Eco-Friendly Photocatalytic Treatment of Dyes with Ag Nanoparticles Obtained through Sustainable Process Involving Spirulina platensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalyst
2.2. Characterization
2.3. In Situ Photocatalysis
3. Results and Discussion
3.1. Characterization
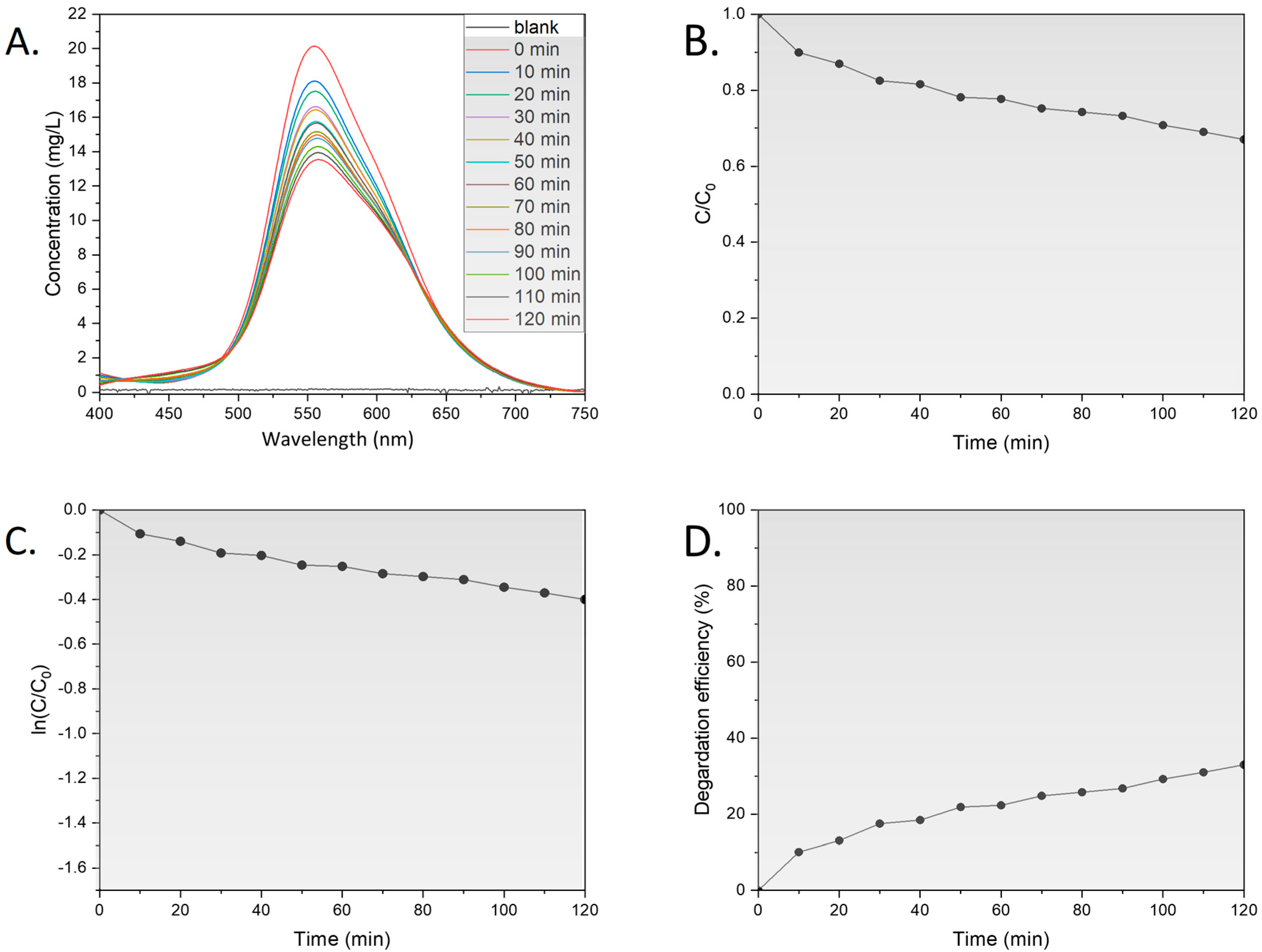
3.2. Photocatalytic Activity
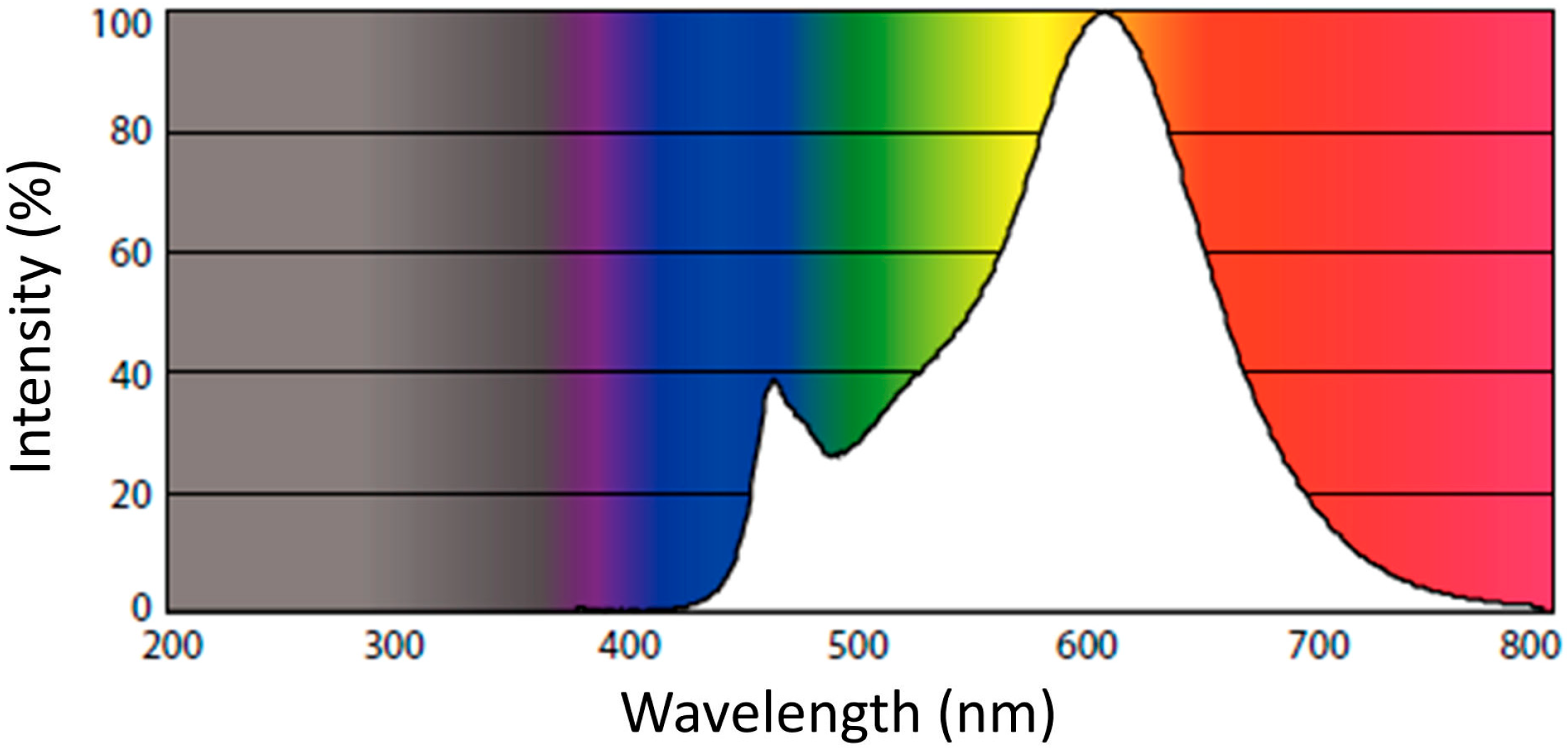
3.2.1. Influence of Light
3.2.2. Influence of Dye Concentration
3.2.3. Influence of Catalyst Dosage
3.2.4. Influence of pH
3.2.5. Influence of Calcination
3.2.6. Mechanism of Dye Degradation

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2022, 30, 9207–9242. [Google Scholar] [CrossRef] [PubMed]
- Tomar, T.; Kahandawala, N.; Kaur, J.; Thounaojam, L.; Choudhary, I.; Bera, S. Bioremediation of Synthetic Dyes from Wastewater by Using Microbial Nanocomposites: An Emerging Field for Water Pollution Management. Biocatal. Agric. Biotechnol. 2023, 51, 102767. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating Polymer Network Hydrogels for Removal of Synthetic Dyes: A Comprehensive Review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Iqbal, A.; Yusaf, A.; Usman, M.; Hussain Bokhari, T.; Mansha, A. Insight into the Degradation of Different Classes of Dyes by Advanced Oxidation Processes; a Detailed Review. Int. J. Environ. Anal. Chem. 2023, 1–35. [Google Scholar] [CrossRef]
- Mohod, A.V.; Momotko, M.; Shah, N.S.; Marchel, M.; Imran, M.; Kong, L.; Boczkaj, G. Degradation of Rhodamine Dyes by Advanced Oxidation Processes (AOPs)—Focus on Cavitation and Photocatalysis—A Critical Review. Water Resour. Ind. 2023, 30, 100220. [Google Scholar] [CrossRef]
- Park, S.; Keum, Y.; Park, J. Ti-Based Porous Materials for Reactive Oxygen Species-Mediated Photocatalytic Reactions. Chem. Commun. 2022, 58, 607–618. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Zhao, S.; Li, C.; Wang, R.; Cao, D.; Liu, G. Construction of Bi-Assisted Modified CdS/TiO2 Nanotube Arrays with Ternary S-Scheme Heterojunction for Photocatalytic Wastewater Treatment and Hydrogen Production. Fuel 2022, 322, 124163. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Zhao, Y.; Deng, Y.; Yang, W.; Ye, Y.; Wang, K. Construction of Z-Scheme Bi2O3/CeO2 Heterojunction for Enhanced Photocatalytic Capacity of TiO2 NTs. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2024, 304, 123405. [Google Scholar] [CrossRef]
