Patterns of Soil Stoichiometry Driven by Mixed Tree Species Proportions in Boreal Forest
Abstract
:1. Introduction
2. Materials and Methods
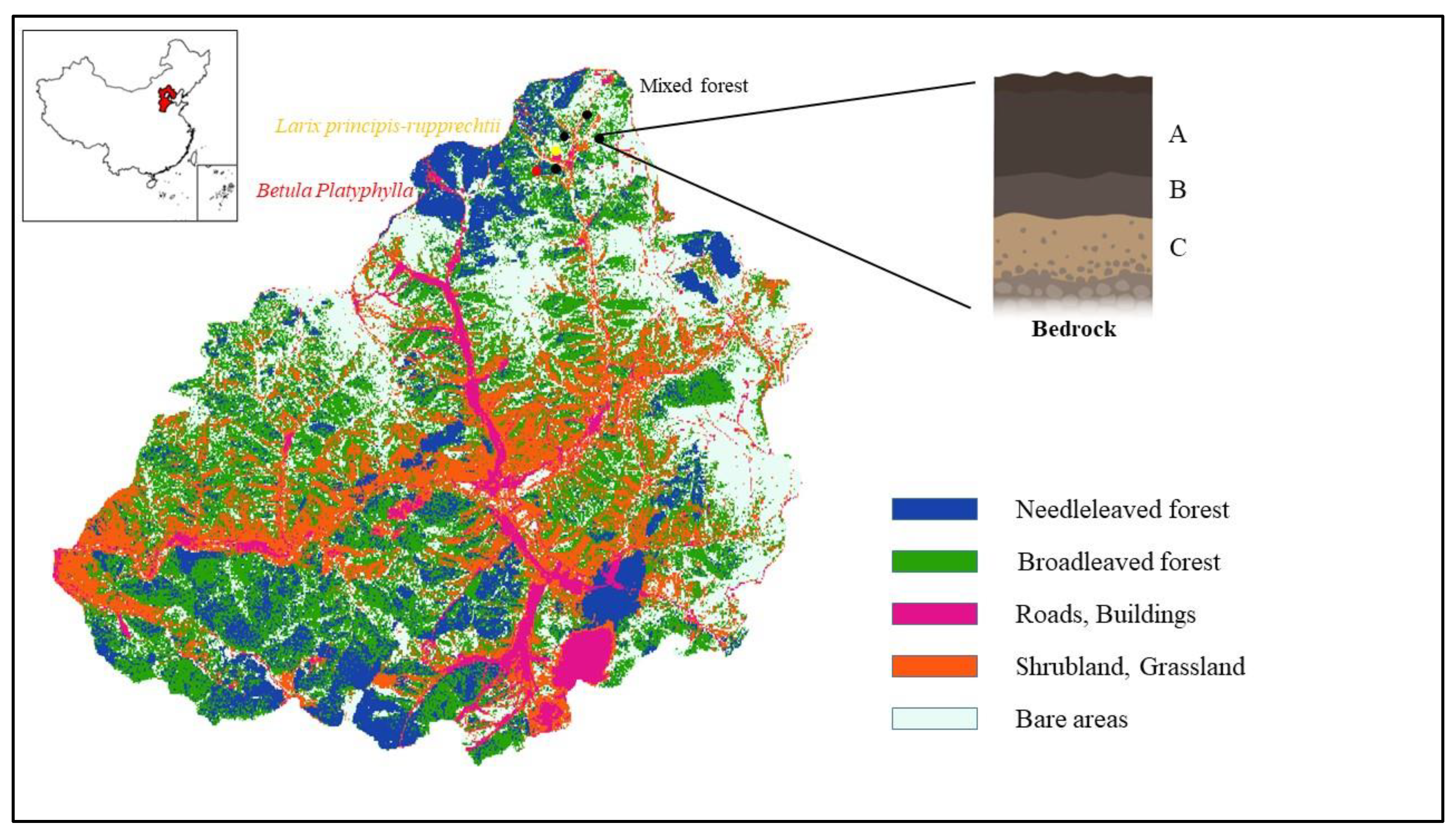
2.1. Study Area
2.2. Experimental Design
2.3. Soil Sample Collection and Chemical Analyses
2.4. Statistical Analysis
3. Results
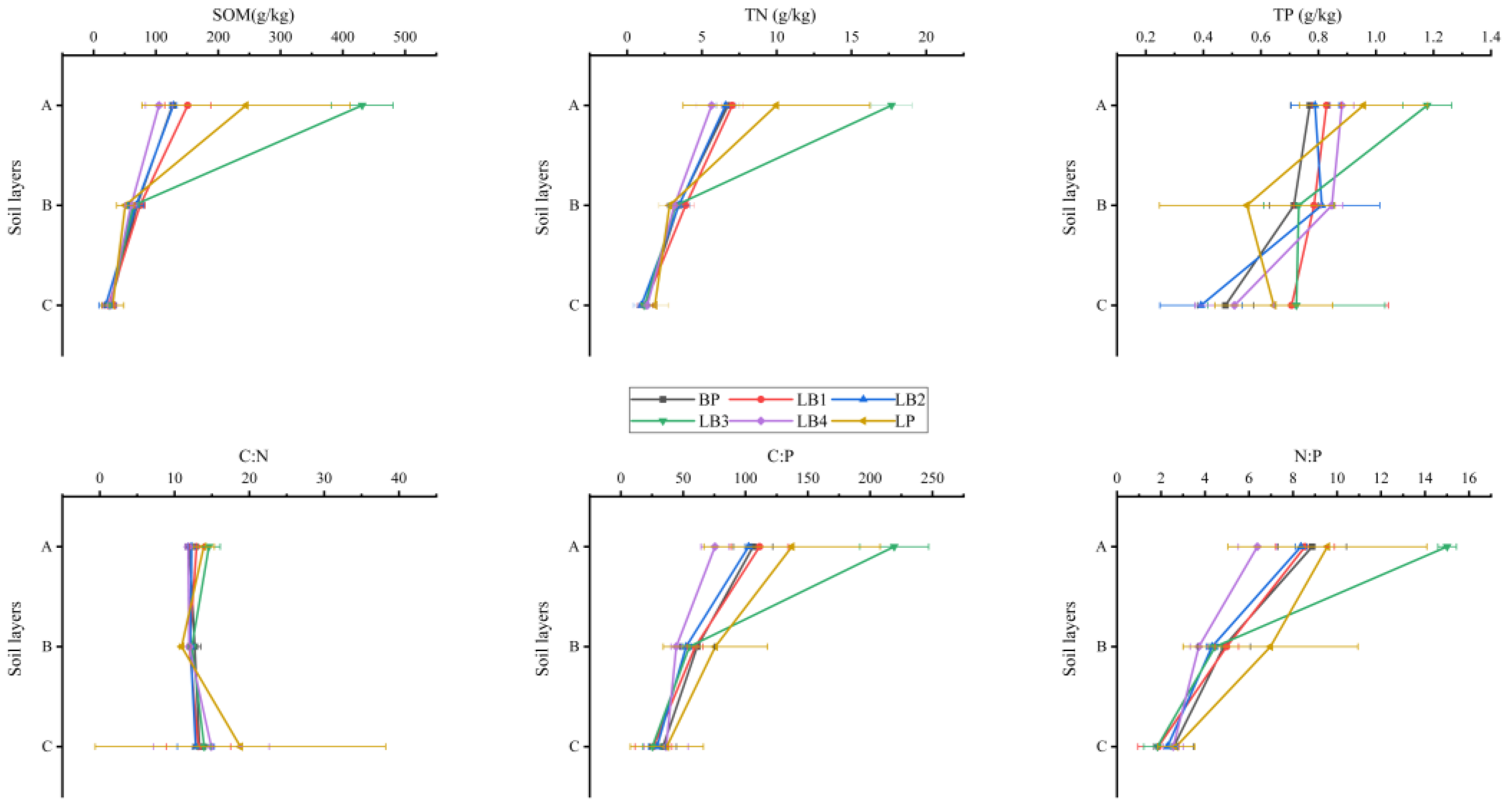
3.1. Variations of Soil C, N, and P Stoichiometry in Soil Profile
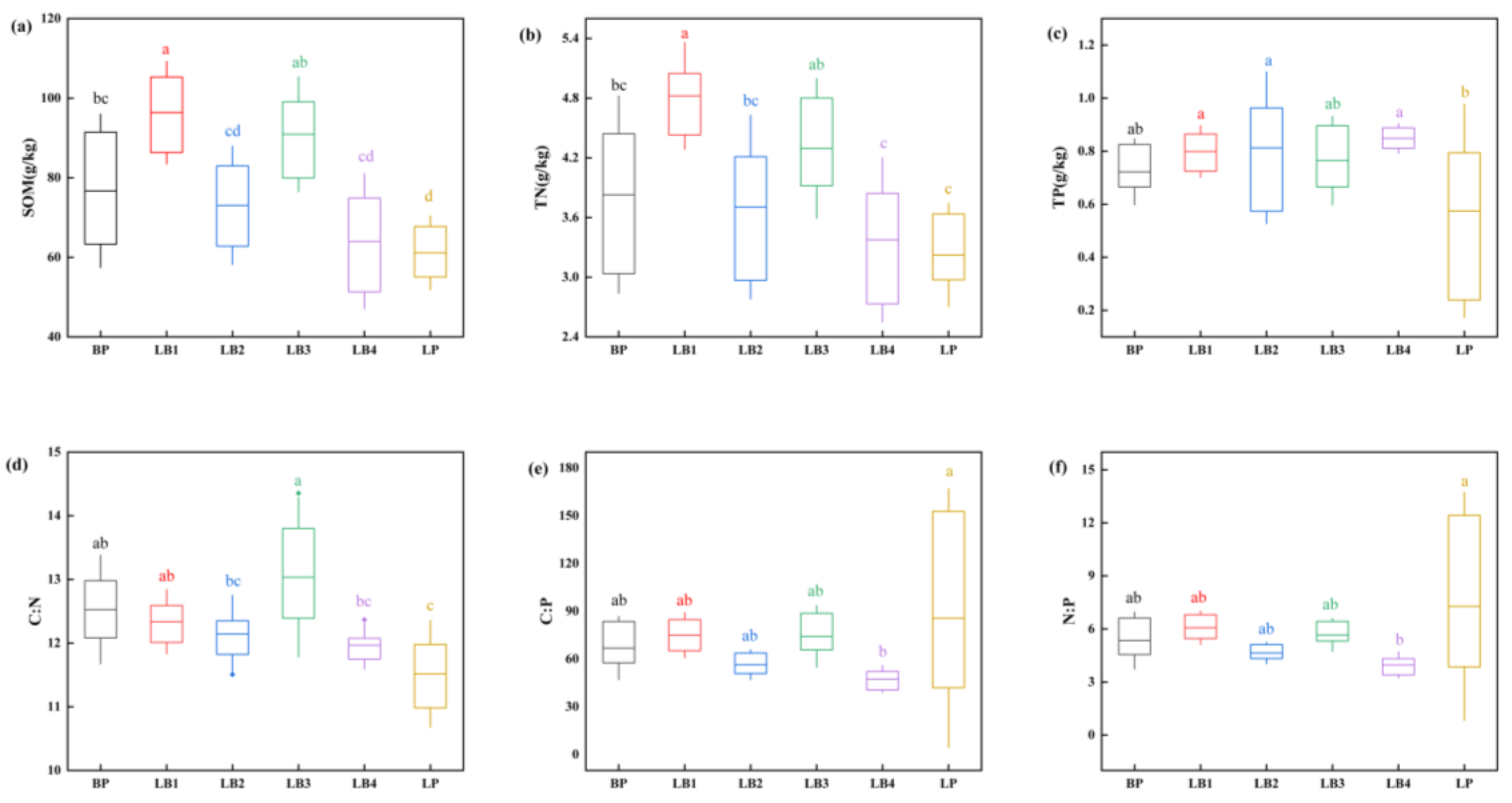
3.2. Patterns of Soil C, N, and P Stoichiometry in Different Mixed Species Proportions
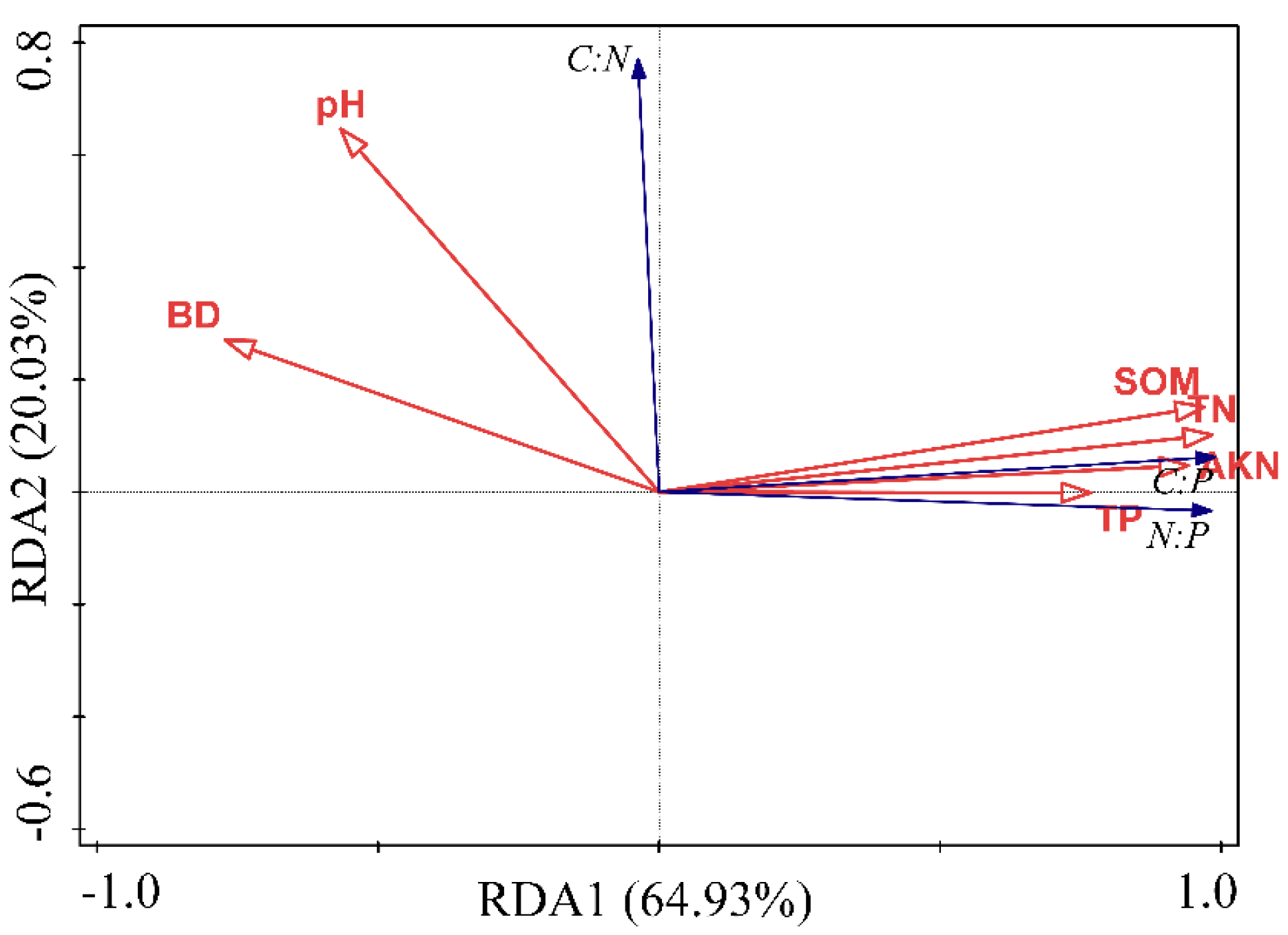
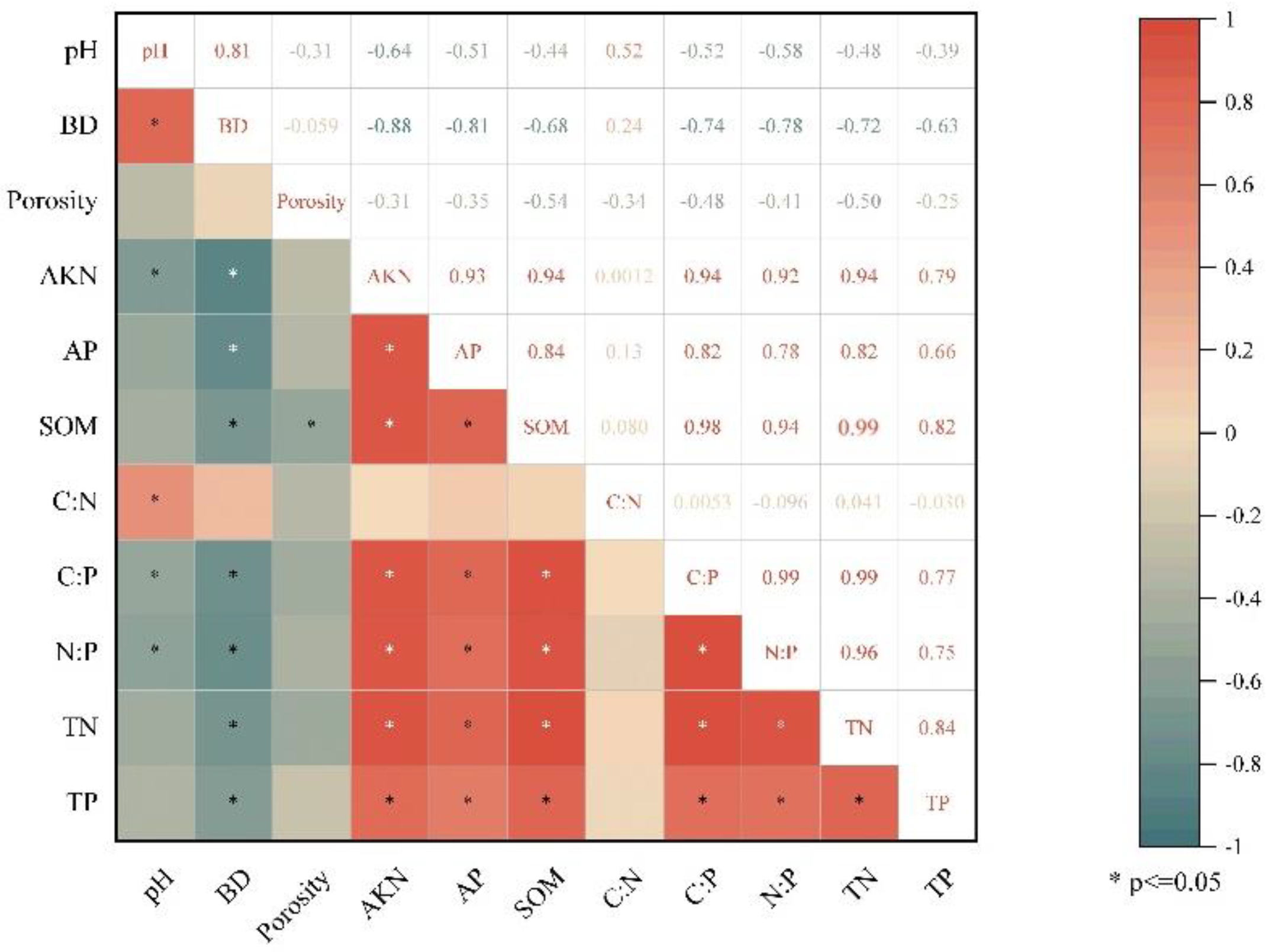
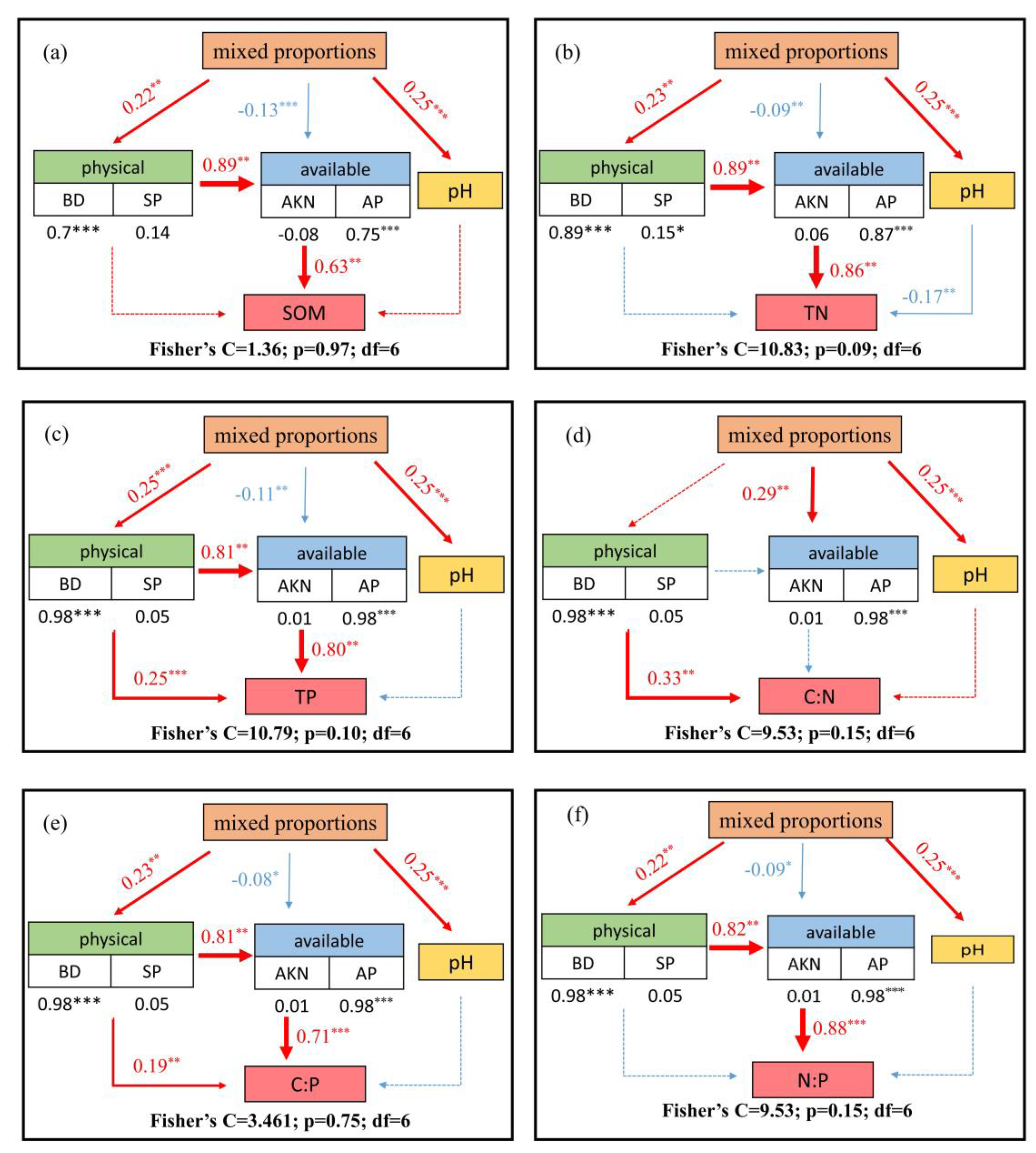
3.3. Relationships between Soil C, N, P Stoichiometry and Related Influential Factors
4. Discussion
4.1. Soil C, N, and P Stoichiometry in Response to Tree Species Proportions
4.2. Driving Factors of Soil C, N, and P Contents and Their Ratios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Hui, D.; Elser, J.; Wang, Y.; Loladze, I.; Zhang, Q.; Dennis, S. Downregulation of tissue N:P ratios in terrestrial plants by elevated CO2. Ecology 2015, 96, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hui, D.; King, A.; Song, X.; Thornton, P.; Zhang, L. Convergence of microbial assimilations of soil carbon, nitrogen, phosphorus, and sulfur in terrestrial ecosystems. Sci. Rep. 2015, 5, 17445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, C.; Ma, Y.; Wang, W.; Huang, C.; Qi, C.; Fu, S.; Chen, H.Y.H. Transition from N to P limited soil nutrients over time since restoration in degraded subtropical broadleaved mixed forests. For. Ecol. Manag. 2021, 494, 119298. [Google Scholar] [CrossRef]
