Vegetation Growth and Physiological Adaptation of Pioneer Plants on Mobile Sand Dunes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Vegetation Survey
2.3. Soil Moisture Measurement
2.4. Plant Photosynthesis, Transpiration, and Water Use Efficiency
2.5. Biomass Measurement
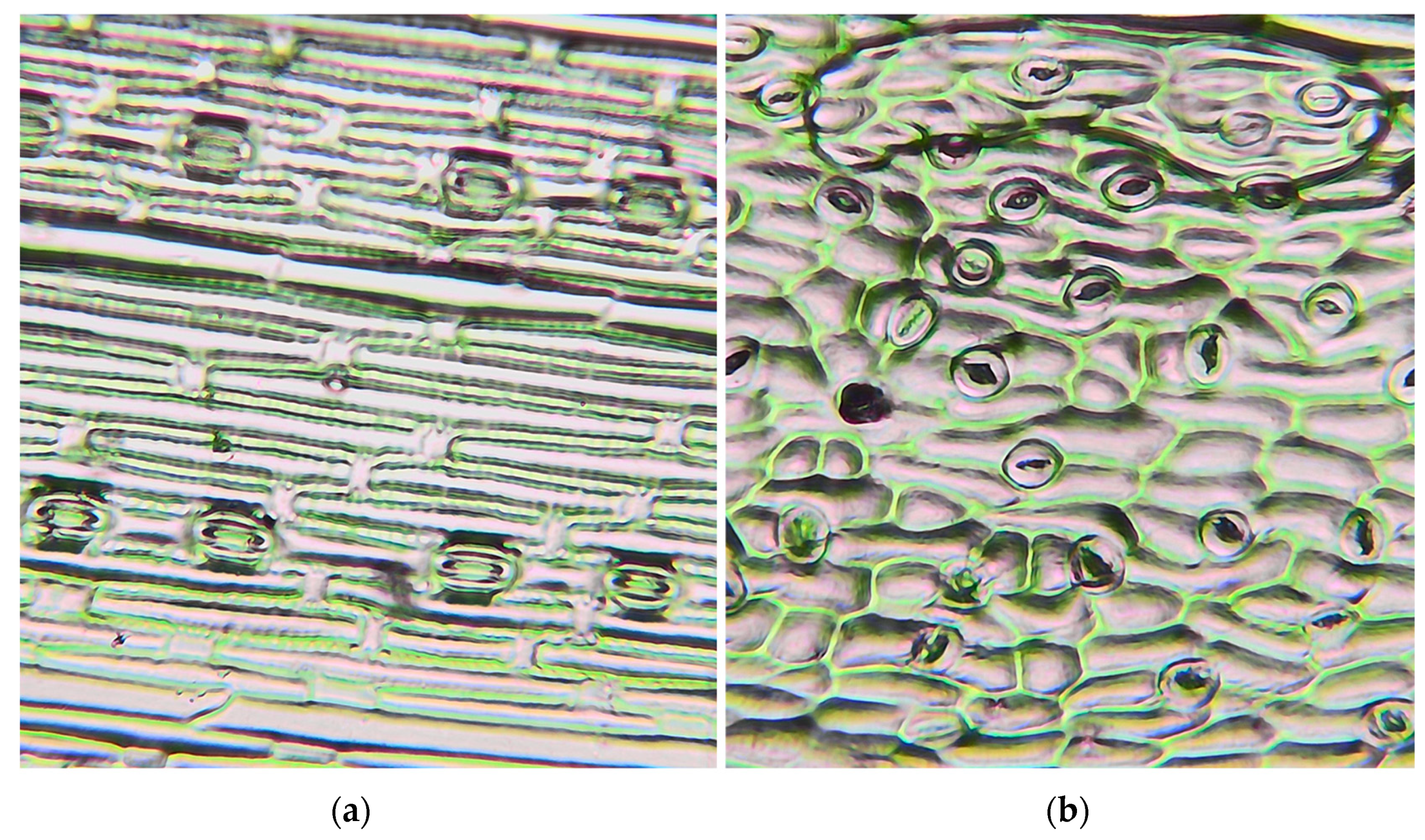
2.6. Stomatal Microscopy Imaging
- (1)
- Stomatal density (SD): We randomly selected nine microscopic visual fields with sizes of about 0.1 mm2 for each sample, measured the field area, and counted the number of stomata in the field. We converted this value into the number of stomata per unit area, which is the SD (cells mm−2).
