ex Chrysanthemum cinerariifolium Vis., and Chrysanthemum coccineum) [14]. Tanacetum cinerariifolium, or “Dalmatian pyrethrum”, in particular, is the plant with the highest pyrethrum content [15]. It is an endemic plant species that is widespread on the eastern coast of the Adriatic Sea (southeastern Europe: Croatia, Bosnia, Montenegro, and Albania) [16]. These plants are currently widely cultivated in East Africa (Kenya, Tanzania, and Rwanda), Australia (Tasmania), Japan, and Southern China for an annual global production of around 10,000 metric tons [17]. Other plant species of the Compositae family also show insecticidal power, but only C. coccineum has reached commercial interest, albeit on a smaller scale than C. cinerariifolium [18].
The insecticidal power of pyrethrum, although already used (as crushed chrysanthemum) in China thousands of years ago (starting from Chou Dynasty), was recognized a long time before its active chemical principles were discovered in the trans-Caucasus area (Asia), around 1800, and imported, with huge success, in Europe as “Persian powder” or “Zacherlin insecticide” or “Bug powder” etc., via the Silk Road, by the Austrian merchant Johann Zacherl (1814–1888). Until the First World War, because the mountainous region of Dalmatia (a region of former Yugoslavia) was the main production area of pyrethrum plants, it was also called “Dalmatian dust” [9]. At the beginning of 1860, Giovanni B. Zampironi (1836–1906), an Italian pharmacist, produced the first mosquito fumigant cone [19], and the invention of the first mosquito coils and sticks was made by Eiichiro Ueyama of Dainippon Jochugiku Co. (Tokyo, Japan) in the first half of 1890 [10].
The pyrethrum, an oleoresin mixture, is extracted from the powder of the dried white Chrysantemum flowers (containing 25% of active ingredients) through solvents, such as acetone, petroleum ether, dichloromethane, nitromethane, methanol, kerosene, or acetic acid [14]. It is stabilized with butylated hydroxy toluene (BHT) to prevent its oxidative degradation and to preserve it for a long time and is sold as a viscous liquid or dry powder [20].
The subsequent refining process to extract pyrethrins from pyrethrum, carefully conducted to avoid degradation by overheating or oxidation, takes place below 60 °C using methyl alcohol, hexane, or supercritical carbon dioxide [21].
Pyrethrins are contact, non-systemic, organic terpenoid insecticides with low vertebrate toxicity (LD50 rat oral 584 to 900 mg/kg bw) [22]. They are all considered “moderately hazardous” (Class II) to humans by the 2019 WHO Classification of Pesticides [23] and highly toxic (Categories: H400 and H410) to aquatic organisms by Regulation EC 1272/2008 on Classification, labeling, and packaging of substances (CLP Regulation, incorporating the United Nations Globally Harmonized System of classification and labeling of chemicals).
The first investigation on pyrethrins, conducted in 1909 by Iunichi Fujitani (1868–1939)—a Japanese biologist assistant at the Pharmacological Institute, Kyoto Imperial University (Japan)—allowed to characterize them as ester compounds, even if this analysis did not allow to define their chemical structures [24]. In particular, Fujitani obtained, from the pyrethrum flowers, an active syrup ester having insecticidal activity and to which he gave the name “Pyrethron”. Fujitani also had the important merit of initiating in-depth studies by subsequent scholars on pyrethrum extracts all over the world.
According to O. Textor [25], however, the first studies on the chemical components of pyrethrum powder would date back to the second half of 1870 by Rother and Semenoff, who, independently of each other, dissolve the pyrethrum powder in organic and inorganic solvents to obtain a volatile substance and a resinous acid, insoluble in cold water.
Initial studies focused on elucidating the chemical structure and functional groups of pyrethrum and related natural pyrethrins. These studies identified, into the six insecticidally active lipophilic pyrethrin esters (pyrethrin I, pyrethrin II, cinerin I, cinerin II, jasmolin I and jasmolin II): (1) an acid moiety (one of these cyclopropane carboxylic acids: crysanthemic acid and pyrethric acid) [A]; (2) an ester linkage [B]; (3) and an aromatic alcohol moiety (one of these three cyclopentenolones: pyrethrolone, cinerolone and jasmolone) [C], obtaining a linear native structure of this type: [A–B–C]. Over time, synthetic pyrethroids have undergone modifications that alter the chemical configuration of this basic structure.
In 1922, Ryo Yamamoto, a Japanese agricultural chemist and student of Umetaro Suzuki (1874–1943), a discoverer of vitamin B1, identified a cyclopropane ring in the compound of natural pyrethrins. This can be regarded as an inaugural documented scientific study of these substances. He subjected the Fujitani pyrethron ester to hydrolysis (saponification) and subsequent oxidation (by ozone), thereby extrapolating the aldehyde and the trans-caronic acid. Despite being unable to define the chemical configuration of this acid, he postulated that it was the “pyrethron acid” (3-butenyl-2,2-dimethylcyclopropanecarboxylic acid) [26].
Subsequent research—conducted in 1924 by two Nobel Prize laureates in other fields of chemistry, Hermann Staudinger (1881–1965), a German organic chemistry, and Lavoslav Stjepan Ružička (1887–1976), a Swiss-naturalized Croatian chemist, at ETH (Eidgenössische Technische Hochschule, Zürich)—enabled the isolation of two pyrethrin compounds with the limited analytical tools available at the time: pyrethrin I and II identifying, albeit with some approximations and errors, their active ingredients as esters of two cyclopropane monocarboxylic acid moieties: respectively, chrysanthemic acid and pyrethric acid [27].
Despite the absence of a definitive understanding of the structural configuration of the alcoholic unit, Staudinger and Ružička were able to synthesize hundred distinct pyrethroids, including piperonyl chrysanthemum. However, the commercial viability of these compounds was limited due to their poor insecticidal efficacy, which was markedly inferior to that observed in natural pyrethrins [28].
In subsequent decades, as scientific studies advanced, it was determined that these acid groups (crysanthemic and pyrethric) could be associated with one of the three potential cyclopentenolone alcohols: cinerolone, jasmolone, and pyrethrolone. The distinct conjugation of these chemical groups, comprising two acids and three alcohols, generates six different permutations or isomers (esters): pyrethrin (I and II), cinerin (I and II), and jasmolin (I and II) in a proportion of 72:20:8 [29,30].
In particular, as an example of the chemical composition of pyrethrins, the structure of cinerin I [CAS RN: 25402-06-6; IUPAC name: 3-(2-butenyl)-2-methyl-4-oxo-2-cyclopenten-1-yl2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopentanecarboxylate] [22,31] (Figure 1) is reported, which can be considered the progenitor of many synthetic pyrethroids, especially, as we will see, of those of the first generation.
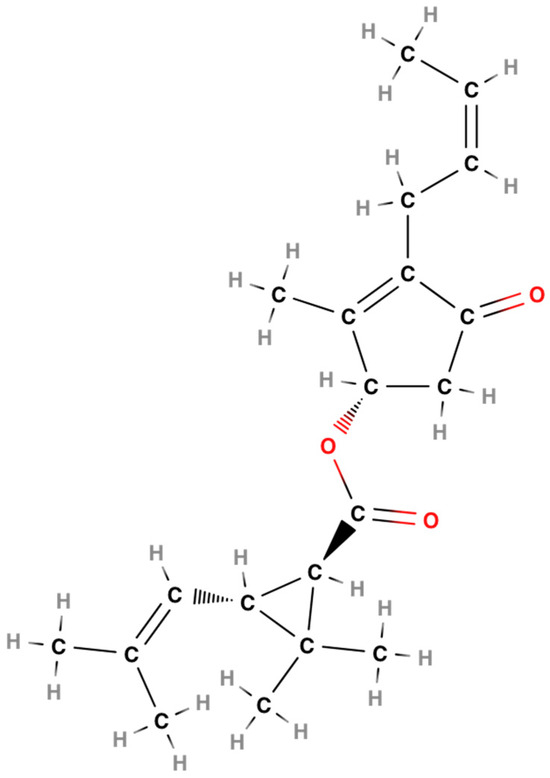
Figure 1.
Cinerin [22,32].
Natural pyrethrins exhibit several key characteristics of eco-compatibility with an ideal insecticide: non-systemic plant activity, potent insecticidal action, low toxicity to mammals and other warm-blooded animals, low insecticidal resistance in pests, and no biomagnification through the food chain [33]. They are among the few insecticidal substances permitted for use in organic agriculture.
A notable drawback of pyrethrins (and similarly of the majority of pyrethroids) is their toxicity to beneficial insects (such as pollinators like bees and predators of other harmful insects) and aquatic organisms (including fish and amphibians) [34]. Nevertheless, pyrethrins are distinguished by their high repellent capacity, even at low concentrations, which minimizes their contact with non-target organisms.
Moreover, they are not persistent in outdoor environments, decomposing rapidly in water and soil, particularly when exposed to sunlight, oxygen, humidity, high temperatures, photosensitizing agents (e.g., fulvic and humic acids), or microorganisms. Indeed, in soil, pyrethrins have a half-life of 1–2 days (in direct sunlight, for a maximum of 5 h) [35] and remain firmly in place due to their tight binding to soil particles [36]. For this reason, synthetic pyrethroids have been developed, and the degradable centers of the molecular structure of pyrethrins have been replaced with chemically stable alternative units.
3. The First Generation of Synthetic Pyrethroids
Following the clarification of the stereochemistry of pyrethrins in the latter half of the 1940s, chemical companies recognized the significant insecticidal potential of pyrethrins and proceeded to modify their molecular structures. This resulted in the creation of new synthetic products that could replicate the action mechanisms of pyrethrum. Consequently, the initial synthetic pyrethroids were identified with no alteration to the cyclopropane carboxylic esters (acid moieties) and appropriate modification to the alcohol moiety, resulting in the following configuration: [A–B–X].
The acid group, which is common to all pyrethroids of this generation and can be considered their basic general structure, is derived from chrysanthemic acid [CAS RN: 10453-89-1; IUPAC name: 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylic acid] [22,31] which is part of the above cinerin I molecule (Figure 2).
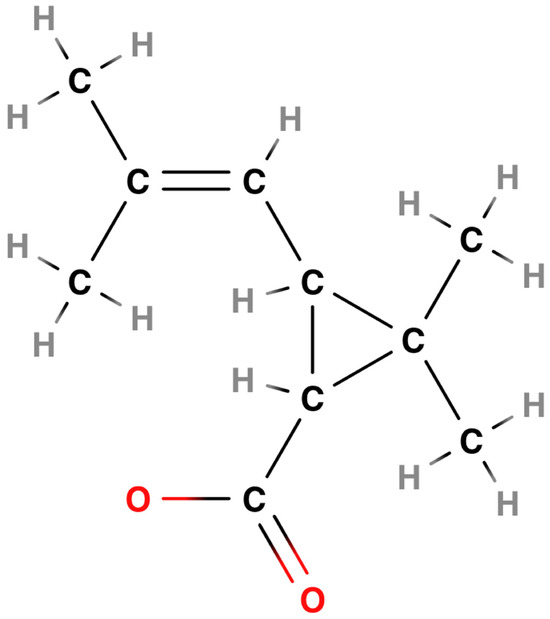
Figure 2.
Acid moiety from chrysanthemic acid [22,32].
In fact, the modification of the alcoholic units of pyrethrins preceded that of the acid parts, as the latter presented significant purification challenges due to their high instability. Additionally, initial attempts by Staudinger and Ruzicka to modify the acid part were unsuccessful [8].
The objective of these compounds, which were patented between 1949 and 1967, was to enhance the metabolic stability of pyrethrins [17]. These compounds are used almost exclusively in household applications because of their susceptibility to photodegradation.
The commercial expansion of this first generation of synthetic pyrethroids was initiated by the National Research Development Corp. (NRDC, New Delhi, India), which was established at the HM Treasury (London, UK) and later became the British Technology Group. By the end of the 1960s, the NRDC had sold its production licenses to six agrochemical industries: Sumitomo, Roussel Uclaf, Mitchell Cotts, Penick, Wellcome Foundation, and FMC [37].
These first pyrethroids, which lack a cyano group, are also referred to as first-generation or type I pyrethroids (such as allethrin, bifenthrin, permethrin, prallethrin, dimethrin, resmethrin, bioresmethrin, tetramethrin, prothrin, proparthrin, and others). All of them exhibit comparable toxic effects (syndrome) in animals, with the same MoA as natural pyrethrin.
The following sections provide a concise overview of the most significant compounds in this group.
3.1. Allethrin
The transient insecticidal efficacy of pyrethrins has prompted scientific investigations into the development of synthetic insecticides with chemical structures analogous to those of pyrethrins. The first commercially available synthetic pyrethroid was allethrin [CAS RN: 584-79-2; IUPAC name: (RS)-3-allyl-2-methyl-4-oxocyclopent-2-enyl (1RS,3RS;1RS, 3SR)-2,2-dimethyl-3-(2-me-thylprop-1-enyl)cyclopropanecarboxylate] [22,31]. In 1948, Milton S. Schechter, Natan Green, and Frederick B. LaForge of the USDA (US Department of Agriculture) Rothamsted Institute in Beltsville, MD, USA synthesized allethrin by reacting chrysanthemic acid with allethrolone [8] (Figure 3A). An analogous chemical process was patented by Schechter and LaForge [38].
