Salt Tolerance of Phragmites australis and Effect of Combing It with Topsoil Filters on Biofiltration of CaCl2 Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soil and Plant Materials
2.2. Experimental Treatments
2.3. Soil Leachate and Plant Growth Measurement
2.4. Statistical Analysis
3. Results
3.1. Soil Leachate and P. australis Growth Characteristics in Response to Increasing De-Icing Salt Concentrations
3.2. Synergistic Effect of Planting P. australis and Adding Topsoil Filters at High Concentration of De-Icing Salts on Salinity Reduction
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunningham, M.A.; Snyder, E.; Yonkin, D.; Ross, M.; Elsen, T. Accumulation of deicing salts in soils in an urban environment. Urban Ecosyst. 2008, 11, 17–31. [Google Scholar] [CrossRef]
- Yang, J.; Yoon, Y.H.; Ju, J.H. Desalinization effect of Pennisetum alopecuroides and characteristics of leachate depending on calcium chloride (CaCl2) concentration. J. People Plants Environ. 2020, 23, 445–453. [Google Scholar] [CrossRef]
- Richburg, J.A.; Patterson, W.A.; Lowenstein, F. Effect of road salt and Phragmites australis invasion on the vegetation of a Western Massachusetts calcareous lake-basin fen. Wetlands 2001, 21, 247–255. [Google Scholar] [CrossRef]
- Delattre, E.; Techer, I.; Reneaud, B.; Verdoux, P.; Laffont-Schwob, I.; Prohin, P. Chloride accumulation in aboveground biomass of three macrophytes (Phragmites australis, Juncus maritimus, and Typha latifolia) depending on their growth stages and salinity exposure: Application for Cl− removal and phytodesalinization. Environ. Sci. Pollut. Res. 2022, 29, 35284–35299. