Relationships between Tree Species Diversity and Aboveground Biomass Are Mediated by Site-Dependent Factors in Northeastern China Natural Reserves on a Small Spatial Scale
Abstract
:1. Introduction
2. Material and Methods
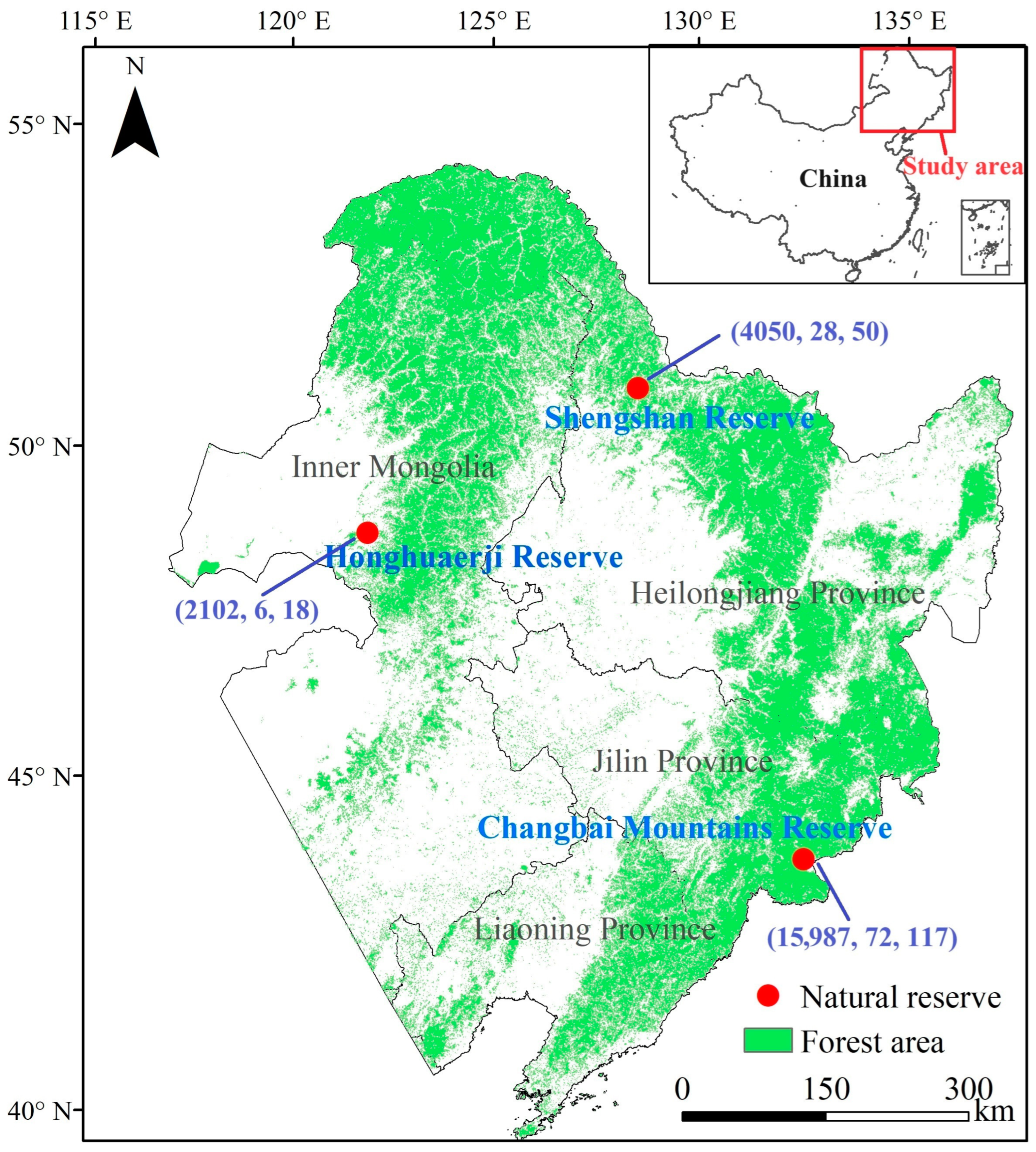
2.1. Description of the Study Area
2.2. Data Acquisition
2.2.1. Ground Survey Data
2.2.2. Environmental Factors Data
2.3. Species Diversity and Forest Productivity
2.3.1. Tree Species Diversity
2.3.2. Aboveground Biomass
2.4. Statistical Analysis
3. Results
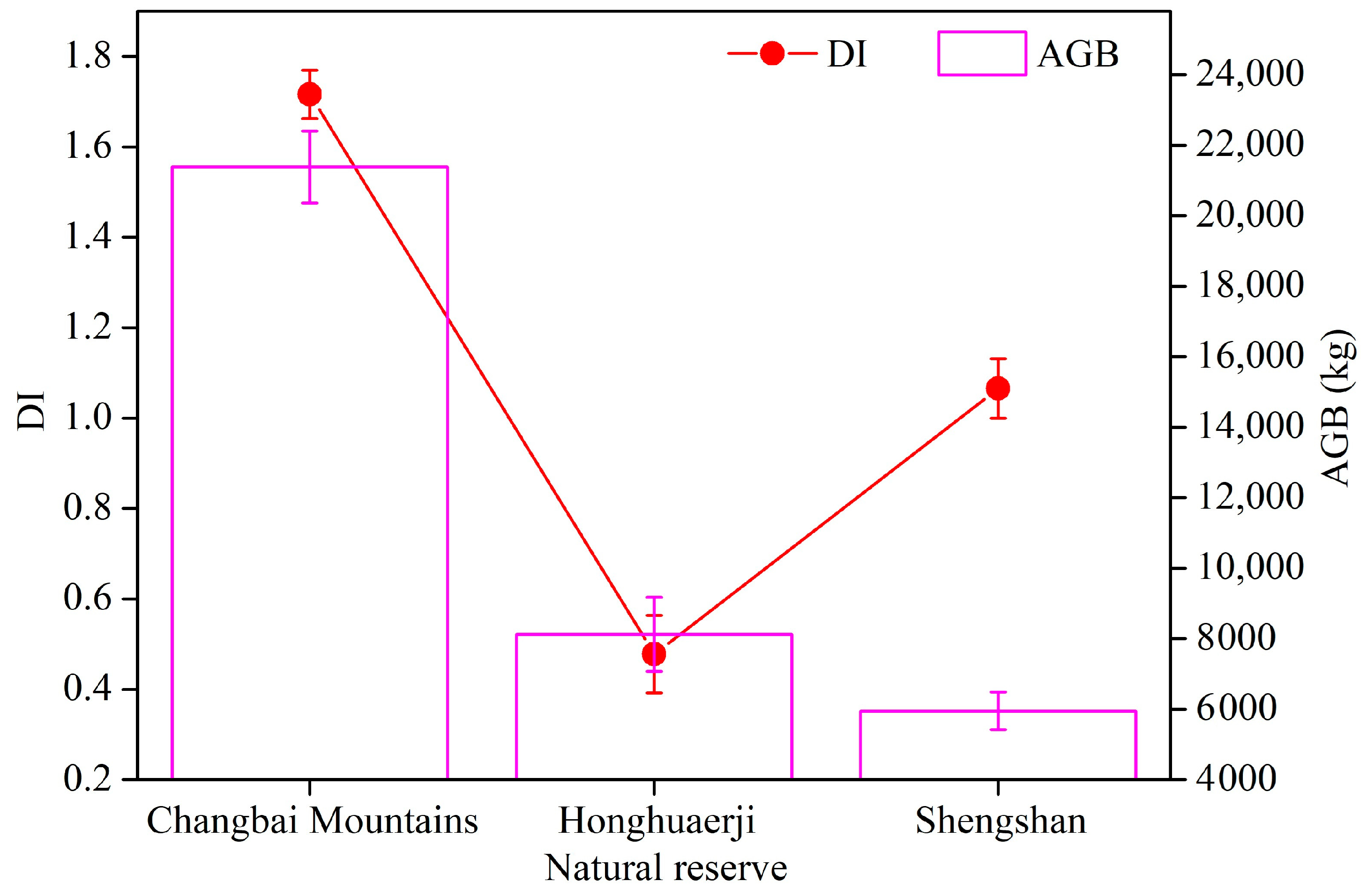
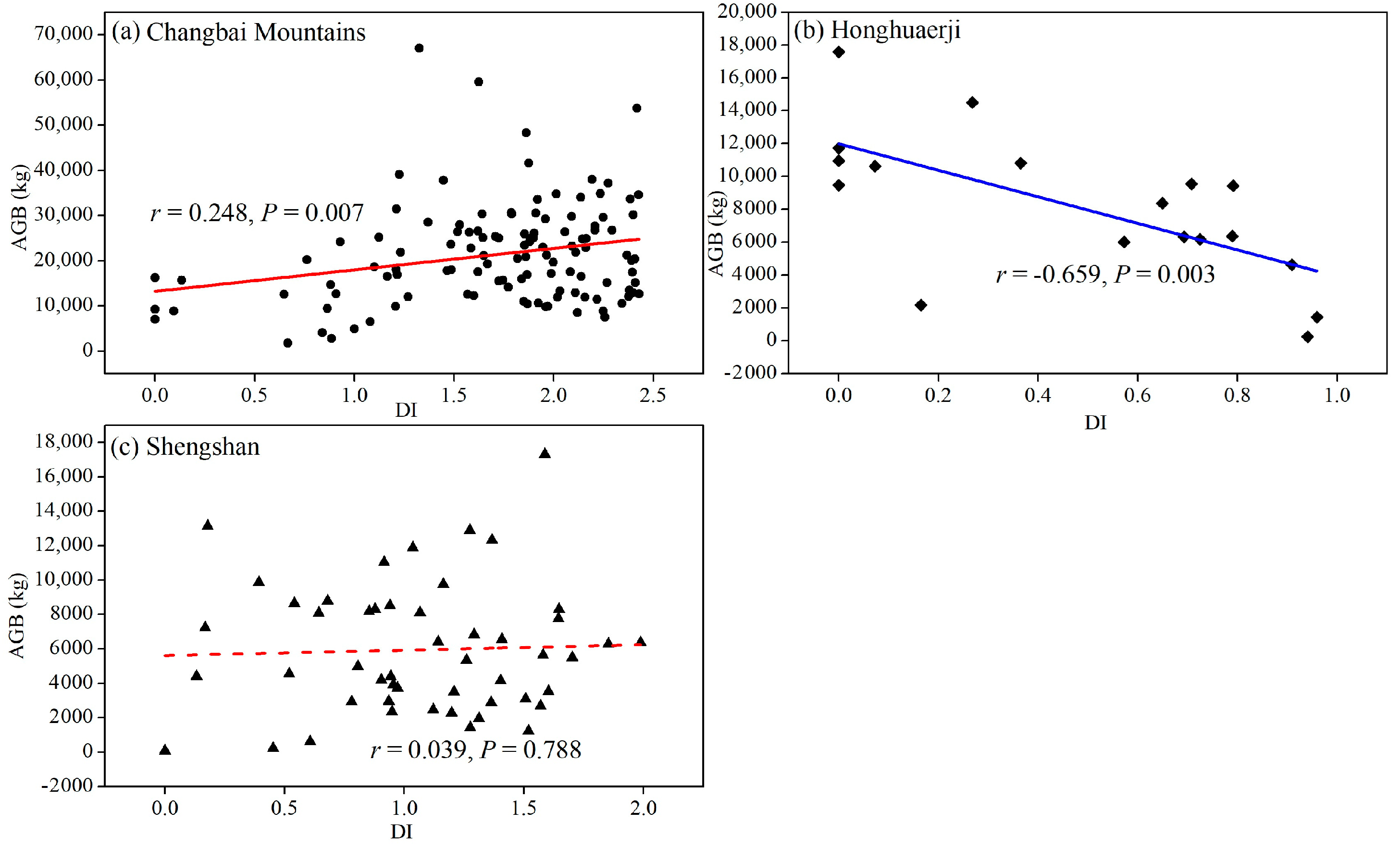
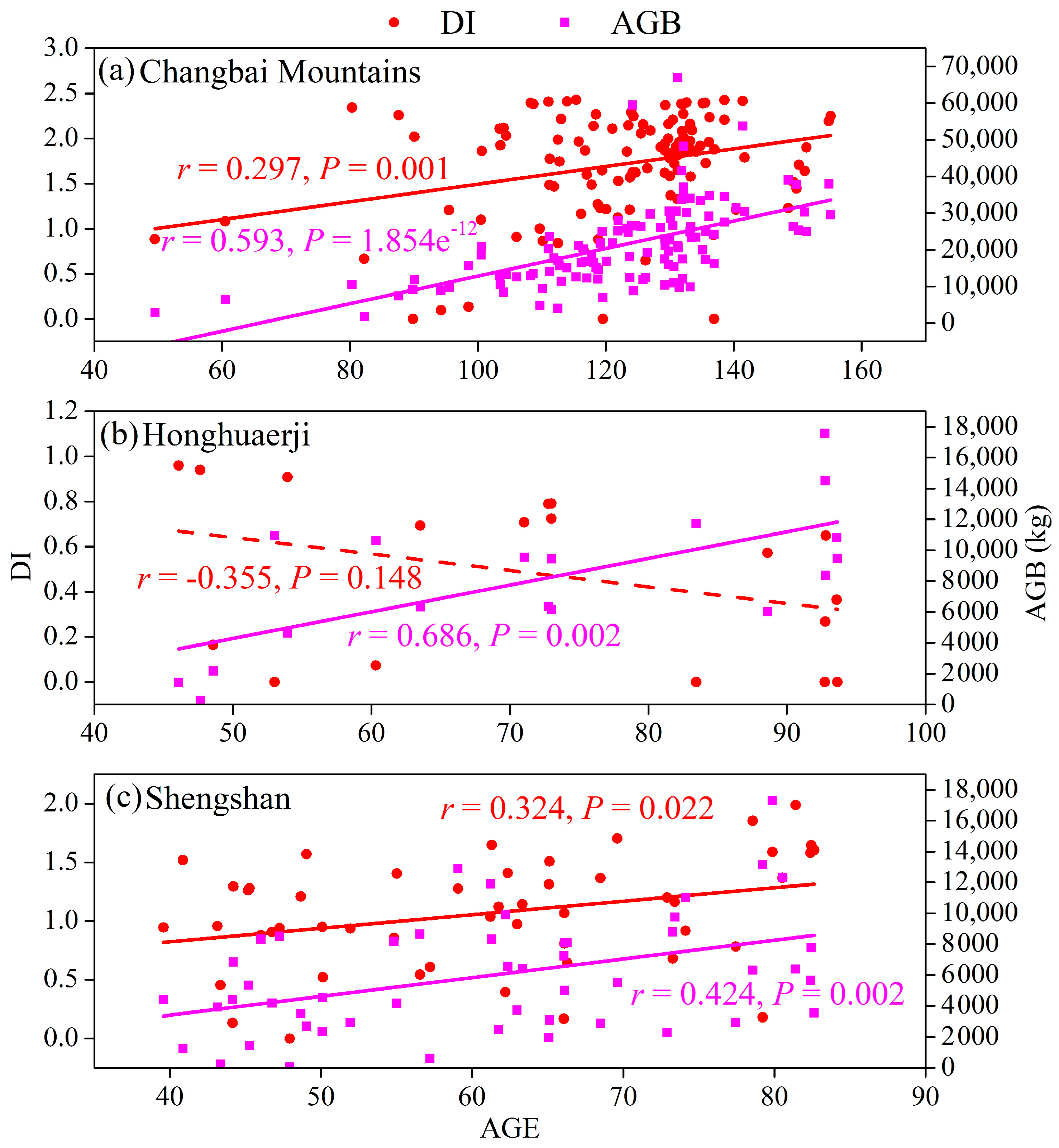
3.1. Changes in Relationships between Species Diversity and Aboveground Biomass
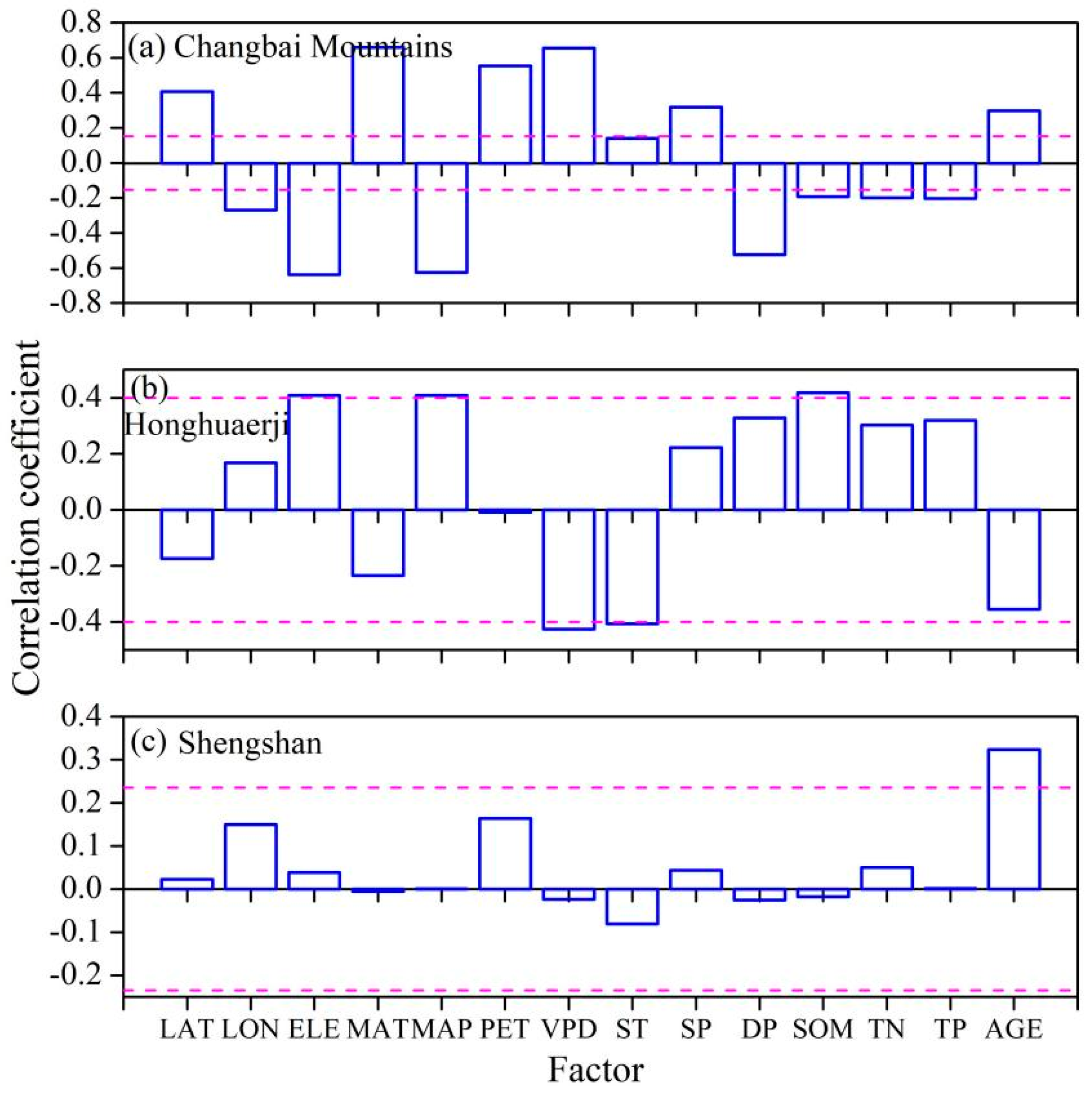
3.2. The Influencing Factors of the Species Diversity–Aboveground Biomass Relationship
4. Discussions
4.1. Changes in the Relationship between Species Diversity and Aboveground Biomass across the Investigated Natural Reserves
4.2. Site-Dependent Factors Modulate the Species Diversity–Aboveground Biomass Relationship on a Small Spatial Scale
4.3. Implications for Forest Management and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.-D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed]
- ter Steege, H.; Pitman, N.C.A.; Killeen, T.J.; Laurance, W.F.; Peres, C.A.; Guevara, J.E.; Salomão, R.P.; Castilho, C.V.; Amaral, I.L.; de Almeida Matos, F.D.; et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 2015, 1, e1500936. [Google Scholar] [CrossRef] [PubMed]
- A Williams, B.; Grantham, H.S.; Watson, J.E.M.; Alvarez, S.J.; Simmonds, J.S.; A Rogéliz, C.; Da Silva, M.; Forero-Medina, G.; Etter, A.; Nogales, J.; et al. Minimising the loss of biodiversity and ecosystem services in an intact landscape under risk of rapid agricultural development. Environ. Res. Lett. 2020, 15, 014001. [Google Scholar] [CrossRef]
