Comprehensive Review of Microbial Inoculants: Agricultural Applications, Technology Trends in Patents, and Regulatory Frameworks
Abstract
:1. Introduction
2. Synthetic Fertilizer and Biofertilizer Overview
| Species Used as Biofertilizer | Biological Division | Culture in Which It Was Applied | Benefits | Reference |
|---|---|---|---|---|
| Chlorella sp. | Chlorophytes (green algae) | Tomato and cucumber | Increased seed growth and germination | [54] |
| Spirulina platensis | Cyanobacteria | Yellow-lupin | Improved pigment production and photosynthetic capacity | [8] |
| Palmaria palmata |
Rhodophyta (red algae) | Pea | Improved concentrations of NH4+ and NO3− in the soil | [55] |
| Laminaria digitata | Pheophyceous (brown algae) | Pea | Improved concentrations of NH4+ and NO3− in the soil | [55] |
| Ascophyllum nodosum | Pheophyceous (brown algae) | Grapevine | Stimulatory effect on fertility, shoot length, shoot diameter, and leaf area | [56] |
| Rhizoglomus irregulare, Funneliformis mosseae, and F. caledonium | Arbuscular mycorrhizal fungi | American ginseng | Alleviated the negative effects of continuous cultivation; promoted the content of nutrients available in the rhizosphere; and decreased the abundance of pathogenic fungi | [9] |
| Macrocystis pyrifera with Azospirillum brasilense | Brown macroalga (kelp type) and plant growth-promoting bacteria, respectively | Lettuce | Increased root and plant growth | [57] |
| Ulva lactuca | Green macroalgae | Canola | Alleviated the harmful effects of salinity under a salinity stress condition and increased antioxidative compounds | [58] |
| Cystoseira sp. | Brown macroalgae | Canola | Alleviated the harmful effects of salinity under a salinity stress condition and increased antioxidative compounds | [58] |
| Gelidium crinale | Red macroalgae | Canola | Alleviated the harmful effects of salinity under a salinity stress condition and increased antioxidative compounds | [58] |
| Bradyrhizobium japonicum | Bacteria | Bambara groundnut | Positively influenced the growth characteristics, biomass yield, and yield traits | [59] |
| Bacillus amyloliquefaciens | Bacteria | Pakchoi | Reduced ammonia volatilization, increased crop yield and nitrogen recovery, inhibited urease activity, and enhanced the potential of ammonia oxidation | [60] |
Types of Inoculant Formulations
3. Beneficial Microbes and Their Multifaceted Agricultural Applications
3.1. Plant Growth Promoters
3.1.1. Mechanisms of Action
3.1.2. Application in Agriculture and Its Benefits
3.2. Nitrogen Fixers
3.3. Phosphorus Solubilizers
3.4. Potassium and Zinc Solubilizers
4. Advances in Inoculants for Agricultural Production
5. Patents: Innovations and Technological Perspectives
5.1. Trends in Patents Applied over Time
5.2. Current Prospects for Innovation
5.3. Current and Future Market Possibilities
6. Regulation and Quality Control of Inoculants in the World
7. Gaps for Applications and Performance in the Field
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Inorganic Fertilizers—FAOSTAT Analytical Background 2000–2021; FAO: Rome, Italy, 2023. [Google Scholar]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Coello, F.; Sardans, J. A Better Use of Fertilizers Is Needed for Global Food Security and Environmental Sustainability. Agric. Food Secur. 2023, 12, 5. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial Biofertilizers: Bioresources and Eco-Friendly Technologies for Agricultural and Environmental Sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Allouzi, M.M.A.; Allouzi, S.M.A.; Keng, Z.X.; Supramaniam, C.V.; Singh, A.; Chong, S. Liquid Biofertilizers as a Sustainable Solution for Agriculture. Heliyon 2022, 8, e12609. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Shedeed, Z.A.; Gheda, S.; Elsanadily, S.; Alharbi, K.; Osman, M.E.H. Spirulina Platensis Biofertilization for Enhancing Growth, Photosynthetic Capacity and Yield of Lupinus Luteus. Agriculture 2022, 12, 781. [Google Scholar] [CrossRef]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.; Guan, Y.; Wu, L.; Zhang, L.; Pan, X.; Zhang, Z.; Zhang, Y.; et al. Arbuscular Mycorrhizal Fungi Biofertilizer Improves American Ginseng (Panax quinquefolius L.) Growth under the Continuous Cropping Regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Schreiber, C.d.S.; Rafacho, A.; Silverio, R.; Betti, R.; Lerário, A.C.; Lotenberg, A.M.P.; Rahmann, K.; de Oliveira, C.P.; Wajchenberg, B.L.; da Luz, P.L. The Effects of Macronutrients Composition on Hormones and Substrates during a Meal Tolerance Test in Drug-Naive and Sitagliptin-Treated Individuals with Type 2 Diabetes: A Randomized Crossover Study. Arch. Endocrinol. Metab. 2022, 66, 312–323. [Google Scholar] [CrossRef]
- Rahman, A.; Ahmad, M.A.; Mehmood, S.; Rauf, A.; Iqbal, A.; Ali, B.; Ullah, M.; Ali, M.; Mohamed, H.I.; Uddin, I. Isolation and Screening of Zn (Zn) Solubilizing Rhizosphere Bacteria from Different Vegetations for Their Ability to Improve Growth, Zn Uptake, and Expression of Zn Transporter Genes in Tomato. Curr. Microbiol. 2024, 81, 83. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Girennavar, B.; Ganapathy, B.A.; Ganapathy, A.B. Bio-Phosphate Fertilizer Formulation with Full of Life, Used for e.g., Crop Nutrition, Comprises Phosphate Solubilizing and Plant Growth Promoting Microorganisms, Rock Phosphate, Bentonite, Minerals, and Macro- and Micro-Nutrients. IN Patent 2,020,410,197,39, 5 March 2021. [Google Scholar]
- May, T.; Zimmermann, J.; Tokovenko, B.; Heinrich, D.C.; Stierl, R.; Herold, A. New Paenibacillus Species Strain Comprising Only One Genetic Locus Comprising Sequence Identity to a Polynucleotide Sequence Used to Control, Suppress or Prevent Fungal Infection of Plants, and Enhance Plant Growth. W.O. Patent 2,023,088,791, 25 May 2023. [Google Scholar]
- Sesma, J.C. Composition Used e.g., for Seed Coating, and for Improving Plant Growth, Comprises Mycorrhizal Fungus, Bacteria of Genus Pseudomonas, Fungus of Genus Trichoderma, Water Retainer, Clay, Adherent, Fauna Deterrent and Organic Matter. W.O. Patent 2,023,246,2, 21 December 2023. [Google Scholar]
