Dose Effect of Drinking Water Nitrate on Health, Feed Intake, Rumen Fermentation and Microbiota, and Nitrogen Excretion in Holstein Heifers for a Sustainable Water Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing, Experimental Design, and Treatments
2.2. Water Quality Measurements
2.3. Water and Feed Intake
2.4. Potential Hazardous Effects Measurements
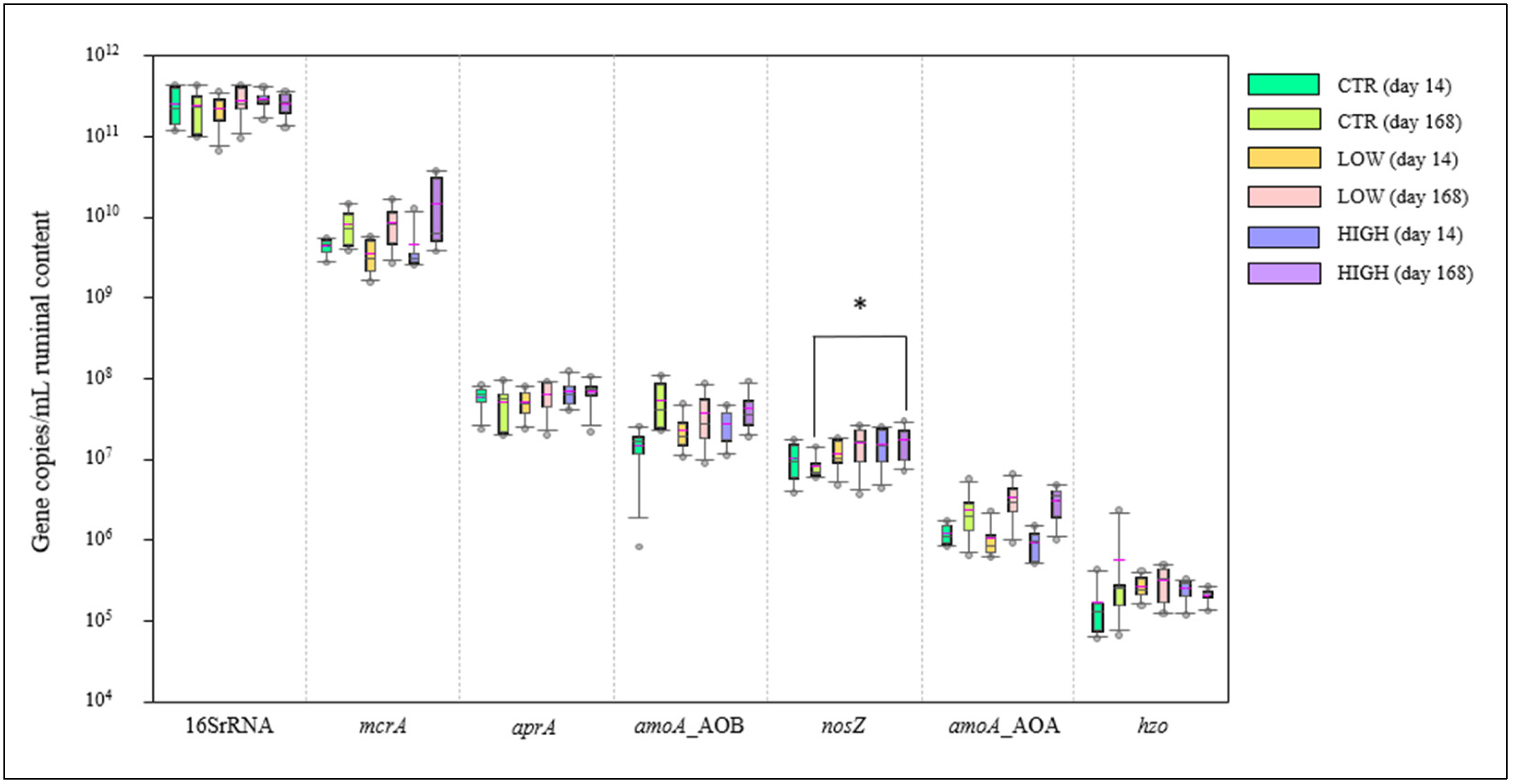
2.5. Rumen Fermentation and Microbiota
2.6. N Excretion and Apparent Total Tract Digestibility
2.7. Chemical Analyses
2.8. DNA Extraction and Bacterial Gene Quantification
2.9. Calculations and Statistical Analysis
3. Results
3.1. Water Quality
3.2. Water and Feed Intake
3.3. Haematological and Biochemical Blood Parameters
3.4. Rumen pH, Volatile Fatty Acids, NO3− and NO2−, and Microbiota
3.5. Nitrogen Excretion and Apparent Total Tract Digestibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meyer, J.A.; Casey, N.H. Establishing risk assessment on water quality for livestock. Anim. Front. 2012, 2, 44–49. [Google Scholar] [CrossRef]
- Tredoux, G. A preliminary investigation of the nitrate content of groundwater and limitation of the nitrate input. In Report to the Water Research Commission (WRC); Report Nº 368/1/93; Water Research Commission: Pretoria, South Africa, 1993. [Google Scholar]
- Holloway, J.M.; Dahlgren, R.A.; Hansen, B.; Casey, W.H. Contribution of bedrock nitrogen to high nitrate concentrations in stream water. Nature 1998, 395, 785–788. [Google Scholar] [CrossRef]
- Tredoux, G.; Engelbrecht, P.; Israel, S. Nitrate in Groundwater. Why is it a hazard and how to control it. In Report to the Water Research Commission (WRC); Report Nº TT 410/09, Natural Resources and the Environment, Stellenbosch; Water Research Commission: Pretoria, South Africa, 2009. [Google Scholar]
- Kaspar, H.F.; Tiedje, J.M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: Nitrous oxide production and effect of acetylene. Appl. Environ. Microbiol. 1981, 41, 705–709. [Google Scholar] [CrossRef]
- Bruning-Fann, C.S.; Kaneene, J.B. The effects of nitrate, nitrite, and N-nitroso compounds on animal health. Vet. Hum. Toxicol. 1993, 35, 237–253. [Google Scholar]
- Stuart, L.D.; Oehme, F.W. Environmental factors in bovine and porcine abortion. Vet. Hum. Toxicol. 1982, 24, 435–441. [Google Scholar]