- Le Provost, G.; Schenk, N.V.; Penone, C.; Thiele, J.; Westphal, C.; Allan, E.; Ayasse, M.; Blüthgen, N.; Boeddinghaus, R.S.; Larissa Boesing, A.; et al. The supply of multiple ecosystem services requires biodiversity across spatial scales. Nat. Ecol. Evol. 2022, 7, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.; Anderegg, L.D.; Kerr, K.L.; Trugman, A.T. Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Glob. Chang. Biol. 2019, 25, 3793–3802. [Google Scholar] [CrossRef]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuchslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S.; et al. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 5066. [Google Scholar] [CrossRef]
- Wright, L.S.; Pessarrodona, A.; Foggo, A. Climate-driven shifts in kelp forest composition reduce carbon sequestration potential. Glob. Chang. Biol. 2022, 28, 5514–5531. [Google Scholar] [CrossRef]
- Arthington, A.H.; Naiman, R.J.; McClain, M.E.; Nilsson, C. Preserving the biodiversity and ecological services of rivers: New challenges and research opportunities. Freshw. Biol. 2010, 55, 1–16. [Google Scholar] [CrossRef]
- Manning, P.; Loos, J.; Barnes, A.D.; Batáry, P.; Bianchi, F.J.J.A.; Buchmann, N.; De Deyn, G.B.; Ebeling, A.; Eisenhauer, N.; Fischer, M.; et al. Transferring biodiversity-ecosystem function research to the management of ‘real-world’ ecosystems. In Advances in Ecological Research; Eisenhauer, N., Bohan, D.A., Dumbrell, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 323–356. [Google Scholar]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Waide, R.B.; Willig, M.R.; Steiner, C.F.; Mittelbach, G.; Gough, L.; Dodson, S.I.; Juday, G.P.; Parmenter, R. The relationship between productivity and species richness. Annu. Rev. Ecol. Evol. Syst. 1999, 30, 257–300. [Google Scholar] [CrossRef]
- Fei, S.; Jo, I.; Guo, Q.; Wardle, D.A.; Fang, J.; Chen, A.; Oswalt, C.M.; Brockerhoff, E.G. Impacts of climate on the biodiversity-productivity relationship in natural forests. Nat. Commun. 2018, 9, 5436. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Ganguly, S.; Tømmervik, H.; Euskirchen, E.S.; Høgda, K.-A.; Karlsen, S.R.; Brovkin, V.; Nemani, R.R.; Myneni, R.B. Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ. Res. Lett. 2016, 11, 084001. [Google Scholar] [CrossRef]
