Enhanced Growth and Productivity of Arthrospira platensis H53 in a Nature-like Alkalophilic Environment and Its Implementation in Sustainable Arthrospira Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultivation Systems
2.2. Growth Monitoring and Biomass Production Analysis
2.3. Calculation of Nutrient Use (NU) and Nutrient Use Efficiency (NUE)
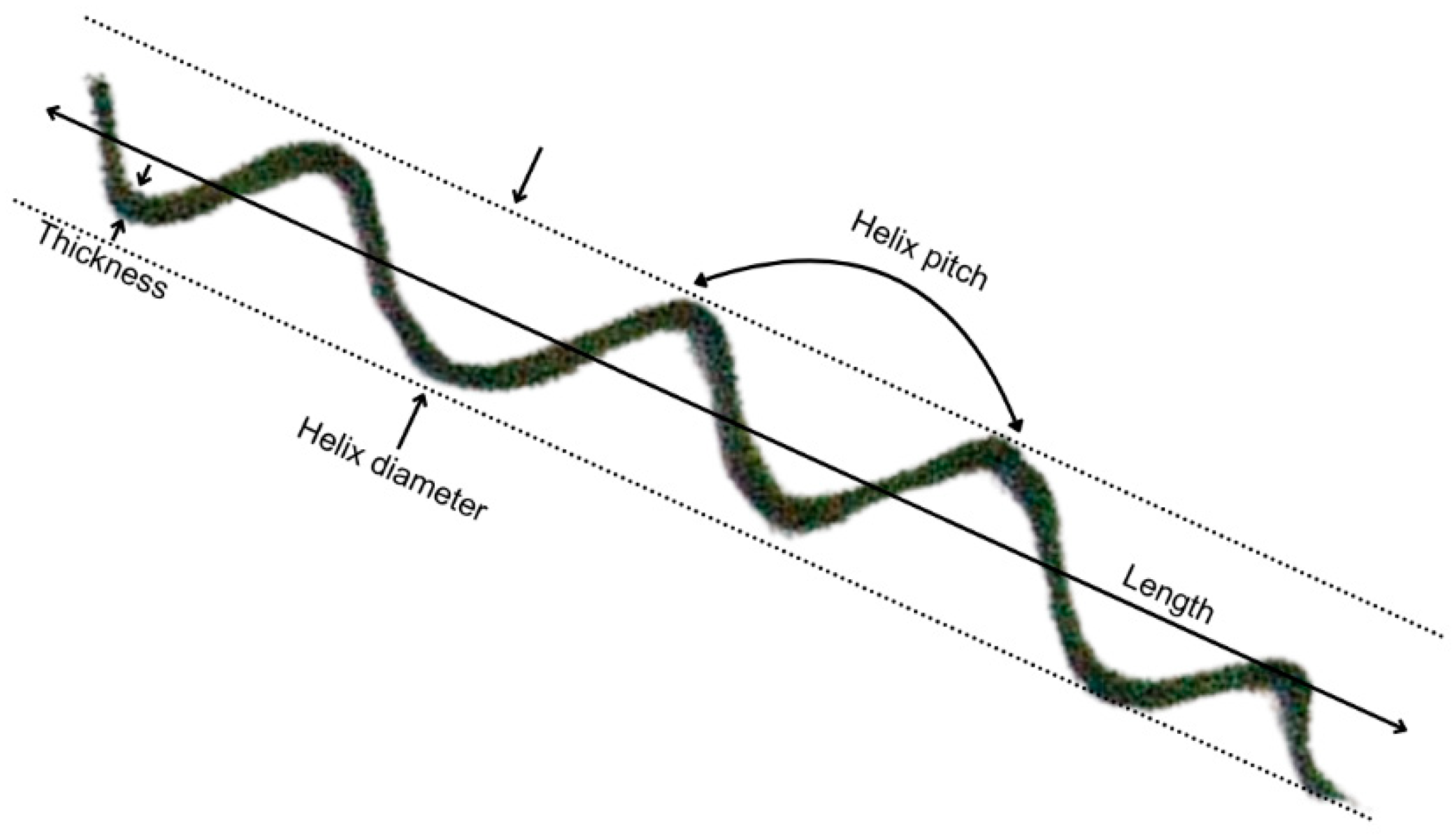
2.4. Microscopic Observations
2.5. DNA Extraction and Amplicon Sequencing
2.6. Microbiota Analysis
2.7. Statistical Analysis
3. Results and Discussion
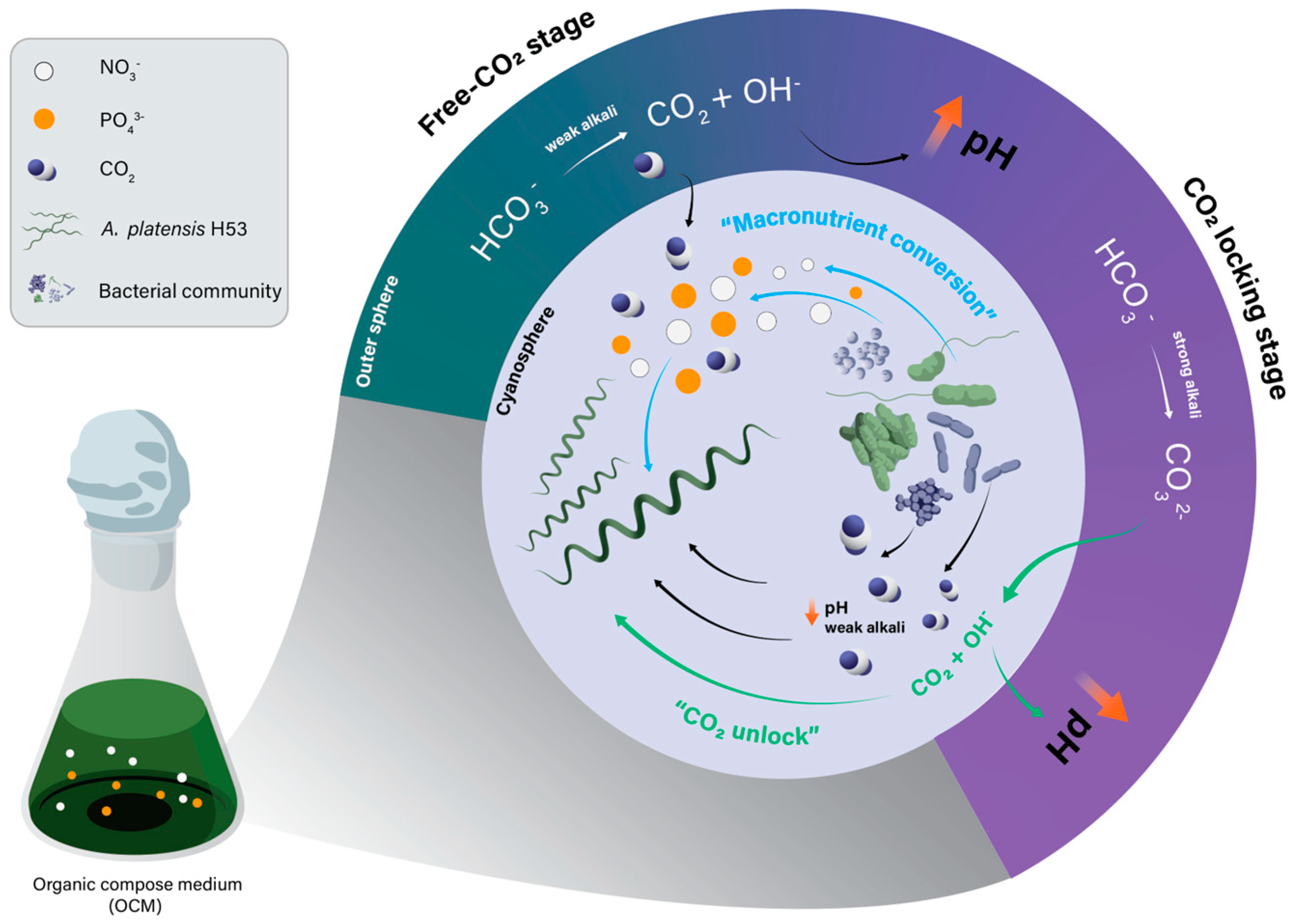
3.1. Growth Profile and pH Determination
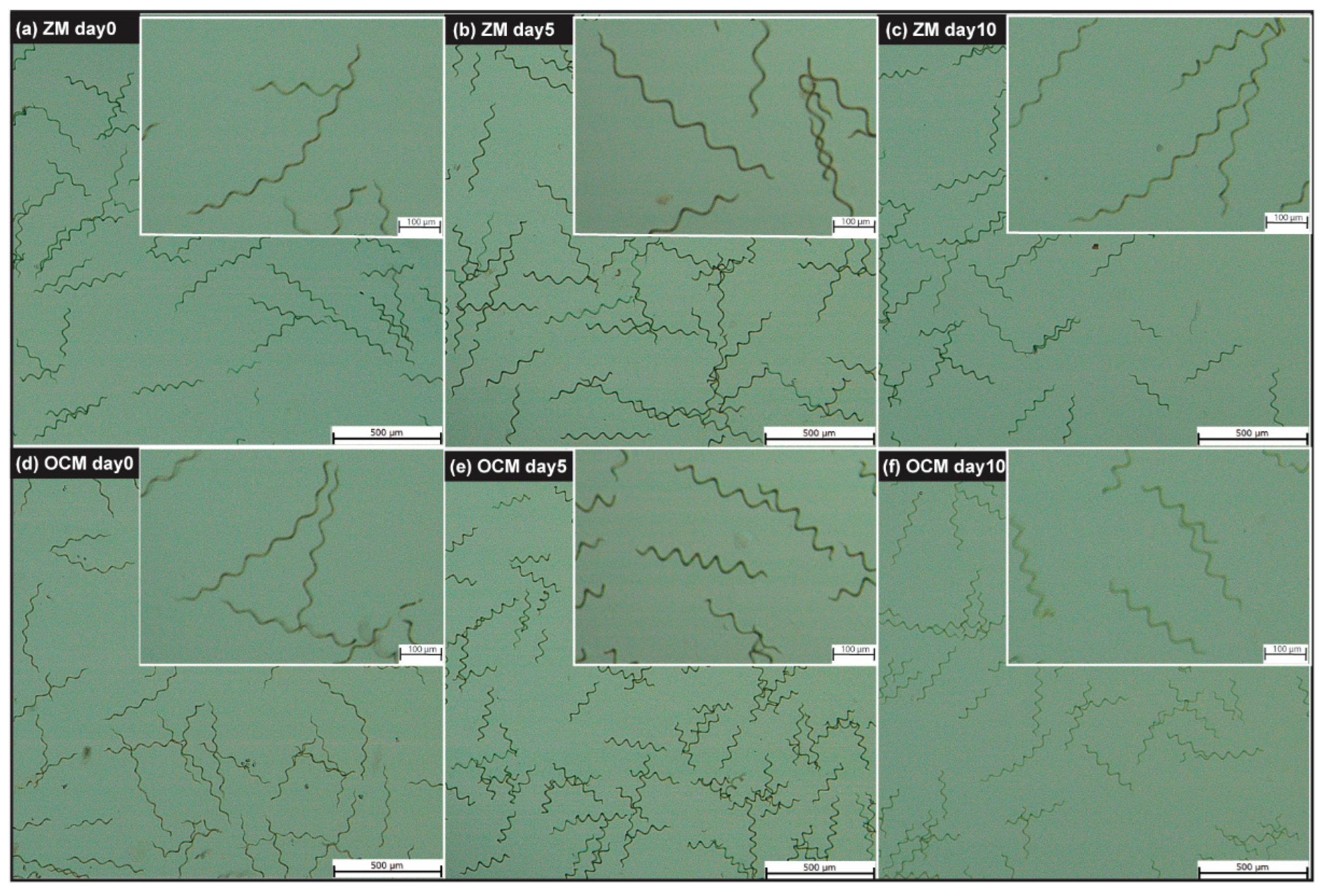
3.2. Cellular Morphological Changes in A. platensis H53
3.3. Dry Biomass Yield, Nutrient Use, and Nutrient Use Efficiency
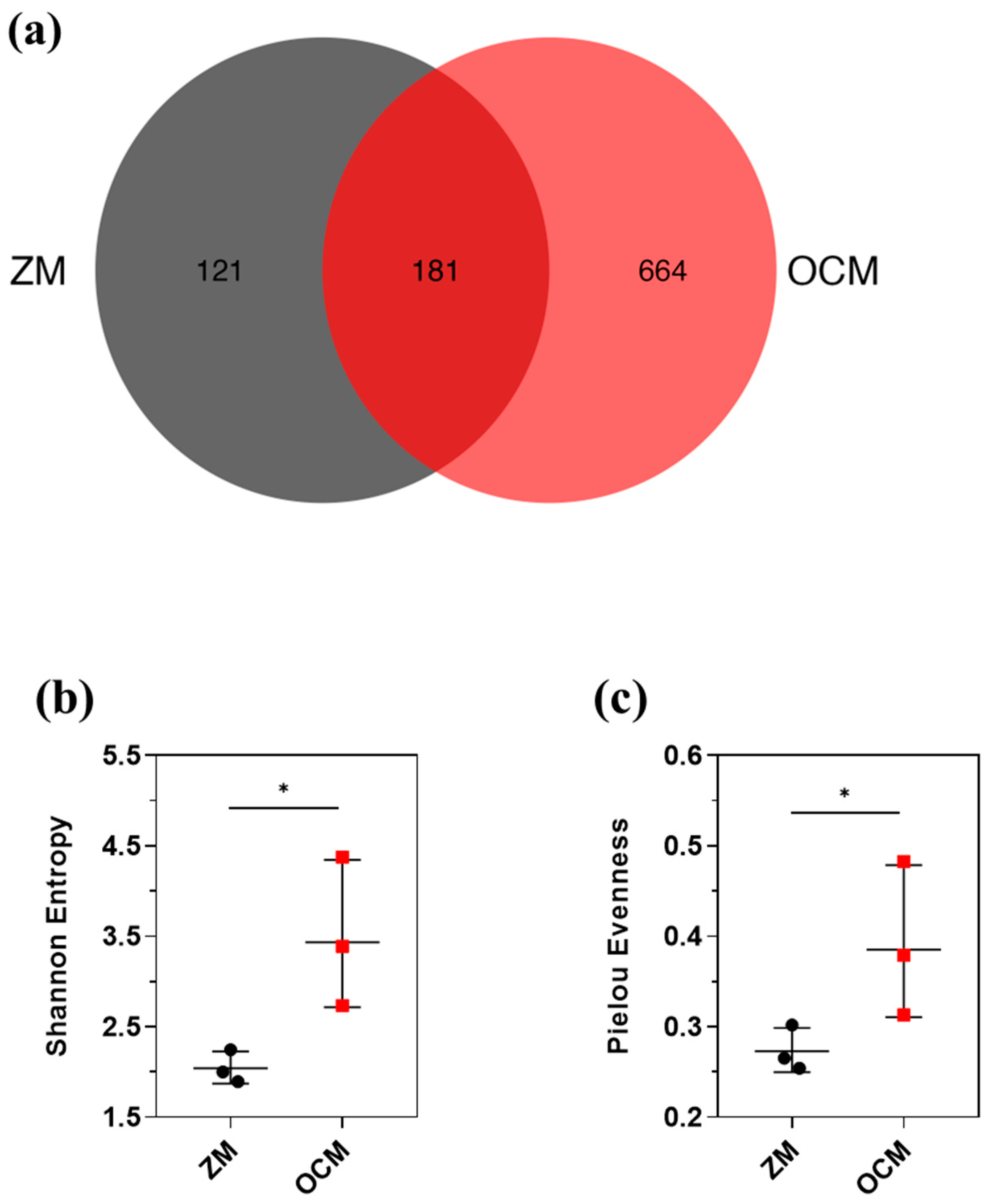
3.4. Microbial Diversity between ZM and OCM Systems
3.5. Composition of the Microbial Community in the ZM and OCM Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, M.M. Study of the edible algae of the Valley of Mexico. Bot. Mar. 1972, 15, 162–166. [Google Scholar]
- Pirie, N. The Spirulina algae. Food Protein Sources 1975, 4, 33–39. [Google Scholar]
- Sarma, A.P.; Petar, P.; Murthy, S. Spirulina as a source of single cell protein. Vegetos 2008, 21, 35–45. [Google Scholar]
- Fuzinato, S.; Fodora, A.; Subakov-Simic, G. Arthrospira fusiformis (Voronichin) Komárek et Lund (Cyanoprokaryota)-a new species for Europe. Algol. Stud. 2010, 134, 17. [Google Scholar] [CrossRef]
- Rahman, K.M. Food and High Value Products from Microalgae: Market Opportunities and Challenges. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 3–27. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etude D’une Cyanobacterie: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina Maxima (Setchell et Gardner) Geitler; University of Paris: Paris, France, 1966. [Google Scholar]
- Belay, A. Spirulina (Arthrospira): Production and quality assurance. In Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–40. [Google Scholar]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of the Micro and Macro Algae for Biofuel Production: A Brief Review. Bioresources 2014, 9, 1606–1633. [Google Scholar] [CrossRef]
- Vonshak, A.; Richmond, A. Mass production of the blue-green alga Spirulina: An overview. Biomass 1988, 15, 233–247. [Google Scholar] [CrossRef]