- Poppeliers, S.W.; Sánchez-Gil, J.J.; de Jonge, R. Microbes to Support Plant Health: Understanding Bioinoculant Success in Complex Conditions. Curr. Opin. Microbiol. 2023, 73, 102286. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.B.; Juárez Tomás, M.S.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of Low-Cost Formulations of Plant Growth-Promoting Bacteria to Be Used as Inoculants in Beneficial Agricultural Technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Gopi, G.K.; Meenakumari, K.S.; Nysanth, N.S.; Subha, P. An Optimized Standard Liquid Carrier Formulation for Extended Shelf-Life of Plant Growth Promoting Bacteria. Rhizosphere 2019, 11, 100160. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring Phosphorus Fertilizers and Fertilization Strategies for Improved Human and Environmental Health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Zonta, E.; Bahiense, J.; Marcos, S.; Pereira, G. Fertilizantes Minerais, Orgânicos e Organominerais. In Recomendações de Calagem e Adubação para Abacaxi, Acerola, Banana, Citros, Mamão, Mandioca, Manga e Maracujá; Borges, A.L., Ed.; Embrapa: Brasília, Brazil, 2021; pp. 263–303. [Google Scholar]
- Prakash Aryal, J.; Bahadur Sapkota, T.; Krupnik, T.J.; Bahadur Rahut, D.; Lal Jat, M.; Stirling, C.M. Factors Affecting Farmers’ Use of Organic and Inorganic Fertilizers in South Asia. Environ. Sci. Pollut. Res. 2021, 28, 51480–51496. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of Biofertilizers in Crop Production and Stress Management for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef]
- ALnaass, N.S.; Agil, H.K.; Ibrahim, H.K. Use of Fertilizers or Importance of Fertilizers in Agriculture. Int. J. Adv. Acad. Stud. 2021, 3, 52–57. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, pp. 1–20. [Google Scholar]
- Pirttilä, A.M.; Tabas, H.M.P.; Baruah, N.; Koskimäki, J.J. Biofertilizers and Biocontrol Agents for Agriculture: How to Identify and Develop New Potent Microbial Strains and Traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef]
- Aoun, M.; Arnaudguilhem, C.; El Samad, O.; Khozam, R.B.; Lobinski, R. Impact of a Phosphate Fertilizer Plant on the Contamination of Marine Biota by Heavy Elements. Environ. Sci. Pollut. Res. 2015, 22, 14940–14949. [Google Scholar] [CrossRef]
- Arjjumend, H.; Koutouki, K.; Neufeld, S. Comparative Advantage of Using Biofertilizers in Indian Agroecosystems: An Analysis from the Perspectives of Stakeholders. Eur. J. Agric. Food Sci. 2021, 3, 26–36. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Fugice, J.; Singh, U.; Lewis, T.D. Development of Fertilizers for Enhanced Nitrogen Use Efficiency–Trends and Perspectives. Sci. Total Environ. 2020, 731, 139113. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-Fertilizers: Between Current Situation and Future Prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, V.; Vijayavel, K.; Ezhilarasibalasubramanian, S.; Balasubramanian, M.P. Impact of Fertilizer (Urea) on Oxygen Consumption and Feeding Energetics in the Fresh Water Fish Oreochromis Mossambicus. Env. Toxicol. Pharmacol. 2005, 19, 351–355. [Google Scholar] [CrossRef]
- Cacialli, P.; Ricci, S.; Frabetti, F.; Ferrando, S.; Franceschini, V. Exposure of Zebrafish Embryos to Urea Affects NOS1 Gene Expression in Neuronal Cells. Environments 2024, 11, 41. [Google Scholar] [CrossRef]
- El-Deeb, F.A.A.; Marie, M.A.S.; Hasheesh, W.S.; Hussein, R.M.A.; Sayed, S.S.M. Biomarkers of Oxidative Stress in Biomphalaria Alexandrina Snails for Assessing the Effects of Certain Inorganic Fertilisers. Molluscan Res. 2017, 37, 289–294. [Google Scholar] [CrossRef]
- Sheir, S.K. The Role of Caselio (Plant Fertilizer) Exposure on Digestive Gland Histology and Heavy Metals Accumulation in the Freshwater Snail, Lanistes Carinatus. J. Biosci. Appl. Res. 2015, 1, 2356–9182. [Google Scholar] [CrossRef]
- Attia, L.; Tine, S.; Tine-Djebbar, F.; Soltani, N. Potential Hazards of an Inorganic Fertilizer (Weatfert) for the Brown Garden Snail (Eobania Vermiculata MÜller, 1774): Growth, Histological and Biochemical Changes and Biomarkers. Appl. Ecol. Environ. Res 2021, 19, 1719–1734. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Krüger, A.P.; Barbosa, W.F.; Guedes, R.N.C. Leaf Fertilizers Affect Survival and Behavior of the Neotropical Stingless Bee Friesella Schrottkyi (Meliponini: Apidae: Hymenoptera). J. Econ. Entomol. 2016, 109, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Sahu, S.K. Lethal Effect of Urea on Soil Biota: A Laboratory Study on Earthworm (Drawida Willsi). J. Biodivers. Environ. Sci. 2014, 64, 64–72. [Google Scholar]
- Hashimova, U.F.; Akhundov, M.M.; Mammadova, S.I. Influence of Fertilizers on Physiological Functions of Fishes. Azerbaijan J. Physiol. 2023, 38, 13–18. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, X.; Zheng, G.; Meng, G.; Dong, Z.; Baek, J.H.; Jeon, C.O.; Yao, Y.; Xuan, Y.H.; Zhang, J.; et al. Development of Biofertilizers for Sustainable Agriculture over Four Decades (1980–2022). Geogr. Sustain. 2024, 5, 19–28. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural Sustainability: Microbial Biofertilizers in Rhizosphere Management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A Nexus between Soil Fertility and Crop Productivity under Abiotic Stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Satapathy, K.C.; Panda, B. Biofertilizers and Nanofertilizers for Sustainable Agriculture: Phycoprospects and Challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P. Cyanobacteria as a Source of Biofertilizers for Sustainable Agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.C.A.; Colla, L.M. Use of Microalgae for the Development of Biofertilizers and Biostimulants. Bioenergy Res. 2023, 16, 289–310. [Google Scholar] [CrossRef]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 2413. [Google Scholar] [CrossRef]
- Seenivasagan, R.; Babalola, O.O. Utilization of Microbial Consortia as Biofertilizers and Biopesticides for the Production of Feasible Agricultural Product. Biology 2021, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, M.; Cordero-Bueso, G.; Ruiz-Muñoz, M.; Cantoral, J.M. Culturable Yeasts as Biofertilizers and Biopesticides for a Sustainable Agriculture: A Comprehensive Review. Plants 2021, 10, 822. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Bumandalai, O.; Tserennadmid, R. Effect of Chlorella Vulgaris as a Biofertilizer on Germination of Tomato and Cucumber Seeds. Int. J. Aquat. Biol. 2019, 7, 95–99. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular Economy Fertilization: Testing Micro and Macro Algal Species as Soil Improvers and Nutrient Sources for Crop Production in Greenhouse and Field Conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Popescu, G.C.; Popescu, M. Effect of the Brown Alga Ascophyllum Nodosum as Biofertilizer on Vegetative Growth in Grapevine (Vitis vinifera L.). Curr. Trends Nat. Sci. 2014, 3, 61–67. [Google Scholar]
