Abstract
Assessing a given product’s design and its recyclability using mass flow analysis based on the material separation and recovery rates of individual recycling processes under realistic conditions can support design decisions promoting better recyclability. EN 45555 defines the calculation of the technical recyclability of electrical and electronic equipment (EEE). However, the lack of specific recycling rates for material or processes often leads to either too small or too high recyclability values. Herein, an extensive database of such recycling rates is presented. Moreover, the quality of recycling is considered. The typical classification into “recycled” and “lost” is expanded into four categories, namely “circular”, “recycled”, “alternate material recovery” and “lost”. The recycling rate database includes yields for all four categories and covers 30 materials for 14 recycling processes relevant in waste EEE (WEEE) treatment. These data enable a detailed calculation of the recyclability of various EEE for multiple recycling scenarios covering the entire WEEE recycling chain. Fraunhofer IZM performed an internal critical review of the data. The recycling rates database can act as a solid foundation for comparing the recyclability of various electronics in different scenarios and recyclability indices. For example, the recyclability of typical smartphones is investigated comparing different dismantling and recycling scenarios highlighting the potential of both database and methodology.
1. Introduction
According to the most recent “Global E-waste Monitor” [1], 2022 saw the highest ever recorded amount of e-waste generated globally rising to 62 billion kg. Moreover, the rate of e-waste generation is outpacing the growth of formal collection and recycling, i.e., sound environmental e-waste management, by a factor 5. These findings highlight the need for action by all stakeholders, i.e., governments, manufacturers, producers and recyclers. On the one hand, the capacities of e-waste collection and recycling have to be increased. Original equipment manufacturers (OEMs) are launching directly managed collection programs to increase the collection of their own equipment. Such programs include ink-jet cartridge collection and recycling, which are offered by most major manufacturers [2,3], as well as electronic trade-in programs [4,5,6] and e-bike battery recycling [7] just to name a few. However, the collection of e-waste must be further improved since only 22.3% of the e-waste generated globally was formally collected and recycled in 2022 [1]. On the other hand, the extraction of metals and other materials from collected WEEE, often referred to as urban mining, remains challenging [8,9]. Material recovery can be improved by investing in new and improved recycling infrastructure while considering material recovery during the product design process. In the European Union (EU), the aspects of ecodesign, design for recycling, and design for circularity are addressed by the ecodesign directive [10] and other related legislation [11]. However, for design for recycling, there is a lack of information about status quo and how to design better products for recycling. To quantify the recyclability of a given EEE, calculations can be made according to EN 45555 “General methods for assessing the recyclability and recoverability of energy related products” [12]. However, these calculations are hampered by the lack of material specific data for the relevant material recovery processes. EN 45555 allows the use of the so-called simplified method when data are missing. Then, unknown material recoverability factors are estimated to be either 1 or 0. Depending on the assumptions made during the simplified method, this yields recyclability values that are either much too small or much too high. Going from such potential recyclability to a more realistic assessment is difficult due to a lack of data [13]. The recycling rates calculated according to EN 45555 can be considered technical recyclability [14]. Ideally, it should be based on large-scale batch tests [13]. Furthermore, calculations according to EN 45555 do not discriminate by the quality of recycling; thus, misleading recyclability factors can be claimed or reported by this method.
Both the challenges of calculating the technical recyclability according to EN 45555, i.e., the lack of recycling rate data, and the lack of consideration of recycling quality, are addressed in this contribution. Firstly, an extensive database of material recovery rates for 30 materials within 14 existing recovery processes based on the relevant literature, expert information, and scientifically sound, well-defined, assumptions has been created. The respective processes represent the best available technologies (BATs) in Europe which meet four minimum requirements: the recovery of a specific material from WEEE input (the facility may also receive non-WEEE input), the technology readiness level (TRL) ≥ 8, >30 employees and a turnover of EUR >10 million. This is in line with EN 45555 [12]. Please note that the BATs selection is based on the current state of the recycling industry, and not the theoretical maximum recycling rate of materials. We expect that, with increased funding and significant research and development efforts, new BATs will become available which will increase material recovery for target materials and improve overall product recyclability. Furthermore, the BAT criteria were intentionally selected to screen technologies for economic viability and process scalability, as these are important factors ensuring that the recycling scenario is based on recovery processes that have a path to wide adoption. The material recovery of a product also depends on the composition of the waste flow within which the product is collected [13]. Electric devices from the WEEE directive’s [15] categories 2 and 6 are the focus of this work, e.g., laptops, tablets, smartphones, et cetera, and consequently, mixed waste flows containing such devices are considered.
Secondly, the four categories “circular”, “recycled”, “alternate material recovery” and “lost” are introduced, discriminating by the quality of the recycling. The “circular” category refers to recovered materials which can be used for the substitution of corresponding virgin material in the original product. Recovered material which can substitute the corresponding virgin material but with a lower quality level fits into the “recycled” category. The “alternate material recovery” refers to a material that is recovered as a product that is not identical to the corresponding virgin material. Materials are considered “lost” if they are used for energy recovery or are landfilled.
Based on these data and this methodology, the recyclability of smartphones is assessed for different end-of-life treatment scenarios in a case study showcasing the potential of both the data and methodology presented herein.
2. Methods
2.1. Calculations of Recyclability
In general and in accordance with EN 45555 [12], the recycling rate R including the different qualities discussed above is calculated as follows:
wherein the following definitions hold:
- n—number of materials;
- —mass of material k;
- —recyclability factor of material k;
- —total mass of device.
In the case of multiple steps, the recyclability factor is the product of the yields or transfer coefficients of all steps. In a simplified example, 85% of copper ends up in the Cu scrap subfraction after mechanical treatment. This is followed by the recycling process copper smelting with a yield of 95% for copper. Thus, the recyclability factor for all copper in the fraction undergoing these two recycling processes is 80.75%. On the more complex level represented by the mass flow model used herein, some copper also ends up in the other subfractions after shredding and sorting. Moreover, other fractions also contain copper. For these, other recyclability factors are calculated. Considering all these aspects for all fractions and all materials yields the final recycling rates at the device level.
2.2. Yields of Recycling Processes
Next to the choice of relevant and adequate recycling processes (discussed below), the most essential parts of the calculations are the recycling yields or recyclability factors per material (). These were gathered from scientific literature, expert information and scientifically based and well-defined assumptions. Firstly, the respective recycling processes were selected—in line with EN 45555—representing the best available technology in Europe. These met four minimum requirements: the recycling of a specific material from WEEE input (the facility may also receive non-WEEE input), the technology readiness level (TRL) ≥ 8, >30 employees and a turnover of EUR >10 million. For these recycling processes, material-specific recycling yields were taken from the literature. In the cases in which more than one value was found and the described processes did not differ significantly, the lowest reported value was taken for the model—following a worst-case scenario approach. In the absence of exact numerical values, estimations had to be made for the recycling processes, facilitating second recycling steps. For processes without subsequent recycling steps, the yield was set to zero where no values were available—despite literature references substantiating that it is non-zero—following the worst-case approach.
During mechanical treatment, the input materials are sorted with certain yields into five subfractions. Here, the data used in the model go beyond the reports in the literature, both in terms of the number of materials and the information on all five subfractions. This also includes yields for materials into other subfractions than intended, e.g., polymers ending up in the ferrous scrap. For the compilation of the used data, the following methodology was used.
Firstly, the losses to dust during shredding were taken from the literature as a starting point [16,17,18,19,20,21,22]. Secondly, the yields for the target materials were taken from the literature [17,18,19,21,23,24], i.e., the materials to the intended subfraction, i.e., copper to “Cu scrap”. The remaining share of the respective material, i.e., what was not in the intended subfraction and not lost, ended up in the other subfractions. The distribution across the four remaining subfractions was estimated on the basis of the four criteria listed below in descending order of priority:
- Literature data: Here, the data are incomplete. For example, mass balances excluding the ferrous fraction are reported or only single materials were quantified. However, literature data were used whenever available.
- Density-based assumptions (for gravity separation) using Table S1.
- Assumptions of electrostatic separation efficiency via the ratio of electric conductivity to density per material according to Table S2.
- Other estimates or calculated differences.
