Drainage and Afforestation More Strongly Affect Soil Microbial Composition in Fens than Bogs of Subtropical Moss Peatlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Site Setting and Sampling
2.3. Determination of Soil Physicochemical Properties
2.4. High-Throughput Sequencing
2.4.1. Soil Bacterial DNA Extraction and PCR Amplification
2.4.2. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Bacterial Community Diversity Indexes
3.3. Correlation between Soil Physicochemical Properties and Soil Bacterial Community Diversity Indexes
3.4. Soil Bacterial Community β Diversity
3.5. Indicator Groups of Soil Bacterial Communities
3.6. Composition of the Main Groups of Soil Bacterial Communities at Different Taxonomic Levels
3.7. Relationship between Soil Bacterial Community Composition and Soil Physicochemical Properties
4. Discussion
4.1. Soil Bacterial Community α Diversity and Its Influencing Factors
4.2. Soil Bacterial Community Composition and Its Influencing Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.-T.; Lei, Y.; Dai, C.; Yang, L.-F.; Li, Z.-Q.; Wang, Z.-X. Effects of both substrate and nitrogen and phosphorus fertilizer on Sphagnum palustre growth in subtropical high-mountain regions and implications for peatland recovery. Wetl. Ecol. Manag. 2018, 26, 651–663. [Google Scholar] [CrossRef]
- Page, S.E.; Baird, A. Peatlands and Global Change: Response and Resilience. Annu. Rev. Environ. Resour. 2016, 41, 35–57. [Google Scholar] [CrossRef]
- Li, T.-T.; Wang, Z.-X.; Bu, G.-J.; Lin, L.-Q.; Lei, Y.; Liu, C.-Y.; Yang, L.-F.; Zheng, C.-L. Effects of microtopography and water table on Sphagnum palustre L. in subtropical high mountains and implications for peatland restoration. J. Bryol. 2019, 41, 121–134. [Google Scholar] [CrossRef]
- Kandel, T.P.; Lærke, P.E.; Elsgaard, L. Annual emissions of CO2, CH4 and N2O from a temperate peat bog: Comparison of an undrained and four drained sites under permanent grass and arable crop rotations with cereals and potato. Agric. For. Meteorol. 2018, 256, 470–481. [Google Scholar] [CrossRef]
- Sloan, T.; Payne, R.J.; Bain, C.; Chapman, S.; Cowie, N.; Gilbert, P.; Lindsay, R.; Mauquoy, D.; Newton, A.; Andersen, R. Peatland afforestation in the UK and consequences for carbon storage. Mires Peat 2018, 23, 1–17. [Google Scholar] [CrossRef]
- Vítovcová, K.; Lipárová, J.; Manukjanová, A.; Vašutová, M.; Vrba, P.; Prach, K. Biodiversity restoration of formerly extracted raised bogs: Vegetation succession and recovery of other trophic groups. Wetl. Ecol. Manag. 2022, 30, 207–237. [Google Scholar] [CrossRef]
- Pospíšilová, P.; Vítovcová, K.; Prach, K. Importance of repeated sampling: Vegetation analyses after 10 years revealed different restoration trends in formerly extracted peatlands. Restor. Ecol. 2023, 31, e13720. [Google Scholar] [CrossRef]
- Prior, L.D.; Nichols, S.C.; Williamson, G.J.; Bowman, D.M. Post-fire restoration of Sphagnum bogs in the Tasmanian Wilderness World Heritage Area, Australia. Restor. Ecol. 2023, 31, e13797. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.-T.; Ran, N.; He, M.-Y.; Jiang, H.-Q.; Wang, Z.-X. Peat swamp biodiversity in the Qizimei Mountain National Nature Reserve, China. Mires Peat 2021, 27, 17–21. [Google Scholar] [CrossRef]
- Andersen, R.; Chapman, S.; Artz, R. Microbial communities in natural and disturbed peatlands: A review. Soil Biol. Biochem. 2013, 57, 979–994. [Google Scholar] [CrossRef]
- Lin, X.; Green, S.; Tfaily, M.; Prakash, O.; Konstantinidis, K.; Corbett, J.; Chanton, J.; Cooper, W.; Kostka, J. Microbial Community Structure and Activity Linked to Contrasting Biogeochemical Gradients in Bog and Fen Environments of the Glacial Lake Agassiz Peatland. Appl. Environ. Microbiol. 2012, 78, 7023–7031. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, Z.; Bárta, J. Microbial community composition and in silico predicted metabolic potential reflect biogeochemical gradients between distinct peatland types. FEMS Microbiol. Ecol. 2014, 90, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.; Leeflang, P.; Gommans, S.; van den Broek, J.; van Mil, S.; Wernars, K. Diversity and Seasonal Fluctuations of the Dominant Members of the Bacterial Soil Community in a Wheat Field as Determined by Cultivation and Molecular Methods. Appl. Environ. Microbiol. 2001, 67, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef]