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.M.; Danko, A.S.; Fiuza, A.; Borges, M.T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.N.; Vicente, O. Wild halophytes: Tools for understanding salt tolerance mechanisms of plants and for adapting agriculture to climate change. Plants 2023, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Matinzadeh, Z.; Akahni, H.; Abedi, M.; Palacio, S. The elemental composition of halophytes correlates with key morphological adaptations and taxonomic groups. Plant Physiol. Biochem. 2019, 141, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Perez-de-Mora, A.; Ortega-Calvo, J.J.; Cabrera, F.; Madejón, E. Changes in enzyme activities and microbial biomass after “in situ” remediation of heavy metal-contaminated soil. Appl. Soil Ecol. 2005, 28, 125–137. [Google Scholar] [CrossRef]
- Epelde, L.; Becerril, J.M.; Mijangos, I.; Garbisu, C. Evaluation of the efficiency of a phytostabilization process with biological indicators of soil health. J. Environ. Qual. 2009, 38, 2041–2049. [Google Scholar] [CrossRef]
- Rozema, E.R.; Gordon, R.J.; Zheng, Y. Harvesting plants in constructed wetlands to increase biomass production and Na+ and Cl− removal from recycled greenhouse nutrient solution. Water Air Soil Pollut. 2016, 227, 136. [Google Scholar] [CrossRef]
- Rozema, E.R.; Gordon, R.J. Plant species for the removal of Na+ and Cl– from greenhouse nutrient solution. HortScience 2014, 49, 1071–1075. [Google Scholar] [CrossRef]