- Vidyasagar, N.; Patel, R.R.; Singh, S.K.; Singh, M. Green Synthesis of Silver Nanoparticles: Methods, Biological Applications, Delivery and Toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Fais, G.; Casula, M.; Borselli, M.; Giannaccare, G.; Locci, A.M.; Lai, N.; Orrù, R.; Cao, G.; Concas, A. Nanoparticles from Microalgae and Their Biomedical Applications. Mar. Drugs 2023, 21, 352. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Margarita, V.; Fais, G.; Pantaleo, A.; Manca, A.; Concas, A.; Rappelli, P.; Fiori, P.L.; Cao, G. Characterization of Nanomaterials Synthesized from Spirulina Platensis Extract and Their Potential Antifungal Activity. PLoS ONE 2022, 17, e0274753. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Steriti, A.; Pisu, M.; Cao, G. Experimental and Theoretical Investigation of the Effects of Iron on Growth and Lipid Synthesis of Microalgae in View of Their Use to Produce Biofuels. J. Environ. Chem. Eng. 2021, 9, 105349. [Google Scholar] [CrossRef]
- Concas, A.; Lutzu, G.A.; Turgut Dunford, N. Experiments and Modeling of Komvophoron Sp. Growth in Hydraulic Fracturing Wastewater. Chem. Eng. J. 2021, 426, 131299. [Google Scholar] [CrossRef]
- Concas, A.; Pisu, M.; Cao, G. Microalgal Cell Disruption Through Fenton Reaction: Experiments, Modeling and Remarks on Its Effect on the Extracted Lipids Composition. Chem. Eng. Trans. 2015, 43, 367–372. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira Platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Ismail, G.A.; Allam, N.G.; El-Gemizy, W.M.; Salem, M.A. The Role of Silver Nanoparticles Biosynthesized by Anabaena Variabilis and Spirulina Platensis Cyanobacteria for Malachite Green Removal from Wastewater. Environ. Technol. 2021, 42, 4475–4489. [Google Scholar] [CrossRef]
- Roja, K.; Mehta, P.; Premalatha, M.; Jeyadheepan, K.; Gopalakrishnan, C.; Meenakshisundaram, N.; Sankaranarayanan, K. Biosynthesized Silver Nanoparticles as Antimicrobial Agents and Photocatalytic Degradation of Methylene Blue. DDesalination Water Treat. 2019, 156, 292–302. [Google Scholar] [CrossRef]
- Gul, A.; Ahmed, D.; Fazil, M.M.; Aslam, T.; Rashid, M.A.; Khan, H.; Ali, A.; Ali, S. Biofabrication of Silver Nanoparticles Using Spirulina Platensis: In Vitro Anti-Coagulant, Thrombolytic and Catalytic Dye Degradation Activity. Microsc. Res. Tech. 2023, 86, 823–833. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Dao, V.D.; Barakat, N.A.M.; Yasin, A.S.; Yousef, A.; Choi, H.S. Efficiency Enhancement of Dye-Sensitized Solar Cells by Use of ZrO2-Doped TiO2 Nanofibers Photoanode. J. Colloid Interface Sci. 2016, 476, 9–19. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, J.; Zhang, X.; Zhao, Y.; Fan, X.; Zhong, S.; Zhang, H.; Yu, X. A Generalized Predictive Model for TiO2–Catalyzed Photo-Degradation Rate Constants of Water Contaminants through Artificial Neural Network. Environ. Res. 2020, 187, 109697. [Google Scholar] [CrossRef]