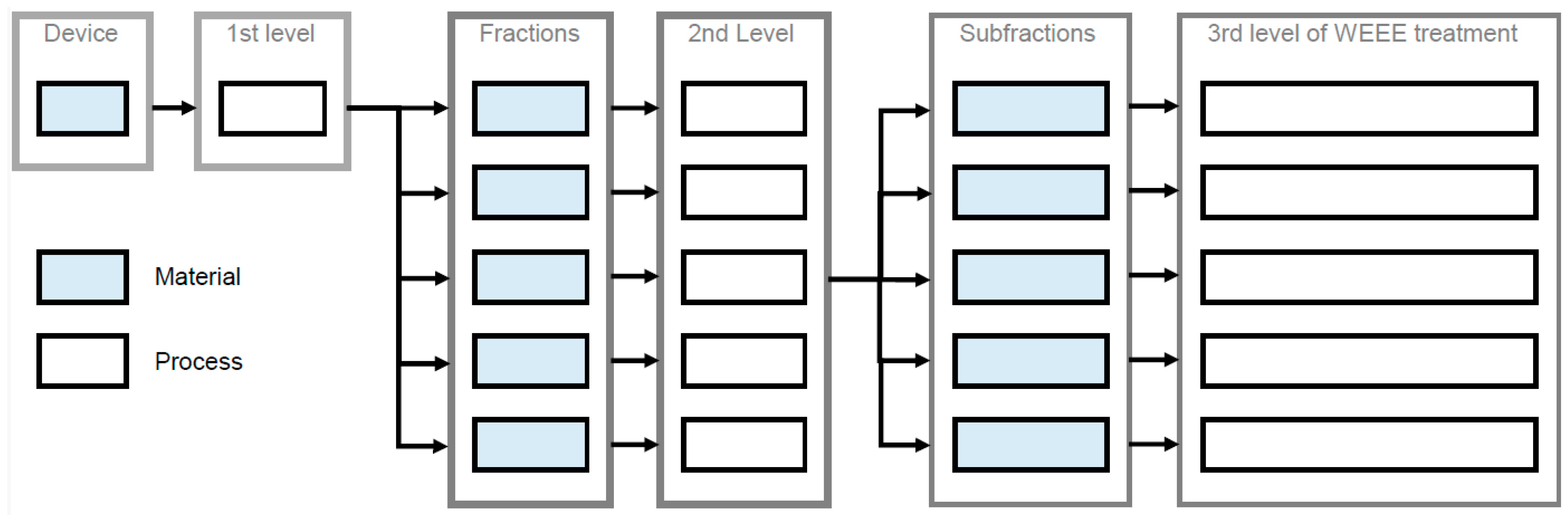
2.3. Nomenclature and Levels of WEEE Treatment
Figure 1 shows the nomenclature used in the following to represent the three levels of WEEE treatment [25]. On the first level, the device is separated in multiple fractions by dismantling. On the second level, mechanical treatment separates each fraction into multiple subfractions. On the third level, material recovery occurs. The second level of WEEE treatment can be bypassed in the case of high-quality dismantling on the first level. For example, PCBs that are separated manually can be directly forwarded to copper smelting without any mechanical treatment. Graphically, material recovery is placed on the second level in these cases—for the sake of clarity (Figure 1). Finally, the fractions’ or subfractions’ materials are turned into secondary raw materials via various recycling processes.
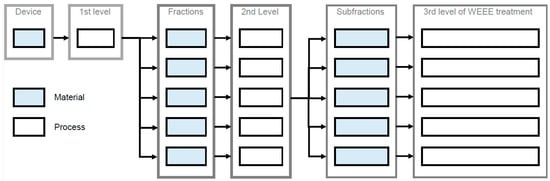
Figure 1.
Nomenclature and three levels of WEEE treatment: Please note that, merely for the third fraction, subfractions are created in this exemplary flow.
2.4. Material Recovery Qualities
The quality and application of the recycled materials can vary strongly. Consequently, the recycling rate is separated into multiple qualities. Four categories are “circular”, “recycled”, “alternate material recovery” and “lost”. The “circular” quality describes the rate of recovered material which can be used for the substitution of the corresponding virgin material of equal quality in the same product. The materials Ag, Au, Co, Cu, Li, Mn, Sn, W, and Zn are considered “circular”. The “recycled” quality describes the rate of recovered material which can substitute virgin material with a lower quality level. For example, aluminum recycled from the WEEE waste stream cannot be used in the form of the same alloy due to a lack of sorting, but it can substitute other virgin aluminum. The “alternate material recovery” quality describes the rate at which a material can replace virgin materials that are not identical to the recovered materials. The material is not directly recovered as the same material but it is eventually used in a new product. An example is aluminum in steel smelting. It is oxidized to Al2O3 and ends up in the slag, which is utilized in road construction. “Downcycling”, e.g., recovering high-quality glass from electronics in the form of foam glass or low-quality polymer regranulates which end up in park benches or pipes, is also included in the “alternate material recycling” (AMR) rate. Materials are considered “lost” if they are used for energy recovery or landfilled.
2.5. Dismantling Scenarios
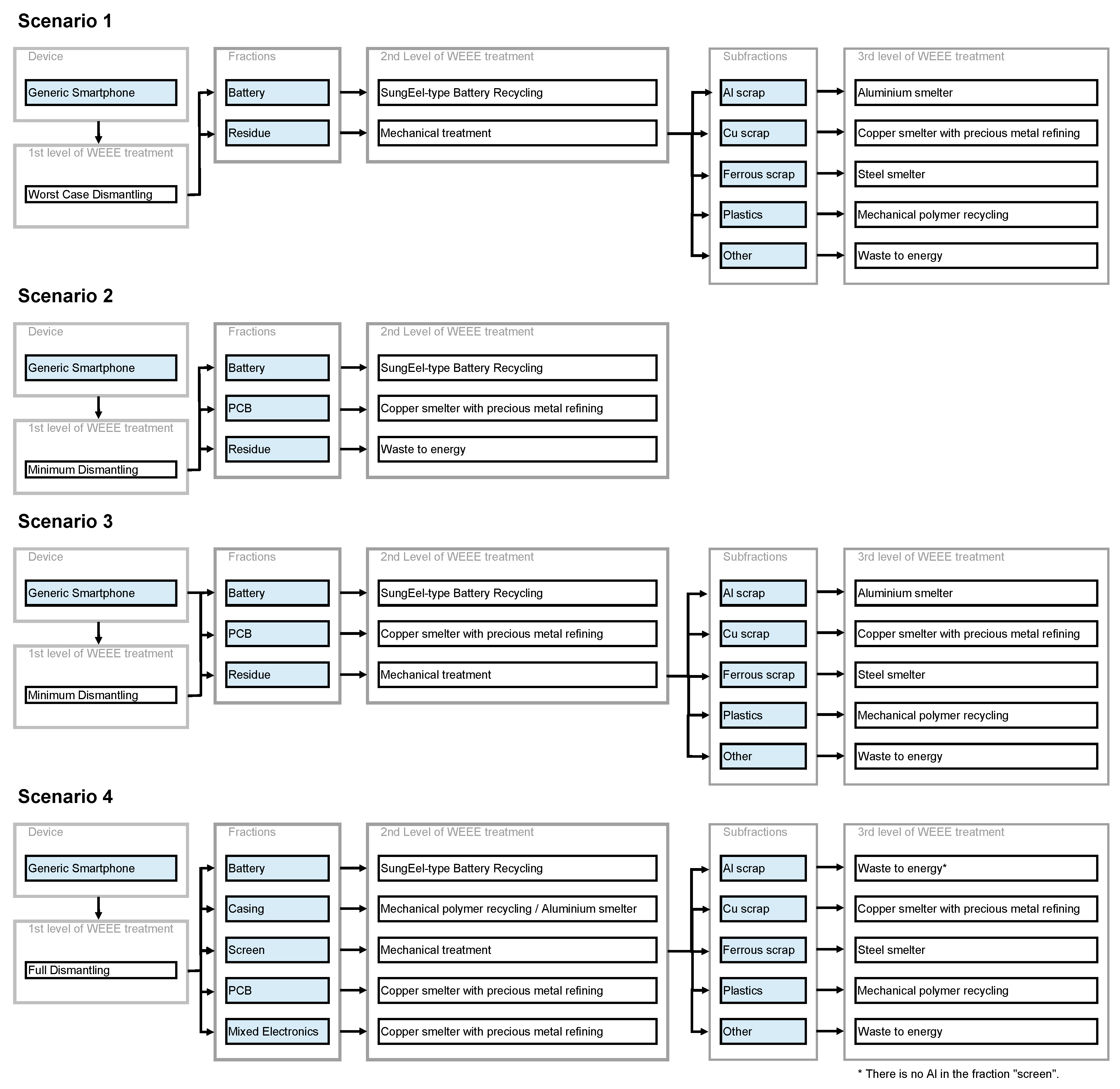
Three dismantling options are investigated herein: Worst case dismantling, minimum dismantling and full dismantling. Note that the first is non-compliant with the WEEE directive. It represents the situation prior to the adaption of the directive with the mere removal of the battery of laptops or phones due to safety reasons. The second option represents the minimum required by the WEEE directive [15], i.e., the removal and separate treatment of batteries, PCBs > 10 cm2 and LCDs > 100 cm2. Full dismantling describes the manual disassembly of the whole device to the deepest practical possible level. For example, this is represented by Apple’s recycler guides [26]. In the case study below, the outputs of full dismantling are battery, casing, screen, PCB and mixed electronics for a generic smartphone.
2.6. Description of Recycling Processes
In the following, the recycling processes relevant for WEEE treatment covered by this contribution are described. The respective recycling rate database is found in the supporting information (Tables S3–S16).
2.6.1. Mechanical Treatment
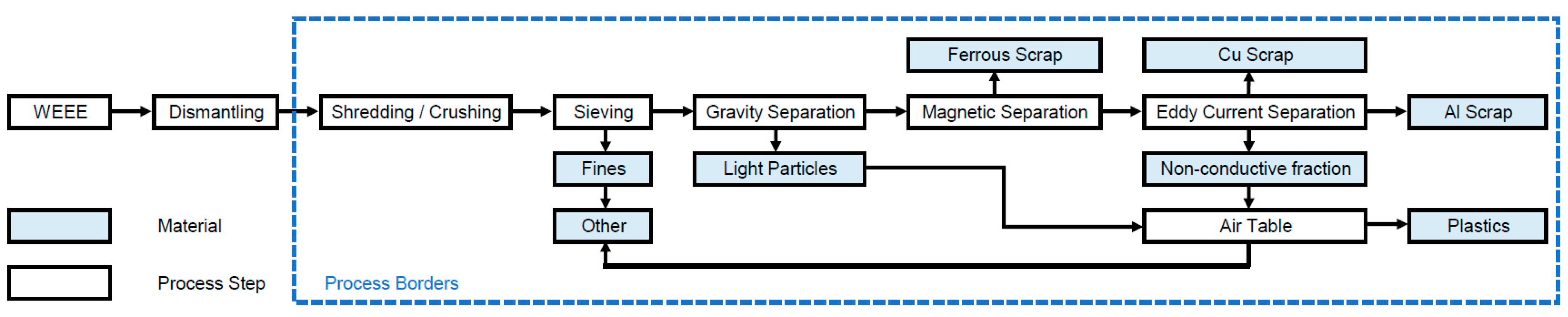
During mechanical treatment, also called shredding and sorting, size reduction to particle sizes below 20 mm [27] by shredders or crushers is followed by sorting. Sorting technologies are sieving, hydrocyclones, air classification, flotation, jigging, electrostatic sorting, magnetic separation and eddy current separation [28]. The flow chart for the mechanical treatment is shown in Figure 2. Separation can be expected to result in 5 subfractions [23,28,29,30,31]: Al scrap, Cu scrap, ferrous scrap, plastics and other (including fines and glass). Naturally, materials can end up in the “wrong” subfractions due to imperfect sorting. This aspect is included in the calculations.