- Cleveland, C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Bui, E.; Henderson, B. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Rivas-ubach, A.; Janssens, I.A. The human-induced imbalance between C, N and P in Earth’s life system. Glob. Change Biol. 2011, 18, 3–6. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; Hu, Y.; Zhang, M.; Wang, G.; Shen, W. Soil Microbial Community and Its Interaction with Soil Carbon Dynamics Following a Wetland Drying Process in Mu Us Sandy Land. Int. J. Environ. Res. Public Health 2020, 17, 4199. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.; Wang, G.; Yu, X. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Gao, Y.; He, N.; Yu, G.; Chen, W.; Wang, Q. Long-term effects of different land use types on C, N, and P stoichiometry and storage in subtropical ecosystems: A case study in China. Ecol. Eng. 2014, 67, 171–181. [Google Scholar] [CrossRef]
- Hu, L.; Ade, L.; Wu, X.; Zi, H.; Luo, X.; Wang, C. Changes in Soil C:N:P Stoichiometry and Microbial Structure along Soil Depth in Two Forest Soils. Forests 2019, 10, 113. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.; Marañón, T. Tree species effects on nutrient cycling and soil biota: A feedback mechanism favoring species coexistence. For. Ecol. Manag. 2013, 2013, 36–46. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, C.; Melillo, J.; Hall, C. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Hui, D.; Yang, X.; Deng, Q.; Liu, Q.; Wang, X.; Yang, H.; Ren, H. Soil C:N:P stoichiometry in tropical forests on Hainan Island of China: Spatial and vertical variations. Catena 2021, 201, 105228. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Williams, L.J.; Paquette, A.; Cavender-Bares, J.; Messier, C.; Reich, P.B. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 2017, 1, 62. [Google Scholar] [CrossRef]