- Szota, C.; Farrell, C.; Livesley, S.J.; Fletcher, F.D. Salt tolerant plants increase nitrogen removal from biofiltration systems affected by saline stormwater. Water Res. 2015, 83, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lim, W.; Hu, J.Y.; Ziegler, A.; Ong, S.L. Comparison of filter media materials for heavy metal removal from urban stormwater runoff using biofiltration systems. J. Environ. Manag. 2015, 147, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Glaister, B.J.; Fletcher, T.D.; Cook, P.L.M.; Hatt, B.E. Interactions between design, plant growth and the treatment performance of stormwater biofilters. Ecol. Eng. 2017, 105, 21–31. [Google Scholar] [CrossRef]
- Owen, J.S.; Warren, S.L.; Bilderback, T.E. Industrial mineral aggregate amendment affects physical and chemical properties of pine bark substrates. HortScience 2007, 42, 1289–1294. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.H.; Lee, W.S.; Koo, N.M.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Park, S.D.; Kim, H.S.; Lee, K.S. Effect of calcium chloride and eco-friendly deicer on the plant growth. J. Korean Soc. Environ. Eng. 2010, 32, 487–498. [Google Scholar]
- Leiva Soto, A.; Culman, S.W.; Herms, C.; Sprunger, C.; Doohan, D. Managing soil acidity vs. soil Ca:Mg ratio: What is more important for crop productivity? Crop Forage Turfgrass Manag. 2023, 9, e20210. [Google Scholar] [CrossRef]
- Phanhwar, Q.A.; Naher, U.A.; Radziah, O.; Shamshuddin, J.; Razi, I.M. Bio-fertilizer, ground magnesium limestone and basalt applications may improve chemical properties of Malaysian acid sulfate soils and rice growth. Pedosphere 2014, 24, 827–835. [Google Scholar] [CrossRef]
- Lee, Y.J.; Sung, J.K.; Lee, S.B.; Lim, J.E.; Song, Y.S.; Lee, D.B.; Hong, S.Y. Plant analysis methods for evaluating mineral nutrient. Korean J. Soil Sci. Fert. 2017, 50, 93–99. [Google Scholar] [CrossRef]