- Bai, Y.-C.; Chang, Y.-Y.; Hussain, M.; Lu, B.; Zhang, J.-P.; Song, X.-B.; Lei, X.-S.; Pei, D. Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef]
- Rahmah, D.M.; Putra, A.S.; Ishizaki, R.; Noguchi, R.; Ahamed, T. A life cycle assessment of organic and chemical fertilizers for coffee production to evaluate sustainability toward the energy–environment–economic nexus in Indonesia. Sustainability 2022, 14, 3912. [Google Scholar] [CrossRef]
- Hailes, O. From guano to green hydrogen: Food security and fertilizer disputes in international energy law. J. Int. Econ. Law 2023, 26, 663–683. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted Ability of Organic Fertilizers to Improve Crop Productivity and Abiotic Stress Tolerance: Review and Perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Al Mahrouqi, H.; Vega, J.; Dobretsov, S.; Abdala Díaz, R.T. The effect of medium concentration and nitrogen source on the productivity and biochemical composition of Arthrospira platensis. Biol. Bull. 2022, 49, 75–84. [Google Scholar] [CrossRef]
- Bari, E.; Cascino, F.C.; Foglio, L.; Proietti, L.; Perteghella, S.; Torre, M.L.; Parati, K. Alternative culture media and cold-drying for obtaining high biological value Arthrospira platensis (Cyanobacteria). Phycologia 2021, 60, 237–246. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- Kumari, A.; Pathak, A.K.; Guria, C. Cost-effective cultivation of Spirulina platensis using NPK fertilizer. Agric. Res. 2015, 4, 261–271. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.; Flores-Hernández, D.I.; Galindez-Mayer, J. Formulation of an alternative culture medium for Arthrospira maxima (Spirulina) based on “tequesquite,” a traditional mineral resource. J. World Aquac. Soc. 2017, 48, 887–897. [Google Scholar] [CrossRef]
- Cid-Ibarra, G.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Belmares, R.; Carlos Contreras-Esquivel, J.; Machado-Cepeda, S.; Cabello-Galindo, A.; Ruiz, H.A. Microalgae Biomass Production from Rice Husk as Alternative Media Cultivation and Extraction of Phycocyanin Using 3D-Printed Ohmic Heating Reactor. Foods 2024, 13, 1421. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.-K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina culture with wastewater into a sustainable circular bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef]
