Abstract
As a clean energy source, hydrogen not only helps to reduce the use of fossil fuels but also promotes the transformation of energy structure and sustainable development. This paper firstly introduces the development status of green hydrogen at home and abroad and then focuses on several advanced green hydrogen production technologies. Then, the advantages and shortcomings of different green hydrogen production technologies are compared. Among them, the future source of hydrogen tends to be electrolysis water hydrogen production. Finally, the challenges and application prospects of the development process of green hydrogen technology are discussed, and green hydrogen is expected to become an important part of realizing sustainable global energy development.
1. Introduction
With the relentless increase in global population and the accelerated pace of economic activities, energy demand has surged significantly, accompanied by severe environmental pollution and climate change issues. The overexploitation and utilization of fossil fuels have exacerbated the strain on energy resources and significantly elevated global greenhouse gas emissions, leading to a cascade of environmental and climatic crises, including global warming, frequent extreme weather events, and biodiversity loss. In response to these challenges, it is imperative to develop cleaner, more efficient, and sustainable energy solutions, such as utilizing renewable energy sources for hydrogen production, to fundamentally shift the energy landscape from dependence on fossil fuels to a sustainable, low-carbon energy system. In this transitional phase, green hydrogen energy, recognized as a clean and efficient energy solution, has garnered significant attention from the international community [1].