- Levesque, M.; Andreu-Hayles, L.; Smith, W.K.; Williams, A.P.; Hobi, M.L.; Allred, B.W.; Pederson, N. Tree-ring isotopes capture interannual vegetation productivity dynamics at the biome scale. Nat. Commun. 2019, 10, 742. [Google Scholar] [CrossRef]
- Hector, A.; Schmid, B.; Beierkuhnlein, C.; Caldeira, M.C.; Diemer, M.; Dimitrakopoulos, P.G.; Finn, J.A.; Freitas, H.; Giller, P.S.; Good, J.; et al. Plant diversity and productivity experiments in European grasslands. Science 1999, 286, 1123–1127. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Isbell, F.I.; Polley, H.W.; Wilsey, B.J. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol. Lett. 2009, 12, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, D.; Xu, K.; Gao, L.M.; Ge, X.J.; Burgess, K.S.; Cadotte, M.W. Biodiversity explains maximum variation in productivity under experimental warming, nitrogen addition, and grazing in mountain grasslands. Ecol. Evol. 2018, 8, 10094–10112. [Google Scholar] [CrossRef]
- Wagg, C.; Roscher, C.; Weigelt, A.; Vogel, A.; Ebeling, A.; de Luca, E.; Roeder, A.; Kleinspehn, C.; Temperton, V.M.; Meyer, S.T.; et al. Biodiversity–stability relationships strengthen over time in a long-term grassland experiment. Nat. Commun. 2022, 13, 7752. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.X.; Fan, C.Y.; Zhang, C.Y.; Zhao, X.H.; von Gadow, K. Anthropogenic disturbance mediates soil water effect on diversity-productivity relationships in a temperate forest region. For. Ecol. Manag. 2022, 525, 120544. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2011, 20, 170–180. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, M.; Tobin, P.C.; McGuire, A.D.; Reich, P.B. Biodiversity influences plant productivity through niche-efficiency. Proc. Natl. Acad. Sci. USA 2015, 112, 5738–5743. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, S.; Wirth, C.; Jucker, T.; van der Plas, F.; Scherer-Lorenzen, M.; Verheyen, K.; Allan, E.; Benavides, R.; Bruelheide, H.; Ohse, B.; et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecol. Lett. 2017, 20, 1414–1426. [Google Scholar] [CrossRef]
