Upcycling PVC and PET as Volume-Enhancing Functional Fillers for the Development of High-Performance Bio-Based Rigid Polyurethane Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection and Preparation of PVC and PET Wastes
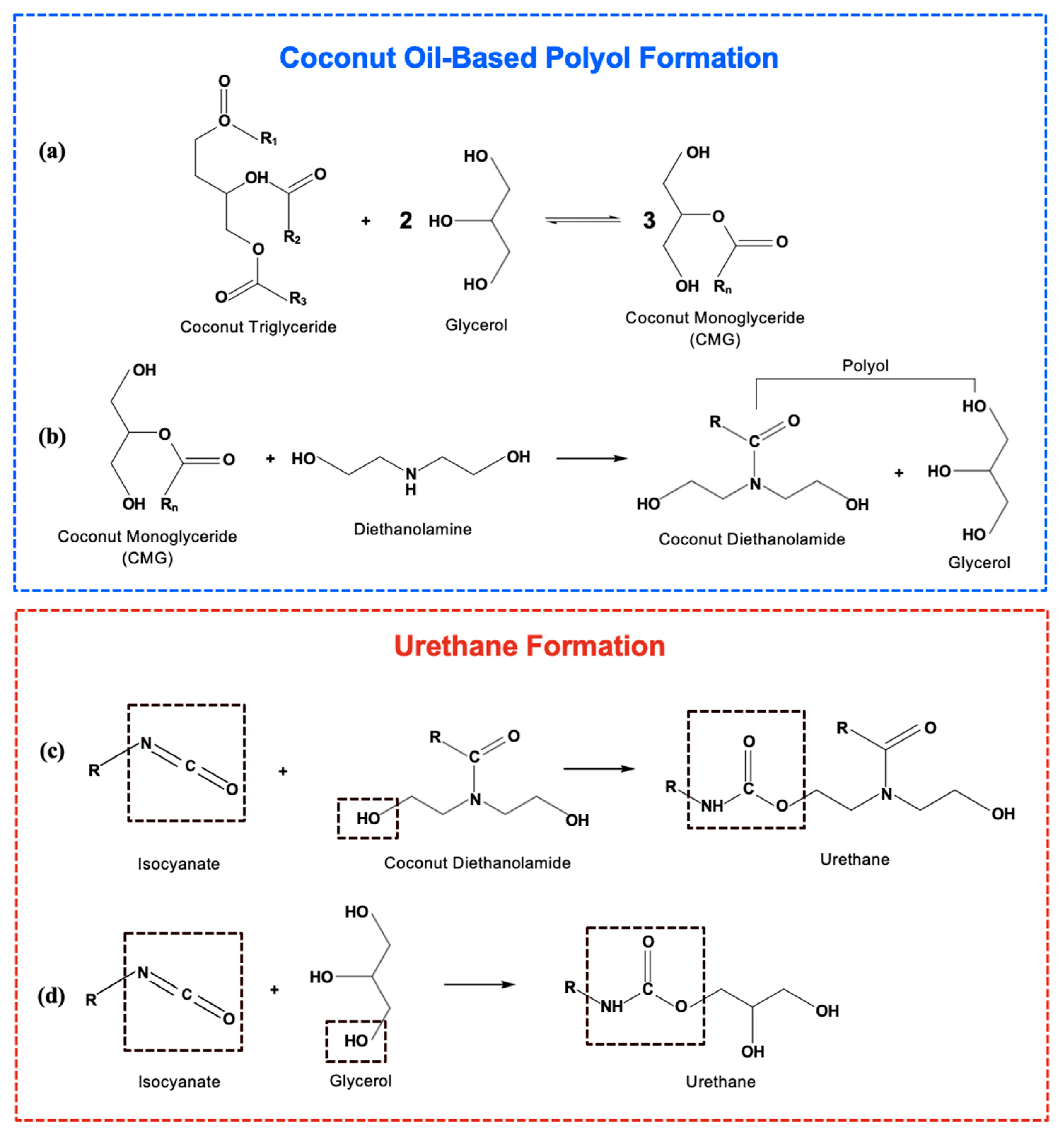
2.3. Preparation of Coconut Oil-Based Polyol
2.4. Foam Formulation
2.5. Polyol Analyses
2.6. Characterization of Rigid Polyurethane Foam
3. Results and Discussion
3.1. Preparation of Polyol
3.2. Viscosities of Polyol and PPW Blends
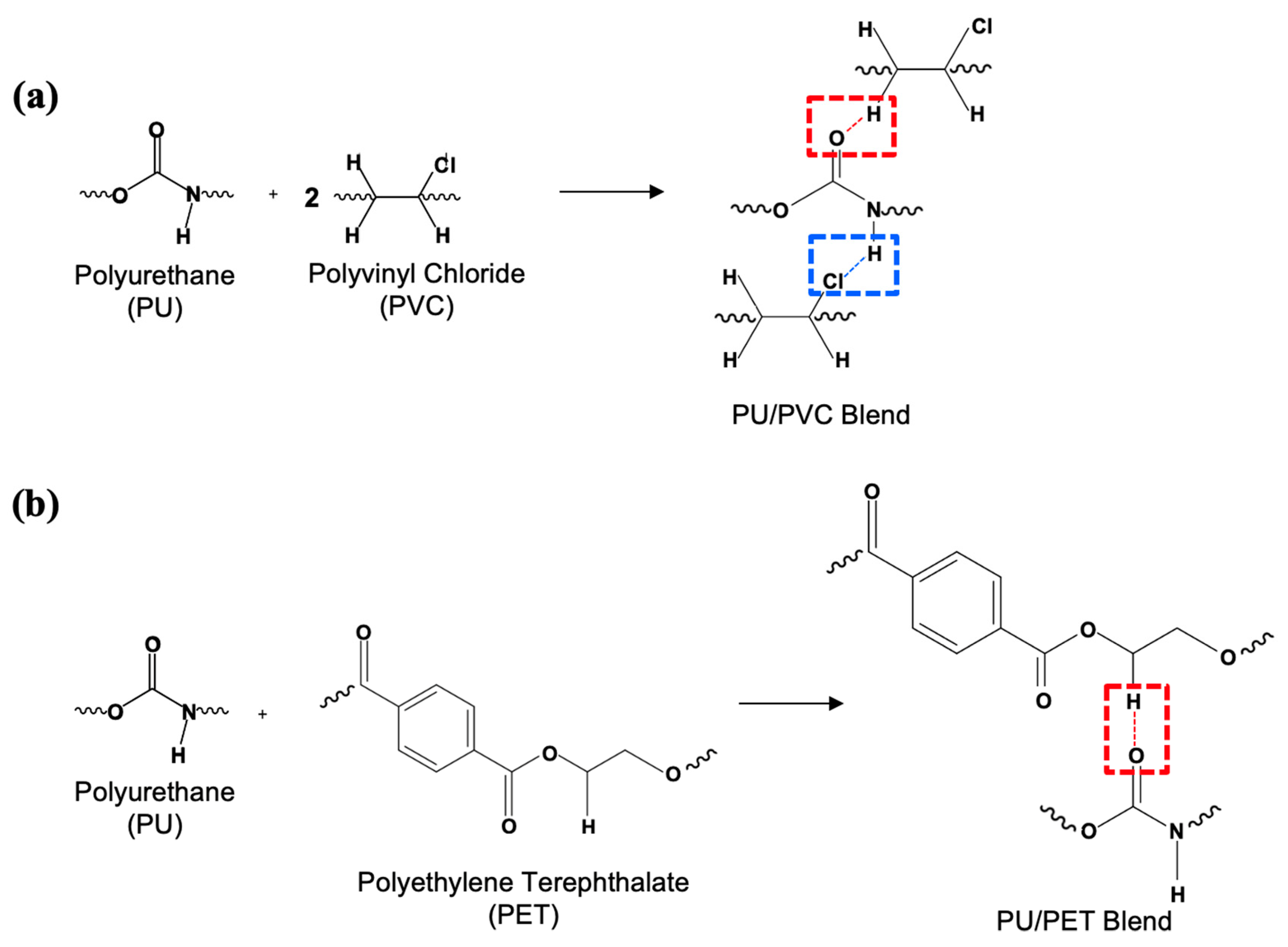
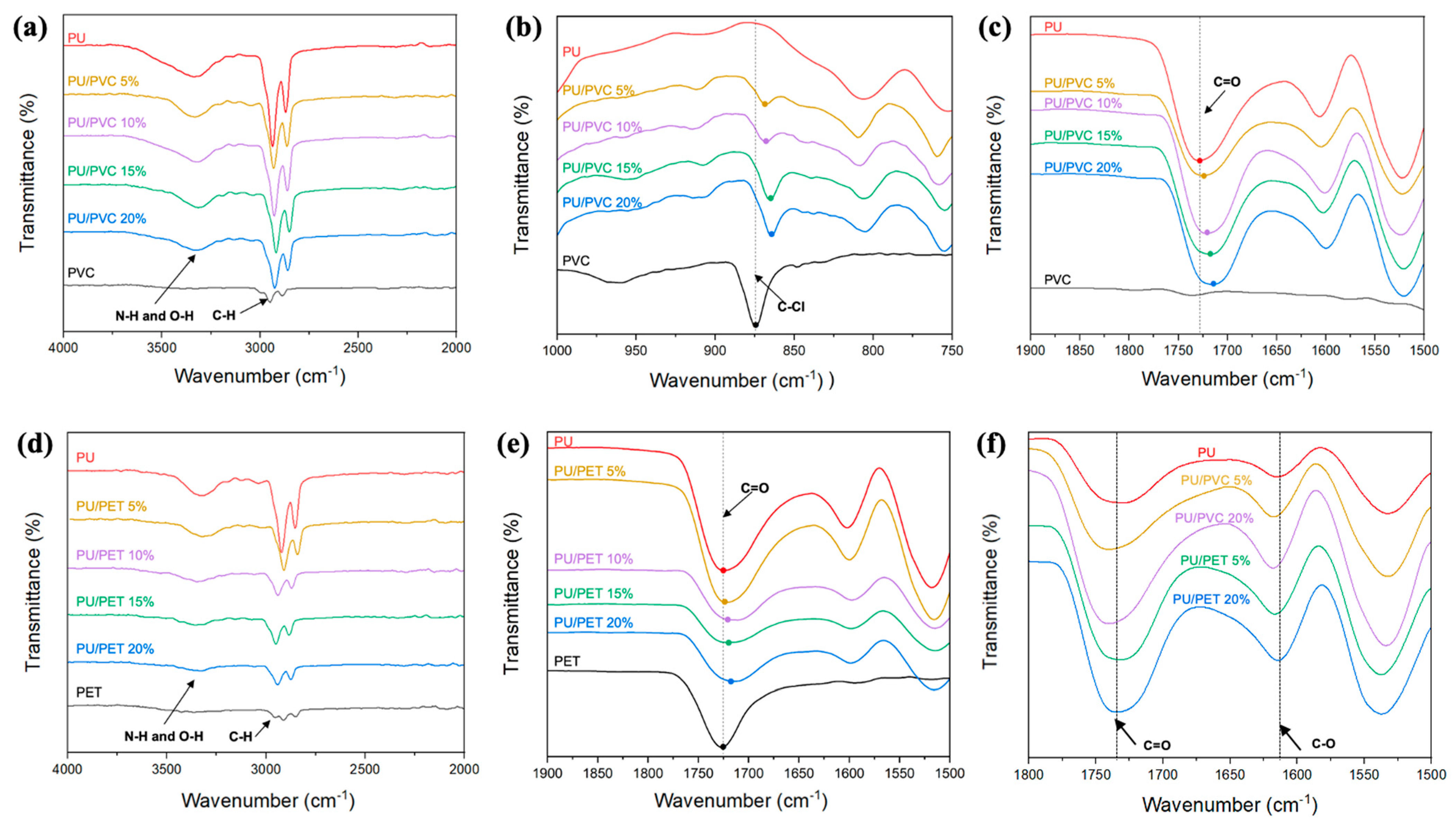
3.3. Chemical Structure of RPUF
3.4. Morphological Structure of RPUF
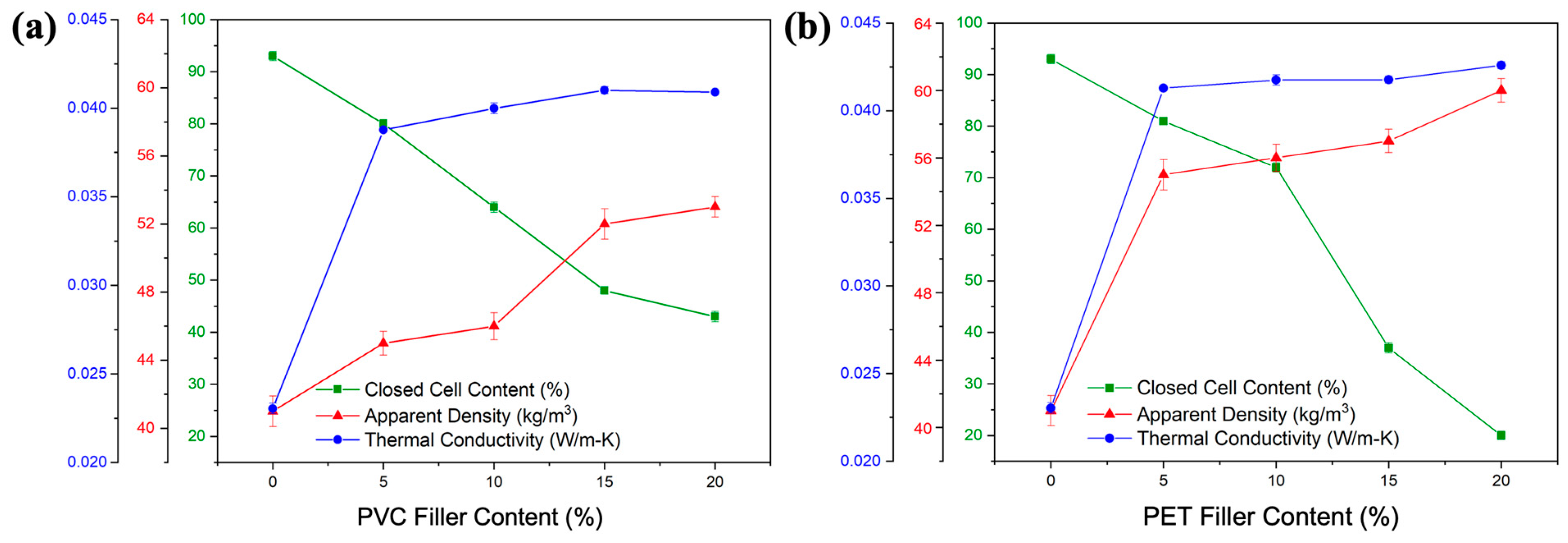
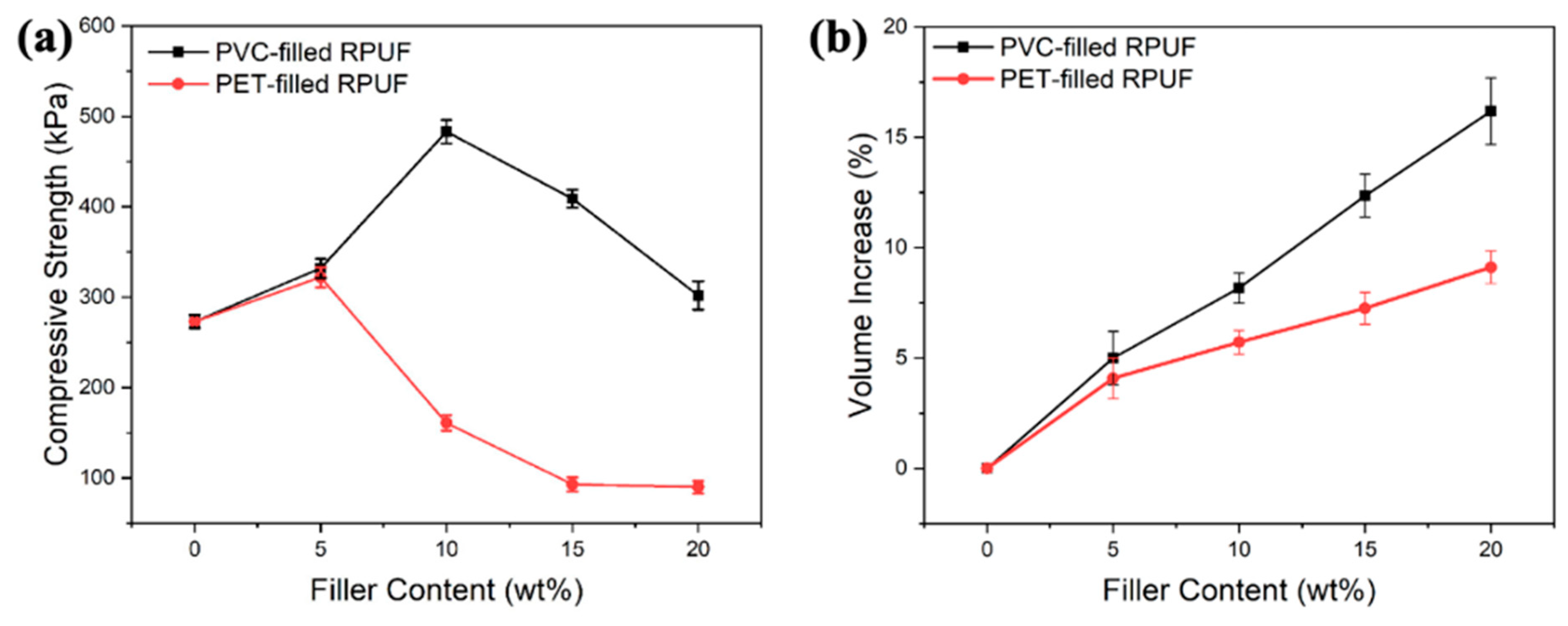
3.5. Physico-Mechanical and Thermal Properties of RPUF
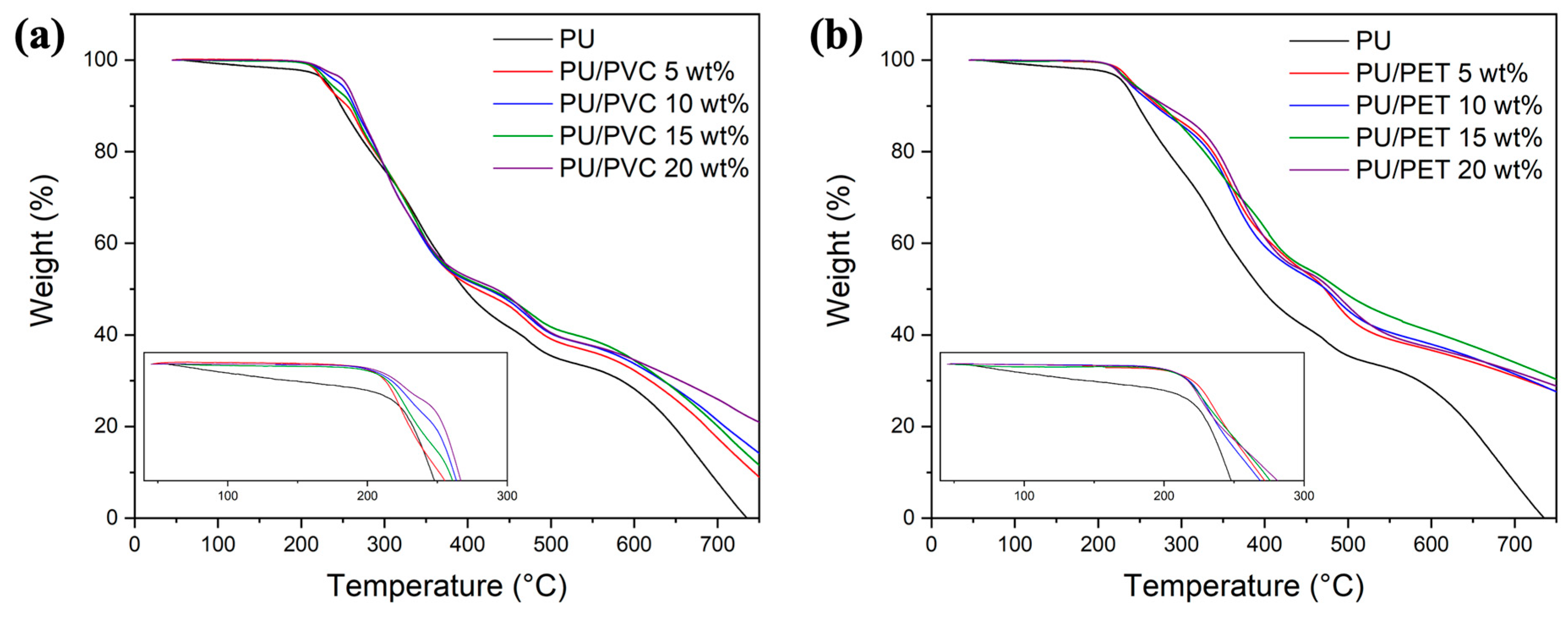
3.6. Thermal Stability of RPUF
3.7. Implications to Sustainability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, B.; Rahul, J.; Singh, P.K.; Rao, V.V.L.K.; Kumar, J.; Dwivedi, N.; Kumar, P.; Singh, D.; Strzałkowski, K. Advancements in PET Packaging: Driving Sustainable Solutions for Today’s Consumer Demands. Sustainability 2023, 15, 12269. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plastic Waste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Jiang, D.-H.; Satoh, T.; Tung, S.H.; Kuo, C.-C. Sustainable Alternatives to Nondegradable Medical Plastics. ACS Sustain. Chem. Eng. 2022, 10, 4792–4806. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Donner, E.; McGrath, S.P.; Tscharke, B.J.; O’Brien, J.W.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef] [PubMed]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Ali, S.M.; Ahmed, S.; Ahmed, H.N.; Sharmin, A.; Rahman, R. Reducing plastic pollutants through catalyzing consumer roles: A novel application of fuzzy total interpretive structural modeling. J. Clean. Prod. 2022, 335, 130327. [Google Scholar] [CrossRef]
- Meng, F.; Brandão, M.; Cullen, J.M. Replacing Plastics with Alternatives Is Worse for Greenhouse Gas Emissions in Most Cases. Environ. Sci. Technol. 2024, 58, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Gondal, A.H.; Bhat, R.A.; Gómez, R.L.; Areche, F.O.; Huaman, J.T. Advances in plastic pollution prevention and their fragile effects on soil, water, and air continuums. Int. J. Environ. Sci. Technol. 2023, 20, 6897–6912. [Google Scholar] [CrossRef]
- Senathirajah, K.; Palanisami, T. Strategies to Reduce Risk and Mitigate Impacts of Disaster: Increasing Water Quality Resilience from Microplastics in the Water Supply System. ACS EST Water 2023, 3, 2816–2834. [Google Scholar] [CrossRef]
- Petrlik, J.; Beeler, B.; Ismawati, Y.; Bell, L. Toxic Contamination Caused by Plastic Waste in Countries of the Global South. In Plast Waste Trade; Gündoğdu, S., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 113–128. [Google Scholar] [CrossRef]
- Bian, H.; Boguta, P.; Huang, J.; Deng, C.; Kong, D.; Zhou, H.; Su, X. Transforming Organic Solid Waste Management: Embracing Humification for Sustainable Resource Recovery. ACS Sustain. Resour. Manag. 2024, 1, 181–198. [Google Scholar] [CrossRef]
- Praveenkumar, T.R.; Sekar, M.; Pasupuleti, R.R.; Gavurová, B.; Arun Kumar, G.; Vignesh Kumar, M. Current technologies for plastic waste treatment for energy recovery, it’s effects on poly aromatic hydrocarbons emission and recycling strategies. Fuel 2024, 357, 129379. [Google Scholar] [CrossRef]