Figure 3.
Allethrin (A); Prallethrin (B); Resmethrin (C); Tetramethrin (D); Imiprothrin (E); Phenothrin (F); Cyphenothrin (G) [22,32].
The commercial availability of allethrin and its various formulations began in Japan in 1954. This was made possible by a lengthy and intricate production process initially developed by Masanao Matsui and colleagues at the Sumitomo Chemical Co., Ltd. (Tokyo, Japan) in the early 1950s [39].
Manufacturers that currently or historically use or have used allethrin (typically in the form of an emulsifiable concentrate) in their products (Alleviate, Pyresin, Pynamin, Exthrin) include the Farnham Co. (Phoenix, AZ, USA), American Cyanamid Co. (Wayne, NJ, USA), Sumitomo Chemicals Co. (Tokyo, Japan), and Fairfield (Blythewood, SC, USA). Due to its heat resistance, it is used almost exclusively in household settings for the control of flying insects in anti-mosquito plates for electric diffusers [40].
As indicated by the Pesticides Properties Database (PPDB) of IUPAC [41], allethrin is a pyrethroid with minimal environmental persistence (soil degradation DT50 = 60 days) due to its rapid photodegradation. It is an active insecticide that acts upon contact with a target organism and is only effective when used in conjunction with other substances. As documented in the aforementioned IUPAC PPDB, this compound exhibits low toxicity to earthworms (acute 14 day LC50 > 1000 mg kg−1) and birds (acute LD50 > 2030 mg kg−1 for Colinus virginianus), moderate toxicity to honeybees (contact acute LD50 > 3.4 μg bee−1 Apis spp.) and fish (acute 96 h LC50 > 19 mg L−1 for Oncorhynchus mykiss), highly toxic to aquatic invertebrates (acute 48 h EC50 > 0.021 mg L−1 for Daphnia magna), moderately acute toxic to mammals (acute oral LD50 = 685 mg kg−1 for rat). Additionally, it is likely to be carcinogenic and an endocrine disruptor in humans.
CLP Regulation 1272/2008 identified this substance as acute, chronic, and very toxic to aquatic life (H400 and H410, according to the GHS categories). Additionally, the 2019 WHO Classification of Pesticides [23] categorized it as moderately hazardous for humans (Class II). It is not approved for use in outdoor plant protection by the EU under Regulation 1107/2009/EC [41].
A review of the Arthropod Pesticide Resistance Database [42] revealed 18 documented cases of insecticide resistance to allethrin in Blattella germanica (4 cases), Cimex hemipterus (5 cases), Cimex lectularius (8 cases), and Culex tritaeniorhynchus (1 case). As is widely recognized, the phenomenon of resistance and cross-resistance pertains to the tolerances exhibited by a population of organisms following exposure to a given toxic substance or another substance that acts in a similar manner. This represents a significant challenge, particularly in the case of synthetic pyrethroids [43].
3.2. Prallethrin
Following the synthesis of allethrin, there was a requirement for compounds with enhanced chemical stability in outdoor environments. This was since allethrin is susceptible to degradation by sunlight.
Replacing the benzyl alcohol ester of chrysanthemic acid, William F. Barthel [44] at the US Department of Agriculture and MGK (McLaughlin Gormley King Co., Chaska, MN, USA), identified dimethrin [CAS RN: 70-38-2; UPAC name: 2,4-dimethylbenzyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate] and barthrin [CAS RN: 70-43-9; IUPAC name: cyclopropanecarboxylicacid, 2,2-dimethyl-3-(2-methyl-1-propen-1-yl)-,(6-chloro-1,3-benzo-dioxol-5-yl)methyl ester] as two promising new insecticides [22,31]. Despite their efficacy against houseflies and low toxicity to fish and mammals, they were ultimately replaced due to their low knockdown activity [41,45].
Barthel’s research, however, had the merit of stimulating other organic chemists to pursue the development of more effective compounds. The next compound to be investigated was prallethrin [CAS RN: 23031-36-9; IUPAC name: 2-methyl-4-oxo-3-(prop-2-ynyl)cyclopent-2-en-1-yl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate] [22,31] (Figure 3B), which was first reported in 1961 by W.A. Gershoff and P.G. Piquett [46] of the USDA Entomology Research Division. Prallethrin was then purified by Noridata Matsuo and his colleagues in 1978 [28] and commercialized in Japan by Sumitomo Chemical in 1988. The insecticidal efficacy of pure prallethrin is four times greater than that of allethrin against Musca domestica [47].
The Godrej Group (Mumbai, India), SC Johnson (Racine, WI, USA), and Sumitomo Chemical Co. are the primary manufacturers that have used or currently use prallethrin (in varying formulations, including oils, foams, and plug-in vaporizers) in their products (Etoc, All Out, and others).
Prallethrin, which exhibits excellent repellent properties, is sold exclusively for domestic use against different house pests (houseflies, mosquitoes, and cockroaches) [48]. Although it is freely commercialized in the USA, it has not been approved in the EU under EC Regulation 1107/2009 for agricultural and outdoor applications [41]. Furthermore, it is considered moderately hazardous (Class II) to humans by the 2019 WHO Classification of Pesticides [23] and acute and chronically very toxic to aquatic life (Categories: H400 and H410) by the CLP Regulation EC 1272/2008.
The toxicity of this chemical is moderate to mammals and humans (acute oral LD50 = 460 mg kg−1 for rats), lowly toxic to birds (acute LD50 > 2000 mg kg−1 for Anas platyrhynchos), and highly toxic to honeybees (contact acute LD50 = 0.026 μg bee−1 for Apis spp.). Furthermore, the substance is highly acute and chronic ecotoxic to aquatic invertebrates (for Daphnia magna: acute 48 h EC50 = 0.0062 mg L−1; chronic 21 day NOEC > 0.00065 mg L−1) and fish (for Oncorhynchus mykiss: acute 96 h LC50 = 0.012 mg L−1; chronic 21 day NOEC > 0.003 mg L−1) [41]. In a study conducted by Yoshio Katsuda [49], it was found that prallethrin exhibited the same minimal cross-resistance as natural pyrethrins.
3.3. Resmethrin and Bioresmethrin
The next challenge was to try to synthesize compounds from the allethrin molecule with higher insecticidal activity than that of natural pyrethrins. At the beginning of the 1960s, Michael Elliott (1924–2007) and his co-workers (1973) at the NRDC (National Research Development Corp., London, UK) developed a new alcohol (5-benzyl-3-furyl-methanol), which, reacting by esterification with chrysanthemic acid, gave resmethrin [CAS RN: 10453-86-8; IUPAC name: 5-benzyl-3-furylmethyl (±)-cis-trans-chrysanthemate] [22,31], a furyl-methyl ester (Figure 3C). It was named “resmethrin” in honor of the Rothamsted Experimental Station (UK), where Elliott himself worked. Remethrin was the first synthetic pyrethroid with higher insecticidal activity than pyrethrins [50].
Michael Elliott, an eminent English chemist who was the recipient of numerous accolades and recognitions, served as the head of several research groups of scholars at the NRDC. During his tenure, he and his colleagues made significant contributions to the field of pyrethroid chemistry by identifying several key compounds, including resmethrin, bioresmethrin, permethrin, deltamethrin, and cypermethrin.
The primary manufacturers and suppliers, both current and historical, utilizing resmethrin in their commercial formulations under various trademarks (including Benzofuroline, Chrysron, Crossfire, Derringer, Pynosect, Raid, Scourge, Vectrin, and numerous others) are Bayer Crop Science (Monheim am Rhein, Germany), Prentiss Inc., Ltd. (St. Louis, MO, USA), and Sumitomo Chemical Co., Ltd.
Remethrin is a contact-active, non-synergizable, and highly photolabile insecticide that is utilized in emulsifiable preparations. It is too moderately persistent in soil (aerobic degradation: DT50 = 30 days) and highly persistent in water (aqueous hydrolysis: DT50 = 485 days at 20 °C and pH 7) [41]. Resmethrin has been identified as an endocrine disruptor and a potential carcinogen in mammals and humans [51]. Nevertheless, it is classified as slightly hazardous (Class III) by the WHO Classification of Pesticides [23].
Due to its broad-spectrum activity and acute high toxicity to honeybees (contact LD50 = 0.063 μg bee−1 for Apis spp.), as well as its high toxicity to fish (acute 96 h LC50 = 0.017 mg L−1 for Lepomis macrochirus; chronic 21 day NOEC = 0.00032 mg L−1 for Oncorhynchus mykiss) and aquatic invertebrates (acute 48 h EC50 = 0.0037 mg L−1 for Daphnia magna), it has not been approved by the EU for agricultural use under EC Regulation 1107/2009 [41]. This substance is currently used indoors, in zootechny, for stored agricultural crops, and in residences for the control of flying and crawling pests [40]. Resmethrin is classified as highly toxic to aquatic life (categories H400 and H410 of the GHS Classification of Chemicals) by the CLP Regulation EC 1272/2008.
The Arthropod Pesticide Resistance Database [42] documents 19 cases of insecticide resistance to resmethrin: Blattella germanica (one case), Culex quinquefasciatus (six cases), Musca domestica (five cases), Plutella xylostella (one case), and Trialeurodes vaporariorum (two cases).
Historically, it was, subsequently, imperative to identify new compounds that were more persistent than resmethrin and less toxic to mammals.
In 1967, M. Elliott, at the French pharmaceutical company Roussel Uclaf SA (Paris, France), initially isolated the pure isomer of chrysanthemic d-trans-acid and then, through esterification, obtained bioresmethrin [CAS RN: 28434-01-7; IUPAC name: (5-bezylfur-3-yl)methyl(1R)-trans-2,2-dimethyl-3-(2-methyl-pro-penyl)cyclopropanecarboylate] [22,31]. It is a trans-isomer of resmethrin. Remethrin and bioresmethrin were the first synthetic pyrethroids that, despite exhibiting comparable insecticidal efficacy to pyrethrin I, showed reduced toxicity to mammals (for resmethrin: acute oral LD50 > 2000 mg kg−1 for rat; for bioresmethrin: acute oral LD50 > 7070 mg kg−1 for rat). This was attributed to the elimination of cyclopentenolone esters [52].
However, they are highly susceptible to light and readily undergo oxidation, limiting their suitability for agricultural applications in outdoor settings.
Resmethrin and bioresmethrin are categorized as acute and chronic and very toxic to aquatic life (categories H400 and H410 of the GHS Classification of Chemicals) by the CLP Regulation EC 1272/2008.
3.4. Tetramethrin
Historically, the identification of new compounds with greater persistence than resmethrin and reduced toxicity to mammals has been of primary importance. During this period, concurrent experimental studies were conducted at the Health & Crop Sciences Research Laboratory (Sumitomo Chemical Co., Ltd., Japan) with the objective of synthesizing pyrethroids with higher knockdown activity than that of dimethrin and barthrin. Among the resulting compounds was tetramethrin (synthesized in 1964 by Takeaki Kato, Kenzo Ueda, and Keimei Fujimoto) [53], imiprothrin (discovered in 1979 by Nobushige Itaya) [17], and, later, dimefluthrin (identified in 2000, by Tatsuya Mori) [54].
Tetramethrin [CAS RN: 7696-12-0; IUPAC name: cyclohex-1-ene-1,2-dicarboximidomethyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate] [22,31] is a racemic compound (Figure 3D) derived from chrysanthemic acid esterified with tetrahydrophthalimidomethyl alcohol [22]. It is an odorless active insecticide and was the first pyrethroid to demonstrate a potent lethal effect. It exhibits high insecticide activity and is relatively stable to light and heat, even in aqueous solutions [41].
Killgerm Chemicals Ltd. (Ossett, UK) and Sumitomo Chemicals Co. are some of the main manufacturers that utilize tetramethrin in a variety of formulations, including emulsifiable concentrates, traps, or coils.
The utilization of tetramethrin in agricultural applications is not authorized within the EU in accordance with Regulation 1107/2009/EC [41]. This is since tetramethrin has been identified as a highly toxic substance to pollinating insects (with an acute toxicity value of LD50 = 0.16 μg bee−1 for Apis spp.), aquatic invertebrates (acute 48 h EC50 = 0.045 mg L−1 for Daphnia magna) and fish (acute 96 h LC50 = 0.016 mg L−1 for Oncorhynchus mykiss). It is categorized as “very toxic to aquatic life” by CLP Regulation EC 1272/2008.