- Padmakumar, K.; Remya, P.; Stephy, K.; Mohan, H.; Preseetha, T.; Arathi, T.; Alan, B.; Abraham, T. Control and management of cynophycean (Spirulina platensis) bloom in Padmatheertham, Thiruvananthapuram, India. Curr. Sci. 2023, 124, 73. [Google Scholar] [CrossRef]
- Schagerl, M.; Burian, A.; Gruber-Dorninger, M.; Oduor, S.O.; Kaggwa, M.N. Algal communities of Kenyan soda lakes with a special focus on Arthrospira fusiformis. Fottea 2015, 15, 245–257. [Google Scholar] [CrossRef]
- Krienitz, L.; Kotut, K. Fluctuating algal food populations and the occurrence of lesser flamingos (Phoeniconaias minor) in three Kenyan rift valley lakes 1. J. Phycol. 2010, 46, 1088–1096. [Google Scholar] [CrossRef]
- Ballot, A. Phylogenetic Relationship of Arthrospira, Phormidium, and Spirulina Strains from Kenyan and Indian Waterbodies; Freie Universität Berlin: Berlin, Germany, 2004. [Google Scholar]
- Ballot, A.; Kotut, K.; Novelo, E.; Krienitz, L. Changes of phytoplankton communities in Lakes Naivasha and Oloidien, examples of degradation and salinization of lakes in the Kenyan Rift Valley. Hydrobiologia 2009, 632, 359–363. [Google Scholar] [CrossRef]
- Ballot, A.; Krienitz, L.; Kotut, K.; Wiegand, C.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Cyanobacteria and cyanobacterial toxins in three alkaline Rift Valley lakes of Kenya—Lakes Bogoria, Nakuru and Elmenteita. J. Plankton Res. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Vijayarasa, J.; Pakeerathan, K.; Thiruchchelvan, N.; Mikunthan, G. Bio-Remediation of Agro-Based Industries’ Wastewater and Mass Production of Spirulina (Spirulina platensis (Gomont) Geitler 1925). Biol. Life Sci. Forum 2021, 3, 24. [Google Scholar] [CrossRef]
- Chotchindakun, K.; Pathom-Aree, W.; Dumri, K.; Ruangsuriya, J.; Pumas, C.; Pekkoh, J. Low Crystallinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Bioproduction by Hot Spring Cyanobacterium Cyanosarcina sp. AARL T020. Plants 2021, 10, 503. [Google Scholar] [CrossRef]
- Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation 2022, 8, 374. [Google Scholar] [CrossRef]
- Khannapho, C.; Phodee, A.; Paithoonrangsarid, K.; Hongsthong, A.; Meechai, A.; Cheevadhanarak, S.; Tanticharoen, M. Effect of dilution rate in continuous cultures of Arthrospira (Spirulina) platensis C1 on nutrient use efficiency and macromolecular- and elemental compositions. J. Appl. Phycol. 2021, 33, 743–754. [Google Scholar] [CrossRef]