- Julia, I.; Oscar, M.; Analía, L.; Guilherme, J.Z.; Virginia, L. Biofertilization with Macrocystis Pyrifera Algae Extracts Combined with PGPR-Enhanced Growth in Lactuca Sativa Seedlings. J. Appl. Phycol. 2020, 4361–4371. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The Potentiality of Marine Macro-Algae as Bio-Fertilizers to Improve the Productivity and Salt Stress Tolerance of Canola (Brassica napus L.) Plants. Agronomy 2019, 9, 146. [Google Scholar] [CrossRef]
- Bitire, T.D.; Abberton, M.; Oyatomi, O.; Babalola, O.O. Effect of Bradyrhizobium Japonicum Strains and Inorganic Nitrogen Fertilizer on the Growth and Yield of Bambara Groundnut (Vigna subterranea (L.) Verdc) Accessions. Front. Sustain. Food Syst. 2022, 6, 3239. [Google Scholar] [CrossRef]
- Xue, L.; Sun, B.; Yang, Y.; Jin, B.; Zhuang, G.; Bai, Z.; Zhuang, X. Efficiency and Mechanism of Reducing Ammonia Volatilization in Alkaline Farmland Soil Using Bacillus Amyloliquefaciens Biofertilizer. Environ. Res. 2021, 202, 111672. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in Plant Growth-Promoting Bacterial Inoculant Technology: Formulations and Practical Perspectives (1998–2013). Plant Soil. 2014, 378, 1–33. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil. Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; Guzmán-Guzmán, P.; Morales-Cedeño, L.R.; Orozco-Mosqueda, M.d.C.; Saucedo-Martínez, B.C.; Sánchez-Yáñez, J.M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for Improving Formulations of Bioinoculants. 3 Biotech. 2020, 10, 199. [Google Scholar] [CrossRef]
- Maitra, P.; Al-Rashid, J.; Mandal, D.; Azam, M.S.; Rasul, N.M. Polyvinylpyrrolidone (PVP) and Na-Alginate Addition Enhances the Survival and Agronomic Performances of a Liquid Inoculant of Bradyrhizobium Japonicum for Soybean (Glycine max (L.) Merr.). Agronomy 2021, 11, 1009. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Temprano, F.J. Use of Sinorhizobium (Ensifer) Fredii for Soybean Inoculants in South Spain. Eur. J. Agron. 2009, 30, 205–211. [Google Scholar] [CrossRef]
- Valetti, L.; Angelini, J.; Taurian, T.; Ibáñez, F.; Muñoz, V.; Anzuay, M.; Ludueña, L.; Fabra, A. Development and Field Evaluation of Liquid Inoculants with Native Bradyrhizobial Strains for Peanut Production. Afr. Crop Sci. J. 2016, 24, 1. [Google Scholar] [CrossRef]
- Manikandan, R.; Saravanakumar, D.; Rajendran, L.; Raguchander, T.; Samiyappan, R. Standardization of Liquid Formulation of Pseudomonas Fluorescens Pf1 for Its Efficacy against Fusarium Wilt of Tomato. Biol. Control 2010, 54, 83–89. [Google Scholar] [CrossRef]
- Amer, G.A.; Utkhede, R.S. Development of Formulations of Biological Agents for Management of Root Rot of Lettuce and Cucumber. Can. J. Microbiol. 2000, 46, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Schoebitz, M.; Simonin, H.; Poncelet, D. Starch Filler and Osmoprotectants Improve the Survival of Rhizobacteria in Dried Alginate Beads. J. Microencapsul. 2012, 29, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, Y.; Chen, L.; Han, Y.; Li, C. Characterization of Raoultella Planticola Rs-2 Microcapsule Prepared with a Blend of Alginate and Starch and Its Release Behavior. Carbohydr. Polym. 2014, 110, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, C.N.; Chatziartemiou, A.; Tsiknia, M.; Karyda, A.G.; Ehaliotis, C.; Gasparatos, D. Calcium- and Magnesium-Enriched Organic Fertilizer and Plant Growth-Promoting Rhizobacteria Affect Soil Nutrient Availability, Plant Nutrient Uptake, and Secondary Metabolite Production in Aloe Vera (Aloe barbadensis Miller) Grown under Field Conditions. Agronomy 2023, 13, 482. [Google Scholar] [CrossRef]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The Role of Microbial Inoculants on Plant Protection, Growth Stimulation, and Crop Productivity of the Olive Tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Wang, X.; Ji, C.; Song, X.; Liu, Z.; Liu, Y.; Li, H.; Gao, Q.; Li, C.; Zheng, R.; Han, X.; et al. Biocontrol of Two Bacterial Inoculant Strains and Their Effects on the Rhizosphere Microbial Community of Field-Grown Wheat. Biomed. Res. Int. 2021, 2021, 8835275. [Google Scholar] [CrossRef]
- Díaz-Ariza, L.A.; Rivera, E.L.; Sánchez, N. Occurrence of Arbuscular Mycorrhizal Fungi in Leaf Litter and Roots of Shaded Coffee Plantations under Organic and Conventional Management. Rev. Bras. Cienc. Solo 2021, 45. [Google Scholar] [CrossRef]
- Mitra, D.; Mondal, R.; Khoshru, B.; Senapati, A.; Radha, T.K.; Mahakur, B.; Uniyal, N.; Myo, E.M.; Boutaj, H.; Sierra, B.E.G.; et al. Actinobacteria-Enhanced Plant Growth, Nutrient Acquisition, and Crop Protection: Advances in Soil, Plant, and Microbial Multifactorial Interactions. Pedosphere 2022, 32, 149–170. [Google Scholar] [CrossRef]
- Nawaz, A.; Qamar, Z.U.; Marghoob, M.U.; Imtiaz, M.; Imran, A.; Mubeen, F. Contribution of Potassium Solubilizing Bacteria in Improved Potassium Assimilation and Cytosolic K+/Na+ Ratio in Rice (Oryza sativa L.) under Saline-Sodic Conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Aczel, M.R. What Is the Nitrogen Cycle and Why Is It Key to Life? Front. Young Minds 2019, 7, 41. [Google Scholar] [CrossRef]
- Marino, R.W.; Howarth, R. Nitrogen Fixation in Freshwater and Saline Waters. Ref. Modul. Earth Syst. Environ. Sci. 2014. [Google Scholar] [CrossRef]
- Lodwig, E.M.; Hosie, A.H.F.; Bourdès, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Amino-Acid Cycling Drives Nitrogen Fixation in the Legume–Rhizobium Symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Vanderleyden, J.; Van Dommelen, A.; Michiels, J. Fix Genes. Encycl. Genet. 2001, 707–709. [Google Scholar] [CrossRef]
- Tao, K.; Kelly, S.; Radutoiu, S. Microbial Associations Enabling Nitrogen Acquisition in Plants. Curr. Opin. Microbiol. 2019, 49, 83–89. [Google Scholar] [CrossRef]
- Parniske, M. Uptake of Bacteria into Living Plant Cells, the Unifying and Distinct Feature of the Nitrogen-Fixing Root Nodule Symbiosis. Curr. Opin. Plant Biol. 2018, 44, 164–174. [Google Scholar] [CrossRef]