- Grossman, J.J.; Vanhellemont, M.; Barsoum, N.; Bauhus, J.; Bruelheide, H.; Castagneyrol, B.; Cavender-Bares, J.; Eisenhauer, N.; Ferlian, O.; Gravel, D.; et al. Synthesis and future research directions linking tree diversity to growth, survival, and damage in a global network of tree diversity experiments. Environ. Exp. Bot. 2018, 152, 68–89. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The effect of biodiversity on tree productivity: From temperate to boreal forests. Glob. Ecol. Biogeogr. 2010, 20, 170–180. [Google Scholar] [CrossRef]
- Fichtner, A.; Härdtle, W.; Bruelheide, H.; Kunz, M.; Li, Y.; von Oheimb, G. Neighbourhood interactions drive overyielding in mixed-species tree communities. Nat. Commun. 2018, 9, 1144. [Google Scholar] [CrossRef] [PubMed]
- Brassard, B.W.; Chen, H.Y.H.; Cavard, X.; Laganière, J.; Reich, P.B.; Bergeron, Y.; Paré, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2012, 101, 210–219. [Google Scholar] [CrossRef]
- Huuskonen, S.; Domisch, T.; Finér, L.; Hantula, J.; Hynynen, J.; Matala, J.; Miina, J.; Neuvonen, S.; Nevalainen, S.; Niemistö, P.; et al. What is the potential for replacing monocultures with mixed-species stands to enhance ecosystem services in boreal forests in Fennoscandia? For. Ecol. Manag. 2021, 479, 118558. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Sardans, J.; Min, Q.; Zeng, C.; Tong, C.; Peñuelas, J. Agricultural land use decouples soil nutrient cycles in a subtropical riparian wetland in China. Catena 2015, 133, 171–178. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.M.; Zhou, X.B. Ecological stoichiometry of surface soil nutrient and its influencing factors in the wild fruit forest in Yili region, Xinjiang, China. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2016, 27, 2239–2248. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Yan, G.; Wang, M.; Jiang, S.; Wang, X.; Xue, J.; Xu, M.; Xing, Y.; Wang, Q. Soil enzyme activities and microbial nutrient limitation during the secondary succession of boreal forests. Catena 2023, 230, 107268. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Mao, Z.J.; Gu, H.Y.; Zhao, Y.X.; Zu, Y.G.; Zou, L. Reproductive strategies and seeds behavior of Betula platyphylla Suk. population. J. For. Res. 2000, 11, 241–244. [Google Scholar] [CrossRef]
- Bronisz, K.; Mehtätalo, L. Mixed-effects generalized height–diameter model for young silver birch stands on post-agricultural lands. For. Ecol. Manag. 2020, 460, 117901. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Frank, K.; Beegle, D.; Denning, J. Phosphorus. In Recommended Chemical Soil Test Procedures; Brown, J.R., Ed.; North Central Regional Research Publication: Ames, IA, USA, 1998; Volume 221, pp. 21–30. [Google Scholar]
- Lu, J.; Feng, S.; Wang, S.; Zhang, B.; Ning, Z.; Wang, R.; Chen, X.; Yu, L.; Zhao, H.; Lan, D.; et al. Patterns and driving mechanism of soil organic carbon, nitrogen, and phosphorus stoichiometry across northern China’s desert-grassland transition zone. Catena 2023, 220, 106695. [Google Scholar] [CrossRef]
- Sheng, H.; Yin, Z.; Zhou, P.; Thompson, M.L. Soil C:N:P ratio in subtropical paddy fields: Variation and correlation with environmental controls. J. Soils Sediments 2022, 22, 21–31. [Google Scholar] [CrossRef]
- Lefcheck, J.S. PiecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2015, 7, 573–579. [Google Scholar] [CrossRef]