- Ozmen, O.; Mor, F.; Unsal, A. Nitrate poisoning in cattle fed Chenopodium album Hay. Vet. Hum. Toxicol. 2003, 45, 83–84. [Google Scholar]
- Ozmen, O.; Mor, F.; Sahinduran, S.; Unsal, A. Pathological and toxicological investigations of chronic nitrate poisoning in cattle. Toxicol. Environ. Chem. 2005, 87, 99–106. [Google Scholar] [CrossRef]
- Lee, C.; Beauchemin, K.A. A review of feeding supplementary nitrate to ruminant animals: Nitrate toxicity, methane emissions, and production performance. Can. J. Anim. Sci. 2014, 94, 557–570. [Google Scholar] [CrossRef]
- Latham, E.A.; Anderson, R.C.; Pinchak, W.E.; Nisbet, D.J. Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front. Microbiol. 2016, 7, 228. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Ungerfeld, E.M.; Zhang, X.M.; Long, D.L.; Mao, H.X.; Deng, J.P.; Bannink, A.; Tan, Z.L. Nitrate improves ammonia incorporation into rumen microbial protein in lactating dairy cows fed a low-protein diet. J. Dairy Sci. 2018, 101, 9789–9799. [Google Scholar] [CrossRef]
- Schütz, K. Effects of providing clean water on the health and productivity of cattle. In Report for Northland Regional Council; Report number RE400/2012/346; Northland Regional Council: Whangarei, New Zealand, 2012. [Google Scholar]
- Willms, W.D.; Kenzie, O.R.; McAllister, T.A.; Colwell, D.; Veira, D.; Wilmshurst, J.F.; Entz, T.; Olson, M.E. Effects of water quality on cattle performance. J. Range Manag. 2002, 55, 452–460. [Google Scholar] [CrossRef]
- Llonch, L.; Verdú, M.; Martí, S.; Medinyà, C.; Riera, J.; Cucurull, J.; Devant, M. Drinking water chlorination in dairy beef fattening bulls: Water quality, potential hazards, apparent total tract digestibility, and growth performance. Animal 2023, 17, 100685. [Google Scholar] [CrossRef]
- Anderson, A.C.; Reimers, R.S.; DeKernion, P. A brief review of the current status of alternatives to chlorine disinfection of water. Am. J. Public Health 1982, 72, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, G.W. A Comparative Review of Water Disinfection Methods Appropriate for Developing Countries and Their Efficacy, Cost-Efficiency, and Usability. Texas Medical Center Dissertations, UMI Number 1479579. 2010. Available online: https://www.proquest.com/docview/746835953 (accessed on 26 August 2024).