- Bellabarba, A.; Fagorzi, C.; DiCenzo, G.C.; Pini, F.; Viti, C.; Checcucci, A. Deciphering the Symbiotic Plant Microbiome: Translating the Most Recent Discoveries on Rhizobia for the Improvement of Agricultural Practices in Metal-Contaminated and High Saline Lands. Agronomy 2019, 9, 529. [Google Scholar] [CrossRef]
- Stroschein, M.R.D.; de Sá, E.L.S.; Machado, R.G.; Cabral, T.d.L.; Bruxel, M.; Giongo, A.; da Fontoura, R.C. Caracterização e Influência de Rizóbios Isolados de Alfafa Na Germinação e Desenvolvimento Inicial de Plântulas de Arroz. Ciência Rural. 2011, 41, 1738–1743. [Google Scholar] [CrossRef]
- Santillana, N.; Freire, J.R.J.; de Sá, E.L.S.; Sato, M. Avaliação de Estirpes de Rizóbio Para a Produção de Inoculantes Para Trevo Vermelho. Rev. Bras. Cienc. Solo 1998, 22, 231–237. [Google Scholar] [CrossRef]
- Stocco, P.; Do Santos, J.C.P.; Vargas, V.P.; Hungria, M. Avaliação Da Biodiversidade de Rizóbios Simbiontes Do Feijoeiro (Phaseolus vulgaris L.) Em Santa Catarina. Rev. Bras. Cienc. Solo 2008, 32, 1107–1120. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Savala, C.E.N.; Muananamuale, C.P.; Malita, C.; Wiredu, A.N.; Chibeba, A.M.; Elia, P.; Chikoye, D. Symbiotic Effectiveness of Bradyrhizobium Strains on Soybean Growth and Productivity in Northern Mozambique. Front. Sustain. Food Syst. 2023, 6, 1084745. [Google Scholar] [CrossRef]
- Capela, D.; Filipe, C.; Bobik, C.; Batut, J.; Bruand, C. Sinorhizobium Meliloti Differentiation During Symbiosis with Alfalfa: A Transcriptomic Dissection. Mol. Plant-Microbe Interact. 2007, 19, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Florentino, L.A.; Moreira, F.M.S. Características Simbióticas e Fenotípicas de Azorhizobium Doebereinerae, Microissimbiote de Sesbania Virgata. Rev. Árvore 2009, 33, 215–226. [Google Scholar] [CrossRef]
- Wolfe, E.R.; Singleton, S.; Stewart, N.U.; Balkan, M.A.; Ballhorn, D.J. F Rankia Diversity in Sympatrically Occurring Red Alder (Alnus rubra) and Sitka Alder (Alnus viridis) Trees in an Early Successional Environment. Tree Struct. Funct. 2022, 36, 1665–1675. [Google Scholar] [CrossRef]
- Bogusz, D.; Franche, C.; Gherbi, H.; Laplaze, L.; Auguy, F.; Duhoux, E. Casuarina-Frankia Symbiosis: Molecular Studies of the Host Plant. In Biological Nitrogen Fixation for the 21st Century: Proceedings of the 11th International Congress on Nitrogen Fixation, Institut Pasteur, Paris, France, 20–25 July 1997; Springer: Dordrecht, The Netherlands, 1998; pp. 359–360. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Chen, J.-S.; Wang, S.-Z.; Johnson, J.L. Nitrogen Fixation Genes of Clostridium Pasteurianum. Nitrogen. Fixat. 1990, 483–490. [Google Scholar] [CrossRef]
- Shameem, M.R.; Sonali, J.M.I.; Kumar, P.S.; Rangasamy, G.; Gayathri, K.V.; Parthasarathy, V. Rhizobium Mayense Sp. Nov., an Efficient Plant Growth-Promoting Nitrogen-Fixing Bacteria Isolated from Rhizosphere Soil. Environ. Res. 2023, 220, 115200. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of Inoculation with Nitrogen-Fixing Bacterium Pseudomonas Stutzeri A1501 on Maize Plant Growth and the Microbiome Indigenous to the Rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef]
- Kaur, S.; Kalia, A.; Sharma, S. Bioformulation of Azotobacter and Streptomyces for Improved Growth and Yield of Wheat (Triticum aestivum L.): A Field Study. J. Plant Growth Regul. 2024, 43, 2555–2571. [Google Scholar] [CrossRef]
- Beheshti, M.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Asadi Rahmani, H.; Noroozi, M. Enriching Periphyton with Phosphate-Solubilizing Microorganisms Improves the Growth and Concentration of Phosphorus and Micronutrients of Rice Plant in Calcareous Paddy Soil. Rhizosphere 2022, 24, 100590. [Google Scholar] [CrossRef]
- Rawat, P.; Shankhdhar, D.; Shankhdhar, S.C. Plant Growth-Promoting Rhizobacteria: A Booster for Ameliorating Soil Health and Agriculture Production. Soil Health 2020, 47–68. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.I.; Pereira, M.C.; de Carvalho, A.M.X.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Pan, F.; Ma, J.; Yang, Z.; Ling, T.; Qin, J.; Lu, S.; Zhong, F.; Song, Z. Alkaline Phosphomonoesterase-Harboring Microorganisms Mediate Soil Phosphorus Transformation With Stand Age in Chinese Pinus Massoniana Plantations. Front. Microbiol. 2020, 11, 571209. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Yadav, N.; Rastegari, A.A.; Singh, B.; Kumar, V.; Yadav, A.N. Phytases from Microbes in Phosphorus Acquisition for Plant Growth Promotion and Soil Health. In New and Future Developments in Microbial Biotechnology and Bioengineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 157–176. [Google Scholar] [CrossRef]
- Kafarski, P.; Kafarski, P. Phosphonates: Their Natural Occurrence and Physiological Role. Contemporary Topics about Phosphorus in Biology and Materials 2019, 1–19. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Wang, C.; Pan, G.; Lu, X.; Qi, W. Phosphorus Solubilizing Microorganisms: Potential Promoters of Agricultural and Environmental Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1181078. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria Sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid. Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes Benefaction Role in Soil and Plant Health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, W.; Ge, Y. Exopolysaccharide: A Novel Important Factor in the Microbial Dissolution of Tricalcium Phosphate. World J. Microbiol. Biotechnol. 2008, 24, 1059–1065. [Google Scholar] [CrossRef]
- De Zutter, N.; Ameye, M.; Vermeir, P.; Verwaeren, J.; De Gelder, L.; Audenaert, K. Innovative Rhizosphere-Based Enrichment under P-Limitation Selects for Bacterial Isolates with High-Performance P-Solubilizing Traits. Microbiol. Spectr. 2022, 10, e02052-22. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K.; Saeid, A. The Effect of Renewable Phosphorus Biofertilizers on Selected Wheat Grain Quality Parameters. Agriculture 2024, 14, 727. [Google Scholar] [CrossRef]
- Bini, D.; Mattos, B.B.; Figueiredo, J.E.F.; dos Santos, F.C.; Marriel, I.E.; dos Santos, C.A.; de Oliveira-Paiva, C.A. Parameter Evaluation for Developing Phosphate-Solubilizing Bacillus Inoculants. Braz. J. Microbiol. 2024, 55, 737–748. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth- Promoting and Biocontrol Fungus Trichoderma Harzianum Rifai 1295-22. In Appl. Environ. Microbiol.; 1999; Volume 65, pp. 2926–2933. [Google Scholar] [CrossRef]