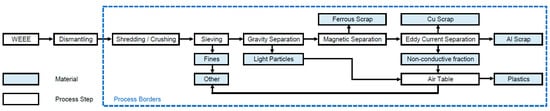
Figure 2.
Flow chart for the recycling process mechanical treatment.
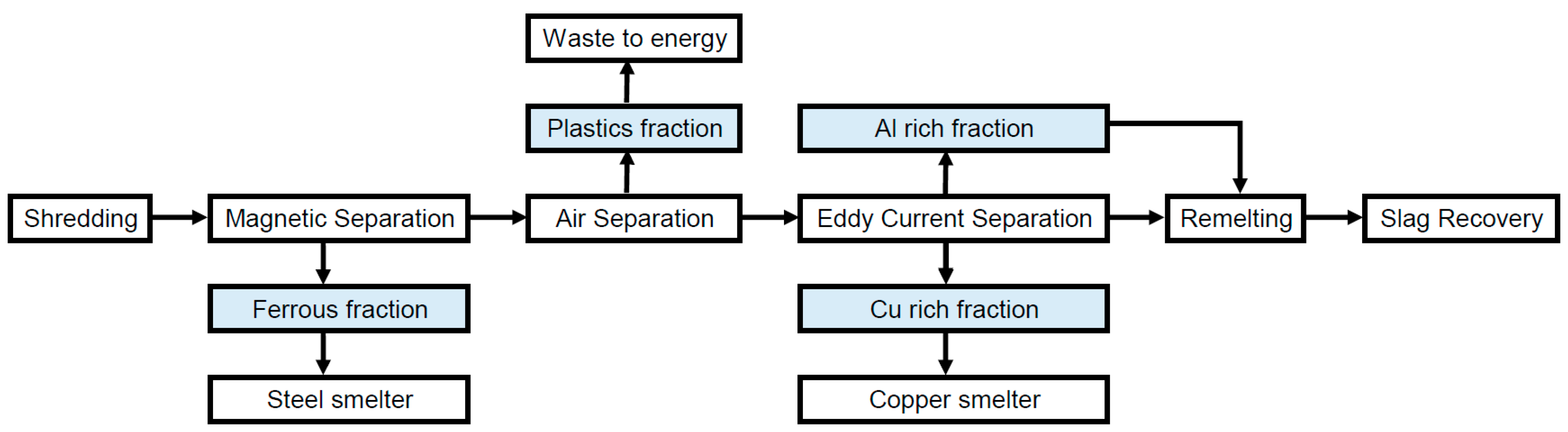
2.6.2. Aluminum Smelter
Aluminum scrap is remelted in aluminum production using scrap instead of raw ore [32]. Secondary aluminum production requires sorting steps prior to that [33,34]. The aluminum output fraction emerging from mechanical treatment or the manual dismantling of WEEE is pretreated prior to smelting. Magnetic and air separation as well as eddy current separation are state of the art [35,36]. Consequently, any ferrous metals and plastics are separated and other metals like Cu, Zn, Au, Ag and others will not end up in the aluminum melt. For the ferrous metal stream, subsequent treatment via the recycling process “steel smelter” and for the Cu-rich stream, subsequent treatment via the recycling process “copper smelter” are assumed in the model. No material recycling is performed for the plastics stream. Figure 3 shows the steps included in the recycling process “aluminum smelter”. Aluminum production generates salt slag. Slag recovery is widely performed due to legal requirements and ecological reasons [35]. The remains are mainly Al2O3 which is used as raw material for cement clinker, ceramics and other applications. All materials that cannot be used as alloy elements in aluminum alloys are expected to end up in the slag. A salt slag recovery rate of 100% is assumed for the calculations [34,37].
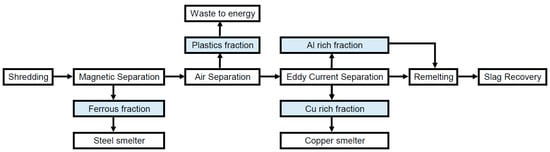
Figure 3.
Flow chart for the recycling process aluminum smelter (details in text).
2.6.3. Copper Smelter with Precious Metal Refining
In the calculations, copper smelting is expected to be followed by precious metal refining for all treated materials. This recycling process is modeled based on the ones performed by Aurubis and Umicore. This includes shredding, smelting, copper leaching, electrowinning and precious metals refining [38,39]. A slag recovery rate of 100% is assumed in the model. Organic materials act as both a reducing agent and fuel in this process [40]. The former is considered an alternate material recovery while the latter represents pure energy recovery and is therefore considered lost. The ratio between these two is set to 50:50 [24].
2.6.4. Steel Smelter
Ferrous scrap, i.e., iron scrap and steel scrap, also from WEEE input, is usually recycled in steel smelting in electric arc furnaces (EAFs) [32]. In contrast to aluminum scrap during the recycling process “aluminum smelter”, ferrous scrap is not commonly subject to further mechanical treatment during the recycling process “steel smelter”. Thus, it can be assumed that every material inserted into the recycling process “steel smelter” enters the smelting process. Steel alloy elements (Al, Co, Cu, Mn, Ni, Si, Ta, Ti, W) are rarely recycled in steel smelting due to a lack of sorting [41,42]. The metals Al, Ga, Li, Mg, Mn, Nd, Ta, Ti and Zn act as reducing agents in steel smelting and end up in slag in their oxidized forms (Al2O3, et cetera) [24]. A slag recovery rate of 91% is assumed herein [43]. Contamination plastics in steel shredding fractions from e-waste treatment may at least in parts act as reducing agents [44]. However, these are considered lost in line with the worst-case approach due to the lack of exact data of the use of plastics as reducing agents.
2.6.5. Battery Recycling
Three main recycling processes operating at the industrial scale (>1000 t/a) can be differentiated for lithium-ion-battery recycling [45,46,47,48]. These are represented by three distinct companies, Umicore, Accurec and SungEel, selected as examples. The respective process steps and the recycled materials are listed in Table 1 and Table 2. Naturally, these processes are only applicable for the input fraction “battery”. Similarly to the pyrometallurgical process “copper smelter with precious metal refining”, a slag recovery rate of 100% is assumed for the pyrometallurgical process steps in battery recycling. So-called direct recycling represents a fourth option for lithium-ion battery recycling [49,50]. It is an emerging technology, which has not been performed on an industrial scale to date and can therefore not be considered the best available technology.

Table 1.
Process steps and techniques in battery recycling processes.

Table 2.
Recycled materials per battery recycling process type.
Umicore-Type Battery Recycling
The Umicore process includes smelting, leaching and precipitation. Iron and copper are recovered as Fe(OH)2 or Fe(OH)3 and CuS or Cu2S, respectively. This is followed by solvent extraction. Then, CoCl2 and Ni(OH)2 are recovered via precipitation from the Co-rich and the Ni-rich stream, respectively [51].
Accurec-Type Battery Recycling
The Accurec process starts with pyrolysis and mechanical separation, after which the steel, Al and Cu fractions are separated. The remainder is treated pyrometallurgically for Ni and Co recycling (including hydrometallurgical final steps) while Li is recycled via hydrometallurgical methods from the produced slag [52]. The final treatments of Al, Fe and Cu in the processes “aluminum smelter”, “copper smelter with precious metal refining” and “steel smelter” are assumed while the yield from the Umicore process for batteries above is used for the final pyro- and hydrometallurgical treatments for both Co and Ni.
SungEel-Type Battery Recycling
The SungEel process includes mechanical pretreatment and hydrometallurgical processing. After grinding into water, the material is separated mechanically into ferrous or non-ferrous metals and active mass. The former two are forwarded to steel and copper smelting facilities, respectively. The latter undergoes an in-house hydrometallurgical process recovering cobalt, nickel, manganese and lithium, mainly as sulfates [53].
2.6.6. Mechanical Polymer Recycling
The recycling process “mechanical polymer recycling” unites multiple recycling processes for specific polymers. The polymers ABS, PC, PC + ABS, TPU and PET can be recycled mechanically in the form of regranulates. However, only the first three are recycled from WEEE to date [20,54]. The sensor-based sorting of the “plastics” waste stream into the respective polymers is included with its respective size limitations [55]. In general, 50% of regranulates are of inferior quality and therefore downcycled [56]. More specific data are only available for pure ABS [20].
2.6.7. Glass Recycling
Clean glass fractions can be used as glass cullet in glass production. Commonly, recycled glass from WEEE is used as foam glass or in other low-value applications [57]. Therefore, all glass recycling from WEEE can be considered downcycling.