- (2)
- Stomatal length (SL): Nine fields with sizes of approximately 0.1 mm2 were randomly selected from each sample, and five stomata were randomly selected from each field. The length of the stomata and the length (μm) of the two guard cells shaping the stomata were measured.
- (3)
- Stomatal area index (SPI): The total stomatal area per unit area was calculated as follows: SPI (%) = SL2 × SD × 10−4.
2.7. Statistic Analysis
3. Results
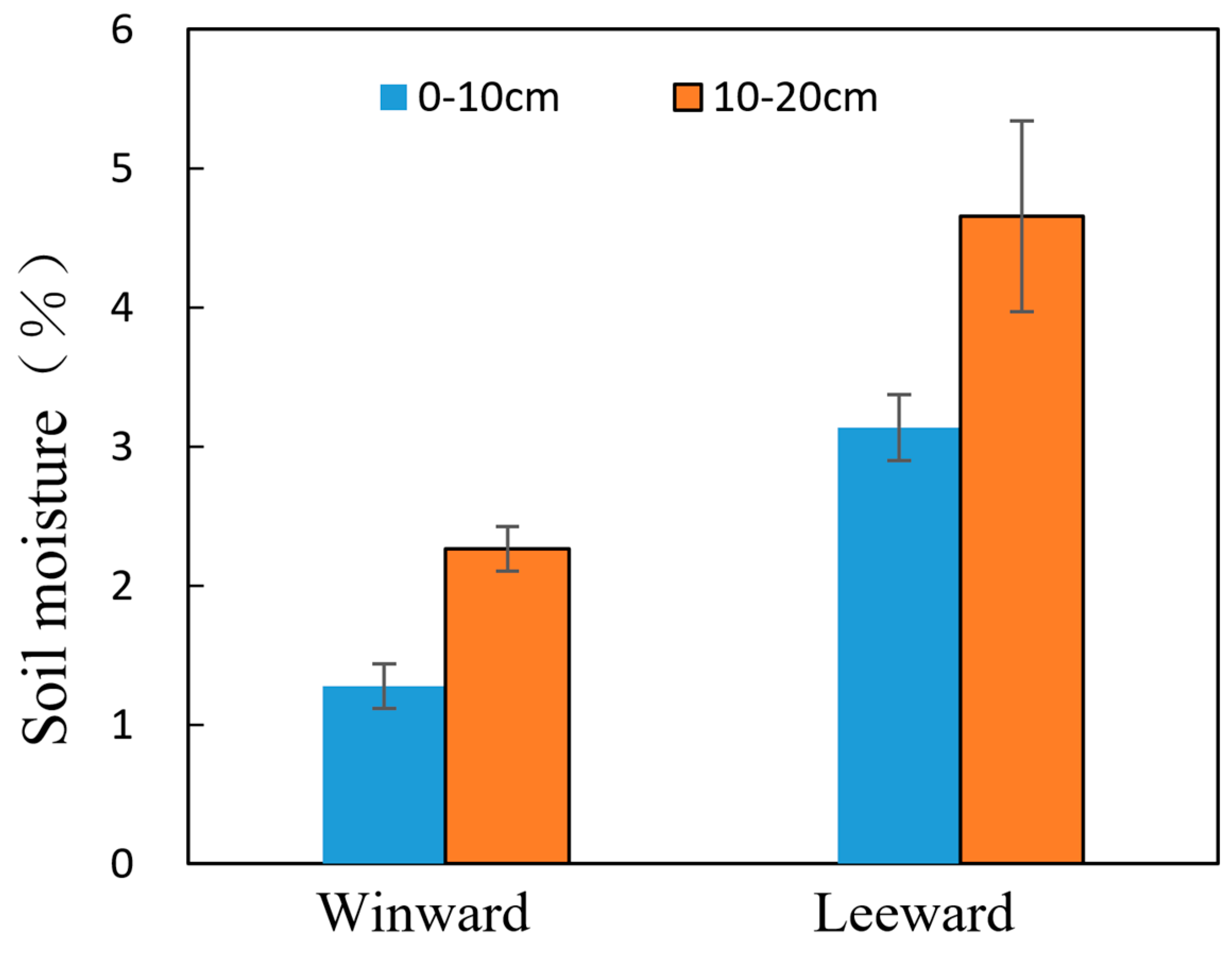
3.1. Soil Properties
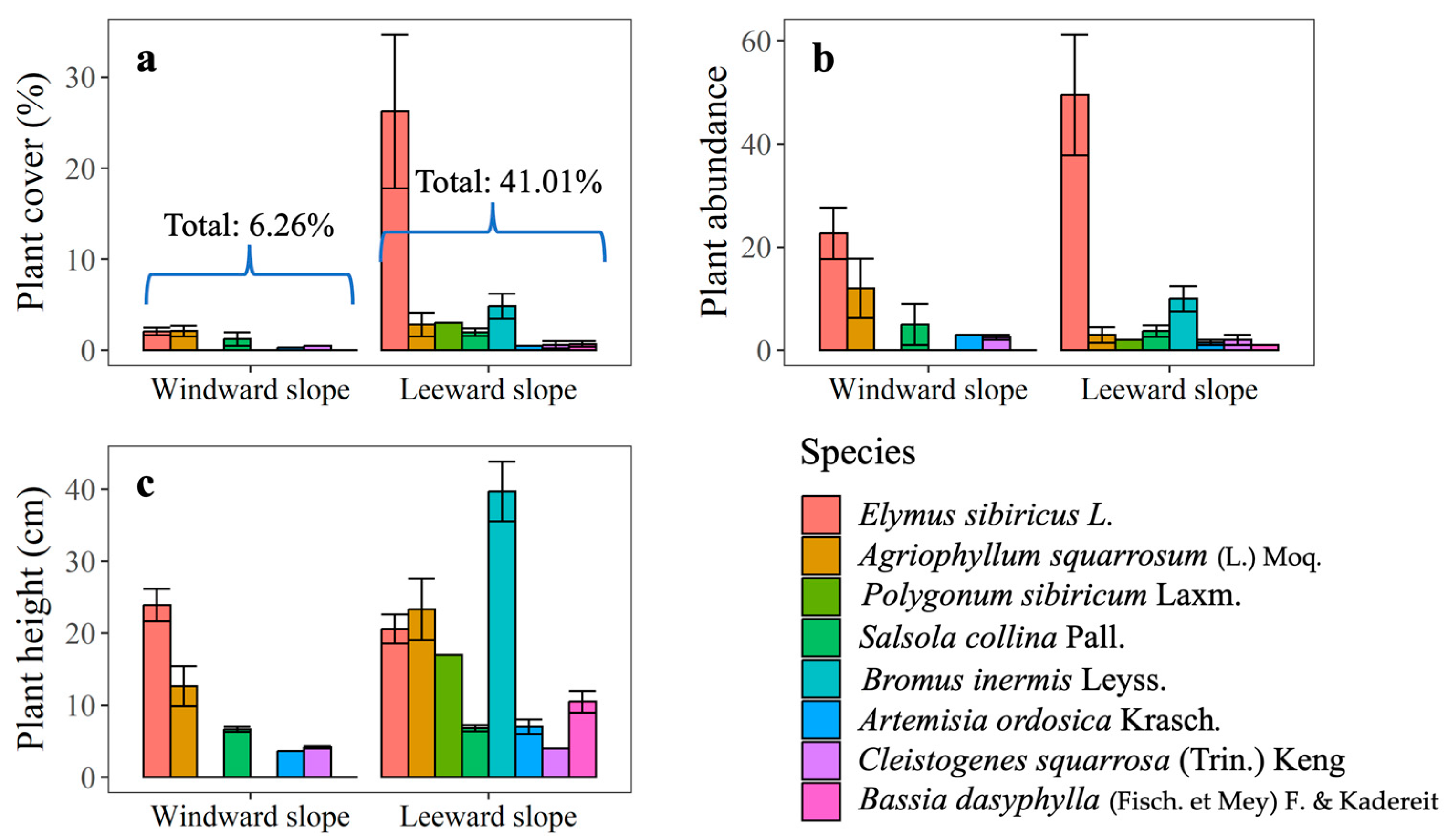
3.2. Species Composition and Community Structure
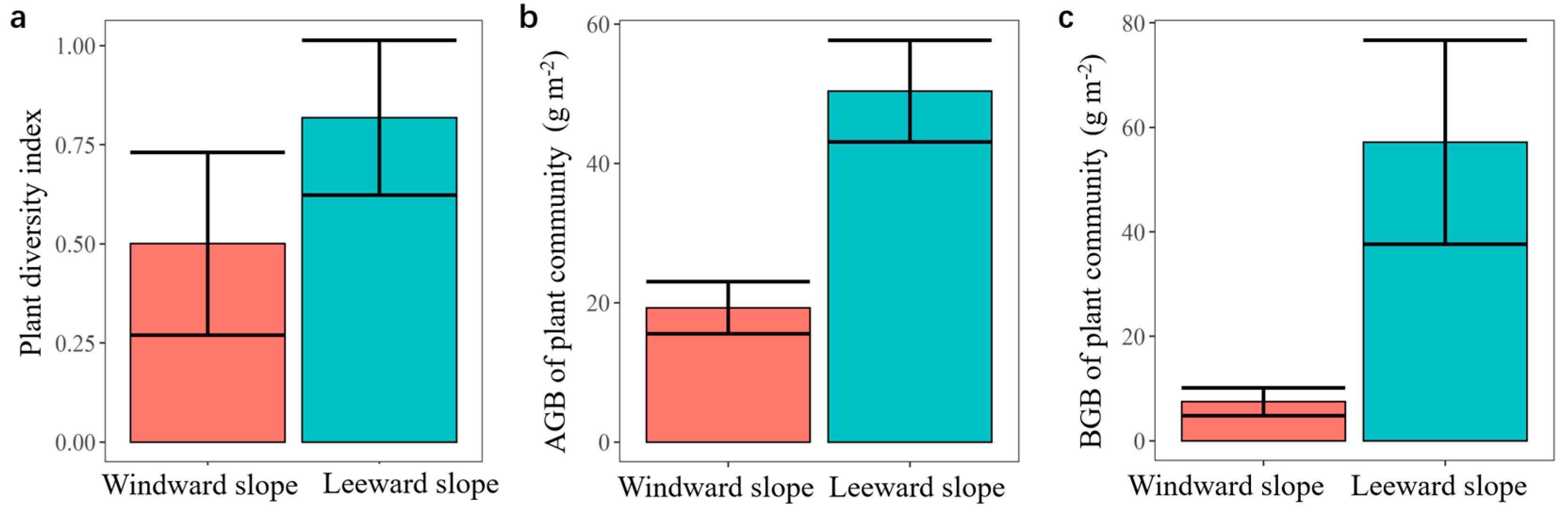
3.3. Vegetation Biomass and Biodiversity
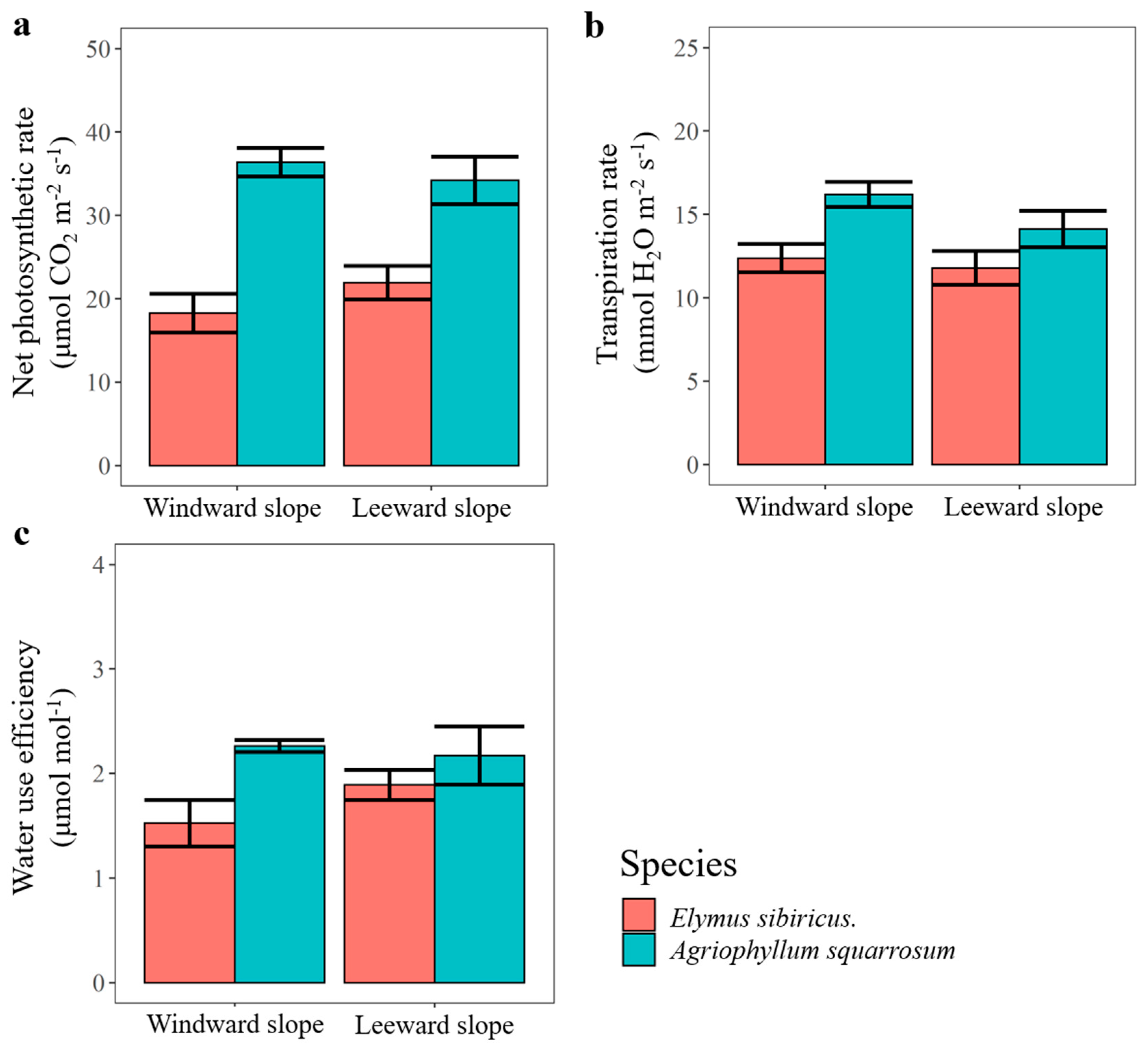
3.4. Photosynthetic Rate, Transpiration Rate, and Water Use Efficiency
3.5. Stomatal Characteristics of Two Pioneer Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, X.; Guo, J.; Wang, X. Detecting the amount of eroded and deposited sand using DInSAR. Terr. Atmos. Ocean. Sci. 2011, 22, 187. [Google Scholar] [CrossRef]
- Yang, T.; Ala, M.; Zhang, Y.; Wu, J.; Wang, A.; Guan, D. Characteristics of soil moisture under different vegetation coverage in Horqin Sandy Land, northern China. PLoS ONE 2018, 13, e0198805. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Guo, Q.; Li, Y.; Li, S. Overview of the measures and techniques used to protect traffic lines against shifting sands in China. J. Resour. Ecol. 2021, 12, 124–135. [Google Scholar]
- Han, Y.; Zhao, W.; Zhou, A.; Pereira, P. Water and wind erosion response to ecological restoration measures in China’s drylands. Geoderma 2023, 435, 116514. [Google Scholar] [CrossRef]