- Cui, K.; Xu, T.; Chen, J.; Yang, H.; Liu, X.; Zhuo, R.; Peng, Y.; Tang, W.; Wang, R.; Chen, L.; et al. Siderophores, a Potential Phosphate Solubilizer from the Endophyte Streptomyces Sp. CoT10, Improved Phosphorus Mobilization for Host Plant Growth and Rhizosphere Modulation. J. Clean. Prod. 2022, 367, 133110. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Meena, R.S. Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–331. [Google Scholar]
- Jalali, M.; Antoniadis, V.; Najafi, S. Assessment of Trace Element Pollution in Northern and Western Iranian Agricultural Soils: A Review. Environ. Monit. Assess. 2021, 193, 823. [Google Scholar] [CrossRef]
- Mali, S.D.; Attar, Y.C. Formulation of Cost-Effective Agro Residues Containing Potassium Solubilizing Bacterial Bio-Inoculants Using Response Surface Methodology. Biocatal. Agric. Biotechnol. 2021, 35, 102113. [Google Scholar] [CrossRef]
- Feng, K.; Cai, Z.; Ding, T.; Yan, H.; Liu, X.; Zhang, Z. Effects of Potassium-solubulizing and Photosynthetic Bacteria on Tolerance to Salt Stress in Maize. J. Appl. Microbiol. 2019, 126, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Kour, D.; Kaur, T.; Devi, R.; Chaubey, K.K.; Yadav, A.N. Co-Inoculation of Nitrogen Fixing and Potassium Solubilizing Acinetobacter Sp. for Growth Promotion of Onion (Allium cepa). Biology 2023, 78, 2635–2641. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Physiological Role of Exogenous Nitric Oxide in Improving Performance, Yield and Some Biochemical Aspects of Sunflower Plant under Zinc Stress. Acta Biol. Hung. 2017, 68, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Mahala, D.M.; Maheshwari, H.S.; Yadav, R.K.; Prabina, B.J.; Bharti, A.; Reddy, K.K.; Kumawat, C.; Ramesh, A. Microbial Transformation of Nutrients in Soil: An Overview. Microorg. Sustain. 2020, 23, 175–211. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Sharma, A. Contemplating the Role of Zinc-Solubilizing Bacteria in Crop Biofortification: An Approach for Sustainable Bioeconomy. Front. Agron. 2022, 4, 903321. [Google Scholar] [CrossRef]
- Suyal, D.C.; Soni, R.; Sai, S.; Goel, R. Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 1: Research Perspectives; Springer: New Delhi, India, 2016; pp. 311–317. [Google Scholar] [CrossRef]
- Prando, A.M.; Barbosa, J.Z.; de Oliveira, A.B.; Nogueira, M.A.; Possamai, E.J.; Hungria, M. Benefits of Soybean Co-Inoculation with Bradyrhizobium Spp. and Azospirillum Brasilense: Large-Scale Validation with Farmers in Brazil. Eur. J. Agron. 2024, 155, 127112. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 171–190. ISBN 9789811090448. [Google Scholar]
- Hungria, M.; Nogueira, M.A.; Campos, L.J.M.; Menna, P.; Brandi, F.; Ramos, Y.G. Seed Pre-Inoculation with Bradyrhizobium as Time-Optimizing Option for Large-Scale Soybean Cropping Systems. Agron. J. 2020, 112, 5222–5236. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial Fertilizers: A Comprehensive Review of Current Findings and Future Perspectives. Span. J. Agric. Res. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Florencio, C.; Bortoletto-Santos, R.; Favaroa, C.P.; Brondia, M.G.; Velloso, C.C.V.; Klaic, R.; Ribeiro, C.; Farinasa, C.S.; Mattoso, L. Advances in the Production and Formulation of Microbial Inoculants for a More Sustainable Agriculture. Quim. Nova 2022, 45, 1133–1145. [Google Scholar]
- Ona, O.; Van Impe, J.; Prinsen, E.; Vanderleyden, J. Growth and Indole-3-Acetic Acid Biosynthesis of Azospirillum Brasilense Sp245 Is Environmentally Controlled. FEMS Microbiol. Lett. 2005, 246, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Starch Industry Wastewater as a Substrate for Antagonist, Trichoderma Viride Production. Bioresour. Technol. 2007, 98, 2154–2162. [Google Scholar] [CrossRef]
- Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Gonzalez-Monterrubio, C.F.; Acevedo-Sánchez, E.V.; Martínez-Salinas, C.; García-Cabrera, R.I.; Gamboa-Suasnavart, R.A.; Marín-Palacio, L.D.; Villegas, J.; Blancas-Cabrera, A. Scale-up from Shake Flasks to Pilot-Scale Production of the Plant Growth-Promoting Bacterium Azospirillum Brasilense for Preparing a Liquid Inoculant Formulation. Appl. Microbiol. Biotechnol. 2013, 97, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.T.; Deaker, R.; Potard, S.; Tran, C.K.T.; Vu, N.T.; Kennedy, I.R. The Survival of Plant Growth Promoting Microorganisms in Peat Inoculant as Measured by Selective Plate Counting and Enzyme-Linked Immunoassay. World J. Microbiol. Biotechnol. 2011, 27, 1649–1659. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Mann, E.W. Treatment of Plants and Seeds with Bacillus Uniflagellatus Increased Crops of Cultures and Plants Esp. in Stressed Conditions. NL Patent 7,315,471, 15 May 1974. [Google Scholar]
- Vandenbergh, P.A.; Gonzalez, C.F. Protection of Growth of Higher Plants against Microorganisms. AU Patent 8,317,489, 10 May 1984. [Google Scholar]
- Tenzer, A.I. Microbial Plant Growth Promoter Prepd. by Mixing Bacterial and Algal Cultures and Incubating. U.S. Patent 4,551,164, 5 November 1985. [Google Scholar]
- Nielsen, S.E.; Soerensen, G.M.; Nielsen, S. New Rhizobium Transformants Used for Treating Seeds of a Non-Legume Plant to Symbiotically Fix Nitrogen. W.O. Patent 1,987,004,182, 16 July 1987. [Google Scholar]
- Triplett, E.W. Recombinant Rhizobium Bacteria for Trifolitoxin Prodn. and Resistance in Plants. W.O. Patent 9,015,138, 13 December 1990. [Google Scholar]
- Bali, A.K.; Blanco, G.; Kennedy, C.K. New Mutant Nitrogen-Fixing Bacterium Has Mutation in NifL or NifL-like Gene and Functional NifA(-like) Gene, Used to Produce Nitrogen-Fixing Plants. GB Patent 2,259,302, 10 March 1993. [Google Scholar]
- Varga, S.S. Process for the Development of Novel Type of Plants with Nitrogen-Fixing Capacity Also in Their Leaves. U.S. Patent 5,664,368, 25 January 1995. [Google Scholar]
- Yadav, R.; Singh, S.; Singh, A.N. Biopesticides: Current Status and Future Prospects. Proc. Int. Acad. Ecol. Environ. Sci. 2022, 2022, 211–233. [Google Scholar]
- Montesinos, E. Development, Registration and Commercialization of Microbial Pesticides for Plant Protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef]
- Raunser, S.; Sitsel, O. Yersinia Entomophaga Bacterium Used for Producing Toxin Composition, and Population of Soldier Cells for Killing Pests, Comprises Modified YenR Gene, Modified YmoA Gene, Disrupted YenDF, Modified Gene Encoding YenTc Subunit or Modified YenTc Subunit Such as Modified YenA2 or Modified YenA2 Gene. E.P. Patent 4,335,866, 13 March 2024. [Google Scholar]