Due to its low environmental persistence and low toxicity for birds (acute LD50 > 2500 mg kg−1 for Colinus virginianus) and mammals (acute oral LD50 > 5000 mg kg−1 for rats), it is exclusively utilized in household applications and, on an exceptional basis, for public health situations to control vector insects that are harmful to humans. Its environmental fate is moderately concerning due to its slight mobility in drain flow and wind, low solubility (in water at 20 °C, solubility = 1.83 mg L−1), and volatility (Henry’s law constant at 25 °C = 1.71 Pa m³ mol−1) [41].
The 2019 WHO Classification of Pesticides [23] classified this substance as “U: unlikely to present acute hazard in normal use”.
The Arthropod Pesticide Resistance Database [42] documents cases of insecticide resistance to tetramethrin in the following pest species: Blattella germanica (3 cases), Dermanyssus gallinae (one case), Helicoverpa armigera (one case), Musca domestica (one case), and Myzus persicae (one case).
3.5. Imiprothrin
Subsequently, research was conducted with the objective of identifying compounds with enhanced knockdown capabilities, such as imiprothrin [CAS RN: 72963-72-5; IUPAC name: 2,5-dioxo-3-(2-propynyl)-1-imidazolidinyl-methyl(1R)-2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropanecarboxylate] [22] (Figure 3E), in order to meet the urgent market demands for indoor applications.
In fact, imiprothrin, which was synthesized in the early 1970s by Sumitomo Chemical Co. and registered in 1996, remains a synthetic pyrethroid with the most potent knockdown activity against cockroaches. It was obtained by N. Itaya through the chemical restructuring of the alcohol moiety of prallethrin, analogous to the process adopted in the synthesis of tetramethrin. Its most prevalent commercial formulation is an aerosol for domestic use against cockroaches [55].
Sumitomo Chemical Co. is the manufacturer that utilizes imiprothrin in its product (Pralle, Supelco).
Due to its moderate mobility by drain flow and solubility in water at 20 °C of 93.5 mg L−1, imiprothrin is highly toxic to honeybees (contact acute LD50 = 0.4 μg bee−1 for Apis spp.), fish (acute 96 h LC50 = 0.038 mg L−1 for Oncorhynchus mykiss), aquatic invertebrates (acute 48 h EC50 = 0.051 mg L−1 for Daphnia magna), and algae (acute 72 h EC50 = 3.1 mg L−1 for Pseudokirchneriella subcapitata growth). It is also moderately toxic to mammals (acute oral LD50 = 900 mg kg−1 in rats). However, it is only used for outdoor applications in Australia and the USA [41].
Its agricultural use in open fields is not approved by the EU under EC Regulation 1107/2009 (repealing 91/414), and it is classified as “moderately hazardous” to humans (Class II) by the 2019 edition of the WHO Classification of Pesticides [23].
No cases of insecticide resistance have been documented in the Arthropod Pesticide Resistance Database for imiprothrin [42].
3.6. Phenothrin and Cyphenothrin
The syntheses of phenothrin [CAS RN 26002-80-2; IUPAC name: 3-phenoxybenzyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate] and cyphenothrin [CAS RN 39515-40-7; IUPAC name: (RS)-α-cyano-3-phenoxybenzyl (1RS,3RS;1RS,3SR)-2,2-dimethyl-3-(2-methyl-prop-1-enyl)cyclopropanecarboxylate] [22,31] were driven by a number of factors, primarily the necessity to develop more efficacious, secure, and selective insecticides. The objective was to develop products that would be effective against a wide variety of insects (especially pests such as fleas, ticks, lice, and mosquitoes) while simultaneously reducing toxicity to mammals. This would allow for the safe use of these substances in environments frequented by humans and pets.
Starting from the research of Katsuzo Kamoshita (1935–2013), who had discovered an herbicidal compound with low insecticidal activity from fenitrothion, in 1968, N. Itaya and collaborators and, in 1973, T. Matsuo and co-workers [56], at Sumitomo Chemical Co., discovered two new alcoholic components: respectively, 3-phenoxybenzyl alcohol and α-cyano-3-phenoxybenzyl acid. The esters of this alcohol, with chrysanthemic acid, yielded phenothrin (a benzyl ester, designated as d-phenothrin or sumithrin) and cyphenothrin (the first pyrethroid of the third generation, with an α-cyano group) (Figure 3F,G), both of which were commercialized by Sumitomo Chemical Co.
Phenothrin and cyphenothrin are highly efficacious insecticides that are effective against a range of arthropods, including houseflies, mosquitoes, fleas, and ticks. They are utilized in non-agricultural applications, such as household, public health, veterinary, and storage facilities [41]. These compounds exhibit high insecticidal activity and demonstrate robust resistance to metabolic degradation by pests. Additionally, they are relatively simple to synthesize, exhibit high photostability, and are less toxic to mammals than pyrethrins [17].
Phenothrin is commercially available from Killgerm Chemicals Ltd., while cyphenothrin is marketed by Sumitomo Chemical Co. and S.C. Johnson & Son, Inc. (Racine, WI, USA) for products such as Gokilaht, S2703 Forte, and Pesguard LG OBA.
These substances are moderately toxic to honeybees (acute LD50 = 2.0 μg bee−1 for Apis mellifera) and highly toxic to aquatic organisms. Furthermore, phenothrin has been demonstrated to be toxic to fish (acute 96 h LC50 > 0.0027 mg L−1 for Oncorhynchus mykiss), aquatic invertebrates (acute 48 h EC50 > 0.0043 mg L−1 for Daphnia magna), and aquatic crustaceans (acute 96 h LC50 > 0.00002 mg L−1 for Americamysis bahia). Cyphenothrin has been demonstrated to possess toxic properties in fish (acute 96 h LC50 = 0.00034 mg L−1 for Oncorhynchus mykiss; chronic 21 day NOEC = 0.000056 mg L−1 for Oncorhynchus mykiss) and aquatic invertebrates (acute 48 h EC50 = 0.00043 mg L−1 for Daphnia magna; chronic 21 day NOEC = 0.00009 mg L−1 for Daphnia magna) [41].
The utilization of these substances in the agricultural sector has been authorized in the USA and Australia; however, it has not been approved in the EU under the provisions of EC Regulation 1107/2009 [41]. Nevertheless, they are considered “U: unlikely to present acute hazard in normal use” by the 2019 WHO Classification of Pesticides [23]. They have been identified as being highly toxic to aquatic life (H400 and H410 of the GHS) by Regulation 1272/2008.
A review of the Arthropod Pesticide Resistance Database [42] reveals that 26 cases of phenothrin resistance have been documented in the following genus species: Aedes aegypti (10 cases), Aeneolamia varia (1 case), Blattella germanica (3 cases), Culex pipiens molestus (1 case), Lipaphis erysimi pseudobrassicae (1 case), Musca domestica (1 case), Myzus persicae (1 case), Pediculus humanus capitis (7 cases), Tribolium castaneum (1 case); while for cyphenothrin, 10 examples of recorded resistance are referred to Bemisia tabaci (1 case) and Musca domestica (9 cases).
While these first synthetic pyrethroids do not induce the same “flushing-out” effect in pests as pyrethrins, they nonetheless exhibit comparable disadvantages. These include UV photodegradation, which results in low outdoor persistence [57]. For these reasons, they are utilized exclusively in domestic settings for the control of flies and mosquitoes and not in outdoor settings for agricultural applications. Moreover, the development of insecticide resistance in pests has been observed with synthetic pyrethroids, a phenomenon that has been documented for the majority of similar compounds.
4. The Second Generation of Synthetic Pyrethroids
The second-generation pyrethroids were developed primarily to address the growing resistance of insects and to provide a more effective solution against resistant pests. In numerous regions, including Asia, Africa, and Latin America, insects have developed resistance to first-generation insecticides and other pesticide types (such as organophosphates and carbamates) that have demonstrated significant loss of efficacy.
The pivotal moment in this process occurred with investigations into the feasibility of synthesizing new acids with the objective of developing chemicals with enhanced insecticidal capabilities. This generation of synthetic pyrethroids follows this scheme: [Y–B–X]. In fact, they are created by modifying the acid and alcohol moieties while maintaining the ester linkage [-B-] of natural pyrethrins. This ester linkage [-C(=O)O-] can be considered the common base of this other compound family.
This was made possible by scientific progress in the manipulation of the chemical structure of pyrethroids, which permitted the modification of the side chain of chrysanthememic acid, addition of halogens (Br and Cl), and replacement of the furyl ring of the alcohol moiety with a benzene ring [10].
This finding led Jacques Martel and Chanh Huynh at the Roussell-Uclaf Laboratories (France) in 1967 to discovery of a new method of synthesizing chrysanthemic acid, which occurs in nature. This method makes it possible for the acid to be produced on an industrial scale.
Prior to 1970, only two attempts had been made to modify the acid part of the compound. The first was by Jiri Farkaš and colleagues at the Czechoslovak Academy of Sciences in Prague [58], and the second was by Takeshi Kitahara and Masanao Matsui at the University of Tokyo [59]. The aforementioned research yielded the synthesis of two analogs of chrysanthemic acid: dichlorovinyl acid and tetramethyl acid.
Modification of the basic chemical structure through the introduction of halogenated groups has allowed us to obtain compounds with greater stability and insecticidal power, but also with greater ecological risks and toxicity for non-target organisms. The evolution of chemical structures has necessitated the introduction of more complex and often divergent regulatory measures, given that these new compounds exhibit higher risk profiles compared to those of previous generations
4.1. Permethrin
In 1973-‘74, M. Elliott and the Rothamsted Scholars Team [60] employed dichlorovinyl acid in the esterification with a phenoxybenzyl alcohol to yield permethrin [CAS RN: 52645-53-1; IUPAC name: m-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate] [22,31] (Figure 4A) and cypermethrin. However, the latter compound is classified as a third-generation synthetic pyrethroid due to its α-cyano group. Permethrin was the first photostable pyrethroid, thereby enabling its outdoor use for agricultural and hygienic applications.
Figure 4.
Permethrin (A); Fenpropathrin (B) [22,32].
The new generation of synthetic compounds differs from those of the previous generation in that chlorine atoms were introduced into the acid side chain instead of methyl groups, thereby preventing the photodegradation of the entire structure. Similar to other pyrethroids, permethrin exists as two enantiomer pairs of stereoisomers: cis-permethrin and trans-permethrin. The cis form exhibits enhanced insecticidal activity relative to the trans-form. It is a residual action pyrethroid, and its insecticidal activity is contingent upon the ratio of the two stereoisomers (cis-permethrin and trans-permethrin) present in the final formulation [61].
Permethrin and analogous halogen-containing pyrethroids (e.g., decamenthrin and fenvalerate) exhibit enhanced insecticidal efficacy, rapidly penetrating the cuticle of insects due to their highly lipophilic nature. Furthermore, these compounds are stable under sunlight and oxygen degradation [62].
Some manufacturers and suppliers, currently or historically utilizing permethrin (in the form of shampoos, fumigants, and spot-on treatments) in a multitude of commercial formulations and associated trademarks (Ambush, Arctic, Corsair, Ectiban, Exmin, Fortefog P Fumer, Kestril, Pynosect), include Agropharm Ltd. (High Wycombe, UK), ICI Plant Protection Ltd. (London, UK), Mitchell Cotts Chemicals Ltd. (London, UK), and FMC Co. (Philadelphia, PA, USA).
Permethrin exhibits moderate persistence in natural ecosystems; in particular, (a) soil degradation (field): DT50 = 42 days; (b) aqueous hydrolysis at 20 °C and pH 7: DT50 = 31 days. Permethrin is highly toxic to pollinators (contact acute LD50: 0.024 for Apis mellifera; 0.022 for Bomus terrestris; 0.0157 for Megachile rotundata; 0.07 for Trigona spinipes) and aquatic organisms (fish—acute 96 h LC50 = 0.0125 mg L−1 for Oncorhynchus mykiss; fish—chronic 21 day NOEC = 0.000093 mg L−1 for Anabas testudineus; aquatic invertebrates—acute 48 h EC50 = 0.0006 mg L−1 for Daphnia magna; aquatic crustaceans—acute 96 h LC50 = 0.00002 mg L−1 for Americamysis bahia; algae growth—chronic 96 h NOEC = 0.0009 mg L−1) [41].
The substance is moderately toxic to mammals (acute oral LD50 > 430 mg kg−1 for rats) and thus falls within Class II (“moderately hazardous to humans”) of the 2019 WHO Recommended Classification of Pesticides. Additionally, it is not approved for plant protection in the EU under EC Regulation 1107/2009. It is currently utilized in domestic applications for the treatment of pets and in Australia and the USA for agricultural applications [41]. It is classified as Category 1 for human skin sensitization and is categorized as highly toxic to aquatic life (Categories: H400 and H410 of the GHS Classification of Chemicals) by EC Regulation 1272/2008.
As documented in the Arthropod Pesticide Resistance Database [42], cases of permethrin resistance have been reported in numerous species (nr. 520 cases for 58 insect species), including Aedes aegypti (84 cases), Culex quinquefasciatus (53 cases), Musca domestica (35 cases), Haematobia irritans (28 cases), Rhipicephalus microplus (27 cases), Pediculus humanus capitis (25 cases), Blattella germanica (19 cases), Culex pipiens (18 cases), Anopheles gambiae (16 cases), Rhipicephalus sanguineus (15 cases), and Aedes albopictus (15 cases) and many others.