- Yi, Y.M.; Oh, C.; Kim, G.; Lee, C.; Sung, K. Changes in the physicochemical properties of soil according to soil remediation methods. J. Soil Groundw. Environ. 2012, 17, 36–43. [Google Scholar] [CrossRef]
- Barbafieri, M.; Bretzel, F.; Scartazza, A.; Baccio, D.D.; Resellini, I.; Grifoni, M.; Pini, R.; Clementi, A.; Franchi, E. Response to hypersalinity of four halophytes growing in hydroponic floating systems: Prospects in the phytomanagement of high saline wastewaters and extreme environments. Plants 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.J. Molecular mechanism of plant adaption to high salinity. Korean J. Plant Biotechnol. 2005, 32, 1–14. [Google Scholar]
- Scheiber, S.M.; Sandrock, D.; Alvarez, E.; Brennan, M.M. Effect of salt spray concentration on growth and appearance of ‘Gracillimus’ maiden grass and ‘Hamelin’ fountain grass. HortTechnology 2008, 18, 34–38. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil 2011, 342, 495–509. [Google Scholar] [CrossRef]
- McSorley, K.; Rutter, A.; Cumming, R.; Zeeb, B.A. Phytoextraction of chloride from a cement kiln dust (CKD) contaminated landfill with Phragmites australis. Waste Manag. 2016, 51, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hartzendorf, T.; Rolletschek, H. Effects of NaCl-salinity on amino acid and carbohydrate contents of Pharagmites australis. Aquat. Bot. 2001, 69, 195–208. [Google Scholar] [CrossRef]
- Seguin, R.; Kargar, M.; Prasher, S.O.; Clark, O.G.; Jutras, P. Remediation montreal’s tree pit soil applying an ash tree-derived biochar. Water Air Soil Pollut. 2018, 229, 84. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Matoh, T.; Matsushita, N.; Takahashi, E. Salt tolerance of the reed plant Phragmites communis. Physiol. Plant. 