2.6.8. Waste to Energy
This recycling process represents the incineration of waste for the generation of electricity and/or heat. Thus, energy is recovered. However, a material recycling rate of 0% (and 0% alternate material recovery) is implemented for this recycling process.
2.6.9. Additional Recycling Processes
Additional recycling processes are described in the supporting information on pages S4 to S6.
3. Results and Discussion
3.1. Recycling Rates Database
Available data for recyclability calculations are scarce. It is in the nature of the recycling industry that, on the one hand, data are very often kept confidential and, on the other hand, the recycling efficiency on the level of single devices and materials does not add relevant data for the optimization of the overall input to output ratio. Even in a few cases of systematic research into the topic [17,18,20,21,22,23,24,27,58,59,60], the results are often limited to a few “important” metals or other specific materials of interest. Consequently, the data are very limited and its public availability is even more restricted. Thus, the recycling rate database presented here is based on an extensive literature study as well as various, well-defined assumptions (Methods: 2. Yields of recycling processes). The database is found in the supporting information (Tables S3–S16). To the best of our knowledge, no similar database has been published so far. The database exceeds any previously published data collection, in terms of both the number of recycling processes and the number of considered materials.
Fraunhofer IZM Dept. Environmental & Reliability Engineering performed an internal critical review and verification of these data and the methodology used, including a promotion of improving data availability and data representativeness. The recycling rate database offers plausible, realistic and appropriate recycling rates to calculate the recyclability of electric devices, especially from the WEEE directive’s categories 2 and 6.
The mass flow-based approach including typical process losses is considered a sufficiently precise approximation for calculating recycling rates. However, it is acknowledged that simulation-based approaches would lead to even more accurate calculations. The use of published separation and recycling rates for the material flow approach enhances the transparency of the methodology justifying such an approach.
3.2. Case Study: Smartphone
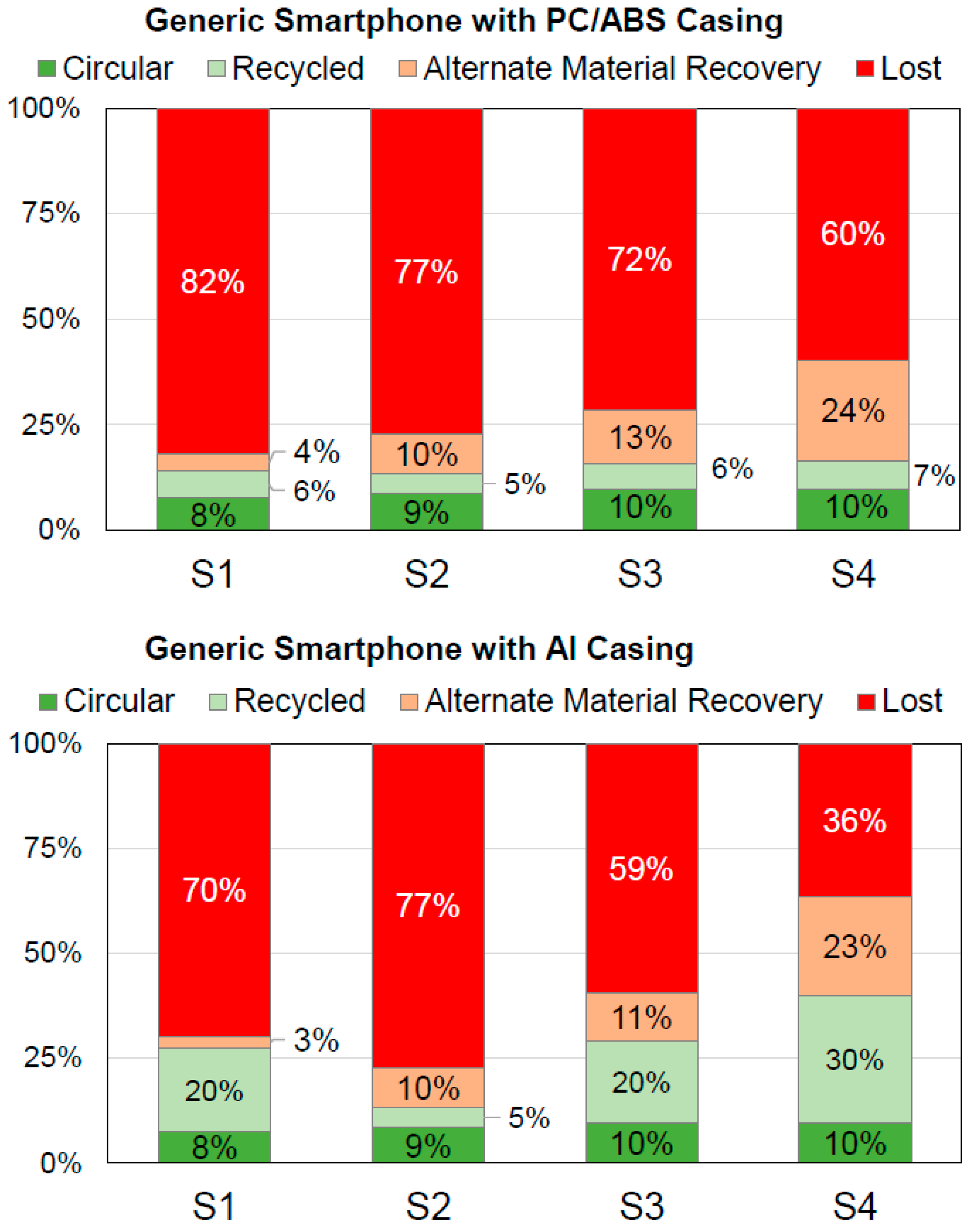
In order to showcase the potential of both the data and methodology presented herein, the recyclability of typical smartphones is calculated as a case study. For a hypothetical generic smartphone, the bill of materials (BOM) was taken from the literature with the battery from [61] and the rest of the smartphone from [62]. This smartphone comprises a casing made of PC/ABS. Additionally, a second smartphone with a casing made of aluminum identical in weight is considered. The respective BOMs are found in Tables S17 and S18. Using these, four different exemplary end of life (EoL) treatment scenarios were investigated and the respective recyclability values were calculated. The four scenarios’ flow charts are shown in Figure 4. Figure 5 depicts the overall recyclability at the device level in the four categories for these scenarios and both generic smartphones. The data for Figure 5 (Tables S19 and S20) and material-specific recyclability values can be found in the supporting information (Figures S1–S8 and Tables S21–S28).
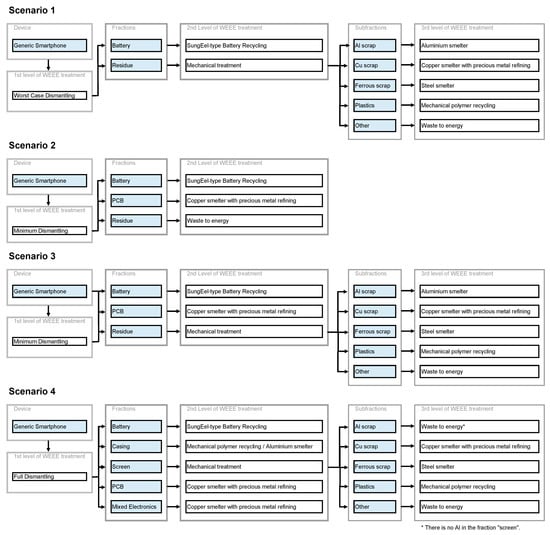
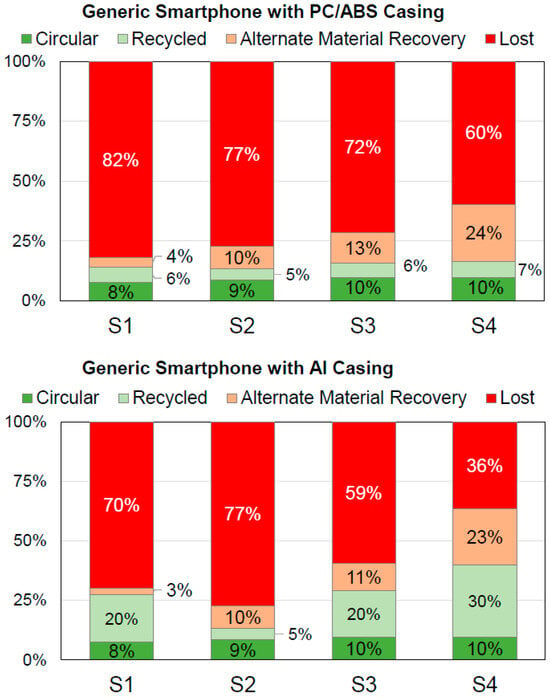
Figure 5.
Recyclability at the device level in the four categories for the four scenarios (S1 to S4) for the generic smartphones with PC/ABS casing (top) and Al casing (bottom).
Scenario S1 represents the situation prior to the adaption of the WEEE directive including formal state-of-the-art treatment and the mere depollution of the battery due to safety issues, while scenarios S2 and S3 stand for the minimum requirements from the WEEE directive. In S2, the residue is not treated further, while the technically possible maximum is used in S3. The former is more likely for low-value electronics or highly heterogeneous waste streams, and can be considered the worst-case scenario when processing in the EU. Scenario S4 represents the best-case with full dismantling and the best available treatment technologies for each fraction.