- Jacobs, C.; Soulliere, K.; Sawyer-Beaulieu, S.; Sabzwari, A.; Tam, E. Challenges to the Circular Economy: Recovering Wastes from Simple versus Complex Products. Sustainability 2022, 14, 2576. [Google Scholar] [CrossRef]
- Sau, D.; Shiuly, A.; Hazra, T. Utilization of plastic waste as replacement of natural aggregates in sustainable concrete: Effects on mechanical and durability properties. Int. J. Environ. Sci. Technol. 2024, 21, 2085–2120. [Google Scholar] [CrossRef]
- Thapa, K.; Vermeulen, W.J.V.; De Waal, M.M.; Deutz, P.; Nguyễn, H.Q. Towards a Just Circular Economy Transition: The Case of European Plastic Waste Trade to Vietnam for Recycling. Circ. Econ. Sustain. 2024, 4, 851–876. [Google Scholar] [CrossRef]
- Habibullah, M.; Manikanta, N.V.V.; Praveen, M.; Ramu, I. Automation of PET & PVC materials segregation system in recycling. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Shaibur, M.R.; Sarwar, S.; Hossain, M.S.; Ambade, B.; Chakraborty, T.K.; Ahmed, F.F. Plastic waste production and management in Jashore municipality and its surrounding areas, Bangladesh: An overview. Phys. Chem. Earth Parts ABC 2024, 134, 103580. [Google Scholar] [CrossRef]
- Cao, X.; Liang, Y.; Jiang, J.; Mo, A.; He, D. Organic additives in agricultural plastics and their impacts on soil ecosystems: Compared with conventional and biodegradable plastics. TrAC Trends Anal. Chem. 2023, 166, 117212. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Khellaf, M.; Raffin, G.; Lebaz, N.; Elaissari, A. Recent advances in polyvinyl chloride ( PVC ) recycling. Polym. Adv. Technol. 2024, 35, e6228. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, B.; Zhu, M. An overview on the recycling of waste poly(vinyl chloride). Green Chem. 2023, 25, 6971–7025. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Wang, Y.; Yu, X.; Li, Y.; Lu, Y.; Zhou, K.; He, J.; Song, Z.; Gao, X. A novel safety treatment strategy of DEHP-rich flexible polyvinyl chloride waste through low-temperature critical aqueous ammonia treatment. Sci. Total Environ. 2020, 708, 134532. [Google Scholar] [CrossRef]
- He, D.; Hu, H.; Jiao, F.; Zuo, W.; Liu, C.; Xie, H.; Dong, L.; Wang, X. Thermal separation of heavy metals from municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2023, 467, 143344. [Google Scholar] [CrossRef]
- Mangold, H.; Von Vacano, B. The Frontier of Plastics Recycling: Rethinking Waste as a Resource for High-Value Applications. Macromol. Chem. Phys. 2022, 223, 2100488. [Google Scholar] [CrossRef]
- Kassab, A.; Al Nabhani, D.; Mohanty, P.; Pannier, C.; Ayoub, G.Y. Advancing Plastic Recycling: Challenges and Opportunities in the Integration of 3D Printing and Distributed Recycling for a Circular Economy. Polymers 2023, 15, 3881. [Google Scholar] [CrossRef] [PubMed]
- Merrington, A. Recycling of Plastics. In Applied Plastics Engineering Handbook; Elsevier: Amsterdam, The Netherlands, 2024; pp. 191–217. [Google Scholar] [CrossRef]
- Buhari, S.B.; Nezhad, N.G.; Normi, Y.M.; Shariff, F.M.; Leow, T.C. Insight on recently discovered PET polyester-degrading enzymes, thermostability and activity analyses. 3 Biotech 2024, 14, 31. [Google Scholar] [CrossRef]