- Islam, M.; Uddin, M.; Islam, K.; Sultana, M. Effect of different sources of water on water quality and growth performance of growing bull. Bang. J. Anim. Sci. 2019, 48, 9–16. [Google Scholar] [CrossRef]
- Akinmoladun, O.F.; Muchenje, V.; Fon, F.N.; Mpendulo, C.T. Small Ruminants: Farmers’ Hope in a World Threatened by Water Scarcity. Animals 2019, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Legesse, G.; Ominski, K.H.; Beauchemin, K.A.; Pfister, S.; Martel, M.; McGeough, E.J.; Hoekstra, A.Y.; Kroebel, R.; Cordeiro, M.R.C.; McAllister, T.A. BOARD-INVITED REVIEW: Quantifying water use in ruminant production. J. Anim. Sci. 2017, 95, 2001–2018. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Devant, M.; Ferret, A.; Calsamiglia, S.; Casals, R.; Gasa, J. Effect of nitrogen source in high-concentrate, low-protein beef cattle diets on microbial fermentation studied in vivo and in vitro. J. Anim. Sci. 2001, 79, 1944–1953. [Google Scholar] [CrossRef]
- Verdú, M.; Marti, S.; Riera, J.; Medinya, C.; Cucurull, J.; Devant, M. PSXIII-10 Effects of nitrate concentration in drinking water on water and feed consumption, total tract digestibility and health implications in Holstein bulls fed high-concentrate diets. J. Anim. Sci. 2021, 99, 462. [Google Scholar] [CrossRef]
- Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA). Necesidades Nutricionales Para Rumiantes de Cebo: Normas FEDNA; FEDNA: Madrid, Spain, 2008. [Google Scholar]
- European Food Safety Authority (EFSA). Technical guidance: Tolerance and efficacy studies in target animals. EFSA J. 2011, 9, 2175. [Google Scholar] [CrossRef]
- Jouany, J.P. Volatile fatty acids and alcohol determination in digestive contents, silage juice, bacterial cultures and anaerobic fermentor contents. Sci. Aliment. 1982, 2, 131–144. [Google Scholar]
- Genís, S.; Verdú, M.; Cucurull, J.; Devant, M. Complete feed versus concentrate and straw fed separately: Effect of feeding method on eating and sorting behavior, rumen acidosis, and digestibility in crossbred Angus bulls fed high-concentrate diets. Anim. Feed Sci. Technol. 2021, 273, 114820. [Google Scholar] [CrossRef]
- Royal Decree (RD) 140/2003 of February 7, 2003. The Sanitary Criteria for the Quality of Water for Human Consumption. State Official Bulletin of Spain Government. 2003, BOE-A-2003-3596, 45, pp. 1–40. Available online: https://www.boe.es/eli/es/rd/2003/02/07/140/con (accessed on 24 September 2024).
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) Nº 152/2009 of January 27, 2009. Laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L 54, 1–130. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:164:0007:0031:EN:PDF (accessed on 24 September 2024).