Regarding the results (Figure 5), multiple aspects are of interest. In the first and shortened assessments, the material flows in the categories “circular” and “recycled” are considered the total recyclability. In general, the smartphone with Al casing shows higher recyclability due to the higher efficiency of aluminum recycling compared to mechanical polymer recycling. Therefore, the use of aluminum as a casing material can be considered a design for recycling approach. However, formal and state-of-the-art WEEE treatment (scenarios S1, S3 and S4) is required. Otherwise, the “real” recyclability is not improved. The best effect is observed for scenario S4 due to the separation of the whole Al casing during dismantling. The total recyclability is increased from 16.3% for the smartphone with PC/ABS casing to 40.0% for the smartphone with Al casing. At this point, it is worth noticing that the recyclability assessment herein is based on the mass-balance of materials, only. It is not possible to draw any direct correlation between higher recyclability and lower environmental impact due to, e.g., differing energy demands for processing and transportation for different recycling scenarios. Consequently, the question of whether the use of an aluminum smartphone casing can be considered not only as a design for recycling, but also an ecodesign approach, would need further information based on assessments of the environmental impact of the respective recycling scenario, e.g., from a life cycle assessment (LCA). This beyond the scope of this case study.
In general, and for both devices, the expected trend of higher recyclability with increased dismantling efforts could be demonstrated (Figure 5). However, formal state-of-the-art mechanical WEEE treatment also has huge potential in terms of increasing the recyclability. This is shown by scenario S1 having higher recyclability rates than S2 for the smartphone with Al casing (total recyclability 27.5% vs. 13.3%). Furthermore, the comparison of S3 with S1 shows the effect of WEEE directive’s requirements of minimum dismantling on recyclability. It is increased from 13.9% to 15.8% for the smartphone with PC/ABS casing and from 27.5% to 29.3% for the smartphone with Al casing. Additionally, the share of the material flow to the “alternate material recovery” increases by 8.6% for both smartphones. This confirms the legitimacy of the directive’s dismantling requirements. A more ambiguous situation is found by comparing S3 and S4. Both scenarios differ in the depth of dismantling followed by equal state-of-the-art downstream processing. More time- and labor-intense full dismantling in S4 leads to minor improvements in the total recyclability of the smartphone with PC/ABS casing (15.8% vs. 16.3%). The components dominating the recyclability are already separated by the dismantling according to the WEEE directive’s minimum requirements. The results for the smartphone with Al casing differ strongly. Here, the recyclability rate is much higher for S4 than S3 (40.0% vs. 29.3%) due to the aluminum casing being treated separately after dismantling in the former and since the casing accounts for almost 30% of total weight of this smartphone.
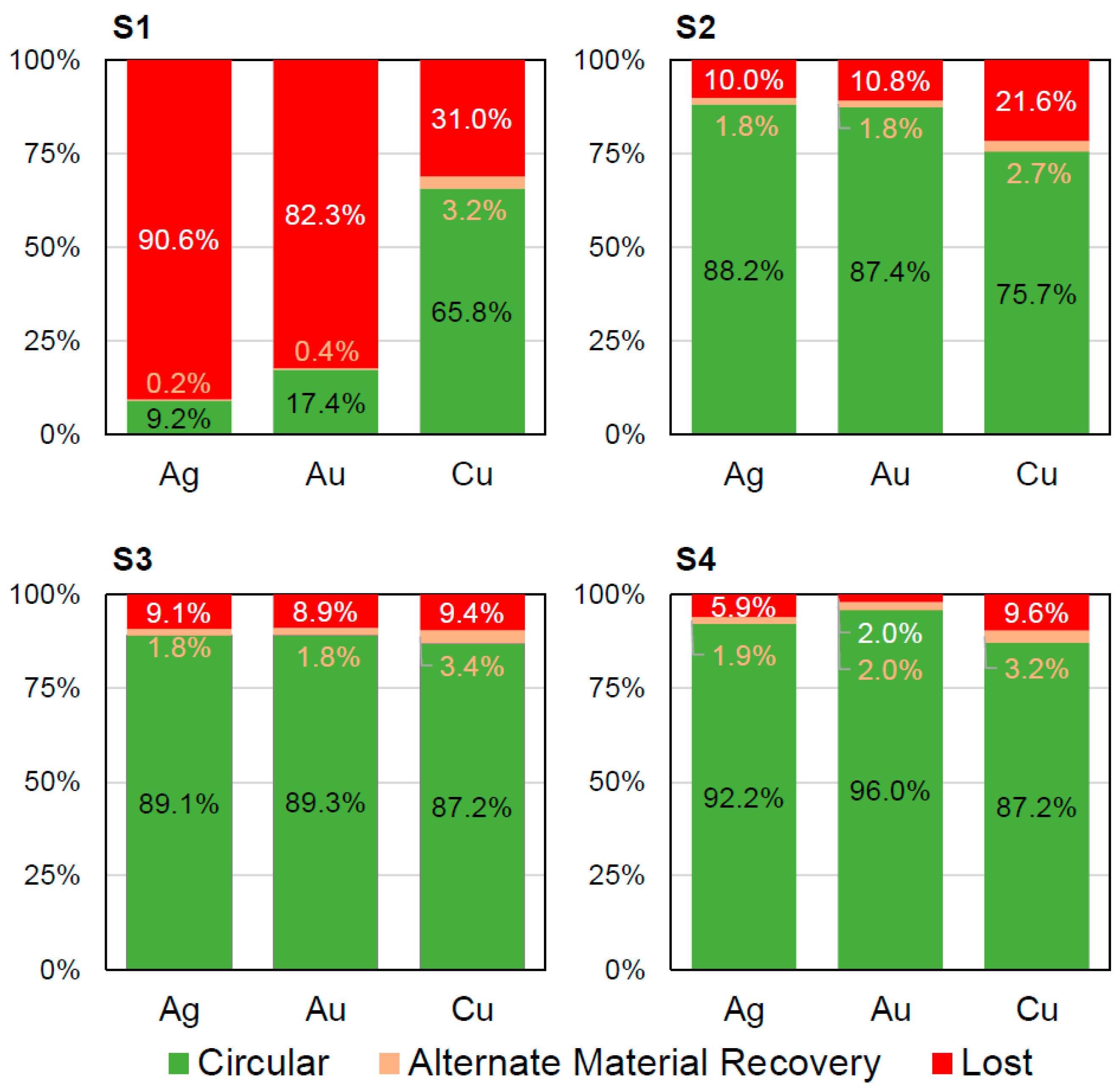
Overall, the material flow in the “circular” category is below 10% for all cases at the device level (Figure 5). It is only slightly increased from 8.6% for scenario S2 with minimal dismantling and no formal mechanical WEEE treatment to 9.7% for both scenarios S3 and S4 including formal mechanical treatment. The additional full dismantling in scenario S4 has no effect on the material flow in the “circular” category when compared to scenario S3. This highlights the huge room for improvements in the design for circularity and improved formal recycling infrastructure. Increasing the material flow in the “circular” category is essential on the way towards a circular economy for smartphones and, thus, should be in the interest of all stakeholders. At the material level (Figures S1–S8), strongly varying values are found for different materials. Figure 6 shows that metals like silver, gold and copper have materials flows of over 80% in the “circular” category in the case of formal state-of-the-art mechanical WEEE treatment (scenarios S2–S4). The lack of the separate treatment of the PCB in scenario S1 decreases the recyclability of silver and gold dramatically. In contrast, over 90% of the polymer’s material flows (namely PC, PC/ABS, PET and PMMA) are in the “lost” category in all four scenarios (Figures S1–S8). This highlights that the database and methodology presented herein can act as a powerful tool in design for recycling and for optimizing the technical recyclability of dedicated materials.
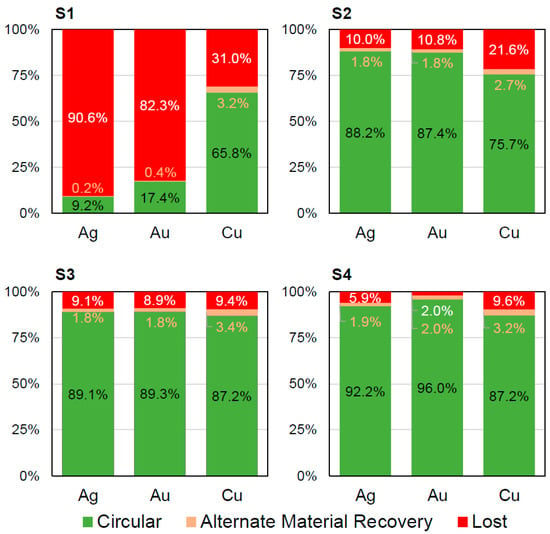
Figure 6.
Recyclability at the material level for the metals silver, gold and copper for the four scenarios (S1 to S4) for the generic smartphones: Please note that all three metals are considered “circular”. Thus, there is no “recycled” category. This level of material-specific recyclability values is found for all considered materials in the supporting information (Figures S1–S8 and Tables S21–S28).
Yet, technical recyclability does not account for collection rates and the fact that, based on size, scale, hazardous properties and economic feasibility, recyclers may not have the incentive to dismantle and send materials to all available facilities. A discussion on the challenges of the real recyclability to meet the technical recyclability could follow building on the results from the methodology, database and case study presented herein. However, this is beyond the scope of this work.
4. Conclusions
In this contribution, both a methodology and a corresponding database are presented to improve the calculation of the recyclability of EEE beyond EN 45555. The methodology is based on the existing recyclability calculation from EN 45555, but introduces four recycling categories, namely “circular”, “recycled”, “alternate material recovery” and “lost”, which are created to emphasize the quality of the recovered material. The extensive database, developed to cover the 30 most common materials used in electronics and 14 different recycling processes representing the best-available technologies (BATs), supports this methodology by providing the required data needed for calculation. The calculated technical recyclability or material recoverability values can aid product designers in their material selection and design decisions as one factor to consider in the more holistic product ecodesign spectrum.