- Tian, P.; Liu, S.; Zhao, X.; Sun, Z.; Yao, X.; Niu, S.; Crowther, T.W.; Wang, Q. Past climate conditions predict the influence of nitrogen enrichment on the temperature sensitivity of soil respiration. Commun. Earth Environ. 2021, 2, 251. [Google Scholar] [CrossRef]
- Stone, M.M.; Plante, A.F. Changes in phosphatase kinetics with soil depth across a variable tropical landscape. Soil Biol. Biochem. 2014, 71, 61–67. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Huang, Z.; Lin, T.C.; Vadeboncoeur, M.A.; Searle, E.B.; Chen, H.Y.H. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China. Glob. Change Biol. 2017, 24, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, Y.; Yang, R. Variation of soil organic carbon, nitrogen, and phosphorus stoichiometry and biogeographic factors across the desert ecosystem of Hexi Corridor, northwestern China. J. Soils Sediments 2019, 19, 49–57. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Zhu, Q.; Fu, S.; Chen, H.Y.H. Rapid increases in fine root biomass and production following cessation of anthropogenic disturbances in degraded forests. Land Degrad. Dev. 2018, 29, 461–470. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Ma, Y.; Fu, S.; Chen, H.Y.H. Increased litterfall contributes to carbon and nitrogen accumulation following cessation of anthropogenic disturbances in degraded forests. For. Ecol. Manag. 2019, 432, 832–839. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.; Ma, W.; Ding, J.; Zhang, B.; Fang, K.; Hu, H.; Yu, J.; Wang, T.; Qin, S.; Zhao, X.; et al. Dryland soils in northern China sequester carbon during the early 2000s warming hiatus period. Funct. Ecol. 2018, 32, 1620–1630. [Google Scholar] [CrossRef]