- Titgemeyer, E.C.; Armendariz, C.K.; Bindel, D.J.; Greenwood, R.H.; Löest, C.A. Evaluation of titanium dioxide as a digestibility marker for cattle. J. Anim. Sci. 2001, 79, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, C.; Guivernau, M.; Viñas, M.; García, J.; Velasco-Galilea, M.; Souza, S.S.; Serezino, P.H.; Ávila, C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018, 141, 185–195. [Google Scholar] [CrossRef]
- Prenafeta-Boldú, F.X.; Guivernau, M.; Gallastegui, G.; Viñas, M.; de Hoog, G.S.; Elías, A. Fungal/bacterial interactions during the biodegradation of TEX hydrocarbons (toluene, ethylbenzene and p-xylene) in gas biofilters operated under xerophilic conditions. FEMS Microbiol. Ecol. 2012, 80, 722–734. [Google Scholar] [CrossRef]
- Casas, M.E.; Guivernau, M.; Viñas, M.; Fernández, B.; Cáceres, R.; Biel, C.; Matamoros, V. Use of wood and cork in biofilters for the simultaneous removal of nitrates and pesticides from groundwater. Chemosphere 2022, 313, 137502. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Chen, X.B.; Grubic, G.; Ørskov, E.R.; Osuji, P. Effect of feeding frequency on diurnal variation and urinary derivatives in steers. Anim. Prod. 1992, 55, 185–191. [Google Scholar] [CrossRef]
- Nolan, J.V.; Godwin, I.R.; de Raphélis-Soissan, V.; Hegarty, H.S. Managing the rumen to limit the incidence and severity of nitrite poisoning in nitrate-supplemented ruminants. Anim. Prod. Sci. 2016, 56, 1317–1329. [Google Scholar] [CrossRef]
- Doornenball, H.; Tong, A.K.; Murray, N.L. Reference values of blood parameters in beef cattle of different ages and stages of lactation. Can. J. Vet. Res. 1988, 52, 99–105. [Google Scholar]
- Joerling, J.; Doll, K. Monitoring of iron deficiency in calves by determination of serum ferritin in comparison with serum iron: A preliminary study. Open Vet. J. 2019, 9, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Shiono, H.; Yagi, Y.; Thongnoon, P.; Kurabayashi, N.; Chikayama, Y.; Miyazaki, S.; Nakamura, I. Acquired methemoglobinemia in anemic cattle infected with Theileria sergenti. Vet. Parasitol. 2001, 102, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Beede, D.K. The Most Essential Nutrient: Water. In Proceedings of the 7th Western Dairy Management Conference, Reno, NV, USA, 9–11 March 2005; pp. 13–32. [Google Scholar]
- Wright, C.L. Management of water quality for beef cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 91–103. [Google Scholar] [CrossRef]
- Lardner, H.A.; Kirychuk, B.D.; Braul, L.; Willms, W.D.; Yarotski, J. The effect of water quality on cattle performance on pasture. Aust. J. Agric. Res. 2005, 56, 97–104. [Google Scholar] [CrossRef]