The material choice and design of the electronic equipment has a strong influence on the final outcome and can alter the importance of the recycling pathway chosen. This indicates that there is no single best sequence of treatment steps for WEEE, but ideally it needs to be recreated for every product separately. Thus, when calculating and comparing the recyclability of EEE, e.g., for a recyclability index, the respective sequence of treatment processes has to be disclosed, since their choice will make a significant impact on the final material recyclability outcome. Consequently, investments in novel recovery technologies leading to new BATs will have a significant impact of the material recovery capabilities of the recycling industry. These aspects become obvious herein during the case study on two generic smartphones. The material selection, depth of dismantling and best available mechanical treatment of WEEE showed the largest influence on the technical recyclability in the case study. The minimum dismantling requirements defined by the WEEE directive cover the majority of components relevant for the overall recyclability of a smartphone, with more in-depth dismantling having only minor effects on recyclability—except in the case of the smartphone with aluminum casing where the recyclability of the casing is strongly increased by in-depth dismantling. These results from the case study showcase the potential of both the database and the methodology and their relevance in terms of design for recycling of electric and electronical devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16198726/s1, Table S1: Density data used for estimations of gravity separation efficiency; Table S2: Ratio of electric conductivity to density per material used for the estimation of electrostatic separation efficiency. Description of 10 additional recycling processes; Tables S3–S16: Recycling rate database; Tables S17 and S18: Bill of materials of generic smartphones used in the case study; Tables S19–S28 and Figures S1–S8: Recyclability results of case study. References [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.H. and K.W.; methodology, M.H.; investigation, M.H.; data curation, M.H.; writing—original draft preparation, M.H.; writing—review and editing, M.H. and K.W.; visualization, M.H.; supervision, K.W.; project administration, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented herein was funded by Apple.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
The authors acknowledge Dragancho Veljanovski (Apple) for his guidance and contributions to this study. A critical review of the presented recycling rate data was performed by Fraunhofer IZM and the authors thank Theresa Aigner, Max Tippner, Otmar Deubzer and Karsten Schischke for their valuable insights.
Conflicts of Interest
The authors declare no conflicts of interest. The funders were involved in the writing of the manuscript by approving the final version of the manuscript and the decision to publish the results was made together with the funders.
References
- Balde, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; D’Angelo, E.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-Dubilllo, E.; Forti, V.; et al. Global E-Waste Monitor 2024; ITU and UNITAR: Geneva, Switzerland; Bonn, Germany, 2024. [Google Scholar]
- Kostenlose Recycling Programme, Brother International GmbH. Available online: https://www.brother.de/recycling (accessed on 15 July 2024).
- Welcome to Epson’s Cartridge Collection & Recycling Programme, Epson. Available online: https://epson-recycling.cycleon.eu/en/home (accessed on 15 July 2024).
- Apple Trade In, Apple Inc. Available online: https://www.apple.com/shop/trade-in (accessed on 15 July 2024).
- Samsung Trade In, SAMSUNG. Available online: https://www.samsung.com/us/trade-in/ (accessed on 15 July 2024).
- Recycle Your Old Phone(s), Fairphone. Available online: https://shop.fairphone.com/recycle (accessed on 15 July 2024).
- E-Bike Battery Recycling. Available online: https://www.hungryforbatteries.org/ (accessed on 15 July 2024).
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Meskers, C.; Worrell, E.; Reuter, M.A. (Eds.) Handbook of Recycling: State-of-the-Art for Practitioners, Analysts, and Scientists, 2nd ed.; Elsevier: San Diego, CA, USA, 2023; ISBN 978-0-323-85514-3. [Google Scholar]
- Directive 2009/125/EC; Ecodesign Directive: Latest Consolidated Vers.: 2012-12-04. European Union: Brussels, Belgium, 2012.
- Regulation (EU) 2024/1781; Ecodesign for Sustainable Products Regulation (ESPR): Latest Consolidated Vers.: 2024-06-28. European Union: Brussels, Belgium, 2024.
- EN 45555:2020; General Methods for Assessing the Recyclability and Recoverability of Energy-Related Products. Slovenian Institute for Standardization: Ljubljana, Slovenia, 2020.
- Mählitz, P.M.; Korf, N.; Chryssos, G.; Rotter, V.S. Contributions of extended batch tests for assessing technical recyclability: A case study of low-value battery flows. J. Ind. Ecol. 2022, 26, 1061–1077. [Google Scholar] [CrossRef]
- Pomberger, R. Über theoretische und reale Recyclingfähigkeit. Österr. Wasser-und Abfallwirtsch. 2021, 73, 24–35. [Google Scholar] [CrossRef]
- Directive 2012/19/EU; Waste Electrical and Electronic Equipment (WEEE): Latest Consolidated Vers.: 2024-04-08. European Union: Brussels, Belgium, 2024.
- Meskers, C.; Hagelüken, C. The impact of different pre-processing routes on the metal recovery from PCs. In Twin World Congress and World Resources Forum “Resource Managemant and Technology for Material and Energy Efficience”; Hilty, L.M., Itoh, H., Hayashi, K., Edelmann, X., Eds.; EMPA Materials Science and Technology: Davos, Switzerland, 2009. [Google Scholar]
- Marra, A.; Cesaro, A.; Belgiorno, V. Separation efficiency of valuable and critical metals in WEEE mechanical treatments. J. Clean. Prod. 2018, 186, 490–498. [Google Scholar] [CrossRef]
- Marra, A.; Cesaro, A.; Belgiorno, V. WEEE Mechanical Treatments: Recovery Effectiveness of Critical Materials. In Proceedings of the 14th International Conference on Environmental Science and Technology, Rhodes, Greece, 3–5 September 2015. [Google Scholar]
- Morf, L.; Taverna, R.; Stengele, M. Metallische und Nichtmetallische Stoffe im Elektronikschrott; BUWAL Schweiz; Schriftenreihe Umwelt No. 374; BUWAL: Bern, Switzerland, 2004. [Google Scholar]
- Cardamone, G.F.; Ardolino, F.; Arena, U. About the environmental sustainability of the European management of WEEE plastics. Waste Manag. 2021, 126, 119–132. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Geiping, J.; Zamzow, M.; Flamme, S.; Rotter, V.S. Assessment of element-specific recycling efficiency in WEEE pre-processing. Resour. Conserv. Recycl. 2017, 124, 25–41. [Google Scholar] [CrossRef]
- Westphal, L. Entwicklung eines Technischen Konzepts zum Recycling von Neodym-Eisen-Bor-Magneten am Beispiel von Elektro- und Elektronikaltgeräten. Doctoral Dissertation, TU Hamburg-Harburg, Hamburg, Germany, 2015. [Google Scholar]
- Horta Arduin, R.; Grimaud, G.; Martínez Leal, J.; Pompidou, S.; Charbuillet, C.; Laratte, B.; Alix, T.; Perry, N. Influence of scope definition in recycling rate calculation for European e-waste extended producer responsibility. Waste Manag. 2019, 84, 256–268. [Google Scholar] [CrossRef]
- Chancerel, P.; Marwede, M.; Mathieux, F.; Talens Peiró, L. Feasibility Study for Setting-Up Reference Values to Support the Calculation of Recyclability/Recoverability Rates of Electr(on)ic Products; EUR 27922 EN; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Goyal, N.; Goyal, D. Exploring E-waste Management: Strategies and Implications. In Handbook of Solid Waste Management: Sustainability through Circular Economy, 1st ed.; Baskar, C., Ramakrishna, S., Baskar, S., Sharma, R., Chinnappan, A., Sehrawat, R., Eds.; Imprint Springer: Singapore, 2022; pp. 1559–1572. ISBN 9789811642302. [Google Scholar]
- Apple Recycler Guides, Apple Inc. Available online: https://www.apple.com/recycling/recycler-guides/ (accessed on 15 July 2024).
- Cesaro, A.; Marra, A.; Belgiorno, V.; Guida, M. Effectiveness of WEEE mechanical treatment: Separation yields and recovered material toxicity. J. Clean. Prod. 2017, 142, 2656–2662. [Google Scholar] [CrossRef]
- Bruch, J.-R.; Bokelmann, K.; Grimes, S.M. Process development options for electronic waste fractionation to achieve maximum material value recovery. Waste Manag. Res. 2022, 40, 54–65. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.E.; Song, S. Recycling Technologies—Physical Separation. In Electronic Waste: Recycling and Reprocessing for a Sustainable Future; Holuszko, M.E., Kumar, A., Espinosa, D.C.R., Eds.; Wiley: Weinheim, Germany, 2022; pp. 95–134. ISBN 9783527344901. [Google Scholar]
- de Oliveira, C.M.; Bellopede, R.; Tori, A.; Zanetti, G.; Marini, P. Gravity and Electrostatic Separation for Recovering Metals from Obsolete Printed Circuit Board. Materials 2022, 15, 1874. [Google Scholar] [CrossRef]
- Batinic, B.; Vaccari, M.; Savvilotidou, V.; Kousaiti, A.; Gidarakos, E.; Marinkovic, T.; Fiore, S. Applied WEEE pre-treatment methods: Opportunities to maximizing the recovery of critical metals. Glob. NEST J. 2019, 20, 706–711. [Google Scholar] [CrossRef]
- Manfredi, S.; Nuss, P.; Passarini, F.; Ciacci, L. Material Flow Analysis of Aluminium, Copper, and Iron in the EU-28; Publications Office: Luxembourg, 2018; ISBN 978-92-79-85744-7. [Google Scholar]
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Liesegang, M.; Bookhagen, B. Status Quo des Recyclings bei der Metallerzeugung und-Verarbeitung in Deutschland: Recyclingatlas für die Metallerzeugung; Datenstand: August 2023; Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe (BGR): Berlin, Germany, 2023; ISBN 978-3-948532-79-6. [Google Scholar]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving aluminum recycling: A survey of sorting and impurity removal technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Yontem, E.; Ozdogru, Z. D1.3: Inventory of Raw materials, Waste, and Energy Flows in Industrial Sectors (Metallurgic, Ceramic, Glass, Chemical, and Natural Stone) Considered in a Dynamic Scenario. Public Executive Summary, Horizon 2020 Project FISSAC, 2016. Available online: https://fissacproject.eu/wp-content/uploads/2018/06/FISSAC-D1.3-Inventory-of-raw-materials-waste-and-energy-flows-in-industrial-sectors-Summary.pdf (accessed on 12 September 2023).