- Farmer, S.; Alibek, K. Composition for Controlling Agricultural Pests, e.g., Nematodes or Fungi, Comprises Hydrolysate of Biochemical-Producing Microorganism, and Biochemicals (e.g., Biosurfactants and/or Enzymes) Produced by Microorganism during Cultivation. W.O. Patent 2,020,142,366, 9 July 2020. [Google Scholar]
- Tamsir, A.; Bloch, S.; Reisinger, M.; Sanders, E.; Broglie, R.; Clark, R.; Temme, K.; Brock, S.; Reissinger, M.; Krack, R.; et al. Providing Fixed Atmospheric Nitrogen to Cereal Plant, Involves Providing Non-Intergeneric Remodeled Bacteria, and Cereal Plants to Locus. W.O. Patent 2,020,014,498, 16 January 2020. [Google Scholar]
- Temme, K.; Tamsir, A.; Bloch, S.; Shah, N.; Johnson, J.; Ozaydin, B.; Eskiyenenturk, B.O. Genetically Engineered Bacterium Used in Composition for Providing Fixed Nitrogen to Plants, Comprises Modification in Gene Selected from NAC, GltA, Pga, PtsH. W.O. Patent 2,020,219,932, 29 October 2020. [Google Scholar]
- Parnell, J.J.; Ridge, G.; Kluber, L.A.; Baker, E.C.; Kirkeng, S.E.; Hall, C.; Marin, C.; Maloney, G.S.; Thompson, D.A. New Isolated Microbacterium Trichothecenolyticum Strain Useful for Enhancing Plant Growth or Yield of Plant Grown, or Enhancing Chlorophyll Production or Accumulation or Content in Plant or Plant Part. W.O. Patent 2,021,086,695, 6 May 2021. [Google Scholar]
- Sharma, H.; Kumar, N.; Swani, A.; Venkateshwarulu, G. Pseudomonas Species Formulation Useful as Antifungal and Growth Promoting Activity Agent, Isolated from Rhizopsheric Zone of Plant Belonging to a Plant Group Comprising Selected Abelmoschus Plant. IN Patent 2,022,110,198,35, 8 April 2022. [Google Scholar]
- Sairam, K.; Biswa, P.; Bijja, R.; Rajashekhar, B. Gel-Based Agrobiological Composition Used as Biofertilizer, or Biocontrol Agent, Comprises Carrier Comprises Water-Soluble or Biodegradable Polymer, and Microbiological Entity. IN Patent 2,022,410,276,81, 27 May 2022. [Google Scholar]
- Breakfield, N.; Jimenez, D.R.; Flack, D.; Neumann, A. Mitigating Methane Gas in Agricultural Field by Applying Composition Comprising at Least One Methanotroph to Soil, Field, Plant, Plant Part or Seed, and Growing Methanotroph. W.O. Patent 2,024,015,850, 4 April 2024. [Google Scholar]
- Vaghela, N.R.; Gohel, S.D. Liquid Formulation of Indole Acetic Acid-Producing Halo Alkali Tolerant to Improve Growth Parameters in Crops, Comprises Plant Growth-Promoting Rhizobacterial Strain, Where Rhizobacteria Strain Is Priestia Filamentosa KhEc 69 to Improve Growth Parameters in Crops. IN Patent 2,023,210,643,54, 3 May 2024. [Google Scholar]
- Mehata, D.K.; Kattel, I.; Sapkota, P.; Ghimire, N.P.; Mehta, R.K. Biofertilizers: A Sustainable Strategy for Organic Farming That Would Increase Crop Production and Soil Health. Plant Physiol. Soil. Chem. 2023, 3, 49–53. [Google Scholar] [CrossRef]
- Latha, S.; Assistant, A.; John, S.; Aggani, S.L. Development of Bio-Fertilizers and Its Future Perspective. Sch. Acad. J. Pharm. 2013, 2, 327–332. [Google Scholar]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Ferreira, E.; Nogueira, M.A.; Hungria, M. Manual de Análises de Bioinsumos Para. Uso Agrícola Inoculantes; Embrapa: Brasília, Brazil, 2024; Volume 1, ISBN 9786554670296. [Google Scholar]
- Deaker, R.; Hartley, E.; Gemell, G.; Herridge, D.F.; Karanja, N. Inoculant production and Quality Control. In Working with Rhizobia; Howieson, J.G., Dilworth, M.J., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2016; Volume 1, p. 312. ISBN 9781925436174. [Google Scholar]
- National Centre of Organic Farming Biofertilizers and Organic Fertilizers in Fertilizer (Control) Order. 1985. Available online: https://www.jaivikkheti.in/DMS/Biofertilizer%20and%20Organic%20Farming%20in%20FCO.pdf (accessed on 3 July 2024).
- Canadian Food Inspection Agency Revised Trade Memorandum T-4-109: Requirements for Rhizobial Inoculants and Pre-Inoculated Seed under the Fertilizers Act—Draft. Available online: https://epe.lac-bac.gc.ca/100/206/301/cfia-acia/2011-09-21/inspection.gc.ca/english/plaveg/fereng/tmemo/rev-t-4-109e.shtml (accessed on 2 July 2024).
- URUGUAY Decreto 546/981 Del 28 de Octubre de 1981. Available online: https://www.impo.com.uy/bases/decretos/546-1981 (accessed on 3 July 2024).
- BRASIL Instrução Normativa SDA No 13, de 24 de Março de 2011. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-sda-13-de-24-03-2011-inoculantes.pdf/view (accessed on 12 July 2024).
- Herrmann, L.; Lesueur, D. Challenges of Formulation and Quality of Biofertilizers for Successful Inoculation. Appl. Microbiol. Biotechnol. 2013, 97, 8859–8873. [Google Scholar] [CrossRef] [PubMed]
- Bullard, G.K.; Roughley, R.J.; Pulsford, D.J. The Legume Inoculant Industry and Inoculant Quality Control in Australia: 1953–2003. Aust. J. Exp. Agric. 2005, 45, 127–140. [Google Scholar] [CrossRef]
- Malusá, E.; Vassilev, N. A Contribution to Set a Legal Framework for Biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Lupwayi, N.Z.; Olsen, P.E.; Sande, E.S.; Keyser, H.H.; Collins, M.M.; Singleton, P.W.; Rice, W.A. Inoculant Quality and Its Evaluation. Field Crops Res. 2000, 65, 259–270. [Google Scholar] [CrossRef]
- MERCOSUR Resolución Mercosur /GMC/RES No 28/98. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/sites/ministerio-ganaderia-agricultura-pesca/files/2020-07/res_028-1998_es_disp-rel_comercio_inoculantes_acta_2_988372346_1.pdf (accessed on 3 July 2024).
- BRASIL Decreto No 8.384, de 29 de Dezembro de 2014. Available online: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2014/decreto/d8384.htm#:~:text=Altera%20o%20Anexo%20ao%20Decreto,ou%20biofertilizantes%20destinados%20%C3%A0%20agricultura (accessed on 16 July 2024).
- BRASIL Lei No 6.894, de 16 de Dezembro de 1980. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/lei-6894-de-16-12-80-alterada-pela-lei-12890-2013.pdf (accessed on 2 July 2024).
- ANPII Inoculantes. Available online: https://www.anpii.org.br/wp-content/uploads/2020/06/Global-Fert-Inoculantes.pdf (accessed on 3 July 2024).
- Faverin, V. Inoculantes Foram Utilizados Em 85% Da Safra de Soja 2022/23. Available online: https://www.canalrural.com.br/agricultura/projeto-soja-brasil/inoculantes-foram-utilizados-em-85-da-safra-de-soja-22-23/ (accessed on 13 July 2023).