A study conducted by the WHO revealed that the average comparative cost of technical-grade permethrin, excluding operational costs, is approximately 3.75 times higher than that of DDT [63].
Permethrin, as well as cyhalothrin, deltamethrin, cypermethrin, and cyfluthrin (which have an altered isobutenyl group attached to the cyclopropane moiety), is significantly more effective than the second-generation synthetic pyrethroids but is also much more stable to sunlight and oxygen. For instance, permethrin remains 60% undecomposed for a minimum of twenty days. It is a residual action pyrethroid [64].
4.2. Fenpropathrin
Tetramethyl acid esterified with 3-phenoxibenzyl alcohol was utilized by N. Matsuo of Sumitomo Chemical for the synthesis, in 1973, of fenpropathrin (a tetramethyl cyclopropanecarboxylic acid ester) [CAS RN: 39515-41-8; IUPAC name: α-cyano-3-phenoxybenzyl 2,2,3,3-tetramethylcyclopropanecarboxylate] [22,31,47] (Figure 4B).
This compound has a relatively simple chemical structure and exhibits high insecticidal activity. Indeed, fenpropathrin possesses several evident advantages over permethrin, including efficacy against a more extensive range of harmful insects, superior persistence, and resistance to environmental factors. This pyrethroid has paved the way for the development of other compounds with similar desirable properties.
Fenpropathrin is utilized as a non-systemic insecticide and acaricide to control many different pests, including loopers, mites, whiteflies, and aphids, in a range of crops, including fruits, vegetables, cotton, and maize [41]. The utilization of this chemical for agronomic applications is permitted in the USA; however, it is prohibited in the EU under the provisions of EC Regulation 1107/2009. This chemical is included in Class II (“moderately hazardous to humans”) of the WHO Classification of Pesticides [23]. Furthermore, it is categorized as “very acute toxic” (H400) and “chronic very toxic” (H410) to aquatic life according to CLP Regulation 1272/2008.
Examples of manufacturers of different commercial products (such as Danitol Fenthrin Platino, Rody, and Meothrin) that have utilized fenpropathrin (as emulsifiable concentrate) include the Agro-Care Chemical Industry Group Ltd. (Wuxi, China), Fortune Ag. Co. Ltd. (Shenzhen, China), Valent USA Corp. (Saint Ramon, CA, USA), and Sumitomo Chemicals Co.
Fenpropathrin exhibits moderate persistence in soil (aerobic degradation DT50 = 34 days) and high persistence in water (aqueous hydrolysis (at 20 °C and pH 7) DT50 = 1130 days). The substance is highly toxic to honeybees (contact acute LD50 > 0.05 μg bee−1 for Apis mellifera), fish (acute 96 h LC50 > 0.0023 mg L−1 for Oncorhynchus mykiss), and aquatic invertebrates (acute 48 h EC50 > 0.00053 mg L−1 for Daphnia magna). It is also moderately toxic to earthworms (acute 14 day LC50 = 184 mg kg−1 for Eisenia phoetida), birds (acute LD50 = 1089 mg kg−1 for Anas platyrhynchos), and mammals (acute oral LD50 = 870 mg kg−1 for rats) [41].
Cases of insecticide resistance to fenpropathrin in the Arthropod Pesticide Resistance Database [42] have been documented in different genus species (nr. 78 cases for 15 pest species), such as Spodoptera exigua (14 cases), Panonychus citri (12 cases), Spodoptera litura (10 cases), Liriomyza sativae (9 cases), and Bemisia tabaci (7 cases).
5. The Third Generation of Synthetic Pyrethroids
The necessity to obtain pyrethroids that are not only more environmentally persistent and resistant to degradation by sunlight and heat, but also possess greater toxicological potency, prompted global agrochemical companies to develop a novel category of synthetic pyrethroids.
In order to significantly enhance the insecticidal efficacy of a compound, an α-cyano group was introduced to the 3-phenoxybenzyl alcohol group, leading to the development of the third generation or Type II pyrethroids (such as cyphenothrin, cypermethrin, cyfluthrin, cyhalothrin, deltamethrin, fenpropathrin, fenvalerate, esfenvalerate, flucythrinate, flumethrin, and tralomethrin). This structural modification, while retaining the same schematic structure as the previous generation (i.e., [Y–B–X]), permitted an increase in the insecticidal activity of these new pyrethroids by up to 3–6 fold compared to non-cyano compounds.
The cyano group allows for the classification of synthetic pyrethroids into two sub-classes: Type I and Type II compounds, which exhibit different and characteristic toxicological profiles.
The cyano derivatives of permethrin (such as deltamethrin, cypermethrin, and fenvalerate) represent the third generation. In these compounds, the acid moiety remains dichlorovinylcyclopropane (in deltamethrin, dibromovinylcyclopropane), while the alcohol moiety incorporates a cyano group, forming an α-cyano-3-phenoxybenzyl-alcohol. For the dichlorovinyl group in their molecules, this generation of pyrethroids is characterized by higher stability than previous pyrethroids. Additionally, the α-cyano group is also characterized by higher insecticidal activity [65].
These properties have contributed to their considerable commercial success as agricultural insecticides. However, they also raise concerns regarding their eco-compatibility and potential toxicity in mammals.
Moreover, the incorporation of the alpha-cyano group in this generation of pyrethroids has further complicated the regulatory landscape. Indeed, the rise in chemical complexity has necessitated the undertaking of new and more comprehensive studies on acute and chronic toxicity, along with other associated environmental risks. This involved a profound and related review of the regulatory provisions in various countries. It is evident that each structural alteration gives rise to new regulatory challenges, as each new pyrethroid introduces a novel set of variables that must be meticulously evaluated to guarantee environmental compatibility and long-term human safety.
5.1. Deltamethrin
Deltamethrin [CAS RN: 52918-63-5; IUPAC name: (S)-α-cyano-3-phenoxybenzyl (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate] [22,31] (Figure 5A) is regarded as the most significant compound among third-generation pyrethroids due to its high efficacy against a broad spectrum of pests and harmful insects in both agricultural and urban contexts. Additionally, it exhibits enhanced chemical stability compared to previous generation pyrethroids, allowing it to retain its efficacy for extended periods, even under adverse environmental conditions such as prolonged sunlight exposure or elevated temperatures. This attribute is crucial for ensuring enduring protection against pests.
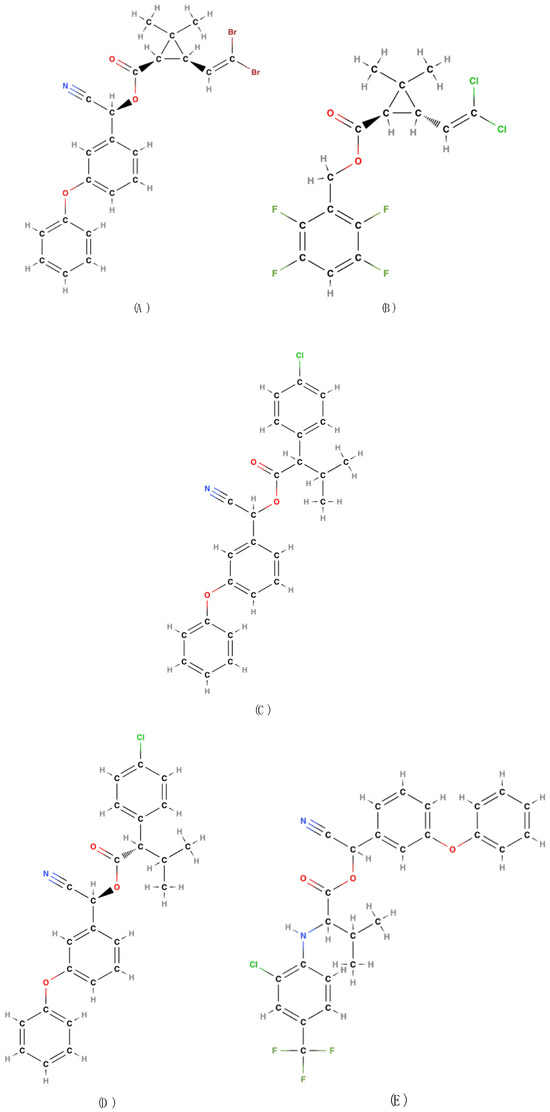
Figure 5.
Deltamethrin (A); Transfluthrin (B); Fenvalerate (C); Esfenvalerate (D); Fluvalinate (E) [22,32].
However, numerous commercial formulations of deltamethrin (used for the control of malaria-spreading mosquitoes) are markedly more toxic than previous pyrethroids, particularly in terms of their impact on the environment and aquatic organisms, with a notable impact on those in the early stages of growth.
Deltamethrin, the progenitor of this group of photostable pyrethroids, was synthesized in 1973 by M. Elliott and colleagues [66]. It is one of the most powerful insecticides sold in Australia, the USA, and the EU (approved under EC Regulation 1107/2009) for agricultural and stored crops and, in traps, even in organic farming against a wide range of pests. It possesses a distinctive attribute: it attracts mites, particularly red spiders [41].
A number of manufacturers and suppliers (e.g., AgriGuard Manufacturing Pvt. Ltd., Nandesari, India; Bayer CropScience; Certis Biologicals, Columbia, MD, USA; Headland Agrochemicals Co., Deeside, UK; and Landgold Ltd., London, UK) have utilized deltamethrin (primarily as an emulsifiable concentrate) in a multitude of commercial formulations (e.g., Bandu, Decis, Delta Gold, and Pearl Micro).
Despite exhibiting minimal environmental persistence and low leachability, it is classified as moderately hazardous (Class II) to humans according to the 2019 WHO Classification of Pesticides [41]. It is highly toxic to animal species, particularly mammals (acute oral LD50 = 87 mg kg−1 and short-term dietary NOEL = 2.5 mg kg−1 for rats), pollinators (contact acute LD50 [μg bee−1]: 0.0015 for Apis meliifera; >0.2 for Bombus terrestris; 0.057 for Osmia bicornis; 0.556 Megachile rotundata), and aquatic organisms (fish—acute 96 h LC50 = 0.00015 mg L−1 for Oncorhynchus mykiss; aquatic invertebrates—acute 48 h EC50 = 0.00056 mg L−1 for Daphnia magna; aquatic crustaceans—acute 96 h LC50 = 0.0000017 mg L−1 for Americamysis bahia; aquatic plants—acute 7 day EC50 > 0.000405 mg L−1 for Lemna gibba) [41].
For these reasons, this chemical is classified under Category 1 (H410) by the CLP Regulation (i.e., “Very toxic to aquatic life with long-term effects”) (Regulation EC 1272/2008) and, in 2013, Greenpeace included it in the list of active ingredients that should be banned from the market to protect bees [67].
As documented in the Arthropod Pesticide Resistance Database [42], numerous cases of deltamethrin resistance have been observed across a diverse range of species (nr. 803 cases for 74 insect species): Rhipicephalus microplus (82 cases), Triatoma infestans (69 cases), Helicoverpa armigera (54 cases), Spodoptera exigua (43 cases), Plutella xylostella (37 cases), Thrips tabaci (35 cases), Culex pipiens pallens (34 cases), and Aedes aegypti (33 cases).
In a cost-comparison hypothesis between different insecticides implemented by the WHO for the treatment of leishmaniasis, deltamethrin (at a dosage with a technical grade of 0.025 g/m2) had a cost ratio of 3.125 compared to DDT [63]. Regarding its utilization for indoor residual spraying (IRS) for the management of malaria, the application cost was 2.7 times higher than that of DDT (the most cost-effective alternative insecticide) [68]. However, in 2007, the cost ratio was equivalent to that of DDT [69].
5.2. Transfluthrin
The necessity for a pyrethroid with high knockdown efficacy and high volatility to permit its utilization in confined environments led to the development of transfluthrin [CAS RN: 118712-89-3; IUPAC name: 2,3,5,6-tetrafluorobenzyl(1R,3S)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate] [22,31] (Figure 5B). Deltamethrin is not particularly volatile and is utilized for surface treatments, in the form of powders, or for agricultural applications.
This compound is derived from permethrin and has been obtained through esterification of tetrafluolobenzyl alcohol with dichlorovinyl chrysantemic acid. It has been in use since 1996 [70]. Currently, it is one of the most widely utilized insecticides (in domestic, public hygiene, and non-crop settings) to its efficacy in repelling flies and mosquitoes. Moreover, transfluthrin has been shown to possess high toxicity, even at low doses, and a pronounced knockdown effect [41].
Bayer Crop Science Ltd. is one of the manufacturers that utilizes transfluthrin for its commercial formulations in products such as Bayothrin and Baygon Mosquito Coil.