- Nocchetti, M.; Pietrella, D.; Antognelli, C.; Di Michele, A.; Russo, C.; Giulivi, E.; Ambrogi, V. Alginate Microparticles Containing Silver@hydroxyapatite Functionalized Calcium Carbonate Composites. Int. J. Pharm. 2024, 661, 124393. [Google Scholar] [CrossRef] [PubMed]
- Cepoi, L.; Zinicovscaia, I.; Rudi, L.; Chiriac, T.; Turchenko, V. Changes in the Dunaliella Salina Biomass Composition during Silver Nanoparticles Formation. Nanotechnol. Environ. Eng. 2022, 7, 235–243. [Google Scholar] [CrossRef]
- Kashyap, M.; Samadhiya, K.; Ghosh, A.; Anand, V.; Lee, H.; Sawamoto, N.; Ogura, A.; Ohshita, Y.; Shirage, P.M.; Bala, K. Synthesis, Characterization and Application of Intracellular Ag/AgCl Nanohybrids Biosynthesized in Scenedesmus Sp. as Neutral Lipid Inducer and Antibacterial Agent. Environ. Res. 2021, 201, 111499. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, M.; Pietroletti, M.; Scarpiniti, M.; Acquistucci, R.; Conti, M.E. Monitoring of Marine Mucilage Formation in Italian Seas Investigated by Infrared Spectroscopy and Independent Component Analysis. Environ. Monit. Assess. 2012, 184, 6025–6036. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Concas, A.; Orrù, R.; Cao, G.; Rupprechter, G. Microalgae-Derived Co3O4 Nanomaterials for Catalytic CO Oxidation. RSC Adv. 2024, 14, 4575–4586. [Google Scholar] [CrossRef]
- Alzahrani, E.A.; Nabi, A.; Kamli, M.R.; Albukhari, S.M.; Althabaiti, S.A.; Al-Harbi, S.A.; Khan, I.; Malik, M.A. Facile Green Synthesis of ZnO NPs and Plasmonic Ag-Supported ZnO Nanocomposite for Photocatalytic Degradation of Methylene Blue. Water 2023, 15, 384. [Google Scholar] [CrossRef]
- Vera, J.; Herrera, W.; Hermosilla, E.; Díaz, M.; Parada, J.; Seabra, A.B.; Tortella, G.; Pesenti, H.; Ciudad, G.; Rubilar, O. Antioxidant Activity as an Indicator of the Efficiency of Plant Extract-Mediated Synthesis of Zinc Oxide Nanoparticles. Antioxidants 2023, 12, 784. [Google Scholar] [CrossRef]
- Kis, B.; Moacă, E.A.; Tudoran, L.B.; Muntean, D.; Magyari-Pavel, I.Z.; Minda, D.I.; Lombrea, A.; Diaconeasa, Z.; Dehelean, C.A.; Dinu, Ș.; et al. Green Synthesis of Silver Nanoparticles Using Populi Gemmae Extract: Preparation, Physicochemical Characterization, Antimicrobial Potential and In Vitro Antiproliferative Assessment. Materials 2022, 15, 5006. [Google Scholar] [CrossRef]