- Yang, C.; Rooke, J.A.; Cabeza, I.; Wallace, R.J. Nitrate and inhibition of ruminal methanogenesis: Microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front. Microbiol. 2016, 7, 132. [Google Scholar] [CrossRef]
- van Zijderveld, S.M.; Gerrits, W.J.J.; Apajalahti, J.A.; Newbold, J.R.; Dijkstra, J.; Leng, R.A.; Perdok, H.B. Nitrate and sulfate: Effective alternative hydrogen sinks for mitigation of ruminal methane production in sheep. J. Dairy Sci. 2010, 93, 5856–5866. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Hulshof, R.B.A.; Berndt, A.; Gerrits, W.J.J.; Dijkstra, J.; van Zijderveld, S.M.; Newbold, J.R.; Perdok, H.B. Dietary nitrate supplementation reduces methane emission in beef cattle fed sugarcane-based diets. J. Anim. Sci. 2012, 90, 2317–2323. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Morgavi, D. The use of direct-fed microbials for mitigation of ruminant methane emissions: A review. Animal 2014, 8, 250–261. [Google Scholar] [CrossRef]
- Liu, L.; Xu, X.; Cao, Y.; Cai, C.; Cui, H.; Yao, J. Nitrate decreases methane production also by increasing methane oxidation through stimulating NC10 population in ruminal culture. AMB Express 2017, 7, 76. [Google Scholar] [CrossRef] [PubMed]
| Items | Treatment 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR | LOW | MOD | HIGH | SEM | T | P | T × P | |
| n | 6 | 6 | 6 | 6 | ||||
| Feed intake (kg DM/day) | ||||||||
| Concentrate | 6.49 | 6.61 | 6.02 | 6.27 | 0.283 | 0.21 | 0.01 | 0.87 |
| Straw | 0.61 | 0.65 | 0.66 | 0.70 | 0.065 | 0.58 | 0.01 | 0.03 |
| Total | 7.10 | 7.26 | 6.69 | 6.97 | 0.285 | 0.26 | 0.01 | 0.48 |
| Water intake (L/day) | 28.4 | 27.0 | 24.4 | 25.9 | 1.99 | 0.27 | 0.01 | 0.75 |
| NO3− intake (mg/day) | ||||||||
| Concentrate | 71 | 73 | 66 | 69 | - | - | - | - |
| Straw | 111 | 118 | 120 | 127 | - | - | - | - |
| Water | 67 | 1330 | 3023 | 6335 | - | - | - | - |
| Total | 249 | 1521 | 3210 | 6532 | - | - | - | - |
| Items | Treatment 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR | LOW | MOD | HIGH | SEM | T | P | T × P | |
| n | 6 | 6 | 6 | 6 | ||||
| WBC (K cells/µL) | 8.83 | 8.40 | 8.23 | 8.83 | 0.832 | 0.84 | 0.79 | 0.49 |
| RBC (×106 cells/µL) | 8.85 | 9.03 | 9.15 | 8.82 | 0.403 | 0.83 | 0.01 | 0.98 |
| Hb (g/dL) | 12.0 | 11.9 | 12.0 | 12.0 | 0.49 | 0.99 | 0.01 | 1.00 |
| MtHb (%) | 2.42 | 2.40 | 2.46 | 2.42 | 0.154 | 0.97 | 0.01 | 0.17 |
| HCT (%) | 32.8 | 32.3 | 32.4 | 32.3 | 1.28 | 0.98 | 0.01 | 0.94 |