It exhibits minimal persistence in the aqueous phase (fast aqueous photolysis: DT50 = 0.6 days; and aqueous hydrolysis: DT50 = 5 days) and high selectivity: low toxicity to mammals (acute oral LD50 > 5000 mg kg−1 for rat), birds (acute LD50 > 1890 mg kg−1 for Colinus virginianus), and pollinators (contact acute LD50 > 2.0 μg bee−1 for Apis spp.). However, it has been demonstrated to be highly toxic to fish (acute 96 h LC50 > 0.0007 mg L−1 for Oncorhynchus mykiss) and aquatic invertebrates (acute 48 h EC50 > 0.0017 mg L−1 for Daphnia magna) [41].
It is classified as “unlikely to present acute hazard in normal use” by the WHO Classification of Pesticides [23]. The product is not approved for use on crops by the EU under EC Regulation 1107/2009. Furthermore, it is considered hazardous to aquatic life, exhibiting high acute (H400) and chronic toxicity (H410) in accordance with the GHS Classification of Chemicals (Regulation EC 1272/2008).
Cases of documented transfluthrin resistance according to the Arthropod Pesticide Resistance Database [42] were observed in Aedes aegypti (one case) and Helicoverpa armigera (one case).
5.3. Fenvalerate
One of the last synthetic pyrethroids in this category is fenvalerate [CAS RN: 51630-58-1; IUPAC name: α-cyano-3-phenoxybenzyl (RS)-2-(4-chlorophenyl)-3-methylbutyrate] [22,31] (Figure 5C), which was discovered by Nobuo Ohno and colleagues in 1973 [71] at the Pesticide Research Department of Sumitomo Chemical Co. and subsequently commercialized in 1978 [72].
Fenvalerate, which is primarily utilized in the agricultural sector, exhibits inferior performance characteristics (efficacy and persistence) in comparison to those of deltamethrin and transfluthrin. While deltamethrin is more potent and persistent, and transfluthrin is more volatile and suitable for rapid control indoors, fenvalerate is positioned as an intermediate solution, effective but with lower persistence and potency than deltamethrin. However, its chemical structure possesses a distinctive quality that renders it a valuable compound. In contrast with the prevailing view of Staudinger and Ruzicka, and other chemists, the absence of the cyclopropane ring in the acid moiety is not essential for the achievement of significant insecticidal activity [8]. It is notable that fenvalerate is the first pyrethroid to lack a cyclopropane ring in its acid moiety.
Due to its relatively low cost in comparison to other pyrethroids, it has been extensively utilized for the control of pests affecting cotton and soybeans, as well as a multitude of other pests, particularly those exhibiting resistance to organochlorine, organophosphate, and carbamate insecticides [73].
It has been approved for outdoor agronomic applications in Australia and the USA, but not in the EU under Regulation EC 1107/2009. It is classified as moderately hazardous to humans according to the WHO Classification of Pesticides [23]. Additionally, it has been categorized as Group 1 for skin sensitization (H317: “May cause an allergic skin reaction”) in humans and as extremely toxic to aquatic life (H400 and H410) by the CLP Regulation 1272/2008.
Several manufacturers and suppliers, including Sumitomo Chemicals Co., Shell Chemicals (Houston, TX, USA), and SDS Biotech K.K. (Tokyo, Japan), have utilized fenvalerate (as emulsifiable concentrates, granules, and powders) in a variety of commercial formulations, such as Sumicidin, Pydrin, Belmark, and Ectrin.
It is characterized by high persistence in soil (aerobic degradation: DT50 = 40 days) and water (aqueous hydrolysis: DT50 = 115 days at 20 °C and pH 7). Fenvalerate is highly toxic to honeybees (contact acute LD50 = 0.23 μg bee−1 for Apis spp.), fish (acute 96 h LC50 = 0.0036 mg L−1 for Oncorhynchus mykiss), and aquatic invertebrates (acute 48 h EC50 = 0.001 mg L−1 for Daphnia magna), and moderately toxic to mammals (acute oral LD50 = 451 mg kg−1 for rat), earthworms (acute 14 day LC50 = 40 mg kg−1), and other pollinators (acute LD50 = 1.09 μg insect−1 for Trigona spinipes) [41].
In the Arthropod Pesticide Resistance Database [42], several cases (179 cases for 37 genus species) of insecticide resistance have been observed for fenvalerate: Helicoverpa armigera (60 cases), Heliothis assulta (20 cases), Aphis gossypii (13 cases), Haematobia irritans (7 cases), Helicoverpa assulta (7 cases), Plutella xylostella (7 cases), and Spodoptera litura (7 cases), among others.
5.4. Esfenvalerate
Fenvalerate has four stereoisomers, one of which, esfenvalerate [CAS RN: 66230-04-4; IUPAC name: (S)-α-cyano-3-phenoxybenzyl (S)-2-(4-chlorophenyl)-3-methylbutyrate] [22,31] (Figure 5D), is biologically highly active. It is one of the most preferred pyrethroids due to the low doses required for application.
Esfenvalerate was developed primarily to enhance the efficacy and safety of fenvalerate, from which it is derived. The primary objective was to create a more potent and selective insecticide while reducing the dosage required for effective pest control. Indeed, it offers more targeted efficacy with a potentially reduced environmental impact, as it requires lower dosages and exhibits greater specificity of action. It is an efficacious insecticide that acts rapidly upon ingestion and contact, and is effective against Coleoptera, Diptera, and Hemiptera. It was first commercialized in 1987 and subsequently approved for outdoor applications in Australia, the USA, and the EU under EC Regulation 1107/2009 [41]. It is classified as “moderately hazardous to humans” by the 2019 WHO Classification of Pesticides [23].
Several manufacturers and suppliers have utilized esfenvalerate (in emulsifiable concentrates and spray products) in their diverse commercial formulations, including Sumi-Alpha, Hounddog, Sven, Asana, and Esfenvalerate 5EC. These include BASF Chemicals Ltd. (Ludwigshafen, Germany), Green Crop Chemicals Ltd. (Vehari, Pakistan), and Standon Chemicals Ltd. (London, UK).
It is moderately persistent in soil (soil degradation DT50 = 66.6 days) but very persistent in water (aqueous hydrolysis DT50 = 428 days at 20 °C and pH 7). Furthermore, it is highly toxic to fish (for Oncorhynchus mykiss: acute 96 h LC50 = 0.0001 mg L−1; chronic 21 day NOEC = 0.00025 mg L−1), aquatic invertebrates (for Daphnia magna: acute 48 h EC50 = 0.00027 mg L−1; chronic 21 day NOEC = 0.000052 mg L−1), algae (acute 72 h EC50 = 0.0065 mg L−1 for Pseudokirchneriella subcapitata), pollinators (for Apis mellifera: contact acute LD50 = 0.07 μg bee−1; oral acute LD50 = 0.21), and mammals (acute oral LD50 = 88.5 mg kg−1 for rat) [41].
Many cases (91 cases documented in 15 insect species) of insecticide resistance to esfenvalerate have been reported in the Arthropod Pesticide Resistance Database [42]. These cases predominantly concern: Leptinotarsa decemlineata (28 cases), Earias vittella (16 cases), Blattella germanica (10 cases), and Spodoptera litura (9 cases).
5.5. Fluvalinate
Another non-cyclopropanecarboxylic acid ester, such as esfenvalerate, is fluvalinate [CAS RN: 69409-94-5; IUPAC name: (RS)-α-cyano-3-phenoxybenzyl N-(2-chloro-α,α,α-trifluoro-p-tolyl)-DL-valinate] [22,31], which is used against crop pests. Fluvalinate is particularly valuable in apiculture due to its low toxicity to bees for the control of mites, particularly Varroa destructor, a devasting parasite for bees. Instead, esfenvalerate is a broad-spectrum insecticide utilized primarily in agricultural applications and for pest control and has a toxicity profile that requires greater caution when handling it in proximity to bees and other beneficial insects. Obtained by replacing the acid part of fenvalerate with a valine group (Figure 5E), it was patented in 1978 by Clive A. Henrick and Barbara A. Garcia at Zoëcon Co., Ltd., Palo Alto, CA, USA [74].
This insecticide is non-persistent (soil degradation DT50 = 7 days) and rapidly photodegradable (aqueous photolysis DT50 = 1 day). It is used to protect assorted crops (cereals, vegetables, grapes, and cotton) from a multitude of pests (aphids, moths, thrips, and others). However, it has not been approved as a plant protector in the EU under EC Regulation 1107/2009. It is categorized as “moderately hazardous to humans” (Class II) for handling use by the WHO Classification of Pesticides [23] and “highly toxic to aquatic life” (H400 and H410) by the CLP Regulation 1272/2008.
Nippon Soda Co. Ltd. (Tokyo, Japan), and Zoëcon Co. Ltd. (Palo Alto, CA, USA) are the manufactures of fluvalinate (as emulsifiable concentrates and suspensions), which are commercialized under the brand names Apistan, Klartan, Minadox, Yardex, and Mavrik.
Fluvalinate is an efficacious miticide that is also well tolerated by honeybees and other pollinators due to its lack of toxicity and repellency, which is attributed to the presence of the valine amino acid in its chemical structure. Indeed, it is commonly utilized in honeybee colonies without detrimental effects on bees [75].
Nevertheless, this pyrethroid has been demonstrated to possess moderate toxicity to mammals (acute oral LD50 = 261 mg kg−1 for rats) and high toxicity to aquatic organisms (fish: acute 96 h LC50 = 0.0009 mg L−1 for Lepomis macro-chirus and chronic 21 day NOEC = 0.000033 mg L−1 for Pimephales promelas; aquatic invertebrates: acute 48 h EC50 = 0.074 mg L−1 for Daphnia magna; aquatic crustaceans: acute 96 h LC50 = 0.0029 mg L−1 for Americamysis bahia). Regarding its environmental fate, this compound is rapidly photodegradable (aqueous photolysis DT50 = 1 day at pH 7) and exhibits low persistence in soil (aerobic degradation DT50 = 7 days). Additionally, it has low solubility (0.002 mg L−1 in water at 20 °C) and low volatility (vapor pressure = 0.013 mPa at 20 °C) [41].
Cases of insecticide resistance to fluvalinate, according to the Arthropod Pesticide Resistance Database [42], have been documented for Dermanyssus gallinae (one case), Spodoptera frugiperda (one case), and Varroa destructor (one case).
6. The Last Generation of Synthetic Pyrethroids
The development of this generation of pyrethroids was primarily driven by the imperative to overcome the constraints associated with previous pyrethroids, particularly in terms of insect resistance, and to enhance the safety profile of the environment and humans while maintaining the efficacy of pest control measures. These compounds represent a new generation of non-ester pyrethroids distinguished by modified chemical structures that confer a range of benefits, including broader applicability in agriculture, households, and public health.
Synthetic pyrethroids of this latest generation have been developed through the implementation of complex and simultaneous modifications to the alcohol and acid moieties and ester linkage, resulting in the following structural configuration: [Y–Z–X]. In general, the synthesis of these compounds is based on the possibility of changing the ester linkage to an ether linkage to obtain compounds with higher insecticidal activities.
From a technical standpoint, these compounds, despite exhibiting the same MoA on sodium channels, cannot be classified as pyrethroid-type insecticides due to their distinct structural differences from pyrethrins. For this reason, they are referred to as “pyrethroid-like compounds”, “pseudopyrethroids”, “synthetic pyrethroid analogs”, or “no-ester pyrethroids”. These substances are primarily used as agricultural insecticides and termicides.
Fenvalerate has opened the doors for the synthesis of this new group of non-cyclopropane pyrethroids, such as etofenprox [76], silafluofen [77], and flufenprox [78].
6.1. Etofenprox
As previously stated, the synthesis of this pesticide is driven by the need to develop molecules that are effective and low in mammalian toxicity and, at the same time, have lower cross-resistance. This enables its use in areas where pyrethroid resistance has become a significant issue.
Etofenprox [CAS RN: 80844-07-1; IUPAC name: 2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzyl ether] [22,31] was discovered in 1981 by Kiyoshi Nakatami and colleagues at Mitsui Toatsu Chemicals Inc. (Tokyo, Japan) [76] is a diphenyl compound that, technically, is not a pyrethroid (Figure 6A). However, its behavior is like that of pyrethroids because it alters the physiological functionality of sodium transport channels by contact or ingestion (Regulation EU 528/2012).
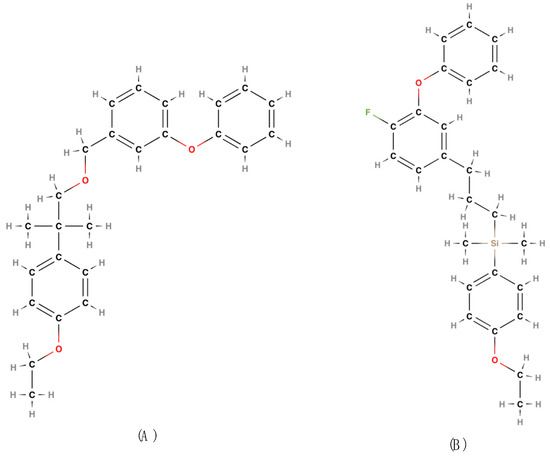
Figure 6.