- Hong, C.; Chenchen, L.; Xueyong, Z.; Huiru, L.i.; Liqiang, K.; Bo, L.; Li, J. Wind erosion rate for vegetated soil cover: A prediction model based on surface shear strength. Catena 2020, 187, 104398. [Google Scholar] [CrossRef]
- Lee, J.T.; Yen, L.Z.; Chu, M.Y.; Lin, Y.S.; Chang, C.C.; Lin, R.S.; Chao, K.H.; Lee, M.J. Growth characteristics and anti-wind erosion ability of three tropical foredune pioneer species for sand dune stabilization. Sustainability 2020, 12, 3353. [Google Scholar] [CrossRef]
- Li, S.; Chang, X.; Zhao, X. Study of Agriophyllum squarrosum-pioneering plant on shifting sand. J. Arid Land Resour. Environ. 1992, 6, 63–70. [Google Scholar]
- Wang, S.; Zhang, B.; Xie, G.; Zhai, X.; Sun, H. Vegetation cover changes and sand-fixing service responses in the Beijing-Tianjin sandstorm source control project area. Environ. Dev. 2020, 34, 100455. [Google Scholar] [CrossRef]
- Sigren, J.M.; Figlus, J.; Armitage, A.R. Coastal sand dunes and dune vegetation: Restoration, erosion, and storm protection. Shore Beach 2014, 82, 5–12. [Google Scholar]
- Rahmonov, O.; Skreczko, S.; Rahmonov, M. Changes in Soil Features and phytomass during vegetation succession in sandy areas. Land 2021, 10, 265. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, D.; Jiang, S. Study of the drought resistance of thirteen sand-fixing plants in Horqin Sand Land, China. Int. J. Agric. Biol. 2018, 20, 1717–1724. [Google Scholar]
- Cui, Y.; Wang, Z.; Zhang, D.; Fan, G. Effects of soil water content and light on photosynthetic characteristics of Sabina vulgari Ant. Ecol. Sci. 2022, 41, 77–83. [Google Scholar]