Green hydrogen is an ideal clean energy carrier, characterized by nearly zero greenhouse gas emissions during production and the release of only water upon use. As such, it is regarded as a pivotal technology for achieving global sustainable energy development and the “Carbon Neutral” goals. Moreover, green hydrogen demonstrates unique advantages in enhancing the flexibility and stability of energy systems. Given the intermittent and unpredictable nature of a renewable energy supply, green hydrogen can effectively balance energy supply and demand by converting surplus renewable energy into hydrogen for storage and transportation, thereby improving the overall stability and efficiency of the energy system [2].
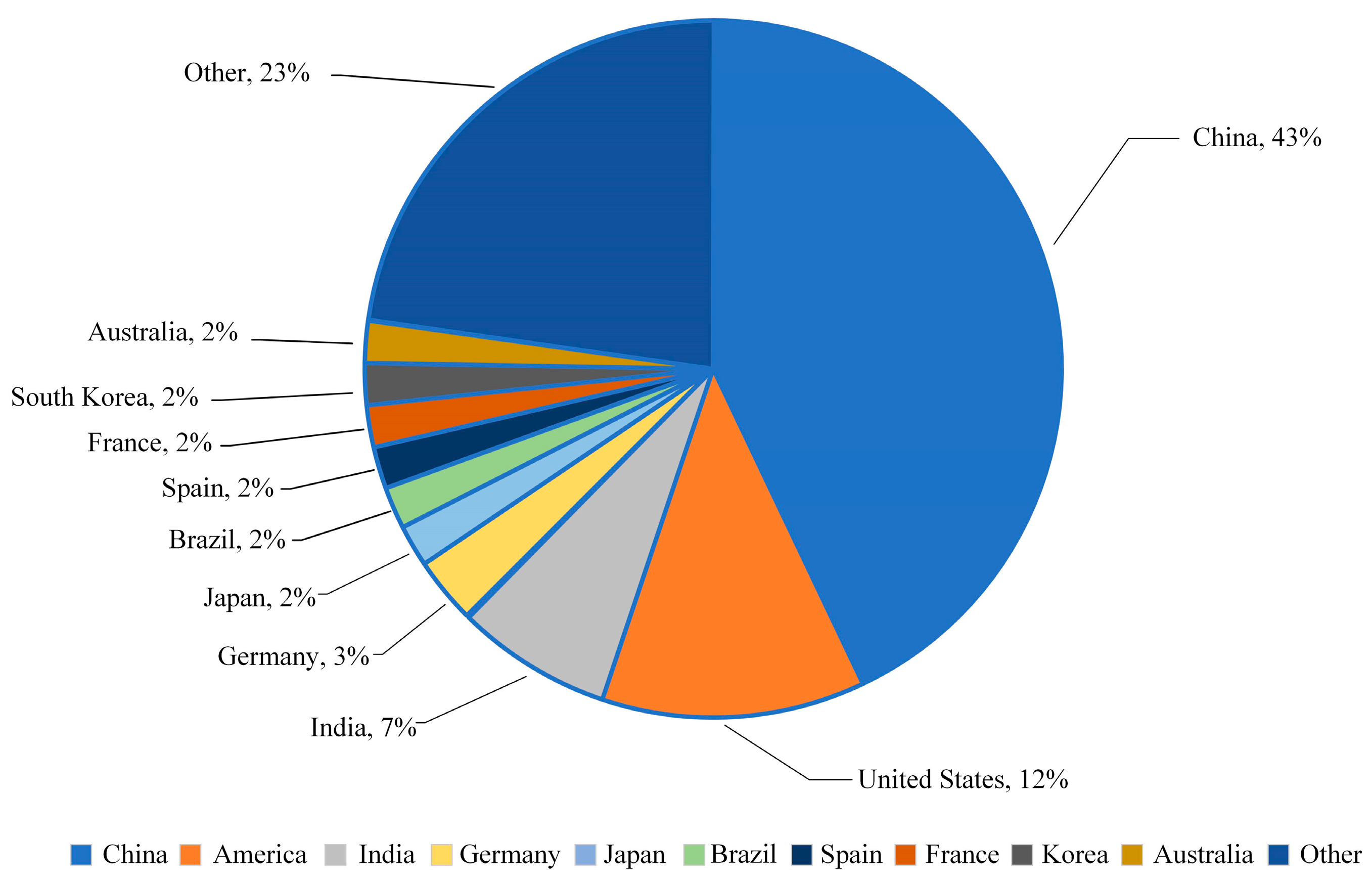
According to the “Renewable Energy Report 2021” published by the International Energy Agency (IEA) [3], during the five-year forecast period from 2021 to 2026, as shown in Figure 1, the installed capacity of the world’s top ten renewable energy-producing countries is expected to account for approximately 80% of the global total installed capacity. China is projected to contribute over 43% of this capacity, followed by the United States and India at 12% and 7%, respectively.
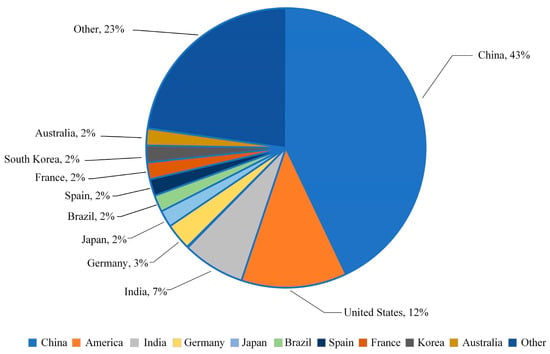
Figure 1.
Projected share of total installed renewable energy capacity in major countries by 2060.