| MCV (fL) | 33.4 | 32.5 | 32.0 | 33.0 | 1.38 | 0.77 | 0.01 | 0.46 |
| MCHC 2 (g/dL) | 36.7 b | 36.8 b | 37.1 ab | 37.4 a | 0.23 | 0.03 | 0.01 | 0.99 |
| RDW (%) | 22.9 | 22.8 | 22.6 | 22.5 | 0.51 | 0.84 | 0.01 | 0.71 |
| HDW (g/dL) | 2.33 | 2.28 | 2.34 | 2.27 | 0.054 | 0.55 | 0.04 | 0.61 |
| NEU (K cells/µL) | 2.65 | 2.61 | 2.41 | 2.48 | 0.437 | 0.94 | 0.01 | 0.28 |
| LYM (K cells/µL) | 4.73 | 4.39 | 4.48 | 4.85 | 0.477 | 0.75 | 0.01 | 0.90 |
| MONO (K cells/µL) | 1.01 | 1.00 | 0.99 | 0.99 | 0.106 | 1.00 | 0.01 | 0.94 |
| EOS (K cells/µL) | 0.30 | 0.22 | 0.22 | 0.37 | 0.117 | 0.52 | 0.06 | 0.71 |
| L-LYM (K cells/µL) | 0.04 | 0.03 | 0.03 | 0.03 | 0.007 | 0.34 | 0.01 | 0.28 |
| BASO (K cells/µL) | 0.10 | 0.10 | 0.11 | 0.12 | 0.014 | 0.58 | 0.01 | 0.59 |
| PLT (K cells/µL) | 408 | 475 | 512 | 449 | 67.5 | 0.49 | 0.58 | 0.07 |
| MPV 3 (fL) | 7.03 a | 6.64 b | 6.41 b | 6.50 b | 0.163 | 0.01 | 0.01 | 0.85 |
| MPC (g/dL) | 24.4 | 23.7 | 24.2 | 23.7 | 0.61 | 0.53 | 0.04 | 0.25 |
| PDW (g/dL) | 6.53 | 6.53 | 6.57 | 6.49 | 0.108 | 0.91 | 0.01 | 0.48 |
| Items | Treatment 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR | LOW | MOD | HIGH | SEM | T | P | T × P | |
| n | 6 | 6 | 6 | 6 | ||||
| Ammonia (µmol/L) | 58.7 | 56.3 | 57.9 | 59.1 | 5.94 | 0.81 | 0.01 | 0.02 |
| Albumin (g/dL) | 3.22 | 3.29 | 3.31 | 3.26 | 0.126 | 0.80 | 0.01 | 0.92 |
| ALT (U/L) | 19.0 | 17.8 | 18.5 | 18.4 | 1.81 | 0.89 | 0.52 | 0.47 |
| Amylase (U/L) | 126 | 125 | 124 | 124 | 23.1 | 1.00 | 0.84 | 0.22 |
| AST (U/L) | 78.8 | 71.2 | 74.3 | 72.8 | 8.49 | 0.73 | 0.01 | 0.38 |
| Total bilirubin (mg/dL) | 0.15 | 0.15 | 0.15 | 0.14 | 0.010 | 0.21 | 0.01 | 0.39 |
| Calcium (mg/dL) | 10.5 | 10.4 | 10.6 | 10.5 | 0.17 | 0.49 | 0.01 | 0.76 |
| Creatine kinase (U/L) | 179 | 188 | 185 | 182 | 121.8 | 1.00 | 0.35 | 0.74 |
| Chloride (mmol/L) | 98.3 | 98.0 | 97.5 | 97.6 | 1.11 | 0.79 | 0.01 | 0.81 |
| Cholesterol (mg/dL) | 123 | 129 | 123 | 123 | 15.8 | 0.94 | 0.02 | 0.86 |
| Creatinine (mg/dL) | 0.87 | 0.83 | 0.84 | 0.84 | 0.069 | 0.91 | 0.01 | 0.52 |
| ALP (U/L) | 285 | 214 | 274 | 240 | 44.0 | 0.27 | 0.01 | 0.37 |
| Phosphorous (mg/dL) | 9.03 | 9.10 | 8.85 | 8.93 | 0.429 | 0.79 | 0.05 | 0.32 |
| GGTP (U/L) | 19.6 | 20.9 | 17.4 | 18.6 | 3.94 | 0.68 | 0.01 | 0.68 |
| Glucose (mg/dL) | 92.1 | 90.7 | 88.2 | 88.2 | 5.36 | 0.57 | 0.01 | 0.61 |
| LDH (U/L) | 2856 | 2792 | 2774 | 2673 | 284.2 | 0.69 | 0.01 | 0.60 |
| Magnesium (mg/dL) | 2.86 | 2.58 | 2.71 | 2.54 | 0.235 | 0.38 | 0.01 | 0.73 |
| Potassium (mg/dL) | 4.65 | 4.48 | 4.45 | 4.49 | 0.152 | 0.20 | 0.01 | 0.48 |