- Amalia, L.; Chang, C.-Y.; Wang, S.S.-S.; Yeh, Y.-C.; Tsai, S.-L. Recent advances in the biological depolymerization and upcycling of polyethylene terephthalate. Curr. Opin. Biotechnol. 2024, 85, 103053. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Weldekidan, H.; Mohanty, A.K.; Misra, M. Upcycling of Plastic Wastes and Biomass for Sustainable Graphitic Carbon Production: A Critical Review. ACS Environ. Au 2022, 2, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, F.; Wei, X.; Yang, Y.; Xu, S.; Deng, D.; Wang, Y.-Z. From trash to treasure: Chemical recycling and upcycling of commodity plastic waste to fuels, high-valued chemicals and advanced materials. J. Energy Chem. 2022, 69, 369–388. [Google Scholar] [CrossRef]
- Abdelsattar, D.E.; El-Demerdash, S.H.; Zaki, E.G.; Dhmees, A.S.; Azab, M.A.; Elsaeed, S.M.; Kandil, U.F.; Naguib, H.M. Effect of Polymer Waste Mix Filler on Polymer Concrete Composites. ACS Omega 2023, 8, 39730–39738. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.-Q.; Jiao, Y.; Li, Y.; Sun, B.; Xiao, D.; Wang, M.; Ma, D. Co-upcycling of polyvinyl chloride and polyesters. Nat. Sustain. 2023, 6, 1685–1692. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, F.; Yu, F.; Zhang, J.; Zhang, J. Methyl Chloride Synthesis over Metal Chlorides-Modified Mesoporous Alumina Catalyst. Catalysts 2018, 8, 99. [Google Scholar] [CrossRef]
- Srivastava, R.; Fujita, S.-I.; Okamura, S.; Arai, M. Alkylation of aromatic compounds with multi-component Lewis acid catalysts of ZnCl2 and ionic liquids with different organic cations. React. Kinet. Catal. Lett. 2009, 96, 55–64. [Google Scholar] [CrossRef]
- Nisar, M.; Gondal, H.Y.; Cheema, Z.M.; Abbasskhan, A. Lewis Acid-Catalyzed Synthesis of Alkoxymethylhalides for MultipurposeMixed Acetals; Scope and Limitations. Lett. Org. Chem. 2022, 19, 750–756. [Google Scholar] [CrossRef]
- Saint-Michel, F.; Chazeau, L.; Cavaillé, J.-Y. Mechanical properties of high density polyurethane foams: II Effect of the filler size. Compos. Sci. Technol. 2006, 66, 2709–2718. [Google Scholar] [CrossRef]
- Bhagia, S.; Gallego, N.C.; Hiremath, N.; Harper, D.P.; Lowden, R.A.; Lowden, R.R.; Pu, Y.; Vaidya, U.; Ozcan, S.; Ragauskas, A.J. Fine grinding of thermoplastics by high speed friction grinding assisted by guar gum. J. Appl. Polym. Sci. 2021, 138, 50797. [Google Scholar] [CrossRef]
- Hipulan, L.N.A.; Dingcong, R.G.; Estrada, D.J.E.; Dumancas, G.G.; Bondaug, J.C.S.; Alguno, A.C.; Bacosa, H.P.; Malaluan, R.M.; Ludguban, A.A. Development of High-Performance Coconut Oil-Based Rigid Polyurethane-Urea Foam: A Novel Sequential Amidation and Prepolymerization Process. ACS Omega 2024, 9, 13112–13124. [Google Scholar] [CrossRef]
- Dingcong, R.G.; Malaluan, R.M.; Alguno, A.C.; Estrada, D.J.E.; Lubguban, A.A.; Resurreccion, E.P.; Dumancas, G.G.; Al-Moameri, H.H.; Ludguban, A.A. A novel reaction mechanism for the synthesis of coconut oil-derived biopolyol for rigid poly(urethane-urea) hybrid foam application. RSC Adv. 2023, 13, 1985–1994. [Google Scholar] [CrossRef]
- Bondaug, J.C.S.; Dingcong, R.G.; Hipulan, L.N.; Ochigue, P.C.; Dumancas, G.G.; Alguno, A.C.; Malaluan, R.M.; Ludguban, A.A.; Bacosa, H.P. Development of a Catalyst System for Enhanced Properties of Coconut Diethanolamide-Based Rigid Poly(urethane-urea) Foam. ACS Appl. Polym. Mater. 2024, 6, 6875–6887. [Google Scholar] [CrossRef]