- Rocchetti, L.; Amato, A.; Beolchini, F. Printed circuit board recycling: A patent review. J. Clean. Prod. 2018, 178, 814–832. [Google Scholar] [CrossRef]
- Hagelueken, C. Recycling of Electronic Scrap at Umicore Precious Metals Refining. Acta Metall. Slovaca 2006, 12, 111–120. [Google Scholar]
- Brusselaers, J.; Mark, F.E.; Tange, L. Using Metal-Rich WEEE Plastics as Feedstock/Fuel Substitute for and Integratedmetals Smelter; Technical report produced by PlasticsEurope incooperation with Umicore and EFRA; PlasticsEurope: Brussels, Belgium, 2006. [Google Scholar]
- Bowyer, J.; Bratkovich, S.; Fernholz, K.; Frank, M.; Groot, H.; Howe, J.; Pepke, E. Understanding Steel Recovery and Recycling Rates and Limits to Recycling; Dovetail Partners Inc.: Minneapolis, MN, USA, 2015. [Google Scholar]
- Ohno, H.; Matsubae, K.; Nakajima, K.; Kondo, Y.; Nakamura, S.; Fukushima, Y.; Nagasaka, T. Optimal Recycling of Steel Scrap and Alloying Elements: Input-Output based Linear Programming Method with Its Application to End-of-Life Vehicles in Japan. Environ. Sci. Technol. 2017, 51, 13086–13094. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef]
- Devasahayam, S.; Raman, R.K.S.; Chennakesavulu, K.; Bhattacharya, S. Plastics-Villain or Hero? Polymers and Recycled Polymers in Mineral and Metallurgical Processing-A Review. Materials 2019, 12, 655. [Google Scholar] [CrossRef]
- Wagner-Wenz, R.; van Zuilichem, A.-J.; Göllner-Völker, L.; Berberich, K.; Weidenkaff, A.; Schebek, L. Recycling routes of lithium-ion batteries: A critical review of the development status, the process performance, and life-cycle environmental impacts. MRS Energy Sustain. 2022, 10, 1–34. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Kaya, M. State-of-the-art lithium-ion battery recycling technologies. Circ. Econ. 2022, 1, 100015. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Xu, P.; Tan, D.H.S.; Jiao, B.; Gao, H.; Yu, X.; Chen, Z. A Materials Perspective on Direct Recycling of Lithium-Ion Batteries: Principles, Challenges and Opportunities. Adv. Funct. Mater. 2023, 33, 2213168. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A comprehensive review and classification of unit operations with assessment of outputs quality in lithium-ion battery recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Sojka, R.; Pan, Q.; Billmann, L. Comparative Study of Li-Ion Battery Recycling Processes; ACCUREC Recycling GmbH: Krefeld, Germany, 2020. [Google Scholar]
- Hool, A.; Schrijvers, D.; van Nielen, S.; Clifton, A.; Ganzeboom, S.; Hagelueken, C.; Harada, Y.; Kim, H.; Ku, A.Y.; Meese-Marktscheffel, J.; et al. How companies improve critical raw material circularity: 5 use cases. Miner. Econ. 2022, 35, 325–335. [Google Scholar] [CrossRef]
- Haarman, A.; Magalini, F.; Courtois, J. Study on the Impacts of Brominated Flame Retardants on the Recycling of WEEE Plastics in Europe. Available online: https://www.bsef.com/news/sofiesreport/ (accessed on 23 February 2024).
- Maisel, F.; Chancerel, P.; Dimitrova, G.; Emmerich, J.; Nissen, N.F.; Schneider-Ramelow, M. Preparing WEEE plastics for recycling—How optimal particle sizes in pre-processing can improve the separation efficiency of high quality plastics. Resour. Conserv. Recycl. 2020, 154, 104619. [Google Scholar] [CrossRef]
- Bendix, P.; Berg, H.; Sebestyén, J.; Ritthoff, M.; Perschel, L.; Eckert, D.; Kocina, R.; Achenbach, H. Promoting the high-Quality Recycling of Plastics from Demolition Waste and Enhancing the Use of Recycled Materials in Construction Products in Accordance with the European Plastics Strategy; Umweltbundesamt TEXTE 152/2021; Umweltbundesamt: Dessau-Roßlau, Germany, 2021. [Google Scholar]
- Wang, R.; Xu, Z. Recycling of non-metallic fractions from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2014, 34, 1455–1469. [Google Scholar] [CrossRef]
- Chancerel, P.; Meskers, C.; Hagelueken, C.; Rotter, S. E-scrap: Metals too precious to ignore. Recycl. Int. 2008, 31, 42–45. [Google Scholar]
- Nieberl, M.; Hornung, A.; Sajdak, M.; Majewski, A.J.; Ouadi, M. Application and recycling of tantalum from waste electric and electronic equipment–A review. Resour. Conserv. Recycl. 2023, 190, 106866. [Google Scholar] [CrossRef]
- Peelman, S.; Venkatesan, P.; Abrahami, S.; Yang, Y. Recovery of REEs from End-of-Life Permanent Magnet Scrap Generated in WEEE Recycling Plants. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 2619–2631. ISBN 978-3-319-95021-1. [Google Scholar]
- Roithner, C.; Cencic, O.; Rechberger, H. Product design and recyclability: How statistical entropy can form a bridge between these concepts—A case study of a smartphone. J. Clean. Prod. 2022, 331, 129971. [Google Scholar] [CrossRef]
- Gómez, M.; Grimes, S.; Qian, Y.; Feng, Y.; Fowler, G. Critical and strategic metals in mobile phones: A detailed characterisation of multigenerational waste mobile phones and the economic drivers for recovery of metal value. J. Clean. Prod. 2023, 419, 138099. [Google Scholar] [CrossRef]
- de Tommaso, J.; Dubois, J.-L. Risk Analysis on PMMA Recycling Economics. Polymers 2021, 13, 2724. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hirao, M.; Ookubo, T.; Sasaki, A. Design of recycling system for poly(methyl methacrylate) (PMMA). Part 1: Recycling scenario analysis. Int. J. Life Cycle Assess. 2014, 19, 120–129. [Google Scholar] [CrossRef]
- Böni, H.; Wäger, P.; Thiebaud, E.; Du, X.; Figi, R.; Nagel, O.; Bunge, R.; Stäubli, A.; Spörry, A.; Wolfensberger-Malo, M.; et al. Rückgewinnung von Kritischen Metallen aus Elektronikschrott am Beispiel von Indium und Neodym; Schlussbericht: St. Gallen, Switzerland, 2015. [Google Scholar]
- Auerbach, R.; Bokelmann, K.; Stauber, R.; Gutfleisch, O.; Schnell, S.; Ratering, S. Critical raw materials—Advanced recycling technologies and processes: Recycling of rare earth metals out of end of life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy. Miner. Eng. 2019, 134, 104–117. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Xia, L.; Wei, X.; Wang, H.; Ye, F.; Liu, Z. Valuable metal recovery from waste tantalum capacitors via cryogenic crushing-alkaline calcination-leaching process. J. Mater. Res. Technol. 2022, 16, 1637–1646. [Google Scholar] [CrossRef]
- Luidold, S. Herausforderungen beim Recycling wolframhaltiger Schrotte. In Recycling und Rohstoffe; Thomé-Kozmiensky, K.J., Thiel, S., Thomé-Kozmiensky, E., Goldmann, D., Eds.; TK-Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2017; pp. 155–164. ISBN 978-3-944310-34-3. [Google Scholar]
- Aromaa, R.; Rinne, M.; Lundström, M. Comparative Life Cycle Assessment of Hardmetal Chemical Recycling Routes. ACS Sustain. Chem. Eng. 2022, 10, 10234–10242. [Google Scholar] [CrossRef]
- Schischke, K.; Proske, M.; Ballester, M.; Reinhold, J.; Lang, K.-D.; Regenfelder, M. Strategies for More Circularity in the Life Cycle of Mobile Information Technology. In Proceedings of the Conference: Care Innovation 2018, Vienna, Austria, 26–29 November 2018. [Google Scholar]
- Frequently Asked Questions about BASF’s ChemCycling projectTM. Available online: https://www.basf.com/global/en/who-we-are/sustainability/we-drive-sustainable-solutions/circular-economy/mass-balance-approach/chemcycling/FAQ_ChemCycling.html#accordion_v2-fb6236637e-item-3ef8b61ec6 (accessed on 15 July 2024).