- Vilà, M.; Vayreda, J.; Comas, L.; Ibáñez, J.J.; Mata, T.; Obón, B. Species richness and wood production: A positive association in Mediterranean forests. Ecol. Lett. 2007, 10, 241–250. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.A. Hidden treatments in ecological experiments: Re-evaluating the ecosystem function of biodiversity. Oecologia 1997, 110, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Jucker, T.; Avacariței, D.; Barnoaiea, I.; Duduman, G.; Bouriaud, O.; Coomes, D.A. Climate modulates the effects of tree diversity on forest productivity. J. Ecol. 2016, 104, 388–398. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Shan, Y.X.; Burgess, K.S.; Ge, X.J. Biotic and abiotic factors determine species diversity-productivity relationships in mountain meadows. J. Plant Ecol. 2021, 14, 1175–1188. [Google Scholar] [CrossRef]
- Vilà, M.; Inchausti, P.; Vayreda, J.; Barrantes, O.; Gracia, C.; Ibáñez, J.J.; Mata, T. Confounding factors in the observational productivity-diversity relationship in forests. In Forest Diversity and Function: Temperate and Boreal Systems; Scherer-Lorenzen, M., Körner, C., Schulze, E.-D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 65–86. [Google Scholar]
- Ammer, C. Diversity and forest productivity in a changing climate. New Phytol. 2019, 221, 50–66. [Google Scholar] [CrossRef]
- Jactel, H.; Gritti, E.S.; Drössler, L.; Forrester, D.I.; Mason, W.L.; Morin, X.; Pretzsch, H.; Castagneyrol, B. Positive biodiversity-productivity relationships in forests: Climate matters. Biol. Lett. 2018, 14, 20170747. [Google Scholar] [CrossRef]
- Nguyen, H.; Herbohn, J.; Firn, J.; Lamb, D. Biodiversity–productivity relationships in small-scale mixed-species plantations using native species in Leyte province, Philippines. For. Ecol. Manag. 2012, 274, 81–90. [Google Scholar] [CrossRef]