- Tian, W.; Wang, H.; Xiang, X.; Wang, R.; Xu, Y. Structural Variations of Bacterial Community Driven by Sphagnum Microhabitat Differentiation in a Subalpine Peatland. Front. Microbiol. 2019, 10, 1661. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Serkebaeva, Y.M.; Kulichevskaya, I.S.; Liesack, W.; Dedysh, S.N. Substrate-induced growth and isolation of Acidobacteria from acidic Sphagnum peat. ISME J. 2008, 2, 551–560. [Google Scholar] [CrossRef]
- Elliott, D.R.; Caporn, S.J.; Nwaishi, F.; Nilsson, R.H.; Sen, R. Bacterial and Fungal Communities in a Degraded Ombrotrophic Peatland Undergoing Natural and Managed Re-Vegetation. PLoS ONE 2015, 10, e0124726. [Google Scholar] [CrossRef]
- Alam, M.J.; Rengasamy, N.; bin Dahalan, M.P.; Halim, S.A.; Nath, T.K. Socio-economic and ecological outcomes of a community-based restoration of peatland swamp forests in Peninsular Malaysia: A 5Rs approach. Land Use Policy 2022, 122, 106390. [Google Scholar] [CrossRef]
- Garcin, Y.; Schefuß, E.; Dargie, G.C.; Hawthorne, D.; Lawson, I.T.; Sebag, D.; Biddulph, G.E.; Crezee, B.; Bocko, Y.E.; Ifo, S.A.; et al. Hydroclimatic vulnerability of peat carbon in the central Congo Basin. Nature 2022, 612, 277–282. [Google Scholar] [CrossRef]
- Wilkinson, S.; Andersen, R.; Moore, P.; Davidson, S.J.; Granath, G.; Waddington, J. Wildfire and degradation accelerate northern peatland carbon release. Nat. Clim. Chang. 2023, 13, 456–461. [Google Scholar] [CrossRef]
- Galand, P.; Fritze, H.; Conrad, R.; Yrjala, K. Pathways for Methanogenesis and Diversity of Methanogenic Archaea in Three Boreal Peatland Ecosystems. Appl. Environ. Microbiol. 2005, 71, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Lee, S.-H.; Freeman, C.; Fenner, N.; Kang, H. Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biol. Biochem. 2008, 40, 2874–2880. [Google Scholar] [CrossRef]
- Urbanová, Z.; Picek, T.; Bárta, J. Effect of peat re-wetting on carbon and nutrient fluxes, greenhouse gas production and diversity of methanogenic archaeal community. Ecol. Eng. 2011, 37, 1017–1026. [Google Scholar] [CrossRef]
- Gupta, V.; Smemo, K.A.; Yavitt, J.B.; Basiliko, N. Active Methanotrophs in Two Contrasting North American Peatland Ecosystems Revealed Using DNA-SIP. Microb. Ecol. 2012, 63, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Urbanová, Z.; Bárta, J. Effects of long-term drainage on microbial community composition vary between peatland types. Soil Biol. Biochem. 2016, 92, 16–26. [Google Scholar] [CrossRef]
- Cui, H.-J.; Zhang, Y.; Tian, K.; Xiao, D.-R.; Wang, K.; Guo, Y.; Li, L.-J. Characteristics of moss bog plant community and interspecific relationships of dominantspecies in Niangniang mountain wetland, Guizhou. Chin. J. Ecol. 2018, 37, 2619–2626. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Cao, W.; Abalori, T.A.; Liu, Y.; Xin, Y.; Wang, S.; Zhang, D. Soil bacterial community responses to short-term grazing exclusion in a degraded alpine shrubland–grassland ecotone. Ecol. Indic. 2021, 130, 108043. [Google Scholar] [CrossRef]
- Wang, H.-C.; Qi, J.-F.; Xiao, D.-R.; Wang, Y.; Shi, W.-Y.; Wang, H. Bacterial community diversity and underlying assembly patterns along vertical soil profiles in wetland and meadow habitats on the Zoige Plateau, China. Soil Biol. Biochem. 2023, 184, 109076. [Google Scholar] [CrossRef]
- Shao, T.; Zhao, J.; Liu, A.; Long, X.; Rengel, Z. Effects of soil physicochemical properties on microbial communities in different ecological niches in coastal area. Appl. Soil Ecol. 2020, 150, 103486. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, R.; Guo, X.; Fu, Q.; Fan, F.; Liu, S. Yak excreta-induced changes in soil microbial communities increased the denitrification rate of marsh soil under warming conditions. Ecology 2021, 165, 103935. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 16 June 2023).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R package version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 2 September 2023).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-6. 2023. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 2 September 2023).
- Wei, T.; Simko, V. R Package ’corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 25 September 2023).