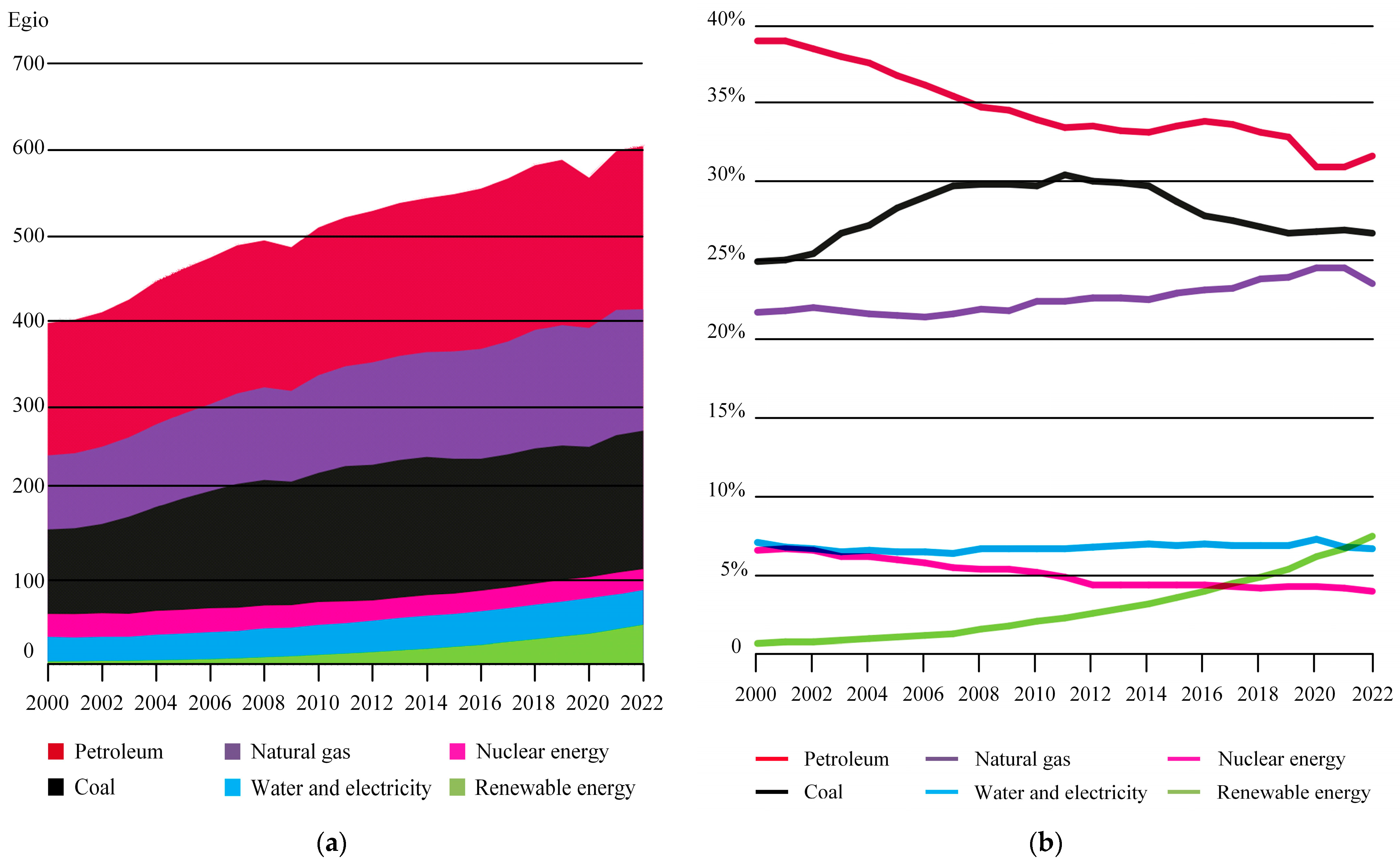
Global energy demand is anticipated to continue its upward trajectory in the coming decades, thereby intensifying the pressure on energy supply and environmental sustainability. According to the World Energy Statistical Yearbook (2023 Edition) [4], the global primary energy consumption for 2022 is illustrated in Figure 2a. Among the energy sources, the consumption of fossil fuels such as oil, gas, and coal remains high and continues to increase, while the consumption of renewable energy shows promising growth. As depicted in Figure 2b, the share of global primary energy consumption indicates that although oil consumption experiences fluctuations, the overall trend is declining. In contrast, renewable energy sources exhibit a persistent upward trend.
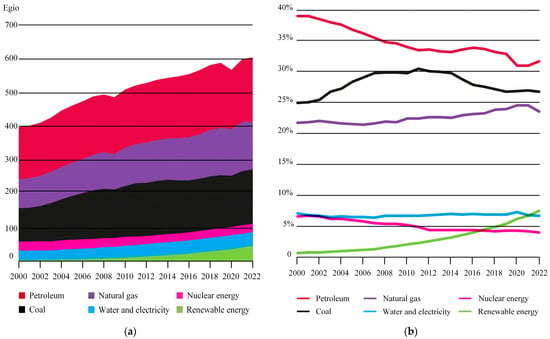
Figure 2.
Global consumption of primary energy. (a) Global consumption; (b) Share of global primary energy consumption.