Etofenprox (A); Silafluofen (B) [22,32].
It is a broad-spectrum insecticide that acts rapidly, by contact or ingestion, even against pests that have developed resistance. It is utilized in Japan, the USA, and the EU (approved under EC Regulation 1107/2009) to control a diverse range of pests (moths, aphids, and leaf miners) on fruits, vegetables, and paddy fields. Etofenprox has been approved in Japan for the control of mosquito larvae due to its ability to overcome resistance to temephos and other anti-larval agents [41]. It is classified as “unlikely to present hazard” (Class “U”) to humans by the WHO Classification of Pesticides [23].
The manufacturers and suppliers that have utilized etofenprox (typically in the form of an emulsifiable concentrate) in their commercial formulations (Boxer, Trebon, and Punkaso) include Certis Europe B.V., Isagro S.p.A. (Milan, Italy), Landis Research Group (Madison, WI, USA), and Mitsui Chemicals Inc. (Tokyo, Japan).
Etofenprox is a non-persistent chemical (aerobic soil degradation rate DT50 = 11 days). It is well tolerated by mammals (acute oral LD50 > 2000 mg kg−1 for rats), birds (acute LD50 > 2000 mg kg−1 for Anas platyrhynchos), and earthworms (acute 14 days LC50 > 24.6 mg kg−1). However, it is highly toxic to pollinators (contact acute LD50 (μg bee−1): 0.177 for Osmia bicornis and 0.051 for Megachile rotundata), fish (acute 96 h LD50 = 0.0027 mg L−1 for Oncorhynchus mykiss), and aquatic invertebrates (acute 48 h EC50 = 0.0012 mg L−1 for Daphnia magna) [41].
As reported by the Arthropod Pesticide Resistance Database [42], 75 cases of etofenprox resistance have been documented in 16 species of pests. These include Aedes aegypti (27 cases), Anopheles gambiae (14 cases), Anopheles funestus (7 cases), Culex tritaeniorhynchus (5 cases), and Culex pipiens molestus (4 cases).
A calculation of the costs of etofenprox in IRS applications for malaria control, excluding operational components, reveals that it is approximately 1.8 times higher than that of lambda-cyhalothrin used for the same purpose [68].
6.2. Silafluofen
These compounds are designed with a particular focus on their application in agriculture and pest control at the industrial or professional level. The chemical structures of these compounds confer properties that may be more suitable than those of etofenprox for use in outdoor environments, on different surfaces, or in situations where greater resistance to degradation and greater persistence are required.
Silafluofen [CAS RN: 105024-66-6; IUPAC name: (4-ethoxyphenyl)[3-(4-fluoro-3-phenoxyphenyl)propyl](dimethyl)silane] [22,31] was first identified by Yoshio Katsuda in 1986 at Dainihon Jochugiku Co., Ltd. (Osaka, Japan) is a derivative of etofenprox. The name is derived from the presence of a silicon atom in the molecule (Figure 6B).
It is an organosilicon insecticide utilized for the management of soil-borne pests (such as termites) that affect tea, wood, rice, and oil palm crops. It is widely utilized in India, China, Vietnam, and Japan; however, it has not been approved for use in crop protection in the EU under the provisions of EC Regulation 1107/2009. Silafluofen has been classified as “unlikely to present a hazard” (Class “U”) to humans by the WHO Classification of Pesticides [23].
The current or historical manufacturers and suppliers of commercial formulations that have utilized silafluofen (typically as an emulsifiable concentrate at 0.10 to 0.15%) in different products (such as 5Joker, Neophan, Silatop, and Silonen) are Bayer CropScience and Jiangsu Yangnong Chemical Co., Ltd. (Yangzhou, China).
This compound possesses notable advantages: high insecticidal and repellent activity and low toxicity to mammals (acute oral LD50 > 5000 mg kg−1 for rat), birds (acute LD50 > 2000 mg kg−1 for Anas platyrhynchos), and aquatic organisms (fish: acute 96 h LC50 > 100 mg L−1 for Cyprinus carpio; aquatic invertebrates: acute 48 h EC50 = 7.7 mg L−1 for Daphnia magna) [41].
In the Arthropod Pesticide Resistance Database [42], there are no cases of insecticide resistance to this substance.
The most significant attribute of silafluofen is its low toxicity in fish, which has been fully elucidated [79]. Consequently, further investigation is warranted to develop alternative potential compounds with analogous characteristics.
Flufenprox [CAS RN: 107713-58-6; IUPAC name: 3-(4-chlorophenoxy)benzyl (RS)-2-(4-ethoxyphenyl)-3,3,3-trifluoropropyl ether] and protrifenbute [CAS RN: 119544-94-4; IUPAC name: (RS)-5-[4-(4-chlorophenyl)-4-cyclopropylbutyl]-2-fluorophenyl phenyl ether] [22,31], which were first manufactured in 1992 by the Zeneca Group PLC and in 1994 by the FMC Corporation, are widely used against a variety of pests to protect rice and other crops [80]. These insecticides are now considered obsolete and have been largely abandoned in all countries. This is due to their high toxicity to aquatic organisms and honeybees, as well as their classification as neurotoxicants (Cramer Class III) and endocrine disruptors in humans [41].
7. Discussion
This analysis allows us to make indicative attributions regarding the specific uses and limitations of each generation of pyrethroids examined based on their common distinctive characteristics. In the table below (Table 1), the most important details of the above reviewed generation of pyrethroids are summarized.

Table 1.
Main details of different generations of pyrethroids (author’s own elaboration).
The evolutionary path of various generations of synthetic pyrethroids has been driven by the necessity to enhance their efficacy, stability, and safety in comparison to natural pyrethrins. Pyrethrins have significant limitations, such as photodegradability and low environmental persistence.
The primary objective of the first generation of pyrethroids was to essentially emulate the insecticidal characteristics of natural pyrethrins, increasing, albeit with slight improvements, their knockdown effect against insects and maintaining low toxicity to mammals. These first compounds, which are highly susceptible to photodegradation, are predominantly utilized in indoor applications, particularly in household environments.
The second generation of pyrethroids was developed with the objective of enhancing the environmental stability of the previous generation and addressing the emergence of insect resistance. Due to their aforementioned properties, these compounds are extensively utilized in outdoor applications, particularly in agriculture, for the management of pests on economically significant crops (such as corn, soybeans, and cotton), and in public health initiatives to control the transmission of disease vectors.
The introduction of the alpha-cyano group has significantly enhanced the efficacy and environmental persistence of third-generation pyrethroids, making them highly suitable for outdoor applications, including the control of pests in extensive crops and post-harvest protection, as well as public hygiene programs.
The non-ester pyrethroids of the latest generation have been synthesized with the objective of overcoming a significant limitation of the pyrethroids of previous generations, namely insect resistance, while maintaining their safety profile for mammals and non-target species. The primary application of these compounds is in outdoor settings, where they are used to control pests in agriculture and combat mosquitoes.
Over the course of a century, scientific research on synthetic pyrethroids has undergone various evolutions to respond to ever-changing challenges. Their use has progressively increased due to their efficacy against a wide range of parasites and their relatively low toxicity to mammals.
Nevertheless, the environmental issues associated with their extensive use remain evident. These include the growing and widespread tolerance and resistance among target insect populations, toxicity to non-target organisms, and regulatory challenges posed by an increasingly numerous and complex group of substances.
The increase in tolerance represents a significant challenge that has driven the evolution of the development of pyrethroids and remains unresolved. This phenomenon, as stated above, has driven chemical research to focus on the synthesis of more toxic compounds and, presumably, on the incremental application of these compounds, both in terms of dosage and frequency. The need to use more potent formulations or higher doses to achieve the same pest control results may have amplified their environmental impact and significantly increased operational costs for farmers, particularly in relation to increased pesticide applications, the purchase of new, more advanced, and expensive formulations, and the potential loss of crop yield due to less effective pest control.
Conversely, with respect to the economic assessments associated with potential benefits such as increased agricultural yields and crop protection, it is indisputable that the efficacious utilization of pyrethroids can result in a substantial increase in crop yields and agricultural production, thereby contributing to global food security and the overall sustainability of agricultural practices. Nevertheless, a comprehensive and objective economic assessment must also consider hidden environmental and social costs, which are not always readily discernible or quantifiable. These include potential adverse effects on human health, ecosystems, non-target species, and biodiversity. Consequently, a comprehensive assessment of costs and benefits necessitates the incorporation of not only a direct economic analysis but also a detailed examination of the immediate and long-term environmental and health impacts.
Even though synthetic pyrethroids have been developed with the intention of being safer than other insecticides, they are not immune from the potential for adverse effects, whether lethal or sublethal, on non-target organisms. In particular, they have been shown to impact pollinators and aquatic organisms by disrupting the equilibrium of ecosystems.
These compounds, though less environmentally persistent than other insecticides such as organophosphates and organochlorines, have been demonstrated to have a negative impact on aquatic and terrestrial ecosystems. Indeed, despite their low solubility in water, these compounds can contaminate aquatic ecosystems, causing significant damage to the fauna present therein, such as fish and invertebrates. While pyrethroids tend to degrade rapidly in sunlight and oxygen, degradation in water and sediments can be significantly slowed down, increasing the risk of chronic exposure for non-target aquatic organisms [34,81].
Moreover, the increasing complexity of the chemical composition of pyrethroids represents a substantial challenge for regulatory authorities in various countries, necessitating meticulous and comprehensive assessments. The advent of novel chemical structures and the enhancement of insecticidal potency have resulted in a notable increase in the complexity of the regulatory framework governing these compounds. This complexity is evident in the assessment of their toxicological effects and the management of their environmental impact. Another significant challenge to regulatory activity is the diversity of the effects that these compounds can have on non-target organisms. Moreover, the differential toxicity and the different persistence in the environment exhibited by the numerous and diverse pyrethroids necessitate the undertaking of specific studies for each compound, resulting in a regulatory process that is not only more laborious but also longer and more expensive. Ultimately, the pervasive expansion of pyrethroids across diverse sectors and applications has resulted in indiscriminate proliferation and overlapping of regulatory competencies between disparate government bodies and agencies. This has led to uncertainty in the application of regulations, even within the same country.
Pyrethrins and pyrethroids are classified as not specific insecticides (more accurately, as “general-use” or “restricted use” pesticides, according to the EPA, based on the concentration of the active ingredient). The MoA of these agents is essentially a rapid and effective “knockdown effect” on target pests, which results in the alteration of their ganglionic nervous functions, leading to their death [43].
In accordance with their chemical structure and toxicological activity, as previously stated, they have been historically classified into two principal categories: Type I pyrethroids (such as allethrin, resmethrin, tetramethrin, bioresmethrin, permethrin, and all the other compounds containing the basic cyclopropane carboxylic acid), and Type II pyrethroids (such as deltamethrin, cyfluthrin, cypermethrin, cyphenothrin, fenvalerate, and fluvalinate). These two groups are characterized by the absence or presence of an α-cyano group at the phenyl-benzyl alcohol moiety, respectively [82]. The presence of the α-cyano group confers a higher level of insecticidal activity to Type II pyrethroids (cyano-containing) than to Type I pyrethroids (cyano-lacking).
Type I pyrethroids (without the α-cyano group) have a prevalent (faster, flushing, but reversible) activity on peripheral and sensory nervous structures (knockdown effect), while those of Type II (with α-cyano) predominantly and irreversibly act on the central nervous system (killing effect). All pyrethroids inhibit sodium ion channels, but those of Type II, containing the α-cyano group, also interfere with the functions of the GABA (gamma-amino-butyric acid) neurotransmitter, resulting in neurotoxicity in mammals because, at high concentrations, they alter the voltage-gated calcium channels (VGCCs) and chloride channels on GABA receptors [83,84].
Although it is the same in insects and higher animals, such as mammals, MoA exhibits heightened toxicity in insects (approximately 2250 times greater than in mammals). This is attributed to their higher sensitivity in sodium channels, smaller body size, and lower body temperature. Furthermore, mammals possess the ability to rapidly detoxify pyrethrins and synthetic pyrethroids into non-toxic metabolites, facilitating their excretion from the body [85].
The specific mechanism of action that interferes with the sodium channels of nerve cells represents a significant challenge in terms of environmental sustainability. This MoA is not species-specific for the target insects and can, therefore, result in the same neurotoxic effects in other species that are beneficial to ecosystems, such as pollinators (bees and bumblebees), natural predators of parasites, and other forms of aquatic life [86,87].
The same MoA and the spread diffusion into the environment of the photostable synthetic pyrethroids (starting from those of the second generation) have caused a much greater extent of persistence than that observed for the photolabile pyrethrins, particularly in pyrethroids of the first generation (i.e., those with similar chemical structures to pyrethrins, such as d-allethrin and prallethrin) and pyrethroids with a cyclopentenolone ring [79]. The progressive increase in knockdown resistance (KDR) and insecticide cross-resistance by mosquitoes, flies, cockroaches, and many other species of pests are further concerns [88].