- Lv, G.; Jin, J.; He, M.; Wang, C. Soil moisture content dominates the photosynthesis of C3 and C4 plants in a desert steppe after long-term warming and increasing precipitation. Plants 2023, 12, 2903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Jiang, G.; Niu, S.; Liu, M.; Gao, L.; Peng, Y.; Jiang, C. Traits of chlorophyll fluorescence in 99 plant species from the sparse-elm grassland in Hunshandak Sandland. Photosynthetica 2004, 42, 243–249. [Google Scholar] [CrossRef]
- Danin, A. Plant adaptations in desert dunes. J. Arid Environ. 1991, 21, 193–212. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, G.; Wan, S.; Li, Y.; Gao, L.; Liu, M. A sand-fixing pioneer C3 species in sandland displays characteristics of C4 metabolism. Environ. Exp. Bot. 2006, 57, 123–130. [Google Scholar] [CrossRef]
- Mohanta, T.; Mohanta, Y.; Kaushik, P.; Kumar, J. Physiology, genomics, and evolutionary aspects of desert plants. J. Adv. Res. 2023, 58, 63–78. [Google Scholar] [CrossRef]
- Dong, X.; Hao, Y.; Xin, Z.; Duan, R.; Zhang, R.; Ma, Y.; Liu, F. Comparative study on sand-fixing capability of three typical shrubs in Otindag Sandy Land. For. Res. 2020, 33, 76–83. [Google Scholar]
- Li, Q.X.; Zhu, Y.J.; Jia, Z.Q.; Wang, Y.S.; Yang, Y.U. Effects of sand dune slope on soil nutrients and plant community for Caragana intermedia plantation. For. Res. 2014, 27, 677–682. [Google Scholar]
- Tao, Y.; Wu, G.L.; Zhang, Y.M. Dune-scale distribution pattern of herbaceous plants and their relationship with environmental factors in a saline-alkali desert in Central Asia. Sci. Total Environ. 2017, 576, 473–480. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Vatén, A.; Bergmann, D.C. Mechanisms of stomatal development: An evolutionary view. EvoDevo 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sack, L.; Li, Y.; Zhang, J.; Yu, K.; Zhang, Q.; Yu, G. Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 2023, 14, 6629. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhong, Y.; Shangguan, Z. Contrasting responses of leaf stomatal characteristics to climate change: A considerable challenge to predict carbon and water cycles. Glob. Change Biol. 2017, 23, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, N.; Zhang, J.; Li, Y.; Wang, Q.; Sack, L.; Yu, G. Variation of stomatal traits from cold temperate to tropical forests and association with water use efficiency. Funct. Ecol. 2018, 32, 20–28. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; An, L.; Ji, C. Leaf stomatal characters of kidney-shaped stomata and dumb-bell-shaped stomata and their relationships with environmental factors in the typical plants of the Tibetan and Inner Mongolian Plateau. Acta Bot. Boreali-Occident. Sin. 2018, 38, 1048–1057. [Google Scholar]
- Guo, K.; Dong, X.; Liu, Z. Characteristics of soil moisture content on sand dunes in Mu Us Sandy Grassland: Why artiemisia ordosica declines on old fixed sand dunes. Acta Phytoecol. Sinca 2000, 124, 275–279. [Google Scholar]
- Liu, M.Z.; Jiang, G.M.; Li, Y.G.; Niu, S.L.; Gao, L.M.; Ding, L.; Peng, Y. Leaf osmotic potentials of 104 plant species in relation to habitats and plant functional types in Hushandak Sandland, Inner Mongolia, China. Trees 2003, 17, 554–560. [Google Scholar] [CrossRef]
- Tian, L.; Wu, H.; Wang, H.; Zhang, D.; He, X.; Wang, Q. Comparing water use patterns of sand-binding species in an alpine semi-arid desert. Hydrol. Process. 2023, 37, e14798. [Google Scholar] [CrossRef]