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The Use of Microbial Inoculants for Biological Control, Plant Growth Promotion, and Sustainable Agriculture: A Review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial Bacteria of Agricultural Importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. The Potential Contribution of Plant Growth-Promoting Bacteria to Reduce Environmental Degradation–A Comprehensive Evaluation. Appl. Soil. Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
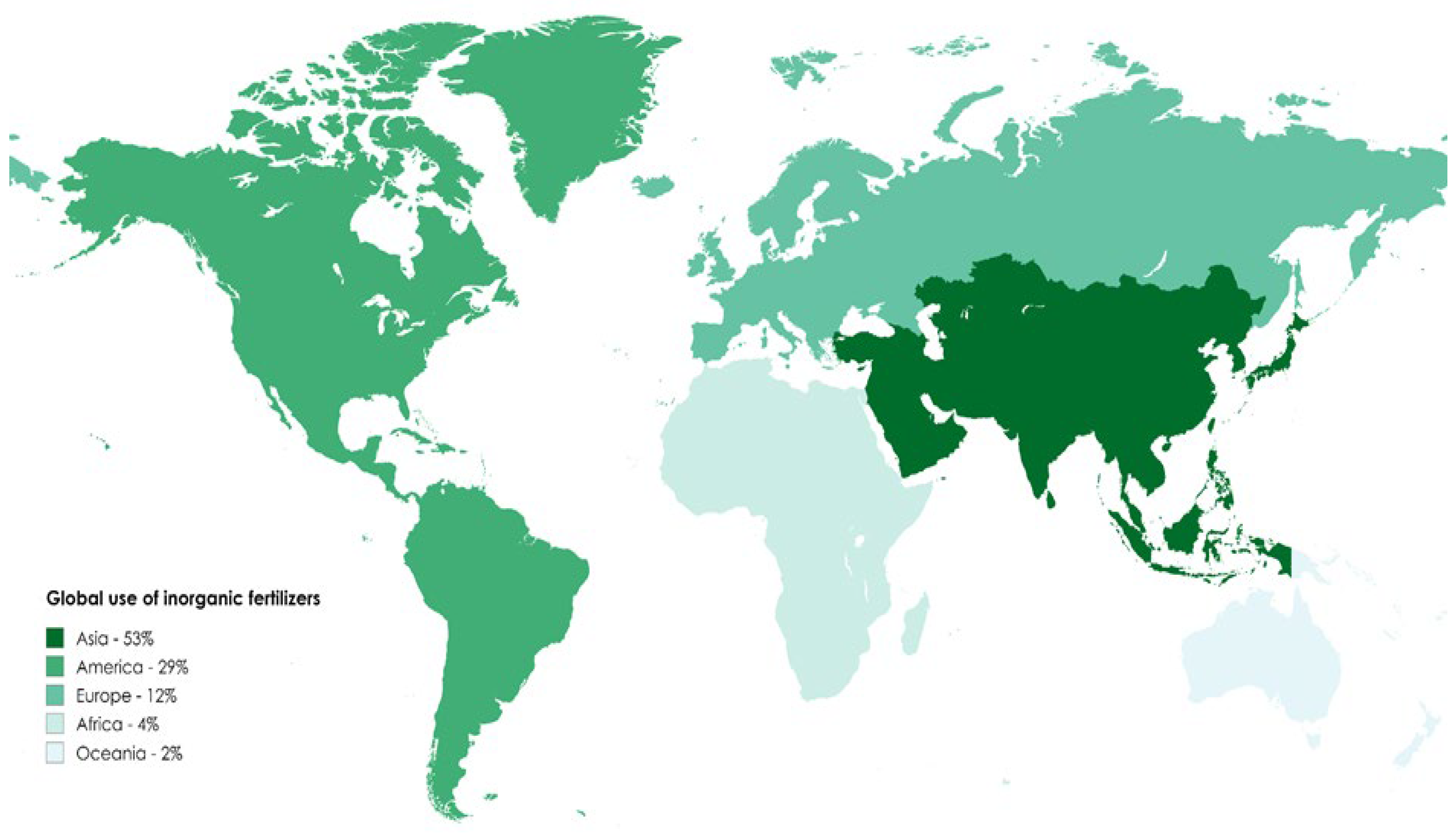
| Fertilizer | Type | Experimental Conditions | Reported Environmental Impacts | Reference |
|---|---|---|---|---|
| Urea | Nitrogen | Exposure of Oreochromis mossambicus to different concentrations of urea for 24, 48, 72, and 96 h | Urea concentrations of 22,000 and 38,000 mg L−1 were sublethal and lethal, respectively; decreased feeding rate and growth rate were observed | [33] |
| Urea | Nitrogen | Exposure of Danio rerio to different urea concentrations (0, 10, 50, and 100 mM) from 4 to 96 h post-fertilization (hpf) | Affected cell proliferation and the number of cells that express nos1, a gene involved in the formation of neuronal cells during embryonic development | [34] |
| NPK (3 different ratios) | Nitrogen, phosphorus, potassium | Exposure of Biomphalaria alexandrina to sublethal concentrations (1/10 LC50, ¼ LC50, and ½ LC50) of each fertilizer for 24 h | Decreased the growth rate of juvenile snails; activation of the antioxidant system | [35] |
| Ceselio | Phosphorus | Exposure of Lanistes carinatus to 200 and 600 µL L−1 of fertilizer for 0, 1, 3, and 7 days | Increase in lipid peroxidation; tissue necrosis; increase in lipofuscin pigment (related to aging); and bioaccumulation of metals in the shell and organs | [36] |
| Weatfert (NPK) | Nitrogen, phosphorus, and potassium (higher phosphorus content) | Exposure of Eobania vermiculata to the recommended agricultural dose (D1 = 500 mg/400 cm2) and the recommended agricultural dose × 2 (D2 = 1000 mg/400 cm2) | Histological and biochemical alterations in the digestive gland; negative impact on growth rate | [37] |
| Copper sulfate | Copper sulfate | Exposure of Friesella schrottkyi 5.0 g L−1 for 72 h | Compromised the survival of bees mainly via oral exposure | [38] |
| 5% S, 5% Zn, 3% Mn, 0.6% Cu, 0.5% Be, 0.06% Mo | Micronutrient mixture | Exposure of Friesella schrottkyi 2.0 mL L−1 for 72 h | Reduction in respiratory rate | [38] |
| Urea | Nitrogen | Exposure of Drawida willsi to 100, 200, 300, 400, 500, 600, 700, and 800 mg of urea kg−1 of dry soil for 96 h | At a dose of 800 mg of urea kg−1 of dry soil, 100%, 76%, and 52% mortality were observed for juvenile, immature, and adult earthworms, respectively | [39] |
| Ammophos | Phosphorus | Exposure of Cyprinus carpio to 97.21 mL L−1 of fertilizer for 96 h | Behavioral and hematological changes | [40] |
| Kristalon (NPK) | Nitrogen, phosphorus, and potassium | Exposure of Cyprinus carpio to 265.18 mL L−1 of fertilizer for 96 h | Behavioral and hematological changes were observed | [40] |
| Inoculant Type | Vehicle | Microorganism | Microbial Concentration | Plant Species | Positive Aspects of Inoculant | References |
|---|---|---|---|---|---|---|
| Liquid | Polyvinylpyrrolidone (1.8%) and sodium alginate (0.3%) | Bradyrhizobium japonicum | 1.93 × 109 cells mL−1 | Soybean | Increase in the number of nodules on plants, number of pods per plant, and number of seeds per pod | [65] |
| Polyvinylpyrrolidone, Fe-EDTA | Several rhizobia, Bradyrhizobium japonicum and Bacillus megaterium | 106–108 CFU/mL | Soybean | Increase in the number of nodules on plants and seeds produced per plant | [66] | |
| Arabic gum | Bradyrhizobium sp. J-81 and Bradyrhizobium sp. J-237 | 1010 CFU/mL | Peanut (Arachis hypogaea) | Increased yield in peanut pods by up to 44% | [67] | |
| Trehalose (2%), polyvinylpyrrolidone (10 mM), and glycerol (10 mM) | Pseudomonas fluorescens | 3 × 1010 CFU/mL | Tomato | Increased yield in tomato | [68] | |
| Solid | Vermiculite—carboxymethylcellulose | Bacillus subtilis | About 108 CFU/mL | Cucumber, lettuce, and American ginseng | Significant increase in weight and roots | [69] |
| Alginate and starch | Raoultella terrigena, Azospirillum brasilense | Between 109 and 1011 CFU/mL | n.s. | n.s. | [70] | |