- Wickam, H. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Kaštovská, E.; Straková, P.; Edwards, K.; Urbanová, Z.; Bárta, J.; Mastný, J.; Šantrůčková, H.; Picek, T. Cotton-Grass and Blueberry have Opposite Effect on Peat Characteristics and Nutrient Transformation in Peatland. Ecosystems 2018, 21, 443–458. [Google Scholar] [CrossRef]
- Perotto, S.; Daghino, S.; Martino, E. Ericoid mycorrhizal fungi and their genomes: Another side to the mycorrhizal symbiosis? New Phytol. 2018, 220, 1141–1147. [Google Scholar] [CrossRef]
- DeGrood, S.H.; Claassen, V.P.; Scow, K.M. Microbial community composition on native and drastically disturbed serpentine soils. Soil Biol. Biochem. 2005, 37, 1427–1435. [Google Scholar] [CrossRef]
- McKinley, V.; Peacock, A.; White, D. Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol. Biochem. 2005, 37, 1946–1958. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Pankratov, T.A.; Belova, S.E.; Kulichevskaya, I.S.; Liesack, W. Phylogenetic Analysis and In Situ Identification of Bacteria Community Composition in an Acidic Sphagnum peat bog. Appl. Environ. Microbiol. 2006, 72, 2110–2117. [Google Scholar] [CrossRef]