- Shen, M.; Li, J.; Dong, Y.; Zhang, Z.; Zhao, Y.; Li, Q.; Dang, K.; Peng, J.; Liu, H. The effects of microbial inoculants on bacterial communities of the rhizosphere soil of maize. Agriculture 2021, 11, 389. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2020, 97, fiaa255. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Narendran, R.; Sampathkumar, P. Cultivation of Spirulina platensis in different selective media. Indian J. Geo Mar. Sci. 2016, 45, 1749–1754. [Google Scholar]
- Shanthi, G.; Premalatha, M.; Anantharaman, N. Potential utilization of fish waste for the sustainable production of microalgae rich in renewable protein and phycocyanin-Arthrospira platensis/Spirulina. J. Clean. Prod. 2021, 294, 126106. [Google Scholar] [CrossRef]
- Richmond, A.; Grobbelaar, J.U. Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 1986, 10, 253–264. [Google Scholar] [CrossRef]
- Ismaiel, M.M.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef]
- González-González, L.M.; De-Bashan, L.E. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef]
- Schäfer, H.; Abbas, B.; Witte, H.; Muyzer, G. Genetic diversity of ‘satellite’bacteria present in cultures of marine diatoms. FEMS Microbiol. Ecol. 2002, 42, 25–35. [Google Scholar]
- Samo, T.J.; Kimbrel, J.A.; Nilson, D.J.; Pett-Ridge, J.; Weber, P.K.; Mayali, X. Attachment between heterotrophic bacteria and microalgae influences symbiotic microscale interactions. Environ. Microbiol. 2018, 20, 4385–4400. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotechnol. 2015, 33, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, K. Photoregulation of morphological structure and its physiological relevance in the cyanobacterium Arthrospira (Spirulina) platensis. Planta 2009, 230, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Nosratimovafagh, A.; Esmaeili Fereidouni, A.; Krujatz, F. Effect of light spectrum, salinity, and glucose levels on Spirulina morphology. J. World Aquac. Soc. 2023, 54, 1687–1701. [Google Scholar] [CrossRef]
- van Eykelenburg, C. The ultrastructure of Spirulina platensis in relation to temperature and light intensity. Antonie Van Leeuwenhoek 1979, 45, 369–390. [Google Scholar] [CrossRef]