| Total protein (g/dL) | 6.89 | 7.07 | 6.96 | 6.99 | 0.226 | 0.82 | 0.35 | 0.03 |
| Sodium (mmol/L) | 143 | 143 | 142 | 142 | 0.9 | 0.89 | 0.01 | 0.82 |
| Urea N (mg/dL) | 16.0 | 17.8 | 17.8 | 17.2 | 2.64 | 0.71 | 0.01 | 0.55 |
| Haptoglobin (mg/mL) | 0.27 | 0.19 | 0.20 | 0.20 | 0.164 | 0.56 | 0.67 | 0.17 |
| Items | Treatment 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR | LOW | MOD | HIGH | SEM | T | P | T × P | |
| n | 6 | 6 | 6 | 6 | ||||
| pH | 5.71 | 5.51 | 5.56 | 5.57 | 0.251 | 0.86 | 0.25 | 0.63 |
| Total VFA (Mm) | 158 | 179 | 169 | 161 | 19.9 | 0.65 | 0.01 | 0.87 |
| Acetic | 72.1 | 83.3 | 75.9 | 73.6 | 8.86 | 0.52 | 0.01 | 0.82 |
| Propionic | 63.4 | 72.3 | 68.6 | 63.8 | 10.87 | 0.79 | 0.01 | 0.96 |
| i-Butyric | 0.44 | 0.46 | 0.41 | 0.45 | 0.093 | 0.95 | 0.01 | 0.82 |
| n-Butyric | 12.4 | 13.6 | 14.4 | 13.4 | 1.92 | 0.77 | 0.01 | 0.51 |
| i-Valeric | 0.56 | 0.72 | 0.65 | 0.75 | 0.202 | 0.77 | 0.01 | 0.49 |
| n-Valeric | 3.46 | 3.53 | 4.56 | 3.42 | 0.811 | 0.47 | 0.01 | 0.25 |
| i-Caproic | 3.95 | 3.95 | 3.67 | 3.96 | 0.346 | 0.81 | 0.01 | 0.91 |
| n-Caproic | 1.16 | 0.95 | 1.06 | 0.99 | 0.354 | 0.92 | 0.01 | 0.75 |
| n-Heptanoic | 0.17 | 0.15 | 0.18 | 0.15 | 0.058 | 0.94 | 0.01 | 0.62 |
| Acetic:Propionic | 1.30 | 1.29 | 1.35 | 1.43 | 0.237 | 0.92 | 0.01 | 0.69 |
| NO3− (ppm) | 1.75 | 1.67 | 1.18 | 1.42 | 0.390 | 0.47 | 0.21 | 0.31 |
| NO2− (ppm) | 22.2 | 17.7 | 16.1 | 21.2 | 6.33 | 0.75 | 0.01 | 0.79 |
| Items | Treatment 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| CTR | LOW | MOD | HIGH | SEM | T | P | T × P | |
| n | 6 | 6 | 6 | 6 | ||||
| Intake | ||||||||
| Concentrate (kg DM/day) | 6.38 | 6.57 | 6.25 | 6.33 | 0.272 | 0.68 | 0.01 | 0.89 |
| Straw (kg DM/day) | 0.56 | 0.60 | 0.61 | 0.65 | 0.081 | 0.74 | 0.01 | 0.51 |
| Total (kg DM/day) | 6.95 | 7.17 | 6.86 | 6.98 | 0.259 | 0.68 | 0.01 | 0.87 |
| CP (kg/day) | 0.86 | 0.88 | 0.84 | 0.85 | 0.041 | 0.78 | 0.01 | 0.83 |
| EE (kg/day) | 0.25 | 0.25 | 0.24 | 0.25 | 0.011 | 0.78 | 0.01 | 0.76 |
| OM (kg/day) | 6.58 | 6.79 | 6.50 | 6.61 | 0.244 | 0.68 | 0.01 | 0.87 |
| NDF (kg/day) | 1.64 | 1.70 | 1.65 | 1.70 | 0.079 | 0.81 | 0.01 | 0.70 |
| Starch (kg/day) | 3.21 | 3.30 | 3.13 | 3.17 | 0.157 | 0.76 | 0.01 | 0.83 |
| Faeces | ||||||||
| Total (kg) | 1.82 | 1.96 | 1.88 | 1.97 | 0.180 | 0.81 | 0.01 | 0.34 |
| CP (kg/day) | 0.28 | 0.30 | 0.29 | 0.30 | 0.025 | 0.94 | 0.01 | 0.47 |
| EE (kg/day) | 0.08 | 0.09 | 0.09 | 0.09 | 0.010 | 0.81 | 0.01 | 0.21 |
| OM (kg/day) | 1.65 | 1.77 | 1.70 | 1.78 | 0.163 | 0.85 | 0.01 | 0.34 |
| NDF (kg/day) | 0.97 | 1.09 | 1.04 | 1.07 | 0.109 | 0.72 | 0.01 | 0.30 |