- Kraigher, B.; Stres, B.; Hacin, J.; Ausec, L.; Mahne, I.; van Elsas, J.D.; Mandic-Mulec, I. Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh. Soil Biol. Biochem. 2006, 38, 2762–2771. [Google Scholar] [CrossRef]
- Morales, S.E.; Mouser, P.J.; Ward, N.; Hudman, S.P.; Gotelli, N.J.; Ross, D.S.; Lewis, T.A. Comparison of Bacterial Communities in New England Sphagnum Bogs Using Terminal Restriction Fragment Length Polymorphism (T-RFLP). Microb. Ecol. 2006, 52, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ausec, L.; Kraigher, B.; Mandic-Mulec, I. Differences in the activity and bacterial community structure of drained grassland and forest peat soils. Soil Biol. Biochem. 2009, 41, 1874–1881. [Google Scholar] [CrossRef]
- Pankratov, T.A.; Ivanova, A.O.; Dedysh, S.N.; Liesack, W. Bacterial populations and environmental factors controlling cellulose degradation in an acidic Sphagnum peat. Environ. Microbiol. 2011, 13, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Serkebaeva, Y.M.; Kim, Y.; Liesack, W.; Dedysh, S.N. Pyrosequencing-Based Assessment of the Bacteria Diversity in Surface and Subsurface Peat Layers of a Northern Wetland, with Focus on Poorly Studied Phyla and Candidate Divisions. PLoS ONE 2013, 8, e63994. [Google Scholar] [CrossRef]