1998, 72, 8–14. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Lehmann, J.; Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amason basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]


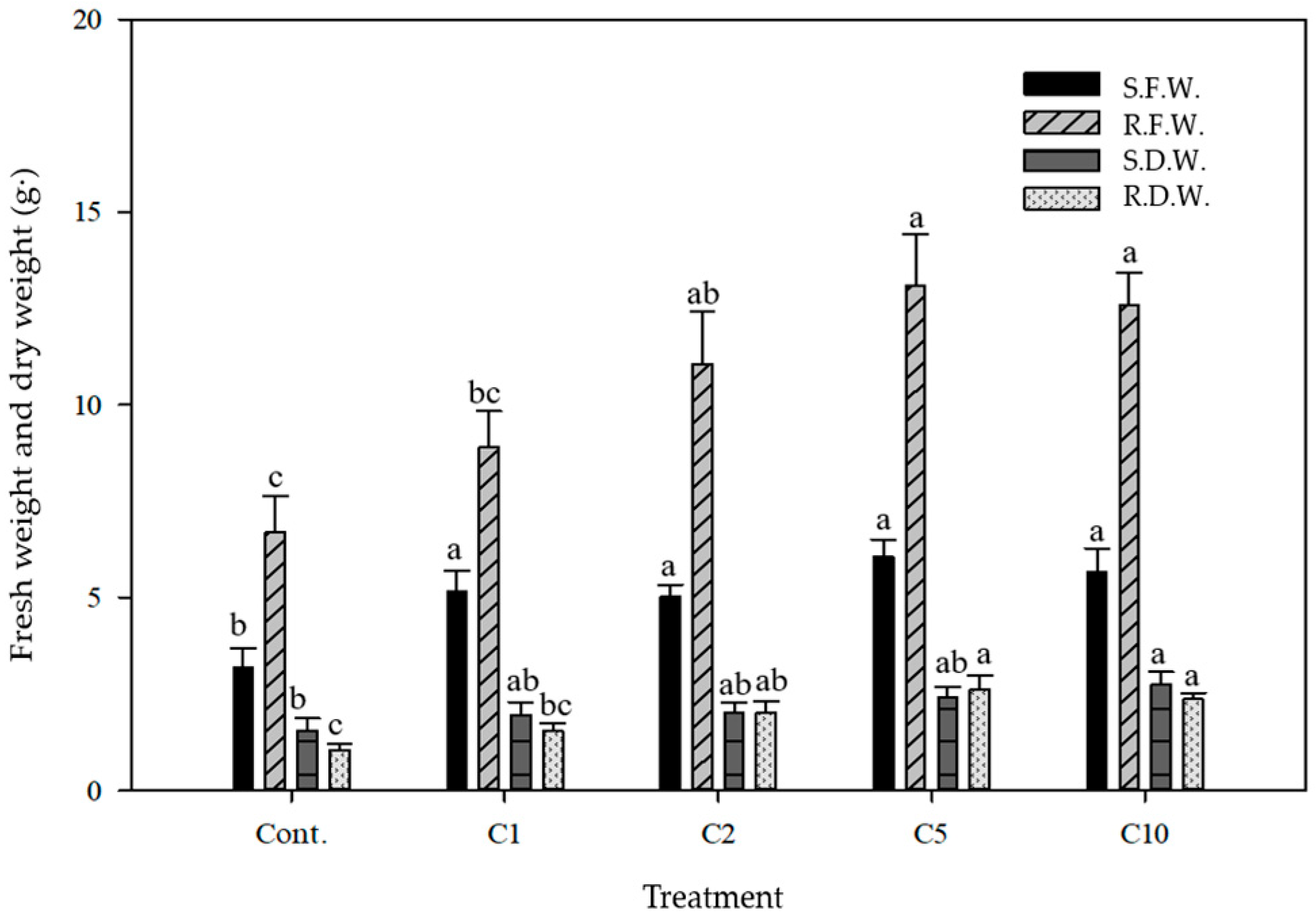
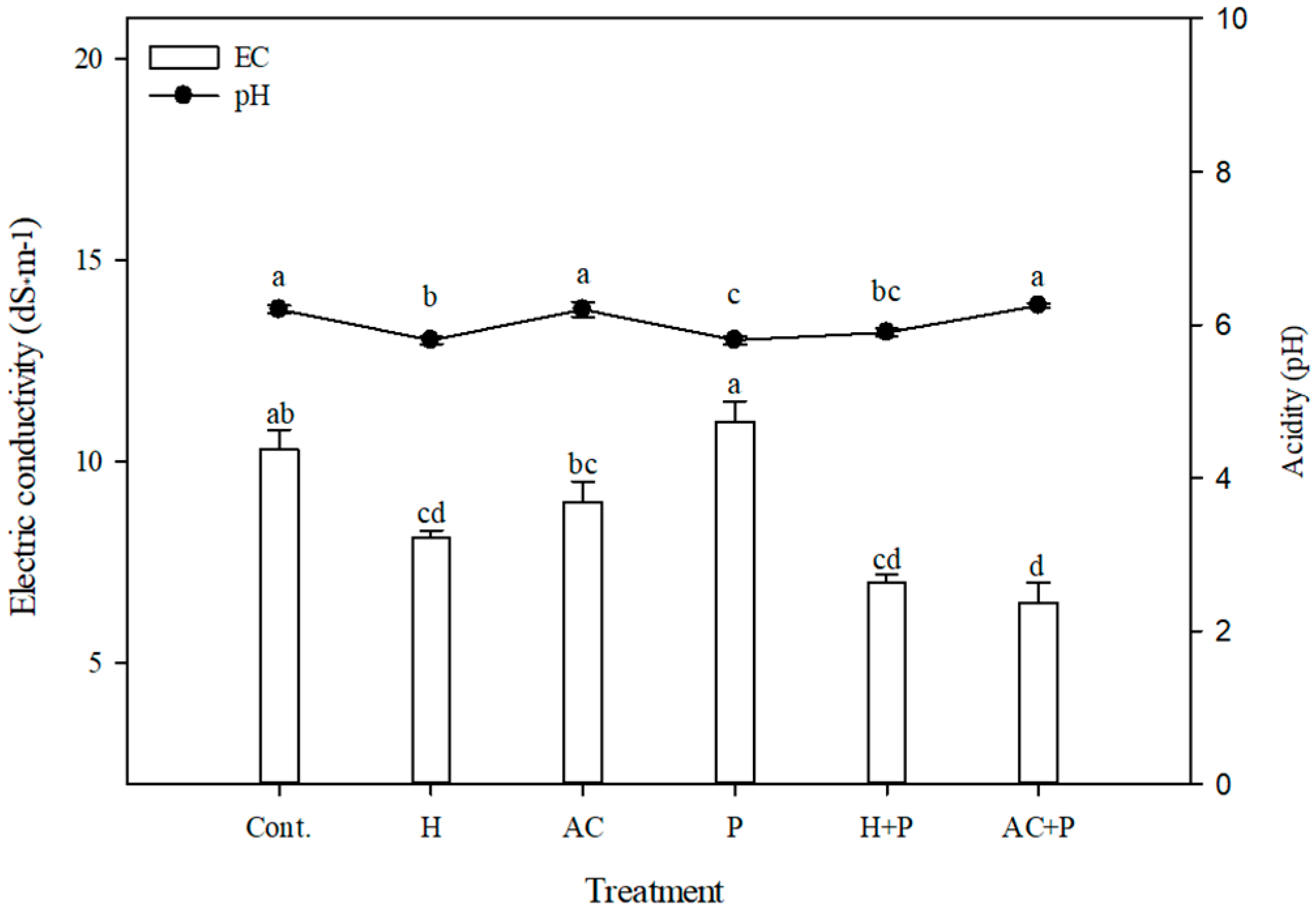
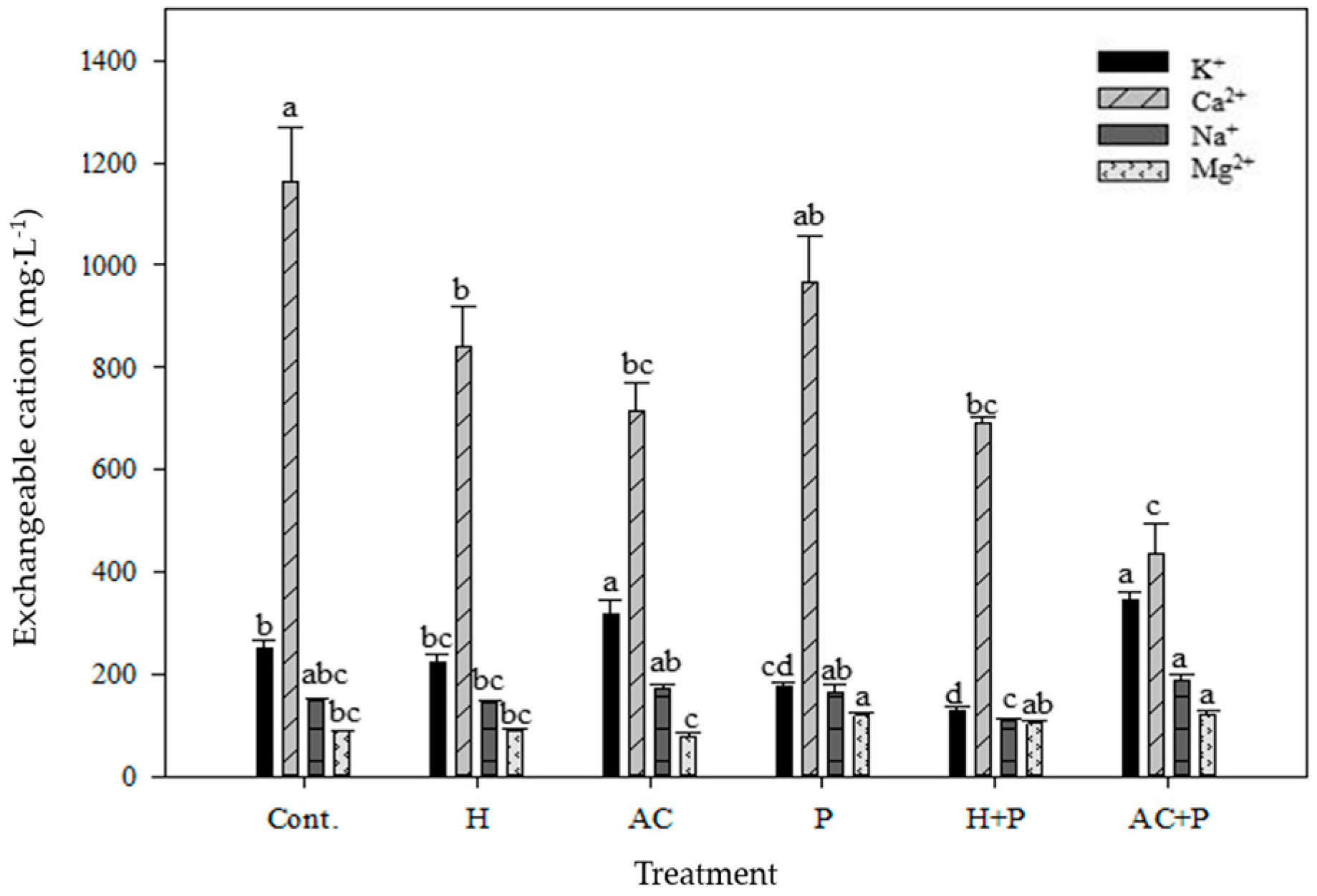

| Treatment z | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | No. of Leaves |
|---|---|---|---|---|
| Cont. | 59.2 b y | 12.54 a | 0.59 a | 25.8 b |
| C1 | 65.0 ab | 11.02 a | 0.53 a | 28.6 ab |
| C2 | 66.8 ab | 11.02 a | 0.57 a | 36.0 ab |
| C5 | 75.4 a | 11.88 a | 0.57 a | 29.5 ab |
| C10 | 71.0 ab | 12.25 a | 0.62 a | 37.3 a |
| Treatment | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) | No. of Leaves |
|---|---|---|---|---|
| P z | 71.0 a y | 12.02 a | 0.6 ab | 37.3 a |
| H + P | 67.7 a | 9.37 b | 0.5 b | 29.3 ab |
| AC + P | 59.5 a | 8.25 b | 0.7 a | 24.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.-H. Salt Tolerance of Phragmites australis and Effect of Combing It with Topsoil Filters on Biofiltration of CaCl2 Contaminated Soil. Sustainability 2024, 16, 8522. https://doi.org/10.3390/su16198522
Ju J-H. Salt Tolerance of Phragmites australis and Effect of Combing It with Topsoil Filters on Biofiltration of CaCl2 Contaminated Soil. Sustainability. 2024; 16(19):8522. https://doi.org/10.3390/su16198522
Chicago/Turabian StyleJu, Jin-Hee. 2024. "Salt Tolerance of Phragmites australis and Effect of Combing It with Topsoil Filters on Biofiltration of CaCl2 Contaminated Soil" Sustainability 16, no. 19: 8522. https://doi.org/10.3390/su16198522