- Taylor, A.R.; Gao, B.; Chen, H.Y.H. The effect of species diversity on tree growth varies during forest succession in the boreal forest of central Canada. For. Ecol. Manag. 2020, 455, 117641. [Google Scholar] [CrossRef]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. The stress-gradient hypothesis does not fit all relationships between plant-plant interactions and abiotic stress: Further insights from arid environments. J. Ecol. 2006, 94, 17–22. [Google Scholar] [CrossRef]
- Armas, C.; Rodriguez-Echeverria, S.; Pugnaire, F.I. A field test of the stress-gradient hypothesis along an aridity gradient. J. Veg. Sci. 2011, 22, 818–827. [Google Scholar] [CrossRef]
- Dohn, J.; Dembele, F.; Karembe, M.; Moustakas, A.; Amevor, K.A.; Hanan, N.P. Tree effects on grass growth in savannas: Competition, facilitation and the stress-gradient hypothesis. J. Ecol. 2013, 101, 202–209. [Google Scholar] [CrossRef]
- Scott, J.M.; Davis, F.W.; McGhie, R.G.; Wright, R.G.; Groves, C.; Estes, J. Nature reserves: Do they capture the full range of America’s biological diversity? Ecol. Appl. 2001, 11, 999–1007. [Google Scholar] [CrossRef]
- Zhao, L.; Du, M.; Du, W.; Guo, J.; Liao, Z.; Kang, X.; Liu, Q. Evaluation of the Carbon Sink Capacity of the Proposed Kunlun Mountain National Park. Int. J. Environ. Res. Public Health 2022, 19, 9887. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhang, J.H.; Wang, J.H.; Gu, Y.; Han, S.J. A linear positive relationship between tree species diversity and forest productivity across forest-dominated natural reserves on a large spatial scale. For. Ecol. Manag. 2023, 548, 121409. [Google Scholar] [CrossRef]
- Friedl, M.; Sulla-Menashe, D. MCD12Q1 MODIS/Terra+Aqua Land Cover Type; Yearly L3 Global 500m SIN Grid V006; NASA EOSDIS Land Processes DAAC: Sioux Falls, SD, USA, 2019. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Poggio, L.; Sousa, L.M.D.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing soil information for the globe with quantified spatial uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Yu, S.; Ma, K.; Xu, C.; Jin, S.; Song, X.; Chen, L. Characteristics of species diversity of Quercus mongolica community under environmental gradients. Chin. J. Ecol. 2004, 24, 2932–2939. (In Chinese) [Google Scholar]