- Sun, H.; Terhonen, E.; Koskinen, K.; Paulin, L.; Kasanen, R.; Asiegbu, F.O. Bacterial diversity and community structure along different peat soils in boreal forest. Appl. Soil Ecol. 2014, 74, 37–45. [Google Scholar] [CrossRef]
- Danilova, O.; Belova, S.; Gagarinova, I.; Dedysh, S. Microbial Community Composition and Methanotroph Diversity of a Subarctic Wetland in Russia. Microbiology 2016, 85, 583–591. [Google Scholar] [CrossRef]
- Philippot, L.; Andersson, S.G.; Battin, T.J.; Prosser, J.I.; Schimel, J.P.; Whitman, W.B.; Hallin, S. The ecological coherence of high bacterial taxonomic ranks. Nat. Rev. Microbiol. 2010, 8, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dedysh, S.N. Cultivating uncultured bacteria from northern wetlands: Knowledge gained and remaining gaps. Front. Microbiol. 2011, 2, 184. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Le Roes-Hill, M.; Khan, N.; Burton, S.G. Actinobacterial Peroxidases: An Unexplored Resource for Biocatalysis. Appl. Biochem. Biotechnol. 2011, 164, 681–713. [Google Scholar] [CrossRef]
- Jaatinen, K.; Fritze, H.; Laine, J.; Laiho, R. Effects of short-and long-term water-level drawdown on the populations and activity of aerobic decomposers in a boreal peatland. Glob. Chang. Biol. 2007, 13, 491–510. [Google Scholar] [CrossRef]