- Wang, X.; Jin, G.; Pan, K.; Zhu, B.; Li, Y. Effects of fluctuating temperature in open raceway ponds on the biomass accumulation and harvest efficiency of Spirulina in large-scale cultivation. Environ. Sci. Pollut. Res. 2021, 28, 20794–20802. [Google Scholar] [CrossRef]
- Kebede, E. Response of Spirulina platensis (= Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J. Appl. Phycol. 1997, 9, 551–558. [Google Scholar]
- Thynn, H.N. Morphological variations of Spirulina under different environmental parameters. Univ. Res. J. 2014, 6, 203. [Google Scholar]
- Ogato, T.; Kifle, D. Morphological variability of Arthrospira (Spirulina) fusiformis (Cyanophyta) in relation to environmental variables in the tropical soda lake Chitu, Ethiopia. Hydrobiologia 2014, 738, 21–33. [Google Scholar] [CrossRef]
- Saygideger, S.D.; Okkay, O. Effect of 2,4-dichlorophenoxyacetic acid on growth, protein and chlorophyll-a content of Chlorella vulgaris and Spirulina platensis cells. J. Environ. Biol. 2008, 29, 175. [Google Scholar]
- Dao, G.-H.; Wu, G.-X.; Wang, X.-X.; Zhuang, L.-L.; Zhang, T.-Y.; Hu, H.-Y. Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour. Technol. 2018, 247, 561–567. [Google Scholar] [CrossRef]
- Lin, B.; Ahmed, F.; Du, H.; Li, Z.; Yan, Y.; Huang, Y.; Cui, M.; Yin, Y.; Li, B.; Wang, M. Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris. J. Appl. Phycol. 2018, 30, 1549–1561. [Google Scholar] [CrossRef]
- Zapata, D.; Arroyave, C.; Cardona, L.; Aristizábal, A.; Poschenrieder, C.; Llugany, M. Phytohormone production and morphology of Spirulina platensis grown in dairy wastewaters. Algal Res. 2021, 59, 102469. [Google Scholar] [CrossRef]
- da Silveira, J.T.; da Rosa, A.P.C.; Costa, J.A.V. Modulating phytohormone supplementation can efficiently increase biomass and lipid production in Spirulina (Arthrospira). BioEnergy Res. 2022, 15, 112–120. [Google Scholar] [CrossRef]
- Ebrahimi-Zarandi, M.; Saberi Riseh, R.; Tarkka, M.T. Actinobacteria as Effective Biocontrol Agents against Plant Pathogens, an Overview on Their Role in Eliciting Plant Defense. Microorganisms 2022, 10, 1739. [Google Scholar] [CrossRef]
- Hong, S.H.; Ham, S.Y.; Kim, J.S.; Kim, I.-S.; Lee, E.Y. Application of sodium polyacrylate and plant growth-promoting bacterium, Micrococcaceae HW-2, on the growth of plants cultivated in the rooftop. Int. Biodeterior. Biodegrad. 2016, 113, 297–303. [Google Scholar] [CrossRef]