- Meier, I.C.; Knutzen, F.; Eder, L.M.; Müller-Haubold, H.; Goebel, M.O.; Bachmann, J.; Hertel, D.; Leuschner, C. The deep root system of Fagus sylvatica on sandy soil: Structure and variation across a precipitation gradient. Ecosystems 2018, 21, 280–296. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, W.; Zhuang, Y. Sand-burial and wind erosion promote oriented-growth and patchy distribution of a clonal shrub in dune ecosystems. Catena 2018, 167, 212–220. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, J.; Tian, L. Responses of belowground bud bank to disturbance and stress in the sand dune ecosystem. Ecol. Indic. 2019, 106, 105521. [Google Scholar] [CrossRef]
- Miao, R.; Liu, Y.; Wu, L.; Wang, D.; Liu, Y.; Miao, Y.; Yang, Z.; Guo, M.; Ma, J. Effects of long-term grazing exclusion on plant and soil properties vary with position in dune systems in the Horqin Sandy Land. Catena 2022, 209, 105860. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hu, X.; Zheng, P.; Hirota, M.; Kamijo, T. Photosynthetic properties of co-occurring pioneer species on volcanically devastated sites in Miyake-jima Island, Japan. Plants 2021, 10, 2500. [Google Scholar] [CrossRef]
- Jafari, M.; Tahmoures, M.; Ehteram, M.; Ghorbani, M.; Panahi, F. Slope stabilization methods using biological and biomechanical measures. In Soil Erosion Control in Drylands; Springer: Cham, Switzerland, 2022; pp. 445–647. [Google Scholar]
- Liu, M.; Zhu, R.; Zhang, Z.; Liu, L.; Hui, R.; Bao, J.; Zhang, H. Water use traits and survival mechanisms of psammophytes in arid ecosystems. Arid. Land Res. Manag. 2016, 30, 166–180. [Google Scholar] [CrossRef]