| Alginate and starch | Raoultella planticola Rs-2 | Between 107 and 1013 CFU/mL | n.s. | n.s. | [71] |
| Country | Biofertilizer | Carrier Material | Storage Condition | Efficacy Time (Minimum) | Seed Size versus Concentration MOs | Viable Cell (Minimum) | pH | Moisture | Shelf Life | Contamination (Dilution Factor) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| India | Rhizobium, Azotobacter, Azospirillum Phosphate-solubilizing bacteria, Mycorrhizal, potassium mobilizing, zinc solubilizing, Acetobacter and consortium | Peat; lignite; peat soil; humus; wood charcoal | No | No | No | 5 × 107 to 5 × 108 | 5.0–7.5 | 8–40% | n.s. | 10−4 a 10−5 | [162] |
| Canada | Only Rhizobium species | n.s. | No | 1 to 2 years | Yes | 103 to 105 | No | n.s. | 6 months | 10−6 | [163] |
| Australia | Only Rhizobium species | Peat; bagasse; manures, sugarcane filter mud; coconut coir dust; perlite; clays | No | No | Yes | 105 | No | n.s. | n.s. | 10−6 | [161] |
| Uruguay | Only Rhizobium species | Peat; others (n.s.) | 4 °C | No | No | 1 × 109 | No | n.s. | n.s. | n.s. | [164] |
| Brazil | Bradyrhizobium, Mesorhizobium, Rhizobium, Sinorhizobium, Azorhizobium Bacillus subtilis, and Frauteria aurantia | Peat; others (n.s.) | No | No | No | 1 × 109 | No | n.s. | 6 months | 1 × 10−5 | [165] |
| Biofertilizer | Carrier Base | Viable Cell (Minimum) | pH | Particles Size | Moisture Percent by Weight |
|---|---|---|---|---|---|
| Rhizobium Azotobacter Azospirillum | Peat, lignite, peat soil, humus, and wood charcoal | 5 × 107 cell/g | 6.5–7.5 | 0.15–0.212 mm IS sieve | 30–40 |
| 1 × 108 cell/mL | |||||
| Phosphate-solubilizing bacteria | Peat, lignite, peat soil, humus, and wood charcoal | 5 × 107 cell/g | 6.5–7.5 | ||
| 1 × 108 cell/mL | 5.0–7.5 | ||||
| Mycorrhizal biofertilizers | n.s. | 100 propagules/g of finished product | 6.0–7.5 | 90% should pass through 250 micron IS sieve | 8–12 |
| Potassium-mobilizing biofertilizers | Peat, lignite, peat soil, humus, and talc | 5 × 107 cell/g | 6.5–7.5 | 0.15 to 0.212 mm IS sieve | 30–40 |
| 1 × 108 cell/mL | 5.0–7.5 | ||||
| Zinc-solubilizing biofertilizers | n.s. | 5 × 107 cell/g | 6.5–7.5 | 0.15 to 0.212 mm IS sieve | 30–40 |
| 1 × 108 cell/mL | 5.0–7.5 | ||||
| Acetobacter | Peat, lignite, peat soil, humus, and wood charcoal | 5 × 107 cell/g | 5.5–6.0 | 0.15 to 0.212 mm IS sieve | 30–40 |
| 1 × 108 cell/mL | 5.5–6.0 | ||||
| Carrier-based consortia | n.s. | 5 × 107 cells/g All microorganisms | n.s. | 0.15 to 0.212 mm IS sieve | 30–40 |
| Liquid consortia | n.s. | 5 × 108 cells/ mL All microorganisms | 5.0–7.0 | n.s. | n.s. |
| Crop | Seed Size | N° of Seeds per kg | Number of Viable Cells per Seed |
|---|---|---|---|
| Alfalfa, clover, Birdsfoot Trefoil | Small | >200,000 | 103 |
| Sainfoin | Medium | 200,000–30,000 | 104 |
| Beans, peas, soybeans | Large | <30,000 | 105 |
| Product | Fresh Count | Expiry Count | Expiry Time (Months) |
|---|---|---|---|
| Peat (CFU/g) | ≥1 × 109 | ≥1 × 108 | 12–18 |
| Liquid (CFU/mL) | ≥5 × 109 | ≥1 × 109 | 6 |
| Granules (MPN/g) | ≥1 × 107 | ≥1 × 106 | 6 |
| Freeze-dried (CFU/vial) | ≥1 × 1012 | ≥5 × 1011 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Reis, G.A.; Martínez-Burgos, W.J.; Pozzan, R.; Pastrana Puche, Y.; Ocán-Torres, D.; de Queiroz Fonseca Mota, P.; Rodrigues, C.; Lima Serra, J.; Scapini, T.; Karp, S.G.; et al. Comprehensive Review of Microbial Inoculants: Agricultural Applications, Technology Trends in Patents, and Regulatory Frameworks. Sustainability 2024, 16, 8720. https://doi.org/10.3390/su16198720
dos Reis GA, Martínez-Burgos WJ, Pozzan R, Pastrana Puche Y, Ocán-Torres D, de Queiroz Fonseca Mota P, Rodrigues C, Lima Serra J, Scapini T, Karp SG, et al. Comprehensive Review of Microbial Inoculants: Agricultural Applications, Technology Trends in Patents, and Regulatory Frameworks. Sustainability. 2024; 16(19):8720. https://doi.org/10.3390/su16198720
Chicago/Turabian Styledos Reis, Guilherme Anacleto, Walter Jose Martínez-Burgos, Roberta Pozzan, Yenis Pastrana Puche, Diego Ocán-Torres, Pedro de Queiroz Fonseca Mota, Cristine Rodrigues, Josilene Lima Serra, Thamarys Scapini, Susan Grace Karp, and et al. 2024. "Comprehensive Review of Microbial Inoculants: Agricultural Applications, Technology Trends in Patents, and Regulatory Frameworks" Sustainability 16, no. 19: 8720. https://doi.org/10.3390/su16198720