- Zhou, G.Y.; Yin, G.C.; Tang, X.L. Carbon Storage-Biomass Equation of Forest Ecosystem in China; Science Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Holgado-Tello, F.P.; Chacon-Moscoso, S.; Barbero-Garcia, I.; Vila-Abad, E. Polychoric versus Pearson correlations in exploratory and confirmatory factor analysis of ordinal variables. Qual. Quant. 2010, 44, 153–166. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. WIRES Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H.H.; Urban, D.L. The sensitivity of some high-latitude boreal forests to climatic parameters. Clim. Change 1990, 16, 9–29. [Google Scholar] [CrossRef]
- Prevéy, J.; Vellend, M.; Rüger, N.; Hollister, R.D.; Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Clark, K.; Cooper, E.J.; Elberling, B.; et al. Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Glob. Chang. Biol. 2017, 23, 2660–2671. [Google Scholar] [CrossRef]
- Liu, J.; Wennberg, P.O.; Parazoo, N.C.; Yin, Y.; Frankenberg, C. Observational constraints on the response of high-latitude northern forests to warming. AGU Adv. 2020, 2, e2020AV000228. [Google Scholar] [CrossRef]
- Chase, J.M.; Leibold, M.A. Spatial scale dictates the productivity–biodiversity relationship. Nature 2002, 416, 427–430. [Google Scholar] [CrossRef]
- Chase, J.M.; Ryberg, W.A. Connectivity, scale-dependence, and the productivity-diversity relationship. Ecol. Lett. 2004, 7, 676–683. [Google Scholar] [CrossRef]
- Belotea, R.T.; Prisley, S.; Jones, R.H.; Fitzpatrick, M.; de Beurs, K. Forest productivity and tree diversity relationships depend on ecological context within mid-Atlantic and Appalachian forests (USA). For. Ecol. Manag. 2011, 261, 1315–1324. [Google Scholar] [CrossRef]
- Kvíderová, J.; Shukla, S.P.; Pushparaj, B.; Elster, J. Perspectives of low temperature biomass production of polar microalgae and biotechnology expansion into high latitudes. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 585–600. [Google Scholar]