- ASTM D4274-23; Test Methods for Testing Polyurethane Raw Materials: Determination of Hydroxyl Numbers of Polyols. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- ASTM D4878-23; Test Methods for Polyurethane Raw Materials: Determination of Viscosity of Polyols. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- ASTM D1622-20; Test Method for Apparent Density of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D6226-21; Test Method for Open Cell Content of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Malakahmad, A.; Abualqumboz, M.S.; Kutty, S.R.M.; Abunama, T.J. Assessment of carbon footprint emissions and environmental concerns of solid waste treatment and disposal techniques; case study of Malaysia. Waste Manag. 2017, 70, 282–292. [Google Scholar] [CrossRef]
- ASTM D1621-16(2023); Test Method for Compressive Properties of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- ASTM C518-21; Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Furtwengler, P.; Avérous, L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Ghodke, S.; Dandekar, P.; Jain, R. Simplified evaluation aided by mathematical calculation for characterization of polyols by hydroxyl value determination. Int. J. Polym. Anal. Charact. 2021, 26, 169–178. [Google Scholar] [CrossRef]
- Onn, M.; Jalil, M.J.; Mohd Yusoff, N.I.S.; Edward, E.B.; Wahit, M.U. A comprehensive review on chemical route to convert waste cooking oils to renewable polymeric materials. Ind. Crops Prod. 2024, 211, 118194. [Google Scholar] [CrossRef]
- Henry, C.; Tindall, G.; Thies, M.C.; Nejad, M. Fractionated and purified hybrid poplar lignins as a polyol replacement in rigid polyurethane/polyisocyanurate foams. J. Appl. Polym. Sci. 2023, 140, e54648. [Google Scholar] [CrossRef]
- Deng, T.; Li, S.; Jia, P.; Yao, N.; Ding, H.; Xu, L.; Zhang, Y.; Yang, X.; Li, M. Self-Plasticized PVC Prepared by Introducing Fatty Acid to the PVC with Triglycidyl Isocyanurate as an Intermediate Bridge. ACS Omega 2022, 7, 35694–35704. [Google Scholar] [CrossRef]
- Chu, M.; Liu, Y.; Lou, X.; Zhang, Q.; Chen, J. Rational Design of Chemical Catalysis for Plastic Recycling. ACS Catal. 2022, 12, 4659–4679. [Google Scholar] [CrossRef]
- Fekete, E.; Földes, E.; Pukánszky, B. Effect of molecular interactions on the miscibility and structure of polymer blends. Eur. Polym. J. 2005, 41, 727–736. [Google Scholar] [CrossRef]
- Hezma, A.M.; Elashmawi, I.S.; Rajeh, A.; Kamal, M. Change Spectroscopic, thermal and mechanical studies of PU/PVC blends. Phys. B Condens. Matter 2016, 495, 4–10. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid Polyurethane Foams with Various Isocyanate Indices Based on Polyols from Rapeseed Oil and Waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef]
- Ali, I.; Ali, A.; Ali, A.; Ramzan, M.; Hussain, K.; Li, X.; Zhan, J.; Dias, O.A.T.; Yang, W.; Li, H.; et al. Highly electro-responsive composite gel based on functionally tuned graphene filled polyvinyl chloride. Polym. Adv. Technol. 2021, 32, 3679–3688. [Google Scholar] [CrossRef]
- Radhakrishnan Nair, M.N.; Gopinathan Nair, M.R. Studies on impact modification and fractography of solution cast blends of PVC and NR/PU block copolymers. Polym. Bull. 2012, 68, 859–877. [Google Scholar] [CrossRef]
- Furhan Ramesan, M.T. Zinc oxide reinforced poly(para-aminophenol) nanocomposites: Structural, thermal stability, conductivity and ammonia gas sensing applications. J. Macromol. Sci. Part A 2022, 59, 675–688. [Google Scholar] [CrossRef]