| Starch (kg/day) | 0.10 | 0.08 | 0.09 | 0.10 | 0.022 | 0.83 | 0.01 | 0.35 |
| Digestibility (%) | ||||||||
| DM corrected | 74.7 | 73.0 | 72.9 | 72.6 | 2.15 | 0.75 | 0.01 | 0.36 |
| CP | 67.4 | 66.7 | 66.5 | 65.9 | 2.62 | 0.95 | 0.01 | 0.46 |
| CP corrected by water N intake | 67.4 | 66.7 | 66.6 | 66.2 | 2.61 | 0.98 | 0.01 | 0.46 |
| EE | 67.8 | 65.7 | 64.8 | 64.5 | 4.63 | 0.89 | 0.01 | 0.33 |
| OM | 75.7 | 74.2 | 74.0 | 73.8 | 2.09 | 0.79 | 0.01 | 0.35 |
| NDF | 41.1 | 35.4 | 36.2 | 37.4 | 6.14 | 0.80 | 0.75 | 0.40 |
| Starch | 96.9 | 97.4 | 97.1 | 97.0 | 0.57 | 0.85 | 0.67 | 0.32 |
| N balance (g/day) | ||||||||
| N intake | ||||||||
| Feed | 138 | 142 | 135 | 137 | 6.6 | 0.77 | 0.01 | 0.83 |
| Water 2 | 0.01 d | 0.31 c | 0.74 b | 1.49 a | 0.060 | 0.01 | 0.01 | 0.01 |
| Total | 138 | 142 | 136 | 138 | 6.6 | 0.82 | 0.01 | 0.82 |
| N excretion | ||||||||
| Faeces | 45.5 | 47.4 | 45.6 | 47.4 | 4.01 | 0.93 | 0.01 | 0.43 |
| Urine | 32.5 | 39.6 | 40.8 | 35.8 | 5.37 | 0.41 | 0.03 | 0.96 |
| Total | 78.0 | 87.0 | 86.4 | 83.2 | 6.18 | 0.46 | 0.01 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llonch, L.; Verdú, M.; Guivernau, M.; Viñas, M.; Martí, S.; Medinyà, C.; Riera, J.; Cucurull, J.; Devant, M. Dose Effect of Drinking Water Nitrate on Health, Feed Intake, Rumen Fermentation and Microbiota, and Nitrogen Excretion in Holstein Heifers for a Sustainable Water Use. Sustainability 2024, 16, 8814. https://doi.org/10.3390/su16208814
Llonch L, Verdú M, Guivernau M, Viñas M, Martí S, Medinyà C, Riera J, Cucurull J, Devant M. Dose Effect of Drinking Water Nitrate on Health, Feed Intake, Rumen Fermentation and Microbiota, and Nitrogen Excretion in Holstein Heifers for a Sustainable Water Use. Sustainability. 2024; 16(20):8814. https://doi.org/10.3390/su16208814
Chicago/Turabian StyleLlonch, Lourdes, Marçal Verdú, Miriam Guivernau, Marc Viñas, Sonia Martí, Carles Medinyà, Joan Riera, Jordi Cucurull, and Maria Devant. 2024. "Dose Effect of Drinking Water Nitrate on Health, Feed Intake, Rumen Fermentation and Microbiota, and Nitrogen Excretion in Holstein Heifers for a Sustainable Water Use" Sustainability 16, no. 20: 8814. https://doi.org/10.3390/su16208814
APA StyleLlonch, L., Verdú, M., Guivernau, M., Viñas, M., Martí, S., Medinyà, C., Riera, J., Cucurull, J., & Devant, M. (2024). Dose Effect of Drinking Water Nitrate on Health, Feed Intake, Rumen Fermentation and Microbiota, and Nitrogen Excretion in Holstein Heifers for a Sustainable Water Use. Sustainability, 16(20), 8814. https://doi.org/10.3390/su16208814