- Righetti, D.; Vogt, M.; Gruber, N.; Psomas, A.; Zimmermann, N.E. Global pattern of phytoplankton diversity driven by temperature and environmental variability. Sci. Adv. 2019, 5, eaau6253. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.G.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Rowe, A.K.; Rutledge, S.A.; Lang, T.J.; Ciesielski, P.E.; Saleeby, S.M. Elevation-dependent trends in precipitation observed during NAME. Mon. Weather Rev. 2008, 136, 4962–4979. [Google Scholar] [CrossRef]
- Dimri, A.P.; Palazzi, E.; Daloz, A.S. Elevation dependent precipitation and temperature changes over Indian Himalayan region. Clim. Dyn. 2022, 59, 1–21. [Google Scholar] [CrossRef]
- Cheng, H.; Sinha, A.; Cruz, F.W.; Wang, X.; Edwards, R.L.; D’horta, F.M.; Ribas, C.C.; Vuille, M.; Stott, L.D.; Auler, A.S. Climate change patterns in Amazonia and biodiversity. Nat. Commun. 2013, 4, 1411. [Google Scholar] [CrossRef]
- Atkinson, S.T.; Cale, D.; Pinder, A.; Chambers, J.M.; Halse, S.A.; Robson, B.J. Substantial long-term loss of alpha and gamma diversity of lake invertebrates in a landscape exposed to a drying climate. Glob. Chang. Biol. 2021, 27, 6263–6279. [Google Scholar] [CrossRef]
- Xu, L.; Myneni, R.B.; Chapin III, F.S.; Callaghan, T.V.; Pinzon, J.E.; Tucker, C.J.; Zhu, Z.; Bi, J.; Ciais, P.; Tømmervik, H.; et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim. Chang. 2013, 3, 581–586. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Peng, C.H.; Xiao, J.F.; Zhou, X.L.; Luo, Y.P.; Zhang, C.C. Divergent responses of autumn vegetation phenology to climate extremes over northern middle and high latitudes. Glob. Ecol. Biogeogr. 2022, 31, 2281–2296. [Google Scholar] [CrossRef]
- Berdanier, A.B.; Klein, J.A. Growing season length and soil moisture interactively constrain high elevation aboveground net primary production. Ecosystems 2011, 14, 963–974. [Google Scholar] [CrossRef]