- Zeller, M.; Netsch, N.; Richter, F.; Leibold, H.; Stapf, D. Chemical Recycling of Mixed Plastic Wastes by Pyrolysis—Pilot Scale Investigations. Chem. Ing. Tech. 2021, 93, 1763–1770. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Lappas, A.A.; Achilias, D.S. Thermo-chemical recycling of plastics retrieved from waste electric and electronic equipment (WEEE) by pyrolysis: Identification of the polymer type, removal of bromine compounds from plastics based on an environmentally-friendly process and characterization of the pyrolysates. Sustain. Chem. Pharm. 2023, 35, 101210. [Google Scholar] [CrossRef]
- Maisels, A.; Hiller, A.; Simon, F.-G. Chemical Recycling for Plastic Waste: Status and Perspectives. ChemBioEng Rev. 2022, 9, 541–555. [Google Scholar] [CrossRef]
- Takeda, O.; Ouchi, T.; Okabe, T.H. Recent Progress in Titanium Extraction and Recycling. Met. Mater. Trans. B 2020, 51, 1315–1328. [Google Scholar] [CrossRef]
- Jing, J.; Guo, Y.; Wang, S.; Chen, F.; Yang, L.; Qiu, G. Recent Progress in Electric Furnace Titanium Slag Processing and Utilization: A Review. Crystals 2022, 12, 958. [Google Scholar] [CrossRef]
- Rotmann, B.; Friedrich, B. Challenges in Titanium Recycling-Do We Need a Specification for Secondary Alloys? In Proceedings of the Poster EMC 2011, Düsseldorf, Germany, 26–28 June 2011. [Google Scholar] [CrossRef]
- Fröhlich, P.; Lorenz, T.; Martin, G.; Brett, B.; Bertau, M. Valuable Metals-Recovery Processes, Current Trends, and Recycling Strategies. Angew. Chem. Int. Ed. 2017, 56, 2544–2580. [Google Scholar] [CrossRef]
- de Oliveira, R.P.; Benvenuti, J.; Espinosa, D. A review of the current progress in recycling technologies for gallium and rare earth elements from light-emitting diodes. Renew. Sustain. Energy Rev. 2021, 145, 111090. [Google Scholar] [CrossRef]
- Wittmer, D.; Erren, M.; Lauwigi, C.; Ritthoff, M.; Dressler, C. Umweltrelevante Metallische Rohstoffe: Meilensteinbericht des Arbeitsschrittes 2.1 des Projekts “Materialeffizienz und Ressourcenschonung“ (MaRess); Wuppertal Institut für Klima: Wuppertal, Germany, 2011. [Google Scholar]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) from waste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Gao, S.; Xing, P.; He, Y.; Yan, S.; Yin, H. Purification and comprehensive utilization of sapphire kerf waste. J. Clean. Prod. 2019, 214, 248–258. [Google Scholar] [CrossRef]
- Zhou, L. Recycling Method of Residual Crystal Scrap after Processing of Sapphire Crystal. CN104131354A, 5 November 2014. [Google Scholar]
- Burlingame, N.H.; Burlingame, S. Method for Recycling of Rare Earth and Zirconium Oxide Materials. US 8,940,256 B2, 27 January 2015. [Google Scholar]
- Saffirio, S.; Pylypko, S.; Fiorot, S.; Schiavi, I.; Fiore, S.; Santarelli, M.; Ferrero, D.; Smeacetto, F.; Fiorilli, S. Hydrothermally-assisted recovery of Yttria- stabilized zirconia (YSZ) from end-of-life solid oxide cells. Sustain. Mater. Technol. 2022, 33, e00473. [Google Scholar] [CrossRef]
- Römer, F.; Elwert, T.; Goldmann, D. Challenges and a Possible Solution for the Recycling of Tantalum from Waste Electronical and Electronic Equipment. In Proceedings of the IMPC 2016: XXVIII International Mineral Processing Congress; IMPC 2016: XXVIII International Mineral Processing Congress, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Buchert, M.; Jenseit, W.; Merz, C.; Schüler, D. Ökobilanz zum “Recycling von Lithium-Ionen-Batterien” (LithoRec); Endbericht: Darmstadt, Germany, 2011. [Google Scholar]
- Feige, R.; Merker, G. SEROX—Ein Synthetischer Al-Glasrohstoff. In Proceedings of the Sitzung des Fachausschusses III “Glasrohstoffe und Glasschmelze” der Deutschen Glastechnischen Gesellschaft (DGG), Würzburg, Germany, 11 October 2006. [Google Scholar]
- Hagelueken, C.; Buchert, M. The Mine above Ground—Opportunities & Challanges to Recover Scarce and Valuable Metals from EOL Electronic Devices; IERC: Salzburg, Austria, 2008. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Tecchio, P.; Ardente, F.; Marwede, M.; Christian, C.; Dimitrova, G.; Mathieux, F. Analysis of Material Efficiency Aspects of Personal Computers Product Group; EUR 28394 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-64943-1. [Google Scholar]
- Huisman, J. QWERTY and Eco-Efficiency Analysis on Cellular Phone Treatment in Sweden; Delft University of Technology: Delft, The Netherlands, 2004. [Google Scholar]
- Deubzer, O. Explorative Study into the Sustainable Use and Substitution of Soldering Metals in Electronics: Ecological and Economical Consequences of the the Ban of Lead in Electronics and Lessons to Be Learned for the Future. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2007. [Google Scholar]
- Schlesinger, M.E.; King, M.J.; Sole, K.C. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-08-096789-9. [Google Scholar]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Lennartsson, A.; Engström, F.; Samuelsson, C.; Björkman, B.; Pettersson, J. Large-Scale WEEE Recycling Integrated in an Ore-Based Cu-Extraction System. J. Sustain. Metall. 2018, 4, 222–232. [Google Scholar] [CrossRef]
- Forsén, O.; Aromaa, J.; Lundström, M. Primary Copper Smelter and Refinery as a Recycling Plant—A System Integrated Approach to Estimate Secondary Raw Material Tolerance. Recycling 2017, 2, 19. [Google Scholar] [CrossRef]
- Reuter, M.A.; van Schaik, A.; Gutzmer, J.; Bartie, N.; Abadías-Llamas, A. Challenges of the Circular Economy: A Material, Metallurgical, and Product Design Perspective. Annu. Rev. Mater. Res. 2019, 49, 253–274. [Google Scholar] [CrossRef]
- Mostaghel, S.; Samuelsson, C. Metallurgical use of glass fractions from waste electric and electronic equipment (WEEE). Waste Manag. 2010, 30, 140–144. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.; Qin, Y.; Li, X. Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate. Materials 2019, 12, 1591. [Google Scholar] [CrossRef]
- Harvey, L.D. Iron and steel recycling: Review, conceptual model, irreducible mining requirements, and energy implications. Renew. Sustain. Energy Rev. 2021, 138, 110553. [Google Scholar] [CrossRef]
- Alaoui Mouayd, A.; Koltsov, A.; Sutter, E.; Tribollet, B. Effect of silicon content in steel and oxidation temperature on scale growth and morphology. Mater. Chem. Phys. 2014, 143, 996–1004. [Google Scholar] [CrossRef]
- Weinmann, K. Factsheet: Electric Mobility and Recycling; Now GmbH: Broderstorf, Germany, 2020. [Google Scholar]
- Accurec GmbH. Battery Recycling Datasheet. 2018. Available online: https://accurec.de/wp-content/uploads/2018/04/Li-ion-RE_2018.pdf (accessed on 12 June 2024).
- Sojka, R. Europe’s First Lithium Recycling Plant. Press Release. 2022. Available online: https://accurec.de/europes-first-lithium-recycling-plant?lang=en (accessed on 15 July 2024).
- Randall, C. Accurec Announces New Proces to Recycle Lithium from Old Batteries, 2022. Available online: https://www.electrive.com/2022/12/09/accurec-announces-new-proces-to-recycle-lithium-from-old-batteries/ (accessed on 15 July 2024).
- Friedrich, B.; Schwich, L.; Stallmeister, C. Effiziente Rückgewinnung von Lithium aus Batterieschrott; BGR-Rohstoffkonferenz: Berlin, Germany, 6 July 2022. [Google Scholar]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 2023, 9, 1–25. [Google Scholar] [CrossRef]
- Won, L.S. Sungeel Hitech Completes Second Battery Recycling Factory in Hungary, 2021. Available online: https://www.thelec.net/news/articleView.html?idxno=3065 (accessed on 15 July 2024).
- Aquafil. Environmental Product Declaration of Econyl Polymer; Rev. 4; Aquafil: Trento, Italy, 2019. [Google Scholar]
- Hischier, R.; Wäger, P.; Gauglhofer, J. Does WEEE recycling make sense from an environmental perspective? Environ. Impact Assess. Rev. 2005, 25, 525–539. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Elordi, G.; Aguado, R.; Olazar, M.; Bilbao, J. Recycling poly-(methyl methacrylate) by pyrolysis in a conical spouted bed reactor. Chem. Eng. Process. Process Intensif. 2010, 49, 1089–1094. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Stanković, V.; Duo, I.; Comninellis, C.; Zonnevijlle, F. Silver electrowinning from silver(I)–calixarene complexes by two-phase electrolysis. J. Appl. Electrochem. 2007, 37, 1279–1286. [Google Scholar] [CrossRef]
- Zeiler, B.; Bartl, A.; Schubert, W.-D. Recycling of tungsten: Current share, economic limitations, technologies and future potential. Int. J. Refract. Met. Hard Mater. 2021, 98, 105546. [Google Scholar] [CrossRef]
- Apple Inc. Internal Information; Apple Inc.: Cupertino, CA, USA, 2024. [Google Scholar]
- Nomura, S. Use of Waste Plastics in Coke Oven: A Review. J. Sustain. Metall. 2015, 1, 85–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).