- Wang, C.; Geng, X.; Chen, J.; Wang, H.; Wei, Z.; Huang, B.; Liu, W.; Wu, X.; Hu, L.; Su, G.; et al. Multiple H-Bonding Cross-Linked Supramolecular Solid–Solid Phase Change Materials for Thermal Energy Storage and Management. Adv. Mater. 2024, 36, 2309723. [Google Scholar] [CrossRef] [PubMed]
- Aparício, R.R.; Santos, G.M.D.; Giacon, V.M.; Silva, C.G.D. Performance of castor oil polyurethane resin in composite with the piassava fibers residue from the Amazon. Sci. Rep. 2023, 14, 6679. [Google Scholar] [CrossRef]
- Ho, Q.B.; Kontopoulou, M. Stabilization of the cellular structure of polypropylene foams and secondary nucleation mechanism in the presence of graphene nanoplatelets. Polymer 2020, 198, 122506. [Google Scholar] [CrossRef]
- Cimavilla-Román, P.; Pérez-Tamarit, S.; Santiago-Calvo, M.; Rodríguez-Pérez, M.Á. Influence of silica aerogel particles on the foaming process and cellular structure of rigid polyurethane foams. Eur. Polym. J. 2020, 135, 109884. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Liu, J.; Han, D.; Rohani, S.; Gao, Z.; Gong, J. Inhibition of Crystal Nucleation and Growth: A Review. Cryst. Growth Des. 2024, 24, 2645–2665. [Google Scholar] [CrossRef]
- Anwar, J.; Zahn, D. Uncovering Molecular Processes in Crystal Nucleation and Growth by Using Molecular Simulation. Angew. Chem. Int. Ed. 2011, 50, 1996–2013. [Google Scholar] [CrossRef]
- Firat-Karalar, E.N.; Welch, M.D. New mechanisms and functions of actin nucleation. Curr. Opin. Cell Biol. 2011, 23, 4–13. [Google Scholar] [CrossRef]
- Camino, J.D.; Gracia, P.; Cremades, N. The role of water in the primary nucleation of protein amyloid aggregation. Biophys. Chem. 2021, 269, 106520. [Google Scholar] [CrossRef]
- Rai, S.K.; Savastano, A.; Singh, P.; Mukhopadhyay, S.; Zweckstetter, M. Liquid–liquid phase separation of tau: From molecular biophysics to physiology and disease. Protein Sci. 2021, 30, 1294–1314. [Google Scholar] [CrossRef]
- Lorusso, C.; Vergaro, V.; Conciauro, F.; Ciccarella, G.; Congedo, P.M. Thermal and mechanical performance of rigid polyurethane foam added with commercial nanoparticles. Nanomater. Nanotechnol. 2017, 7, 184798041668411. [Google Scholar] [CrossRef]
- Matuana, L.M.; Diaz, C.A. Study of Cell Nucleation in Microcellular Poly(lactic acid) Foamed with Supercritical CO 2 through a Continuous-Extrusion Process. Ind. Eng. Chem. Res. 2010, 49, 2186–2193. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Xu, Y.; Nie, Q.; Yang, Z.; Sheng, W.; Qian, G. Municipal solid waste incineration residues recycled for typical construction materials—A review. RSC Adv. 2022, 12, 6279–6291. [Google Scholar] [CrossRef]
- Song, T.; Liu, M.; Tian, J.; Wang, S.; Li, Q. Effect of PLA/TiO2/Lg filler competition and synergy on crystallization behavior, mechanics and functionality of composite foaming materials. Polymer 2023, 271, 125797. [Google Scholar] [CrossRef]
- Xiao, T.; Lu, L.; Peng, W.; Yue, Z.; Yang, X.; Lu, T.J.; Sundén, B. Numerical study of heat transfer and load-bearing performances of corrugated sandwich structure with open-cell metal foam. Int. J. Heat Mass Transf. 2023, 215, 124517. [Google Scholar] [CrossRef]
- Dukarska, D.; Walkiewicz, J.; Derkowski, A.; Mirski, R. Properties of Rigid Polyurethane Foam Filled with Sawdust from Primary Wood Processing. Materials 2022, 15, 5361. [Google Scholar] [CrossRef]