- Li, Y.; Tian, L.; Zhou, H.; Wang, H.; He, X.; Jin, Y.; Zhang, H. Comparison of water use efficiency of sand-binding species along revegetation chronosequence in an alpine desert. Ecol. Indic. 2023, 153, 110475. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, H.; Zhang, T.; Zhao, X.; Drake, S. Community succession along a chronosequence of vegetation restoration on sand dunes in Horqin Sandy Land. J. Arid Environ. 2005, 62, 555–566. [Google Scholar] [CrossRef]
- Shtein, I.; Shelef, Y.; Marom, Z.; Zelinger, E.; Schwartz, A.; Popper, Z.A.; Benny Bar-On, B.; Harpaz-Saad, S. Stomatal cell wall composition: Distinctive structural patterns associated with different phylogenetic groups. Ann. Bot. 2017, 119, 1021–1033. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- McKown, K.H.; Bergmann, D.C. Grass stomata. Curr. Biol. 2018, 28, R803–R825. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Wang, H.; Cui, Z.; Cao, C. Sand-fixation plantation type affects soil phosphorus transformation microbial community in a revegetation area of Horqin Sandy Land, Northeast China. Ecol. Eng. 2022, 180, 106644. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Jarvie, S.; Yan, Y.; Han, P.; Liu, T.; Guo, K.; Ren, L.; Yue, K.; Wu, H.; et al. Ecosystem restoration through aerial seeding: Interacting plant–soil microbiome effects on soil multifunctionality. Land Degrad. Dev. 2021, 32, 5334–5347. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Zhang, Q.; Guo, K.; He, Y.; Chen, D.; Sui, M.; Zhang, G.; Zang, L.; Liu, Q. Aerial seeding promotes the restoration of ecosystem health in Mu Us Sandy Grasslands in China. Agriculture 2022, 12, 1255. [Google Scholar] [CrossRef]