- Blodau, C.; Siems, M. Drainage-induced forest growth alters belowground carbon biogeochemistry in the Mer Bleue bog, Canada. Biogeochemistry 2012, 107, 107–123. [Google Scholar] [CrossRef]
- Mastný, J.; Urbanová, Z.; Kaštovská, E.; Straková, P.; Šantrůčková, H.; Edwards, K.; Picek, T. Soil organic matter quality and microbial activities in spruce swamp forests affected by drainage and water regime restoration. Soil Use Manag. 2016, 32, 200–209. [Google Scholar] [CrossRef]
- Urbanová, Z.; Straková, P.; Kaštovská, E. Response of peat biogeochemistry and soil organic matter quality to rewetting in bogs and spruce swamp forests. Eur. J. Soil Biol. 2018, 85, 12–22. [Google Scholar] [CrossRef]
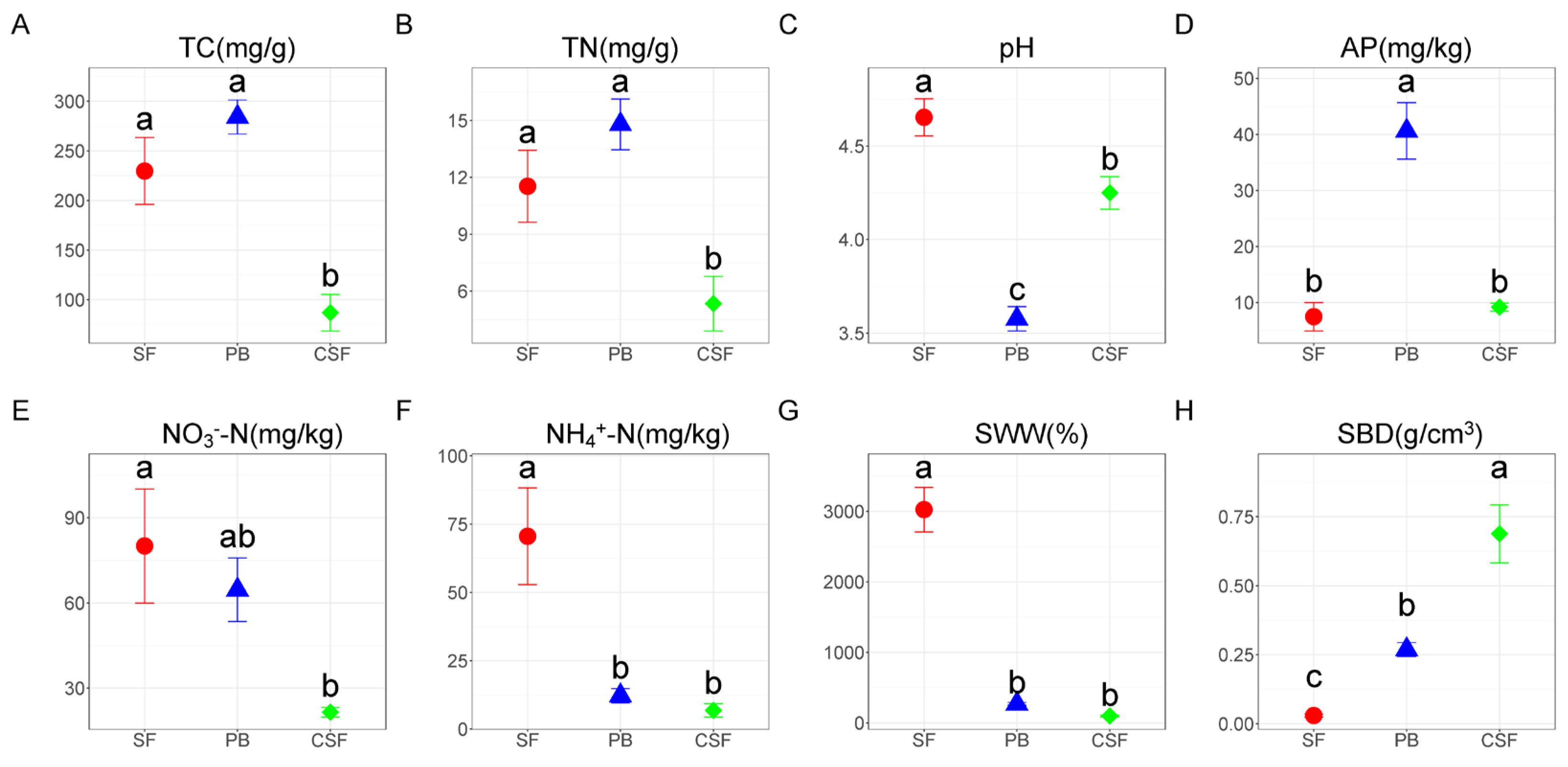
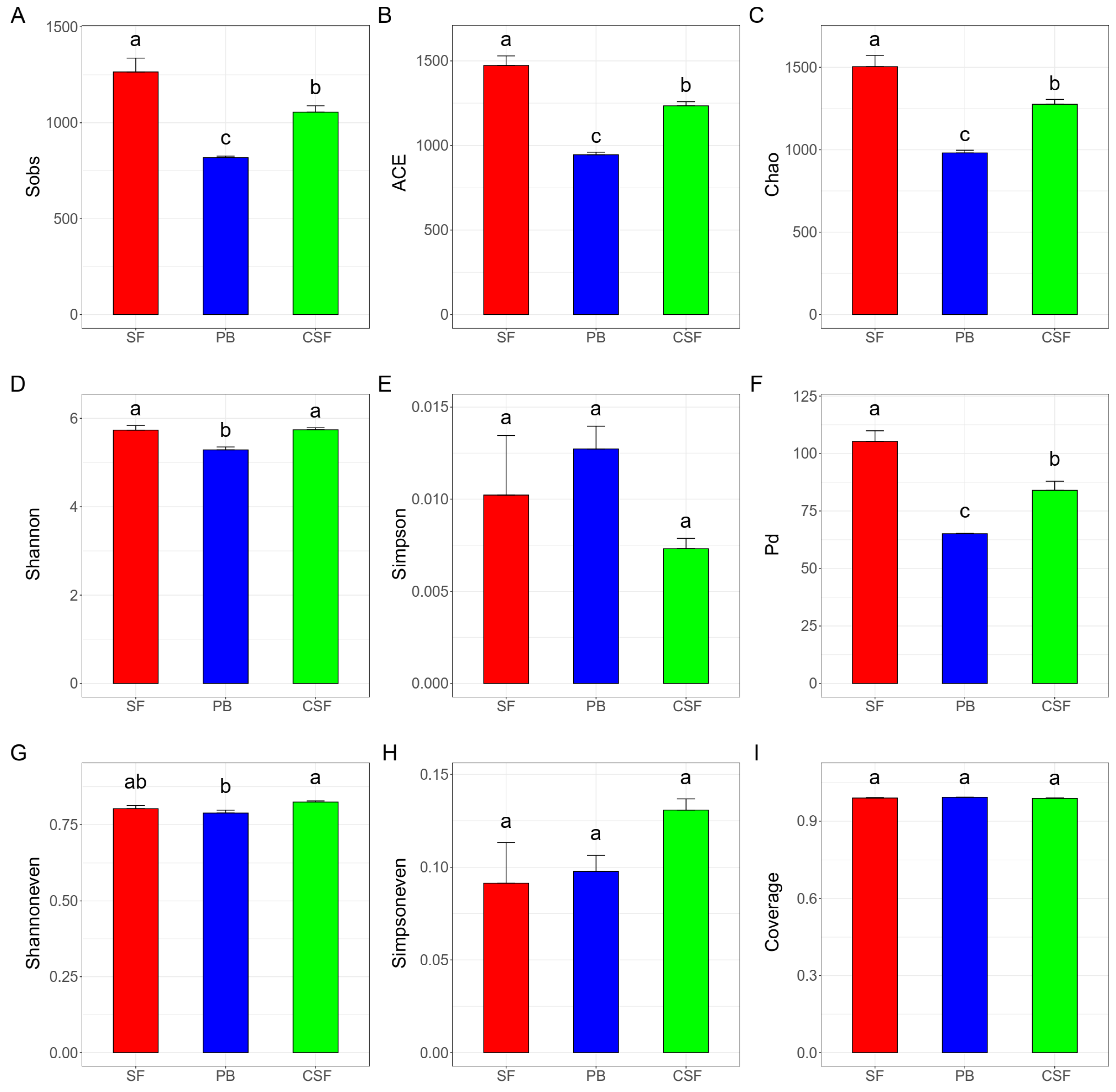
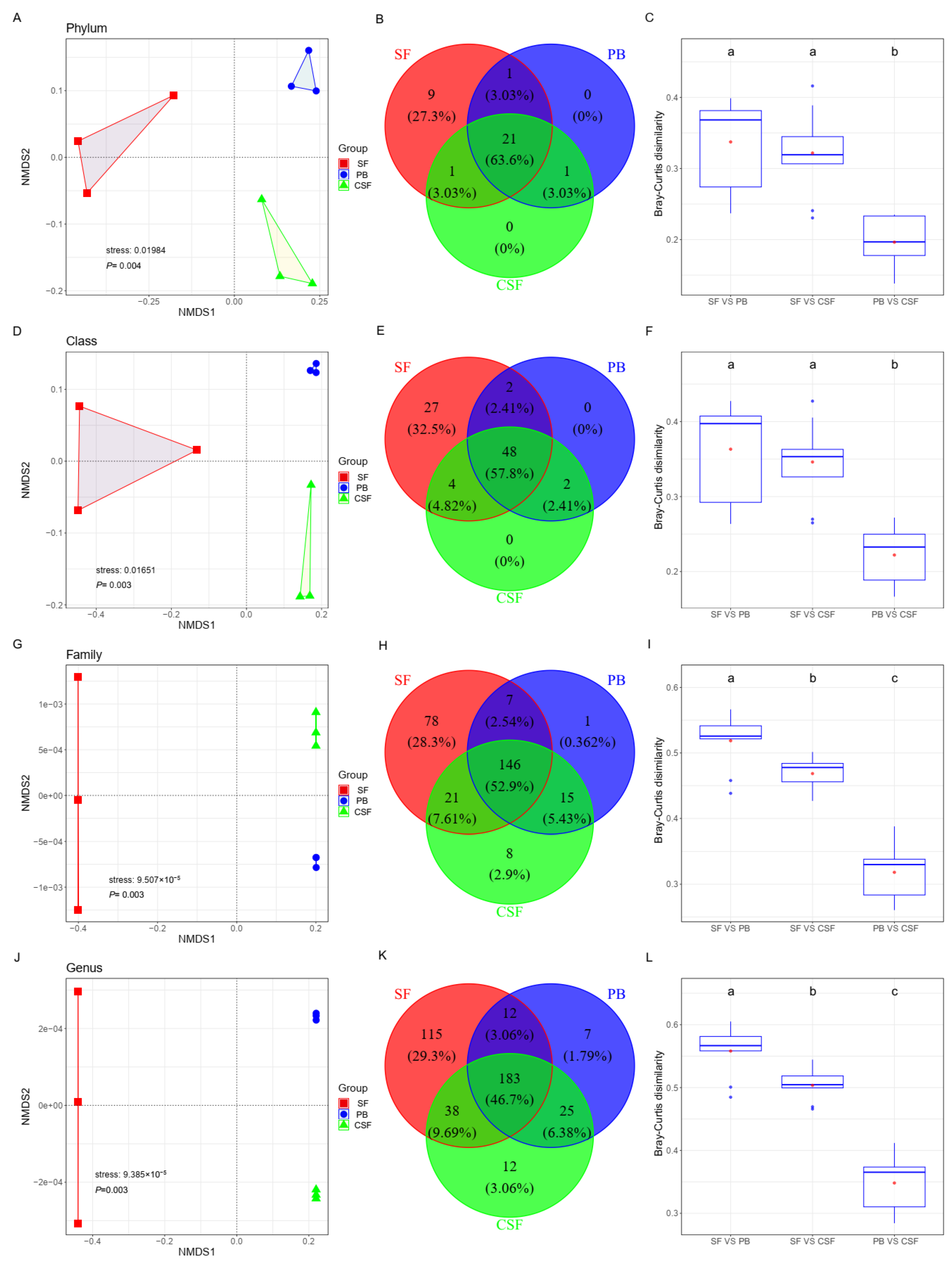

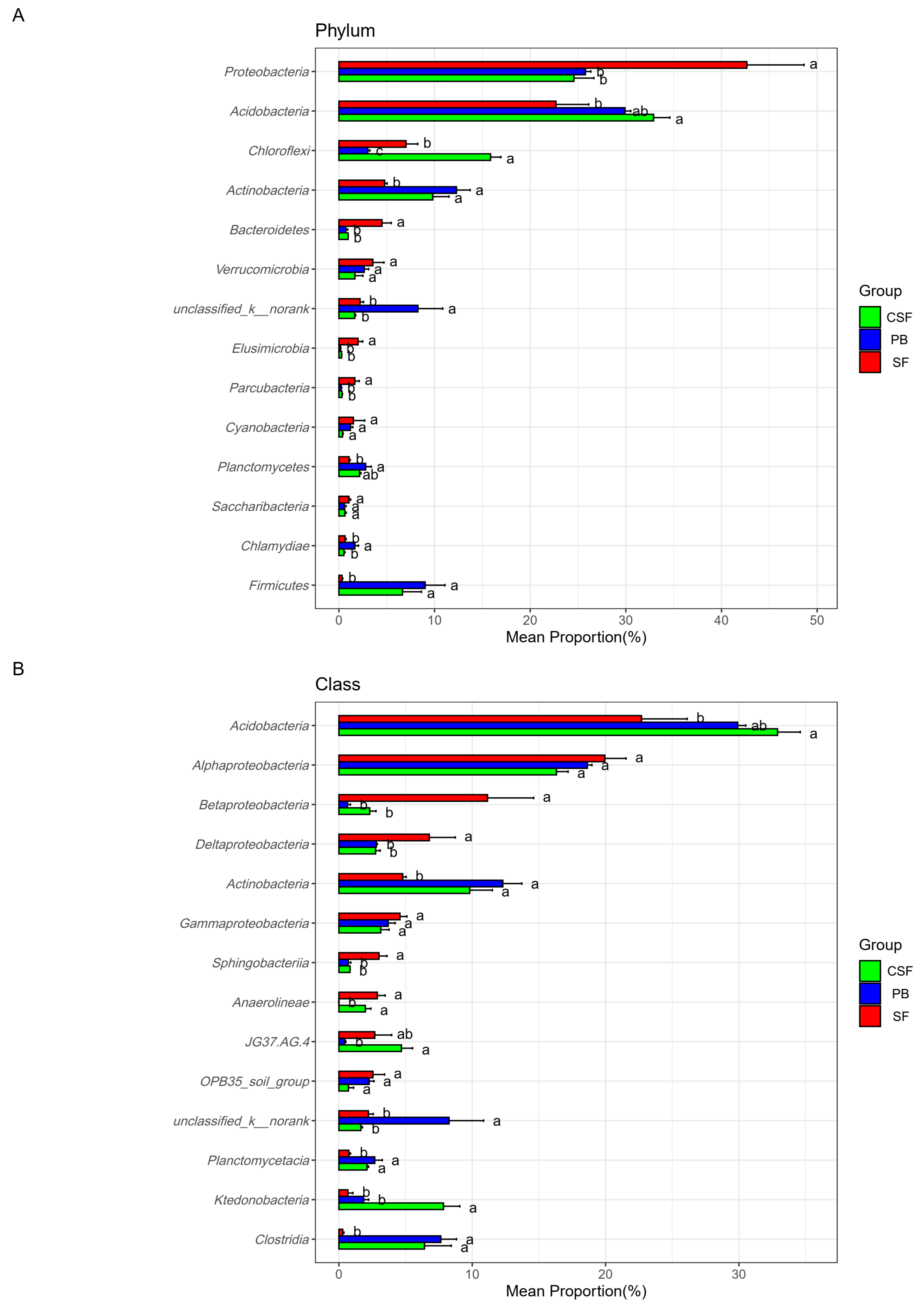
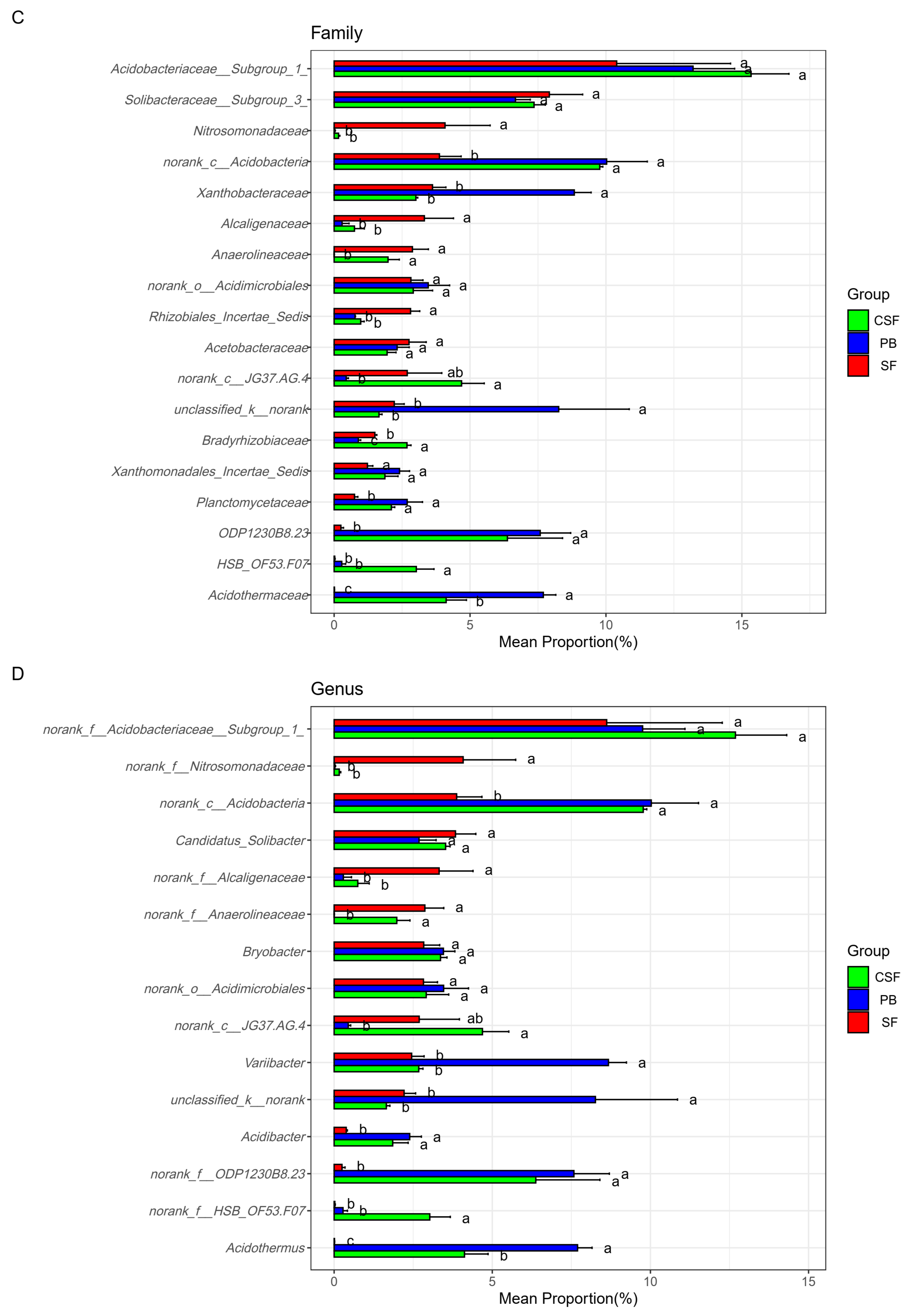
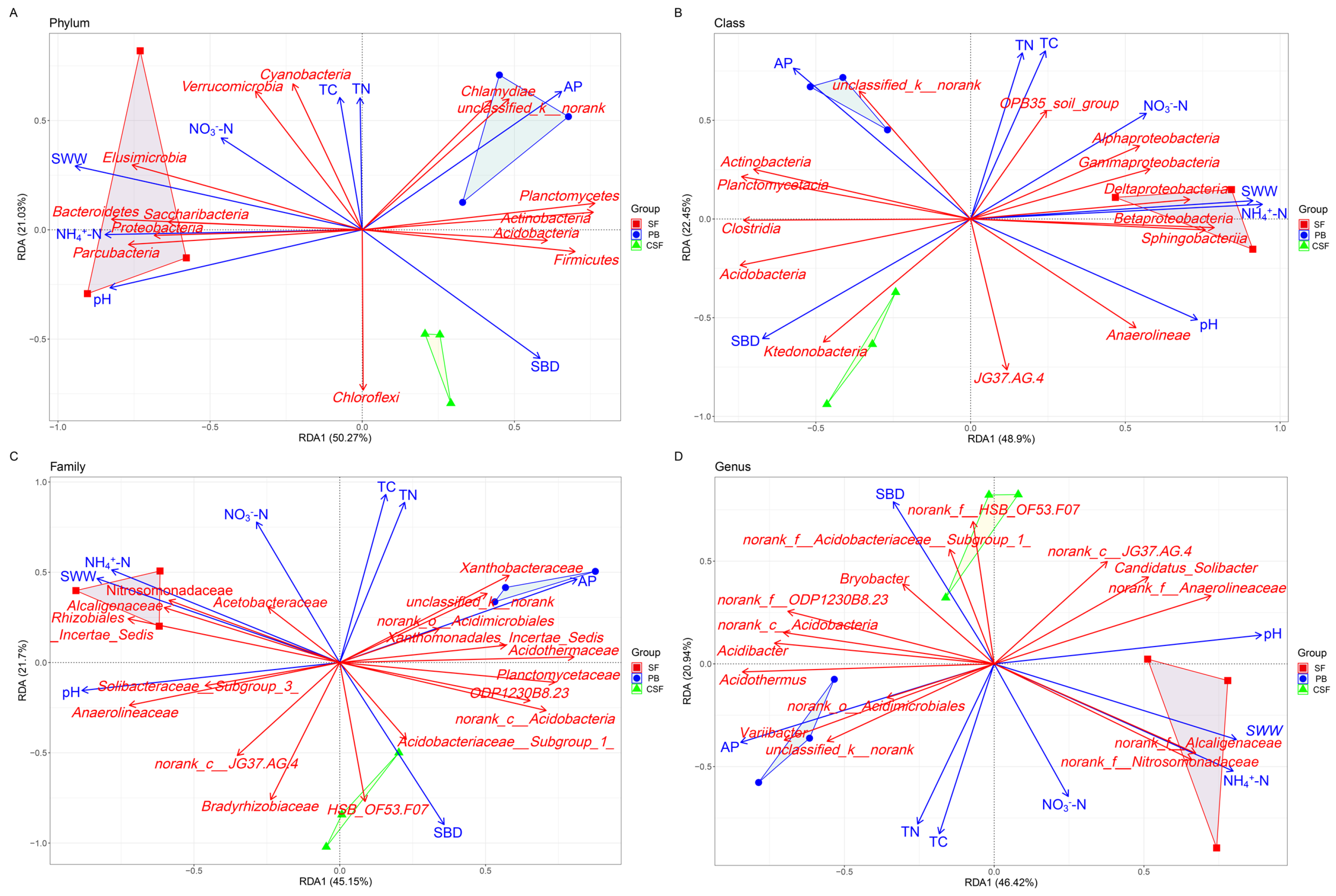
| Sobs | ACE | Chao | Shannon | Simpson | Pd | Shannoneven | Simpsoneven | Coverage | |
|---|---|---|---|---|---|---|---|---|---|
| TC | −0.364 | −0.357 | −0.392 | −0.689 * | 0.731 * | −0.304 | −0.791 * | −0.726 * | 0.365 |