- Muthuraj, R.; Jimenez-Saelices, C.; Grohens, Y.; Seantier, B. Chapter 15: Applications of Polysaccharide and Protein Based Aerogels in Thermal Insulation. In Green Chemistry Series; Thomas, S., Pothan, L.A., Mavelil-Sam, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 261–294. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Vaitkus, S. Composites of rigid polyurethane foams and silica powder filler enhanced with ionic liquid. Polym. Test. 2019, 75, 12–25. [Google Scholar] [CrossRef]
- Paruzel, A.; Michałowski, S.; Hodan, J.; Horák, P.; Prociak, A.; Beneš, H. Rigid Polyurethane Foam Fabrication Using Medium Chain Glycerides of Coconut Oil and Plastics from End-of-Life Vehicles. ACS Sustain. Chem. Eng. 2017, 5, 6237–6246. [Google Scholar] [CrossRef]
- Danowska, M.; Piszczyk, Ł.; Strankowski, M.; Gazda, M.; Haponiuk, J.T. Rigid polyurethane foams modified with selected layered silicate nanofillers. J. Appl. Polym. Sci. 2013, 130, 2272–2281. [Google Scholar] [CrossRef]
- Saleem, F.; Li, S.; Cui, S.; Liu, X.; Xu, T.; Mei, L.; Bian, Y.; Miao, C.; Luo, T. The strain rate and density dependence of the mechanical properties of closed-cell aluminum foam. Mater. Sci. Eng. A 2023, 884, 145568. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M. Improvement in physico-mechanical and structural properties of rigid polyurethane foam composites by the addition of sugar beet pulp as a reactive filler. J. Polym. Res. 2021, 28, 80. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Jiang, X.; Zhao, J.; Zhao, G.; Xie, W.; Zhang, W. Tensile properties of transversely isotropic closed-cell PVC foam under quasi-static and dynamic loadings. J. Sandw. Struct. Mater. 2024, 26, 373–395. [Google Scholar] [CrossRef]
- Bartczak, P.; Siwińska-Ciesielczyk, K.; Haak, N.; Parus, A.; Piasecki, A.; Jesionowski, T.; Borysiak, S. Closed-cell polyurethane spray foam obtained with novel TiO2–ZnO hybrid fillers–mechanical, insulating properties and microbial purity. J. Build. Eng. 2023, 65, 105760. [Google Scholar] [CrossRef]
- Șerban, D.-A.; Linul, E. Fatigue behaviour of closed-cell polyurethane rigid foams. Eng. Fail. Anal. 2023, 154, 107728. [Google Scholar] [CrossRef]
- Li, N.; Shi, J.-F.; Zou, K.-K.; Wang, Y.-Y.; Yan, D.-X. Mechanically Reinforced Rigid Polyimide Foam via Chemically Grafting Isocyanate Acid for Ultrabroad Band Microwave Absorption. ACS Appl. Mater. Interfaces 2023, 15, 25990–25999. [Google Scholar] [CrossRef]
- Saif, M.S.; Shanour, A.S.; Abdelaziz, G.E.; Elsayad, H.I.; Shaaban, I.G.; Tayeh, B.A.; Hammad, M.S. Influence of blended powders on properties of Ultra-High Strength Fibre Reinforced Self Compacting Concrete subjected to elevated temperatures. Case Stud. Constr. Mater. 2023, 18, e01793. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, K.; Zhang, J.; Li, M.; Han, X.; Chen, Z.; Ma, L.; Chang, C. New bio-based polyurethane (PU) foams synthesized using crude glycerol-based biopolyol and humin-based byproducts from biomass hydrolysis. Ind. Crops Prod. 2023, 205, 117548. [Google Scholar] [CrossRef]
- Bokobza, L. Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions. Polymers 2023, 15, 2900. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Mechanical, Morphological and Thermal Properties of Rigid Polyurethane Foam: Effect of the Fillers. Cell. Polym. 2007, 26, 245–259. [Google Scholar] [CrossRef]