- Shaw, N.; Barak, R.S.; Campbell, R.E.; Kirmer, A.; Pedrini, S.; Dixon, K.; Frischie, S. Seed use in the field: Delivering seeds for restoration success. Restor. Ecol. 2020, 28, S276–S285. [Google Scholar] [CrossRef]
- Miao, R.; Song, Y.; Sun, Z.; Guo, M.; Zhou, Z.; Liu, Y. Soil seed bank and plant community development in passive restoration of degraded sandy grasslands. Sustainability 2016, 8, 581. [Google Scholar] [CrossRef]

| Stomatal Index | Elymus sibiricus | Grassland Plant (Dumbbell Type) | Agriophyllum squarrosum | Grassland Plant (Kidney Type) |
|---|---|---|---|---|
| Stomatal density (number/mm2) | 151.24 ± 35.6 | 248.9 | 197.12 ± 43.52 | 206.0 |
| Stomatal length (μm) | 29.93 ± 7.85 | 33.4 | 18.21 ± 4.37 | 23.7 |
| Stomatal area index (%) | 13.18 ± 4.12 | 24.2 | 19.31 ± 2.12 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Xu, H.; Li, Y.; Su, H. Vegetation Growth and Physiological Adaptation of Pioneer Plants on Mobile Sand Dunes. Sustainability 2024, 16, 8771. https://doi.org/10.3390/su16208771
Cao Y, Xu H, Li Y, Su H. Vegetation Growth and Physiological Adaptation of Pioneer Plants on Mobile Sand Dunes. Sustainability. 2024; 16(20):8771. https://doi.org/10.3390/su16208771
Chicago/Turabian StyleCao, Yingfei, Hong Xu, Yonggeng Li, and Hua Su. 2024. "Vegetation Growth and Physiological Adaptation of Pioneer Plants on Mobile Sand Dunes" Sustainability 16, no. 20: 8771. https://doi.org/10.3390/su16208771
APA StyleCao, Y., Xu, H., Li, Y., & Su, H. (2024). Vegetation Growth and Physiological Adaptation of Pioneer Plants on Mobile Sand Dunes. Sustainability, 16(20), 8771. https://doi.org/10.3390/su16208771