- Chun, H.; Liu, Y.; Zhang, C.; Li, Y.; Zhou, T.; Khan, S.; Chen, N.; Zhao, C. Effects of three coniferous plantation species on plant-soil feedbacks and soil physical and chemical properties in semi-arid mountain ecosystems. For. Ecosyst. 2021, 8, 3. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Zhu, P.; Xing, S.; Wang, C. Soil C, N and P contents and their stoichiometry as affected by typical plant communities on steep gully slopes of the Loess Plateau, China. Catena 2022, 208, 105740. [Google Scholar] [CrossRef]
- Luo, W.; Dijkstra, F.; Bai, E.; Feng, J.; Lü, X.-T.; Wang, C.; Wu, H.; Li, M.-H.; Han, X.-G. A threshold reveals decoupled relationship of sulfur with carbon and nitrogen in soils across arid and semi-arid grasslands in northern China. Biogeochemistry 2016, 127, 141–153. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, X.-B.; Zhang, S.-H.; Lu, H.-Y.; Shao, H. Soil nutrient stoichiometry on linear sand dunes from a temperate desert in Central Asia. Catena 2020, 195, 104847. [Google Scholar] [CrossRef]
- Gartner, T.; Cardon, Z. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Song, F.; Fan, X.; Ruiqing, S. Review of mixed forest litter decomposition researches. Acta Ecol. Sin. 2010, 30, 221–225. [Google Scholar] [CrossRef]
- Garnier, E.; Laurent, G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol. 1994, 128, 725–736. [Google Scholar] [CrossRef]
- Haonan, S.; Weipo, L.; Zhihua, L.; Yigui, W.; Xiaozhou, Y. Effects of fir and broad-leaved mixed forest with different mixing proportions on the species diversity and soil nutrients under the forest. J. Cent. South Univ. For. Technol. 2022, 42, 8. [Google Scholar] [CrossRef]