- Ruamcharoen, J.; Phetphaisit, C.W.; Chanlert, P.; Cheming, S.; Ruamcharoen, P. Sago starch and esterified sago starch as eco-friendly fillers for rigid polyurethane foams. J. Cell. Plast. 2024, 60, 23–40. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, C.; Xin, Z.; Li, Y.; Qin, W.; Zhou, S. 13X zeolite as Difunctional nucleating agent regulating the crystal form and improving the Foamability of blocked copolymerized polypropylene in supercritical CO2 foaming process. J. Polym. Res. 2019, 26, 58. [Google Scholar] [CrossRef]
- Muhazeli, N.S.; Nordin, N.A.; Mazlan, S.A.; Rizuan, N.; Abdul Aziz, S.A.; Abd Fatah, A.Y.; Ibrahim, Z.; Dbaidillah, U.; Choi, S.-B. Characterization of morphological and rheological properties of rigid magnetorheological foams via in situ fabrication method. J. Mater. Sci. 2019, 54, 13821–13833. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Liu, X.-M. Mechanical response of composite materials prepared with polyurethane elastomers and polyvinyl chloride films. J. Mech. Behav. Biomed. Mater. 2023, 146, 106006. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-Y. Polyvinyl chloride/engineering polymer blends, interpenetrating polymeric networks, and gels. In Polyvinyl Chloride-Based Blends IPNs Gels; Elsevier: Amsterdam, The Netherlands, 2024; pp. 179–199. [Google Scholar] [CrossRef]
- Pokharel, P.; Choi, S.; Lee, D.S. The effect of hard segment length on the thermal and mechanical properties of polyurethane/graphene oxide nanocomposites. Compos. Part Appl. Sci. Manuf. 2015, 69, 168–177. [Google Scholar] [CrossRef]
- Kamairudin, N.; Abdullah, L.C.; Hoong, S.S.; Biak, D.R.A.; Ariffin, H. Preparation and Effect of Methyl-Oleate-Based Polyol on the Properties of Rigid Polyurethane Foams as Potential Thermal Insulation Material. Polymers 2023, 15, 3028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, X.; Chen, W.; Wang, Y.; Liu, C.; Cui, B.; Li, G.; Song, J.; Zhao, D.; Wang, D.; et al. A simple and green strategy for preparing flexible thermoplastic polyimide foams with exceptional mechanical, thermal-insulating properties, and temperature resistance for high-temperature lightweight composite sandwich structures. Compos. Part B Eng. 2022, 228, 109405. [Google Scholar] [CrossRef]
- Srihanum, A.; Tuan Noor, M.T.; Devi, K.P.; Hoong, S.S.; Ain, N.H.; Mohd, N.S.; Nek Mat Din, N.S.M.; Kian, Y.S. Low density rigid polyurethane foam incorporated with renewable polyol as sustainable thermal insulation material. J. Cell. Plast. 2022, 58, 485–503. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Kumar, S.; Purkait, M.K. Polyurethane Foams as Packing and Insulating Materials. In ACS Symposium Series; Gupta, R.K., Ed.; American Chemical Society: Washington, DC, USA, 2023; Volume 1454, pp. 83–99. [Google Scholar] [CrossRef]
- Horak, Z.; Dvorak, K.; Zarybnicka, L.; Vojackova, H.; Dvorakova, J.; Vilimek, M. Experimental Measurements of Mechanical Properties of PUR Foam Used for Testing Medical Devices and Instruments Depending on Temperature, Density and Strain Rate. Materials 2020, 13, 4560. [Google Scholar] [CrossRef]
- Yun, J.S.; Im, S.H. Porous PEDOT:PSS smart thermal insulators enabling energy harvesting and detection. J. Mater. Chem. A 2024, 12, 7837–7846. [Google Scholar] [CrossRef]