- Lite, M.C.; Constantinescu, R.R.; Tănăsescu, E.C.; Kuncser, A.; Romanițan, C.; Lăcătuşu, I.; Badea, N. Design of Green Silver Nanoparticles Based on Primula Officinalis Extract for Textile Preservation. Materials 2022, 15, 7695. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Liu, Y.; Zheng, X.; Tang, K. Green Synthesis of Silver Nanoparticles Using Soluble Soybean Polysaccharide and Their Application in Antibacterial Coatings. Int. J. Biol. Macromol. 2021, 166, 567–577. [Google Scholar] [CrossRef]
- Baia, L.; Simon, S. UV-VIS and TEM Assessment of Morphological Features of Silver Nanoparticles from Phosphate Glass Matrices. Mod. Res. Educ. Top. Microsc. 2007, 2, 576–583. [Google Scholar]
- Skanda, S.; Bharadwaj, P.S.J.; Datta Darshan, V.M.; Sivaramakrishnan, V.; Vijayakumar, B.S. Proficient Mycogenic Synthesis of Silver Nanoparticles by Soil Derived Fungus Aspergillus Melleus SSS-10 with Cytotoxic and Antibacterial Potency. J. Microbiol. Methods 2022, 199, 106517. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Gurumurthy, M.S.; Thomas, R.; Balachandran, M. Biosynthesized AG Nanoparticles: A Promising Pathway for Bandgap Tailoring. Biointerface Res. Appl. Chem. 2021, 11, 8875–8883. [Google Scholar] [CrossRef]
- Ananda Murthy, H.C.; Desalegn Zeleke, T.; Ravikumar, C.R.; Anil Kumar, M.R.; Nagaswarupa, H.P. Electrochemical Properties of Biogenic Silver Nanoparticles Synthesized Using Hagenia Abyssinica (Brace) JF. Gmel. Medicinal Plant Leaf Extract. Mater. Res. Express 2020, 7, 055016. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.Y.; Gollakota, A.R.K.; Wen, J.C.; Lin, K.Y.A.; Shu, C.M.; Yarramuthi, V.; Basivi, P.K.; Reddy, G.M.; Zyryanov, G.V. Magnetic Fe3O4 Nanoparticles Loaded Guava Leaves Powder Impregnated into Calcium Alginate Hydrogel Beads (Fe3O4-GLP@CAB) for Efficient Removal of Methylene Blue Dye from Aqueous Environment: Synthesis, Characterization, and Its Adsorption Performance. Int. J. Biol. Macromol. 2023, 246, 125675. [Google Scholar] [CrossRef] [PubMed]
- Heredia Deba, S.A.; Wols, B.A.; Yntema, D.R.; Lammertink, R.G.H. Photocatalytic Ceramic Membrane: Effect of the Illumination Intensity and Distribution. J. Photochem. Photobiol. A Chem. 2023, 437, 114469. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters That Affect the Photodegradation of Dyes and Pigments in Solution and on Substrate—An Overview. Dyes Pigments 2023, 210, 110999. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2021, 29, 293–311. [Google Scholar] [CrossRef]
- Chiarello, L.M.; Mittersteiner, M.; de Jesus, P.C.; Andreaus, J.; Barcellos, I.O. Reuse of Enzymatically Treated Reactive Dyeing Baths: Evaluation of the Number of Reuse Cycles. J. Clean. Prod. 2020, 267, 122033. [Google Scholar] [CrossRef]