| TN | −0.397 | −0.400 | −0.447 | −0.668 * | 0.710 * | −0.356 | −0.716 * | −0.659 | 0.446 |
| pH | 0.948 ** | 0.958 ** | 0.952 ** | 0.835 ** | −0.423 | 0.931 ** | 0.436 | 0.087 | −0.349 |
| AP | −0.795 * | −0.836 ** | −0.845 ** | −0.786 * | 0.420 | −0.792 * | −0.484 | −0.134 | 0.625 |
| NO3−-N | 0.070 | 0.084 | 0.027 | −0.300 | 0.656 | 0.135 | −0.581 | −0.778 * | 0.252 |
| NH4+-N | 0.534 | 0.592 | 0.559 | 0.189 | 0.292 | 0.612 | −0.254 | −0.637 | −0.329 |
| SWW | 0.806 ** | 0.804 ** | 0.786 * | 0.424 | 0.015 | 0.836 ** | −0.101 | −0.376 | 0.027 |
| SBD | −0.259 | −0.257 | −0.230 | 0.153 | −0.386 | −0.303 | 0.509 | 0.604 | −0.218 |
| Genus | Family | Class | Phylum | |||||
|---|---|---|---|---|---|---|---|---|
| r2 | p | r2 | p | r2 | p | r2 | p | |
| TC | 0.718 | 0.031 | 0.892 | 0.002 | 0.784 | 0.016 | 0.372 | 0.259 |
| TN | 0.673 | 0.039 | 0.837 | 0.003 | 0.736 | 0.013 | 0.364 | 0.263 |
| pH | 0.816 | 0.009 | 0.806 | 0.014 | 0.797 | 0.016 | 0.758 | 0.020 |
| AP | 0.862 | 0.004 | 0.875 | 0.012 | 0.912 | 0.005 | 0.830 | 0.011 |
| NO3−-N | 0.478 | 0.144 | 0.687 | 0.025 | 0.609 | 0.051 | 0.391 | 0.243 |
| NH4+-N | 0.909 | 0.005 | 0.875 | 0.008 | 0.894 | 0.010 | 0.715 | 0.024 |
| SWW | 0.789 | 0.022 | 0.910 | 0.018 | 0.841 | 0.023 | 0.975 | 0.001 |