- Wu, X.; Fang, H.; Zhao, Y.; Smoak, J.M.; Li, W.; Shi, W.; Sheng, Y.; Zhao, L.; Ding, Y. A conceptual model of the controlling factors of soil organic carbon and nitrogen densities in a permafrost-affected region on the eastern Qinghai-Tibetan Plateau. J. Geophys. Res. Biogeosciences 2017, 122, 1705–1717. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Fang, H.; Zhao, Y.; Smoak, J.M.; Pang, Q.; Ding, Y. Environmental controls on soil organic carbon and nitrogen stocks in the high-altitude arid western Qinghai-Tibetan Plateau permafrost region. J. Geophys. Res. Biogeosciences 2016, 121, 176–187. [Google Scholar] [CrossRef]
- Karak, T.; Paul, R.; Boruah, R.; Sonar, I.; Bordoloi, B.; Dutta, A.; Borkotoky, B. Major Soil Chemical Properties of the Major Tea-Growing Areas in India. Pedosphere 2015, 25, 316–328. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; He, P.; Yin, J.; Yang, J.; Liu, H.; Cai, J.; Shi, Z.; Feng, X.; Dijkstra, F.A.; et al. Intensity and frequency of nitrogen addition alter soil chemical properties depending on mowing management in a temperate steppe. J. Environ. Manag. 2018, 224, 77–86. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Zeng, C.; Zhong, C.; Li, Y.; Peñuelas, J. Responses of soil nutrient concentrations and stoichiometry to different human land uses in a subtropical tidal wetland. Geoderma 2014, 232–234, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, C.; He, B.; Liang, C.; Zhang, Y.; Zhang, Y. Soil nutrient concentrations and stoichiometry under different tree-cropping systems in a purple hillslope in southwestern China. Arch. Agron. Soil Sci. 2018, 65, 741–754. [Google Scholar] [CrossRef]