- Meena, K.K.; Bitla, U.M.; Sorty, A.M.; Singh, D.P.; Gupta, V.K.; Wakchaure, G.C.; Kumar, S. Mitigation of Salinity Stress in Wheat Seedlings Due to the Application of Phytohormone-Rich Culture Filtrate Extract of Methylotrophic Actinobacterium Nocardioides sp. NIMMe6. Front. Microbiol. 2020, 11, 2091. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Cultivation of Arthrospira (Spirulina) platensis in olive-oil mill wastewater treated with sodium hypochlorite. Bioresour. Technol. 2012, 112, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Economou, C.N.; Markou, G.; Nicodemou, A.; Koutinas, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Cultivation of Arthrospira platensis in brewery wastewater. Water 2022, 14, 1547. [Google Scholar] [CrossRef]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Park, Y.-I.; Labrecque, M.; Lavoie, J.-M. Influence of Elevated CO2 and Municipal Wastewater Feed on the Productivity, Morphology, and Chemical Composition of Arthrospira (Spirulina) platensis. ACS Sustain. Chem. Eng. 2013, 1, 1348–1356. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Zeng, X.; Chen, J.; Lu, Y.; Jing, K. Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour. Technol. 2015, 180, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Yao, X.; Wu, Z.; Jiang, X.; Hu, Y.; Tang, X.; Xu, Q.; Gao, G. The bacterial community composition and its environmental drivers in the rivers around eutrophic Chaohu Lake, China. BMC Microbiol. 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Wang, Y.; Li, P.; Zhang, Y.; Zhang, X. Responses of soil microbial communities to nutrient limitation in the desert-grassland ecological transition zone. Sci. Total Environ. 2018, 642, 45–55. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Saurey, S.; Ball, B.A.; Wall, D.H.; Barrett, J.E.; Muscarella, M.E.; Griffin, N.A.; Virginia, R.A.; Barberán, A.; Adams, B.J. Stoichiometric Shifts in Soil C:N:P Promote Bacterial Taxa Dominance, Maintain Biodiversity, and Deconstruct Community Assemblages. Front. Microbiol. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Botlagunta, N.; Babu, S. Growth enhancement and changes in bacterial microbiome of cucumber plants exhibited by biopriming with some native bacteria. Saudi J. Biol. Sci. 2024, 31, 103997. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Khorshidi, M.; Amirahmadi, F.; Amirahmadi, A. Effectiveness of Phosphate and Zinc Solubilizing Paenarthrobacter nitroguajacolicus P1 as Halotolerant Rhizobacterium with Growth-Promoting Activity on Pistacia vera L. Curr. Microbiol. 2023, 80, 336. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, M.; Zhou, X.; Li, S.; Zhao, Y.; Li, L.; Hu, X. Effect of the mineral-microbial complexes on the quality, soil nutrients, and microbial community of tailing substrates for growing potted Rorippa. Microbiol. Res. 2022, 262, 127084. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, X.-C.; Nie, Y.; Luo, X.; Zhou, E.; Zhou, L.; Pan, Y.; Li, W. Pontibacter diazotrophicus sp. nov., a novel nitrogen-fixing bacterium of the family Cytophagaceae. PLoS ONE 2014, 9, e92294. [Google Scholar] [CrossRef]
- Naqqash, T.; Imran, A.; Hameed, S.; Shahid, M.; Majeed, A.; Iqbal, J.; Hanif, M.K.; Ejaz, S.; Malik, K.A. First report of diazotrophic Brevundimonas spp. as growth enhancer and root colonizer of potato. Sci. Rep. 2020, 10, 12893. [Google Scholar] [CrossRef]
- Corsini, A.; Zaccheo, P.; Muyzer, G.; Andreoni, V.; Cavalca, L. Arsenic transforming abilities of groundwater bacteria and the combined use of Aliihoeflea sp. strain 2WW and goethite in metalloid removal. J. Hazard. Mater. 2014, 269, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.d.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Yadav, A.K.; Singh, A. Biodiesel yield optimisation from a third-generation feedstock (microalgae spirulina) using a hybrid statistical approach. Int. J. Ambient Energy 2023, 44, 1202–1213. [Google Scholar] [CrossRef]
- Gonzalez Bautista, E.; Laroche, C. Arthrospira platensis as a Feasible Feedstock for Bioethanol Production. Appl. Sci. 2021, 11, 6756. [Google Scholar] [CrossRef]



| ZM System | |||
| Medium Composition | Amount (g L−1) | Physiochemical Characteristics | Value |
| NaHCO3 | 16.8 | NO3− (mg L−1) | 1989.07 |
| NaNO3 | 2.5 | PO43− (mg L−1) | 291.00 |
| NaCl | 1.0 | pH | 9.26 |
| K2SO4 | 1.0 | ||
| K2HPO4 | 0.5 | ||
| MgSO4·7H2O | 0.2 | ||
| FeSO4·7H2O | 0.01 | ||
| CaCl2·2H2O | 0.04 | ||
| a C10H14N2Na2O8·2H2O | 0.08 | ||
| b trace element mixture A5 | 1 mL | ||
| c trace element mixture B6 | 1 mL | ||
| OCM System | |||
| Medium Composition | Amount (g L−1) | Physiochemical Characteristics | Value |