| SBD | 0.736 | 0.021 | 0.934 | 0.001 | 0.819 | 0.013 | 0.687 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Yang, J.; Cui, H.; Song, W.; Liu, Y.; Shi, X.; Bi, X.; Yuan, S. Drainage and Afforestation More Strongly Affect Soil Microbial Composition in Fens than Bogs of Subtropical Moss Peatlands. Sustainability 2024, 16, 8621. https://doi.org/10.3390/su16198621
Zhang P, Yang J, Cui H, Song W, Liu Y, Shi X, Bi X, Yuan S. Drainage and Afforestation More Strongly Affect Soil Microbial Composition in Fens than Bogs of Subtropical Moss Peatlands. Sustainability. 2024; 16(19):8621. https://doi.org/10.3390/su16198621
Chicago/Turabian StyleZhang, Putao, Junheng Yang, Haijun Cui, Weifeng Song, Yingying Liu, Xunxun Shi, Xiaoting Bi, and Suyao Yuan. 2024. "Drainage and Afforestation More Strongly Affect Soil Microbial Composition in Fens than Bogs of Subtropical Moss Peatlands" Sustainability 16, no. 19: 8621. https://doi.org/10.3390/su16198621
APA StyleZhang, P., Yang, J., Cui, H., Song, W., Liu, Y., Shi, X., Bi, X., & Yuan, S. (2024). Drainage and Afforestation More Strongly Affect Soil Microbial Composition in Fens than Bogs of Subtropical Moss Peatlands. Sustainability, 16(19), 8621. https://doi.org/10.3390/su16198621