- Nawaz, A.; Atif, M.; Khan, A.; Siddique, M.; Ali, N.; Naz, F.; Bilal, M.; Kim, T.H.; Momotko, M.; Haq, H.U.; et al. Solar Light Driven Degradation of Textile Dye Contaminants for Wastewater Treatment—Studies of Novel Polycationic Selenide Photocatalyst and Process Optimization by Response Surface Methodology Desirability Factor. Chemosphere 2023, 328, 138476. [Google Scholar] [CrossRef]
- Javanbakht, V.; Mohammadian, M. Photo-Assisted Advanced Oxidation Processes for Efficient Removal of Anionic and Cationic Dyes Using Bentonite/TiO2 Nano-Photocatalyst Immobilized with Silver Nanoparticles. J. Mol. Struct. 2021, 1239, 130496. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, X.; Lu, L.; Tang, D. Snowflake-like CdS@ZnIn2S4 Heterojunction-Based Photocatalyst-Electrolyte Effect: An Innovative Mode for Photoelectrochemical Immunoassay. Biosens. Bioelectron. 2022, 216, 114679. [Google Scholar] [CrossRef] [PubMed]
- Uma, H.B.; Ananda, S.; Nandaprakash, M.B. High Efficient Photocatalytic Treatment of Textile Dye and Antibacterial Activity via Electrochemically Synthesized Ni-Doped ZnO Nano Photocatalysts. Chem. Data Collect. 2019, 24, 100301. [Google Scholar] [CrossRef]
- Khandan Nasab, N.; Sabouri, Z.; Ghazal, S.; Darroudi, M. Green-Based Synthesis of Mixed-Phase Silver Nanoparticles as an Effective Photocatalyst and Investigation of Their Antibacterial Properties. J. Mol. Struct. 2020, 1203, 127411. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Yu, B.; Ding, X.; Peng, Q.; Shen, Y.; Cong, H. Efficient Photocatalytic Degradation of Toxic Alizarin Yellow R Dye from Industrial Wastewater Using Biosynthesized Fe Nanoparticle and Study of Factors Affecting the Degradation Rate. J. Photochem. Photobiol. B Biol. 2020, 202, 111682. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.X.; Cheng, X.D.; Su, T.; Wang, X.H.; Zhang, W.N.; Lu, Y.M.; Chen, Y. Biodegradation and Detoxification of the Triphenylmethane Dye Coomassie Brilliant Blue by the Extracellular Enzymes from Mycelia of Lactarius Deliciosus. Front. Chem. Sci. Eng. 2021, 15, 421–436. [Google Scholar] [CrossRef]
- Bukallah, S.B.; Rauf, M.A.; Ashraf, S.S. Photocatalytic Decoloration of Coomassie Brilliant Blue with Titanium Oxide. Dyes Pigments 2007, 72, 353–356. [Google Scholar] [CrossRef]