The combustion of fossil fuels not only releases significant quantities of greenhouse gases, including carbon dioxide [5], exacerbating global warming, but also contributes to environmental issues like air pollution, acid rain, and water contamination. These environmental challenges have profound impacts on ecosystems, human health, and economic development. Additionally, rising sea levels and melting glaciers pose significant threats to ecosystems and biodiversity.
The energy crisis has far-reaching implications for the global economy and trade and exacerbates geopolitical instability. According to the “Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC)” [6], the average global surface temperature is now approximately 1.1 °C higher than the average at the end of the 19th century. Continued temperature increases will severely impact agricultural productivity, human health, and species survival. The anthropogenic release of greenhouse gases is the primary driver of global warming, making the global mitigation of climate change an urgent priority. In response to the intensifying global energy crisis and growing environmental awareness, efficient hydrogen production technologies based on renewable energy have emerged as a key focus of global research. These technologies aim to facilitate sustainable energy development by harnessing renewable sources such as wind and solar power for clean and efficient hydrogen production. Concurrently, the development of control strategies ensures that hydrogen production systems operate efficiently and stably, optimizing energy utilization [7].
China is proactively formulating a hydrogen energy development strategy and gradually refining its policy framework related to hydrogen energy. Supported and guided by national and local policies, the domestic hydrogen energy industry has witnessed rapid growth [8]. In 2020, China established the environmental goals of “Carbon Peaking” and “Carbon Neutrality”, as articulated in relevant policy documents such as the “Mid- to Long-Term Plan for the Development of the Hydrogen Energy Industry (2021–2035)” [9], the “14th Five-Year Plan for Renewable Energy Development” [10], and the “Guiding Opinions on Energy Work for 2024” [11]. These policies highlight the strategic significance of hydrogen technology in China’s clean energy transition, focusing on the advancement of large-scale hydrogen production from renewable sources and the transition to a green, low-carbon development model. Notably, the “14th Five-Year Plan for Renewable Energy Development” commits China to achieving approximately 20% of non-fossil energy consumption by 2025 in the short term, while actively promoting the development and utilization of renewable energy power generation. At the 2024 China International Hydrogen Energy and Fuel Cell Industry Exhibition, the “Hydrogen Island” project, a world-class green hydrogen ecological innovation zone, will be officially launched. This project aims to establish a leading renewable energy-based hydrogen production base, spearhead large-scale development of the hydrogen energy industry, and ultimately achieve a green hydrogen production capacity exceeding 700 tons per day [12]. China’s hydrogen energy strategies/plans are given in Table 1.

Table 1.
China’s hydrogen energy strategy/planning.
The National Energy Administration’s 2024 analysis of renewable energy development and construction indicates that by the end of December 2023, China’s total installed capacity of renewable energy power generation reached 1.516 billion kilowatts, representing 51.9% of the nation’s total installed power generation capacity. This constitutes nearly 40% of the global total installed renewable energy capacity. In 2023 alone, China added 305 million kilowatts of new renewable energy capacity, accounting for 82.7% of the country’s newly installed power generation capacity and representing half of the world’s newly installed capacity—exceeding the combined total of the rest of the world [13].