This issue frequently arises with more photostable synthetic pyrethroids, which significantly hinders the development of new pyrethroids and their widespread outdoor applications. Indeed, the resistance of insects to synthetic pyrethroids is far more prevalent than that to natural pyrethrins, which are susceptible to minute natural genetic variations. The uniform molecular structure of synthetic pyrethroids makes them more likely to induce insecticide resistance in pests.
Indeed, as documented in the Arthropod Pesticide Resistance Database [42], 30 cases of insecticide resistance have been observed for pyrethrins, affecting 13 distinct insect species. The majority of these cases involved houseflies (nine cases of Musca domestica) and cockroaches (eight cases of Blattella germanica). In contrast, for synthetic pyrethroids (allethrin, acrinathrin, bifenthrin, bioresmethrin, bioresmethrin PBO, cis-cypermethrin, cycloprothrin, cyfluthrin, cyfluthrin DEF, cyfluthrin-beta, cyhalothrin-beta, cyalothrin- lambda PBO, cyperethrin-alpha, cypermethrin-beta, cypermethrin-theta, cypermethrin-zeta, cyphenothrin, deltamethrin, deltamethrin DEF, deltamethrin DEM, deltamethrin PBO, empenthrin, esfenvalerate, fenpropathrin, fenvalerate, permethrin, permethrin TPP, phenethrinres, bioethrin, tefluthrin, tetramethrin, tetramethrin-[(1R)-isomers], trans-cypermethrin, transfluthrin), there have been 4503 reports of cases of insecticide resistance concerning 163 species of parasitic insects, primarly: Meligethes aeneus (487), Helicoverpa armigera (407), Plutella xylostella (250), Aedes aegypti (223 records), Rhipicephalus microplus (212), Spodoptera litura (206), Musca domestica (140), Blattella germanica (109), Bemisia tabaci (101), Haematobia irritans (97), Aedes abopictus (95), Anopheles gambiae (93).
It can be generally observed that pyrethroids induce genetic resistance or cross-resistance in pests. This can occur in two ways: (a) through an additional decrease in the sensitivity of the sodium channels to the active compound, which is known as “altered target-site resistance” or “non-metabolic resistance” and is not reducible with synergists); and (b) through an improvement in the efficacy of metabolic detoxification by insects, which is referred to as “metabolic resistance”. The latter can be reduced with synergists. In response to this situation, the chemical industry has developed pyrethroids with enhanced insecticidal activity at low concentrations. However, these new pyrethroids have the same MoA as the previous ones and have contributed to the reinforcement of the cross-resistance phenomenon [49].
As stated by Yoshio Katsuda [79] a prominent expert in this field, the different progressive substitutions of single functional groups of natural pyrethrins are the primary cause of the widespread cross-resistance phenomenon observed in pests. To address this challenge, it is essential to re-examine the entire pyrethrin molecule, particularly the homologs of pyrethrin II) rather than focusing on individual groups. The aim of this action is to develop novel synthetic pyrethroids that are more effective and have a low risk of insecticide resistance, according to this new structural type: [A′–B′–C′]. Indeed, the same MoA of pyrethroids and pseudo-pyrethroids indicates that the fundamental unit responsible for the insecticidal activity of these insecticides may be latent, and therefore, requires further investigation in other non-ester structures.
Furthermore, additional research is required to assess the eco-compatibility of these pseudo-pyrethroids and their toxicity, with a particular focus on their impact on aquatic organisms.
These factors underscore the necessity for continued research and related economic investments in the development of new pyrethroids or analogous compounds.
8. Conclusions
The primary distinguishing feature of pyrethroids is their favorable toxicological profile, which is markedly different from that of other categories of insecticides. Indeed, they exhibit high toxicity to pests and minimal toxicity to mammals.
Moreover, pyrethroids are more environmentally compatible than other insecticides. In fact, they are biodegradable by plants and soil. Furthermore, natural pyrethrins and the first generation of synthetic pyrethroids are also readily biodegradable by heat and sunlight.
However, natural pyrethrins and synthetic pyrethroids have been found to have shortcomings. The former has been shown to have poor efficacy and risks to the ecosystem. In light of these considerations, the search for an optimal insecticide cannot be definitively concluded. Further studies are necessary to identify superior chemical compounds that are highly specific to the target pest species and harmless to beneficial insects. Moreover, future pyrethroids or pyrethroid-like compounds must be developed in a way that ensures they are harmless to fish and other aquatic organisms.
It seems plausible to suggest that in light of the considerable demand for environmentally friendly and harmless insecticides for human health, particularly in the context of household and organic farming, the market for pyrethrins and pyrethroids will experience a notable expansion in the coming years.
The global research initiative on novel pyrethroids, which commenced several decades ago, has effectively reached a standstill. This is even though numerous chemical companies have dedicated themselves to this objective for an extended period. This is primarily due to the pervasive emergence of pyrethroid resistance in numerous pest species. Consequently, manufacturers have redirected their research efforts toward alternative insecticides, such as organophosphates, which exhibit different MoAs.
In this regard, it is imperative to enhance our comprehension of the precise molecular mechanisms underlying the MoA of pyrethroids. This will facilitate the reduction of insect resistance to these insecticides and the development of novel compounds that exhibit greater specificity of action and superior environmental compatibility. This will ensure the sustainable utilization of these compounds in pest control while safeguarding ecosystems.
The broad spectrum of action, increasing cross-resistance, and high toxicity to fish and amphibians are the main issues associated with synthetic pyrethroids. In light of these considerations, non-ester pyrethroid-like compounds, such as silafluofen, which do not exhibit these shortcomings, should provide renewed impetus for organic chemists to further investigate the entire structure of natural pyrethrins and the precise roles of their individual functional groups. It is imperative that further studies be conducted to identify additional pyrethroids, such as those belonging to the first generation, including allethrin, that exhibit high insecticidal efficacy, low production costs, and high environmental compatibility, while also demonstrating adequate outdoor persistence.
In conclusion, the subject of pyrethroids remains an area of ongoing scientific inquiry, with several unresolved issues yet to be addressed. First and foremost, there is growing and widespread tolerance and insecticide resistance, toxicity to non-target organisms, and the challenges of regulating substances that are increasingly numerous and complex, with effects that are not easily assessable, particularly in the medium- to long-term. In particular, the increasing complexity of chemical formulations and their widespread use worldwide necessitate continuous monitoring and careful regulation to ensure that the benefits of new pyrethroids are not outweighed by potential risks.
From a sustainability perspective, it would be essential to implement integrated pest management (IPM) strategies as a core approach to addressing these challenges and reducing excessive reliance on chemical pesticides. Such sustainable agricultural practices include the rational use of pyrethroids, which are less environmentally dangerous and persistent than other types of insecticides, such as organophosphates and organochlorines. This should be performed in combination with other cultural and biological control measures. It is imperative that comprehensive guidelines be established for the more prudent utilization of pyrethroids, limiting their applications to situations where they are truly essential, and promoting the use of minimum effective doses. It is also imperative to implement a program of constant monitoring to identify cases of resistance at an early stage and to encourage research into the development of new and safer compounds that can maintain efficacy while minimizing their impact on the environment. The implementation of these strategies would not only assist in the containment of resistance but would also result in a notable reduction in the chemical burden on the environment.
Funding
This research received no external funding.
Conflicts of Interest
The author declares that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ranson, H.; N’Guessan, R.; Lines, J.; Moiroux, N.; Nkuni, Z.; Corbel, V. Pyrethroid resistance in African anopheles’ mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Sharma, R.; Abraham, J.; Sharma, P. Pyrethroids: A natural product for crop protection. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A., Eds.; Springer: Singapore, 2020; pp. 113–130. [Google Scholar] [CrossRef]
- IMARC (International Market Analysis Research and Consulting Group) Global Pyrethroids Market to Reach US$ 5.6 Billion by 2032, Catalyzed by Increasing Applications across Various End Use Industries. 2022. Available online: https://www.imarcgroup.com/global-pyrethroids-market (accessed on 11 August 2024).
- EMR (Expert Market Research) Global Pyrethroids Market Outlook. 2024. Available online: https://www.expertmarketresearch.com/reports/pyrethroids-market (accessed on 11 August 2024).
- Mordor Global Pyrethroid Insecticide Market-Growth, 2017–2022. Trends and Forecasts. 2022. Available online: https://www.mordor-intelligence.com/industry-reports/global-insecticides-market-industry (accessed on 11 February 2022).
- EPA Special Docket Pyrethroids, Pyrethrins, and Synergists. Docket Number EPA-HQ-OPP-2008-0331. 2024. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2008-0331 (accessed on 23 January 2024).
- Aggarwal, R.; Diddee, S. Organophosphate or organochlorines or something else? Indian J. Crit. Care Med. 2009, 13, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. 1980, 34, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.; Vaupel, E. Pyrethrum: History of a bio-insecticide. In Chemie in Unserer Zeit; Wiley-VCH: Weinheim, Germany; Verlag: Breisgau, Germany; GmbH & Co. KGaA: Schütz, Germany, 2018. [Google Scholar] [CrossRef]
- Ujihara, K. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Schleier, J.J.; Peterson, R.K.D. Chapter 3: Pyrethrins and Pyrethroid Insecticides. In Green Trends in Insect Control; López, O., Fernández-Bolaños, J.G., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar]
- Chen, M.; Du, Y.; Zhu, G.; Takamatsu, G.; Ihara, M.; Matsuda, K.; Zhorov, B.; Dong, K. Action of six pyrethrins purified from the botanical insecticide pyrethrum on cockroach sodium channels expressed in Xenopus oocytes. Pestic. Biochem. Physiol. 2018, 151, 82–89. [Google Scholar] [CrossRef]
- Burnett, D.; Farrell, G.; Kiiru, M. Strategies for the Development of a Competitive Pyrethrum-Based Pesticide Sector in Kenya; Final report (NRI report no. 2695). Technical Report; Natural Resources Institute: Chatham, UK, 2002; Available online: https://gala.gre.ac.uk/id/eprint/11840/1/Doc-0342.pdf (accessed on 22 April 2024).
- Metcalf, R.L. Insect control technology. In Kirk-Othmer Encyclopedia of Chemical Technology; Grayson, M., Eckroth, D., Eds.; Wiley: New York, NY, USA, 1995; Volume 13, pp. 533–602. [Google Scholar]
- Head, S.W. A study of the insecticidal constituents in Chrysanthemum cinerariaefolium. Pyrethrum Post 1966, 8, 32–37. [Google Scholar]
- Nikolic, T. Flora Croatica. Acta Bot. Croat. 2021, 80, S1–S3. Available online: https://www.abc.botanic.hr/index.php/abc/article/view/3365/574 (accessed on 20 August 2024).
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proceedings of the Japan Academy. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef]
- Crombie, L.; Elliott, M. Chemistry of the Natural Pyrethrins. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Zechmeister, L., Ed.; Springer: Vienna, Austria, 1961; Volume 19. [Google Scholar] [CrossRef]
- Malavasi, G. Zampironi, un nome non comune. Gente Veneta 2012, 42. Available online: https://web.archive.org/web/20170216060925/http://www.genteveneta.it/public/articolo.php?id=7201 (accessed on 22 January 2022).
- Adedeji, A.K.; Adebanjo, A.S.; Oseghale, E.M. Design and fabrication of a mosquito repellent coil machine. Sci. J. Kurd. Univ. Med. Sci. 2020, 8, 360–372. [Google Scholar]
- Kirimi, K.H.; Camy, S.; Gourdon, C.; Condoret, J.S. Pyrethrin extraction from pyrethrum flowers using carbon dioxide. J. Supercrit. Fluids 2003, 26, 193–200. [Google Scholar] [CrossRef]
- NIH (National Institutes of Health), 2024. PubChem Chemistry DataBase. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 August 2024).
- WHO. Recommended Classification of Pesticides by Hazard and Guidelines to Classification, 2019th ed.; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 27 December 2021).
- Shepard, H.H. The use of the term “Pyrethrin” by entomologists. J. Econ. Entomol. 1933, 26, 997–998. [Google Scholar]
- Textor, O. The Examination of Persian Insect Powder for Its Active Principle. Am. J. Pharm. Educ. 1881, 491. Available online: https://www.proquest.com/openview/0394dff9c0aab4a15723cdbab3c25c41/1?pq-origsite=gscholar&cbl=41445 (accessed on 22 February 2022).
- Yamamoto, R. On the insecticidal principle of Chrysanthemum cinerariaefolium. J. Chem. Soc. Jpn. 1923, 44, 311–330. [Google Scholar]
- Staudinger, H.; Ruzicka, L. Insektentö-tende Stoffe, Part 1–5. Helv. Chim. Acta 1924, 7, 177–259. [Google Scholar] [CrossRef]
- Matsuo, T.; Nishioka, T.; Hirano, M.; Suzuki, Y.; Tsushima, K.; Itaya, N.; Yoshioka, H. Recent topics in the chemistry of synthetic pyrethroids containing certain secondary alcohol moieties. Pestic. Sci. 1980, 11, 202–218. [Google Scholar] [CrossRef]
- Hodgson, E. (Ed.) Toxycology and human environments. In Progress in Molecular Biology and Translational Science, 1st ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, ISBN 9780124159082. [Google Scholar]
- Klaassen, C.D.; Amdur, M.O.; Doull, J. (Eds.) Casarett and Doull’s Toxicology. The Basic Science of Poisons; McGraw-Hill Companies, Inc.: Toronto, ON, Canada, 1996. [Google Scholar]
- ECHA (European Chemical Agency) Substance Infocard. 2024. Available online: https://echa.europa.eu/it/information-on-chemicals (accessed on 11 August 2024).