- Salem, M.S.; Wassel, A.R.; Fedawy, M.; Shaker, A.; Al-Bagawia, A.H.; Aleid, G.M.; El-Mahalawy, A.M. Integration of Biocompatible Coomassie Brilliant Blue Dye on Silicon in Organic/Inorganic Heterojunction for Photodetection Applications. J. Phys. Chem. Solids 2022, 169, 110890. [Google Scholar] [CrossRef]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic Degradation of Organic Dyes in the Presence of Nanostructured Titanium Dioxide: Influence of the Chemical Structure of Dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical Degradation of Coomassie Brilliant Blue: Effect of Frequency, Power Density, PH and Various Additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Ultrasound Based AOP for Emerging Pollutants: From Degradation to Mechanism. Environ. Sci. Pollut. Res. 2017, 24, 6261–6269. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cao, X.; Sun, T.; Miao, H.; Chen, Z.; Xue, W.; Fan, J.; Liu, E. Efficient Photocatalytic H2 Evolution over NiMoO4/Twinned-Cd0. 5Zn0. 5S Double S-Scheme Homo-Heterojunctions. Compos. Part B Eng. 2024, 277, 111389. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Zhang, Z.; Liao, S.; Deng, Y.; Wang, X.; Ye, Q.; Wang, K. Hydrothermal Preparation of Sn3O4/TiO2 Nanotube Arrays as Effective Photocatalysts for Boosting Photocatalytic Dye Degradation and Hydrogen Production. Ceram. Int. 2023, 49, 5977–5985. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Yu, X.; Jia, Y.; Chang, Y.; Gao, S. Morphology Regulated Bi2WO6 Nanoparticles on TiO2 Nanotubes by Solvothermal Sb3+ Doping as Effective Photocatalysts for Wastewater Treatment. Electrochim. Acta 2020, 330, 135167. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorowicz, A.; Fais, G.; Desogus, F.; Loy, F.; Licheri, R.; Lai, N.; Cao, G.; Concas, A. Eco-Friendly Photocatalytic Treatment of Dyes with Ag Nanoparticles Obtained through Sustainable Process Involving Spirulina platensis. Sustainability 2024, 16, 8758. https://doi.org/10.3390/su16208758
Sidorowicz A, Fais G, Desogus F, Loy F, Licheri R, Lai N, Cao G, Concas A. Eco-Friendly Photocatalytic Treatment of Dyes with Ag Nanoparticles Obtained through Sustainable Process Involving Spirulina platensis. Sustainability. 2024; 16(20):8758. https://doi.org/10.3390/su16208758
Chicago/Turabian StyleSidorowicz, Agnieszka, Giacomo Fais, Francesco Desogus, Francesco Loy, Roberta Licheri, Nicola Lai, Giacomo Cao, and Alessandro Concas. 2024. "Eco-Friendly Photocatalytic Treatment of Dyes with Ag Nanoparticles Obtained through Sustainable Process Involving Spirulina platensis" Sustainability 16, no. 20: 8758. https://doi.org/10.3390/su16208758
APA StyleSidorowicz, A., Fais, G., Desogus, F., Loy, F., Licheri, R., Lai, N., Cao, G., & Concas, A. (2024). Eco-Friendly Photocatalytic Treatment of Dyes with Ag Nanoparticles Obtained through Sustainable Process Involving Spirulina platensis. Sustainability, 16(20), 8758. https://doi.org/10.3390/su16208758