- Chapman, S.K.; Langley, J.A.; Hart, S.C.; Koch, G.W. Plants actively control nitrogen cycling: Uncorking the microbial bottleneck. New Phytol. 2005, 169, 27–34. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Kerfahi, D.; Guo, Y.; Dong, K.; Wang, Q.; Adams, J.M. pH is the major predictor of soil microbial network complexity in Chinese forests along a latitudinal gradient. Catena 2024, 234, 107595. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Augusto, L.; Achat, D.L.; Jonard, M.; Vidal, D.; Ringeval, B. Soil parent material—A major driver of plant nutrient limitations in terrestrial ecosystems. Glob. Change Biol. 2017, 23, 3808–3824. [Google Scholar] [CrossRef]
- Porder, S.; Ramachandran, S. The Phosphorus Concentration of Common Rocks—A Potential Driver of Ecosystem Status. Plant Soil 2012, 367, 41–55. [Google Scholar] [CrossRef]
- Cools, N.; Vesterdal, L.; De Vos, B.; Vanguelova, E.; Hansen, K. Tree species is the major factor explaining C:N ratios in European forest soils. For. Ecol. Manag. 2014, 311, 3–16. [Google Scholar] [CrossRef]
- Dan, Z.; Talkner, U.; Spohn, M.; Joergensen, R. Microbial biomass phosphorus and C/N/P stoichiometry in forest floor and a horizons as affected by tree species. Soil Biol. Biochem. 2017, 111, 166–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhang, H.; Wang, Z.; Tian, W.; Liu, Z. Patterns of Soil Stoichiometry Driven by Mixed Tree Species Proportions in Boreal Forest. Sustainability 2024, 16, 8646. https://doi.org/10.3390/su16198646
Wu X, Zhang H, Wang Z, Tian W, Liu Z. Patterns of Soil Stoichiometry Driven by Mixed Tree Species Proportions in Boreal Forest. Sustainability. 2024; 16(19):8646. https://doi.org/10.3390/su16198646
Chicago/Turabian StyleWu, Xiaochang, Huayong Zhang, Zhongyu Wang, Wang Tian, and Zhao Liu. 2024. "Patterns of Soil Stoichiometry Driven by Mixed Tree Species Proportions in Boreal Forest" Sustainability 16, no. 19: 8646. https://doi.org/10.3390/su16198646