| AgentWild™ | 350.0 | NO3− (mg L−1) | 228.15 |
| NaHCO3 | 16.8 | PO43− (mg L−1) | 85.00 |
| pH | 9.24 | ||
| Morphological Features | Day 0 | Day 5 | Day 10 |
|---|---|---|---|
| Length (µm) | |||
| ZM | 618.7 ± 60.7 | 607.8 ± 77.6 | 617.3 ± 74.8 |
| OCM | 616.3 ± 57.3 | 529.6 ± 74.0 | 352.3 ± 28.4 |
| p-Value | ns | *** | *** |
| Thickness (µm) | |||
| ZM | 7.4 ± 1.1 | 8.3 ± 0.7 | 7.6 ± 1.1 |
| OCM | 7.5 ± 1.1 | 10.4 ± 1.4 | 11.6 ± 1.6 |
| p-Value | ns | *** | *** |
| Helix diameter (µm) | |||
| ZM | 29.8 ± 3.2 | 30.2 ± 0.9 | 31.2 ± 3.2 |
| OCM | 30.5 ± 3.1 | 37.4 ± 5.4 | 42.7 ± 5.7 |
| p-Value | ns | *** | *** |
| Helix pitch (µm) | |||
| ZM | 77.2 ±5.7 | 82.2 ± 1.9 | 81.1 ± 4.9 |
| OCM | 78.5 ± 8.9 | 71.4 ± 8.1 | 64.9 ± 7.4 |
| p-Value | ns | *** | *** |
| Systems | Dry Biomass Yield (mg L−1) | Nutrient Use (mg L−1) | Nutrient Use Efficiency | ||
|---|---|---|---|---|---|
| NO3− | PO43− | NO3− | PO43− | ||
| ZM | 436.67 ± 4.99 | 687.09 ± 62.62 | 119.70 ± 3.20 | 0.64 ± 0.06 | 3.65 ± 0.09 |
| OCM | 713.33 ± 61.46 | 191.91 ± 2.18 | 70.60 ± 2.41 | 3.72 ± 0.31 | 10.09 ± 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotchindakun, K.; Buddhasiri, S.; Kuntanawat, P. Enhanced Growth and Productivity of Arthrospira platensis H53 in a Nature-like Alkalophilic Environment and Its Implementation in Sustainable Arthrospira Cultivation. Sustainability 2024, 16, 8627. https://doi.org/10.3390/su16198627
Chotchindakun K, Buddhasiri S, Kuntanawat P. Enhanced Growth and Productivity of Arthrospira platensis H53 in a Nature-like Alkalophilic Environment and Its Implementation in Sustainable Arthrospira Cultivation. Sustainability. 2024; 16(19):8627. https://doi.org/10.3390/su16198627
Chicago/Turabian StyleChotchindakun, Kittipat, Songphon Buddhasiri, and Panwong Kuntanawat. 2024. "Enhanced Growth and Productivity of Arthrospira platensis H53 in a Nature-like Alkalophilic Environment and Its Implementation in Sustainable Arthrospira Cultivation" Sustainability 16, no. 19: 8627. https://doi.org/10.3390/su16198627
APA StyleChotchindakun, K., Buddhasiri, S., & Kuntanawat, P. (2024). Enhanced Growth and Productivity of Arthrospira platensis H53 in a Nature-like Alkalophilic Environment and Its Implementation in Sustainable Arthrospira Cultivation. Sustainability, 16(19), 8627. https://doi.org/10.3390/su16198627