- Molview. 2024. Available online: http://www.molview.org (accessed on 16 September 2024).
- Davies, J.H. The pyrethroids: An historical introduction. In The Pyrethroid Insecticides; Leahey, J.P., Ed.; Taylor & Francis, Ltd.: London, UK, 1985; pp. 1–41. [Google Scholar]
- Li, H.; Cheng, F.; Wei, Y.; Lydy, M.; You, J. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. J. Hazard. Mater. 2017, 324 Pt B, 258–271. [Google Scholar] [CrossRef]
- Crosby, D.G. Environmental fate of pyrethrins. In Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses; Casida, J.E., Quistad, G.B., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 194–213. [Google Scholar]
- Wauchope, R.D.; Buttler, T.M.; Hornsby, A.G.; Augustijn-Beckers, P.W.M.; Burt, J.P. The SCS/ARS/CES Pesticide Properties Database for Environmental Decision-Making. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1992; Volume 123. [Google Scholar] [CrossRef]
- Robbins, S.R.J. Pyrethrum: A Review of Market Trends and Prospects in Selected Countries. Report of the Tropical Development and Research Institute, G185. 1984. Available online: https://gala.gre.ac.uk/id/eprint/11999/1/11999_Robins_Pyrethrum%20a%20review%20of%20market%20trends%20(book)%201984.pdf (accessed on 28 December 2021).
- Schechter, M.S.; LaForge, F.B. Hydroxydketones. U. S. Patent 2574500, 13 November 1951. [Google Scholar]
- Matsui, M.; Kitamura, S.; Kato, T.; Sugihara, S. Synthesis of cinerolone type cyclopentenolone. J. Chem. Soc. Jpn. 1950, 71, 235–236. [Google Scholar]
- Extoxnet (Extension Toxicology Network) Pesticide Information Profiles (PIPs). 2024. Available online: http://extoxnet.orst.edu/ghindex.html (accessed on 27 March 2024).
- IUPAC (International Union of Pure and Applied Chemistry) Pesticides Properties DataBase (PPDB). 2024. Available online: http://sitem.herts.ac.uk/aeru/iupac/index.htm (accessed on 16 September 2024).
- IRAC (Insecticide Resistance Action Committee) Arthropod Pesticide Resistance Database (APRD). 2023. Available online: https://www.pesticideresistance.org/search.php (accessed on 12 December 2023).
- Wickham, J.C.; Chadwick, P.R.; Stewart, D.C. Factors which influence the knockdown effect of insecticide products. Pestic. Sci. 1974, 5, 657–664. [Google Scholar] [CrossRef]
- Barthel, W.F. Dimethylbenzyl Chrysanthemumates as Insecticides. Patent No. 2,857,309, 21 October 1958. [Google Scholar]
- Gersdorff, W.A.; Freeman, S.K.; Piquett, P.G. Insecticidal activity and structure, some barthrin isomers and their toxicity to houseflies in space sprays. J. Agric. Food Chem. 1959, 7, 548–550. [Google Scholar] [CrossRef]
- Gersdorff, W.A.; Piquett, P.G. The relative effectiveness of two synthetic pyrethroids more toxic to houseflies than pyrethrins in kerosene. J. Econ. Entomol. 1961, 54, 1250–1252. [Google Scholar] [CrossRef]
- Matsuo, N.; Miyamot, J. Development of Synthetic Pyrethroids with Emphasis on Stereochemical Aspects. In Phytochemicals for Pest Control; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 658, pp. 183–194. [Google Scholar] [CrossRef]
- WHO Specifications and Evaluations for Public Health Pesticides—Prallethrin. 2004. Available online: https://extranet.who.int/prequal/sites/default/files/vcp-documents/WHOVC-SP_Prallethrin_2004.pdf (accessed on 22 January 2022).
- Katsuda, Y. Progress and future of pyrethroids. Top. Curr. Chem. 2012, 314, 1–30. [Google Scholar] [CrossRef]
- Field, L.M.; Davies, T.G.E.; O’Reilly, A.O.; Williamson, M.S.; Wallace, B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017, 46, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Colborn, T.; Vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M. The pyrethroids: Early discovery, recent advances and the future. Pestic. Sci. 1989, 27, 337–351. [Google Scholar] [CrossRef]
- Kato, T.; Ueda, K.; Fujimoto, K. New Insecticidally Active Chrysanthemates. Agric. Biol. Chem. 1964, 28, 914–915. [Google Scholar] [CrossRef]
- Mori, T.; Sugano, M.; Kubota, S.; Shono, Y. Dimefluthrin: A new pyrethroid insecticide and innovative mosquito control agent. Jpn. J. Environ. Entomol. Zool. 2014, 25, 81–83. [Google Scholar]
- Hirano, M.; Itaya, N.; Ohno, I.; Fujita, Y.; Yoshioka, H. A new pyrethroid-type ester with strong knockdown activity. Pestic. Sci. 1979, 10, 291–294. [Google Scholar] [CrossRef]
- Matsuo, T.; Itaya, N.; Mizutani, T.; Ohno, N.; Fujimoto, K.; Okuno, Y.; Yoshioka, H. 3-Phenoxyszw3-cyanobenzyl esters, the most potent synthetic pyrethroids. Agric. Biol. Chem. 1976, 40, 247–249. [Google Scholar]
- Pinchin, R.; de Oliveira Filho, A.M.; Pereira, A.C.B. The flushing-out activity of pyrethrum and synthetic pyrethroids on Panstrongylus megistus, a vector of Chagas’s disease. Trans. R. Soc. Trop. Med. Hyg. 1980, 74, 801–803. [Google Scholar] [CrossRef]
- Farkas, J.; Kourim, P.; Sorm, F. Relation between chemical structure and insecticidal captivity in pyrethroids. Chem. Listy 1958, 52, 688–694. (In Czech) [Google Scholar]
- Matsui, M.; Kitahara, T. Studies on chrysanthemic acid component related to chrysanthemic acid. Agr. Biol. Chem. 1967, 31, 1143–1150. [Google Scholar]
- Elliott, M.; Farnham, A.W.; Janes, N.F.; Needham, P.H.; Pearson, B.C. Potent Pyrethroid Insecticides from Modified Cyclopropane Acids. Nature 1973, 244, 456–457. [Google Scholar] [CrossRef] [PubMed]
- EMA (European Medicine Agency) Permethrin. Summary Report EMEA/MRL/112/96-FINAL. 1998. Available online: https://medicines.health.europa.eu/veterinary/fr/documents/download/c1628880-d6f3-4b0f-9b68-981f828cf039 (accessed on 22 February 2022).
- Eisler, R. Fenvalerate Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; Contaminant Hazard Reviews Report No. 24; U.S. Fish and Wildlife Service Patuxent Wildlife Research Center: Laurel, MA, USA, 1992. Available online: https://www.pwrc.usgs.gov/CHR_24_Fenvalerate (accessed on 23 March 2022).
- WHO. Control of the Leishmaniases. In Report of a WHO Expert Committee; Technical Report Series no. 793; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- WHO. Permethrin. In Environmental Health Criteria 94; Technical Report EHC 94/1990; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Khambay, B.P.S.; Jewess, P.J. Classification of Pyrethroids. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 978-0-444-51924-5. [Google Scholar]
- Elliott, M.; Farnham, A.W.; Janes, N.F.; Needham, P.H.; Pulman, D.A.; Stevenson, J.H. A photostable pyrethroid. Nature 1973, 246, 169–170. [Google Scholar] [CrossRef]
- Greenpeace The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post-2013. 2017. Available online: https://www.greenpeace.org/static/planet4-international-stateless/2017/01/960a04f9-neonicotinoid-pesticides.pdf (accessed on 18 January 2022).
- Walker, K. Cost-comparison of DDT and alternative insecticides for malaria control. Med. Vet. Entomol. 2000, 14, 345–354. [Google Scholar] [CrossRef]
- Sadasivaiah, S.; Tozan, Y.; Breman, J.G. Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: How can it be used for malaria control? Am. J. Trop. Med. Hyg. 2007, 77, 249–263. [Google Scholar] [CrossRef]
- Pettebone, M.S. Characterization of Transfluthrin Emissions over Time in an Enclosed Space over a Range of Discreet Temperatures; Faculty of the Occupational & Environmental Health Sciences Graduate Program Uniformed Services University of the Health Sciences; Bethesda: Rockville, MD, USA, 2014; Available online: https://apps.dtic.mil/sti/pdfs/AD1012851.pdf (accessed on 20 August 2024).
- Ohno, N.; Fujimoto, K.; Okuno, Y.; Mizutani, T.; Hirano, M.; Yoshioka, H. A new class of pyrethroidal insecticides: α-substituted phenylacetic acid esters. Agric. Biol. Chem. 1974, 38, 881–883. [Google Scholar] [CrossRef]
- WHO Safety of Pyrethroids for Public Health Use. WHO/CDS/WHOPES/GCDPP/2005.10. 2005. Available online: http://apps.who.int/iris/bitstream/handle/10665/69008/WHO_CDS_WHOPES_GCDPP_2005.10.pdf (accessed on 11 May 2024).
- Pohanish, R.P. Sittig’s Handbook of Pesticides and Agricultural Chemicals; William Andrew Publishing Norwich: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Henrick, C.A.; Garcia, B.A. Patent for Fluvalinate Development. U.S. Patent 4243819, 6 January 1981. [Google Scholar]
- Ko, C.Y.; Nai, Y.S.; Lo, W.; Chen, C.T.; Chen, Y.W. Low-level fluvalinate treatment in the larval stage induces impaired olfactory associative behavior of honeybee workers in the field. Insects 2022, 13, 273. [Google Scholar] [CrossRef]
- Nakatani, K.; Numata, S.; Inoue, T.; Kodaka, K.; Ishii, T.; Toyama, T.; Tachibana, H.; Udagawa, T.; Gohbara, M. Nouveaux Ethers-Oxydes et Sulfures Aryl-2 Propyliques, Procede De Leur Preparation, Agents’ Insecticides Et Acaricides Les Contenant Et Procede De Lutte Contre Les Insectes et Les Acariens Utilisant Ces Nouveaux Composes. Patent No. FR2481695A1, 2 May 1980. [Google Scholar]
- Katsuda, Y.; Chikamoto, T.; Inouye, Y. The absolute configuration of naturally derived pyrethrolone and cinerolone. Bull. Agric. Chem. Soc. Jpn. 1958, 22, 184–187. [Google Scholar] [CrossRef]
- Gordon, R.F.S.; Bushell, M.J.; Pascoe, R.; Enoyoshi, T. Flufenprox—A new insecticide for rice. In Proceedings of the Brighton Crop Protection Conference, Pests and Diseases, Brighton, UK, 23–26 November 1992; pp. 81–88. Available online: https://eurekamag.com/research/002/384/002384905.php (accessed on 21 September 2024).
- Katsuda, Y. Development of and future prospects for pyrethroid chemistry. Pestic. Sci. 1999, 55, 775–782. [Google Scholar] [CrossRef]
- Bhupinder, K. Pyrethroid insecticides. Pestic. Outlook 2002, 13, 49–54. [Google Scholar] [CrossRef]
- Méjanelle, L.; Jara, B.; Dachs, J. Fate of Pyrethroids in Freshwater and Marine Environments. The Handbook of Environmental Chemistry. Pyrethroid Insectic. 2020, 2020, 81–107. [Google Scholar] [CrossRef]
- Suppiramaniam, V.; Abdel-Rahman, E.A.; Buabeid, M.A.; Parameshwaran, K. Ion Channels. Compr. Toxicol. (Second. Ed.) 2010, 13, 129–171. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Cage, S.A.; Proudfoot, A.T.; Vale, J.A. Poisoning due to pyrethroids. Arch. Toxicol. 2005, 45, 93–106. [Google Scholar] [CrossRef]
- Gammon, D.W.; Liu, Z.; Chandrasekaran, A.; El-Naggar, S.F.; Kuryshev, Y.A.; Jackson, S. Pyrethroid neurotoxicity studies with bifenthrin indicate a mixed Type I/II mode of action. Pest. Manag. Sci. 2019, 75, 1190–1197. [Google Scholar] [CrossRef]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current research on the safety of pyrethroids used as insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef]
- Casida, J.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Q.; Zhu, F.; Zhang, L. Pyrethroid resistance in mosquitoes. Insect Sci. 2006, 13, 159–166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).