- Gugger, S.; Kesselring, H.; Stöcklin, J.; Hamann, E. Lower plasticity exhibited by high- versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann. Bot. 2015, 116, 953–962. [Google Scholar] [CrossRef]

| Linear Regression Equation | R | F | p |
|---|---|---|---|
| DI = 0.016LN(ELE) + 0.37CLI_PC1 + 0.03SOI_PC1 + 0.76LN(AGE) − 2.06 (0.98) (0.042) (0.52) (0.004) (0.65) | 0.69 | 24.74 | <0.001 |
| LN(AGB) = 0.43LN(ELE) + 0.28CLI_PC1 + 0.009SOI_PC1 + 2.23LN(AGE) − 3.93 (0.49) (0.10) (0.83) (<0.001) (0.34) | 0.75 | 35.22 | <0.001 |
| Linear Regression Equation | R | F | p |
|---|---|---|---|
| DI = −16.13LN(ELE) − 0.51CLI_PC1 + 0.091SOI_PC1 − 0.28LN(AGE) + 110.053 (0.49) (0.35) (0.35) (0.44) (0.48) | 0.61 | 1.88 | 0.17 |
| LN(AGB) = 97.94LN(ELE) + 2.40CLI_PC1 − 0.27SOI_PC1 + 1.94LN(AGE) − 657.71 (0.054) (0.044) (0.184) (0.021) (0.053) | 0.81 | 6.18 | 0.005 |
| Linear Regression Equation | R | F | p |
|---|---|---|---|
| DI = 110.35LN(LAT) − 0.002CLI_PC1 + 0.92LN(AGE) − 433.37 (0.25) (0.99) (0.013) (0.25) | 0.37 | 2.45 | 0.076 |
| LN(AGB) = 12.40LN(LAT) − 0.18CLI_PC1 + 1.42LN(AGE) − 45.83 (0.95) (0.42) (0.064) (0.95) | 0.43 | 3.38 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, J.; Wang, J.; Han, S.; Guo, Z.; Fan, C.; Yu, J. Relationships between Tree Species Diversity and Aboveground Biomass Are Mediated by Site-Dependent Factors in Northeastern China Natural Reserves on a Small Spatial Scale. Sustainability 2024, 16, 8378. https://doi.org/10.3390/su16198378
Yang L, Zhang J, Wang J, Han S, Guo Z, Fan C, Yu J. Relationships between Tree Species Diversity and Aboveground Biomass Are Mediated by Site-Dependent Factors in Northeastern China Natural Reserves on a Small Spatial Scale. Sustainability. 2024; 16(19):8378. https://doi.org/10.3390/su16198378
Chicago/Turabian StyleYang, Leilei, Junhui Zhang, Jiahui Wang, Shijie Han, Zhongling Guo, Chunnan Fan, and Jinghua Yu. 2024. "Relationships between Tree Species Diversity and Aboveground Biomass Are Mediated by Site-Dependent Factors in Northeastern China Natural Reserves on a Small Spatial Scale" Sustainability 16, no. 19: 8378. https://doi.org/10.3390/su16198378