- Bank, M.S.; Swarzenski, P.W.; Duarte, C.M.; Rillig, M.C.; Koelmans, A.A.; Metian, M.; Wright, S.; Provencher, J.F.; Sanden, M.; Jordaan, A.; et al. Global Plastic Pollution Observation System to Aid Policy. Environ. Sci. Technol. 2021, 55, 7770–7775. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.S. The Family as a Farm Institution: Cases in Japan and the Philippines. In Contemporary Perspectives in Family Research; Gregorio, V.L., Batan, C.M., Blair, S.L., Eds.; Emerald Publishing Limited: Bingley, UK, 2023; pp. 205–225. [Google Scholar] [CrossRef]
- Seike, T.; Isobe, T.; Harada, Y.; Kim, Y.; Shimura, M. Analysis of the efficacy and feasibility of recycling PVC sashes in Japan. Resour. Conserv. Recycl. 2018, 131, 41–53. [Google Scholar] [CrossRef]
- Lu, L.; Li, W.; Cheng, Y.; Liu, M. Chemical recycling technologies for PVC waste and PVC-containing plastic waste: A review. Waste Manag. 2023, 166, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, J.; Kim, J.; Ouellet-Plamondon, C.M. Life cycle assessment of emergent masonry blocks. J. Clean. Prod. 2018, 171, 1622–1637. [Google Scholar] [CrossRef]
- Von Der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO 2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Shibamoto, T.; Yasuhara, A.; Katami, T. Dioxin Formation from Waste Incineration. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Whitacre, D.M., Gunther, F.A., Eds.; Springer: New York, NY, USA, 2007; Volume 190, pp. 1–41. [Google Scholar] [CrossRef]





| Foam Formulation | Components | Concentration, php a | |||||
|---|---|---|---|---|---|---|---|
| 0% Filler | 5% Filler | 10% Filler | 15% Filler | 20% Filler | 0% Filler | ||
| B-Side Components | |||||||
| Polyol | Coco Diethanolamide | 100 | 100 | 100 | 100 | 100 | 100 |
| PPW filler | PVC or PET | 0 | 2.5 | 5 | 7.5 | 10 | 0 |
| Catalyst | Polycat 8 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Surfactant | INV 690 | 1 | 1 | 1 | 1 | 1 | 1 |
| Blowing agent | Water | 2 | 2 | 2 | 2 | 2 | 2 |
| A-Side Component | |||||||
| Isocyanate index b of PAPI 27 | 110 | 110 | 110 | 110 | 110 | 110 | |
| Parameter | Value |
|---|---|
| OH number | 332 ± 3 mg KOH/g |
| Acid Number | 1.5 ± 0.2 mg KOH/g |
| Viscosity | 1750 ± 25 mPa·s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochigue, P.C.D.; Dingcong, R.G., Jr.; Bondaug, J.C.S.; Magalong, B.C.G.; Dumancas, G.G.; Gutierrez, C.S.; Alguno, A.C.; Malaluan, R.M.; Lubguban, A.A.; Bacosa, H.P. Upcycling PVC and PET as Volume-Enhancing Functional Fillers for the Development of High-Performance Bio-Based Rigid Polyurethane Foams. Sustainability 2024, 16, 8540. https://doi.org/10.3390/su16198540
Ochigue PCD, Dingcong RG Jr., Bondaug JCS, Magalong BCG, Dumancas GG, Gutierrez CS, Alguno AC, Malaluan RM, Lubguban AA, Bacosa HP. Upcycling PVC and PET as Volume-Enhancing Functional Fillers for the Development of High-Performance Bio-Based Rigid Polyurethane Foams. Sustainability. 2024; 16(19):8540. https://doi.org/10.3390/su16198540
Chicago/Turabian StyleOchigue, Princess Claire D., Roger G. Dingcong, Jr., John Christian S. Bondaug, Brian Christian G. Magalong, Gerard G. Dumancas, Carlo S. Gutierrez, Arnold C. Alguno, Roberto M. Malaluan, Arnold A. Lubguban, and Hernando P. Bacosa. 2024. "Upcycling PVC and PET as Volume-Enhancing Functional Fillers for the Development of High-Performance Bio-Based Rigid Polyurethane Foams" Sustainability 16, no. 19: 8540. https://doi.org/10.3390/su16198540