Developed regions, including Europe, the United States, and Japan, are actively progressing in the research of hydrogen production technologies based on renewable energy [14]. Selected International Hydrogen Strategies/Plans are listed in Table 2. The United States has introduced several initiatives, such as the “Hydrogen Energy Program Development Plan (2020–2030)”, the “National Clean Hydrogen Strategy and Roadmap (Draft)”, and the “National Clean Hydrogen Strategy and Roadmap”, highlighting hydrogen’s pivotal role in the energy transition. Japan has released key plans such as the “Hydrogen/Fuel Cell Strategic Roadmap (2019)” and the “Sixth Energy Basic Plan (2021)”, underscoring the strategic importance of hydrogen technology in the carbon-neutral agenda and accelerating Japan’s transition to a hydrogen-based society. The European Union, recognizing hydrogen as critical to achieving a clean energy transition, published the “EU Hydrogen Strategy” in 2020, outlining a strategic blueprint for the long-term development of hydrogen energy in Europe. Additionally, countries and regions such as the United Kingdom, Australia, South Korea, the Middle East, and North Africa have progressively implemented national hydrogen energy strategies. The International Energy Agency’s 2022 Global Hydrogen Energy Assessment Report [15] indicates that since September 2021, nine countries have adopted national hydrogen energy strategies, and more and more countries are adopting hydrogen energy strategies; the global energy crisis has fueled the momentum of hydrogen energy development in Europe. The completion of the Ningxia Solar Hydrogen Project in China, with an electrolyzer capacity of 150 MW, accounts for almost three-quarters of the growth. The project is now the largest electrolyzer in the world. By 2030, global electrolyzer capacity will exceed 60 GW per year. In 2021, electrolyzed water will account for only about 0.1 percent of global hydrogen production. However, the installed capacity of electrolyzers is expanding rapidly, reaching 510 MW by the end of 2021, a 70% increase from 2020.

Table 2.
Selected international hydrogen strategies/plans.
According to the report of the International Renewable Energy Agency (IRENA), technological research and project implementation of hydrogen production from renewable energy sources have made remarkable progress in countries and regions such as Europe, the United States, Japan, and China. In particular, a series of breakthroughs have been made by research teams in electrolytic water hydrogen production, photocatalytic hydrogen production, and bio-hydrogen production. In the face of increasingly severe energy and environmental challenges, governments and scientific research organizations regard green hydrogen production technology as one of the solutions [16]. Dincer I. [17] categorizes green hydrogen production methods according to the type of energy they drive, which mainly includes electrical, thermal, photovoltaic, and biochemical energy. These forms of energy can come from renewable sources (e.g., solar, wind, geothermal, etc.) or nuclear. In the paper, several major hydrogen production technologies are described in detail: water electrolysis, biomass gasification, photocatalytic water decomposition, and thermochemical cycles for hydrogen production, systematically evaluating the advantages and disadvantages of the different green hydrogen production methods and emphasizing the importance of hydrogen in the future energy system. Squadrito G et al. [18] analyze and compare the unique advantages and shortcomings of different green hydrogen production technologies: biomass pyrolysis and gasification, water electrolysis, etc., with water electrolysis being considered the most suitable technology for large-scale green hydrogen production. Niaz S et al. [19] explore a variety of methods for hydrogen production and storage, discuss in detail the advantages and disadvantages of different storage methods, and make positive predictions for the future development of the hydrogen economy. Fernández-Arias P et al. [20] focus on alkaline electrolyzer, proton exchange membrane electrolyzer, and solid oxide electrolyzer. A bibliometric and technological review of different green hydrogen production technologies and their applications is presented. Dorel S et al. [21] discuss green hydrogen generation from processes such as biomass gasification, pyrolysis, fermentation, and wastewater treatment. Although these technologies can reduce CO2 emissions, the hydrogen often contains impurities such as CO and CO2 and is therefore referred to as “impure hydrogen”. The literature also suggests that electrolysis of water is the dominant method of green hydrogen production and that future reliance on cheap renewable energy sources will be needed to reduce the cost of hydrogen production.
However, comprehensive review articles on green hydrogen production technologies are limited in the literature. In this context, there is a need for a comprehensive review paper that analyzes green hydrogen production technologies in the literature and provides a comprehensive overview of these technologies. The motivation is to explore the future development trend of green hydrogen production technology by comparing the advantages and shortcomings of different green hydrogen production technologies. In this paper, the current status of the domestic and international development of green hydrogen energy is firstly introduced, followed by green hydrogen production technologies, including advanced hydrogen production technologies including solar decomposition of water for hydrogen production, biomass hydrogen production, and electrolysis of water for hydrogen production. Then, the advantages and shortcomings of different green hydrogen production technologies are compared. Among them, the future source of hydrogen tends to be electrolytic water hydrogen production. Finally, the challenges and application prospects for the future development of green hydrogen production technologies are discussed and analyzed.
2. Research Progress of Hydrogen Production Technology
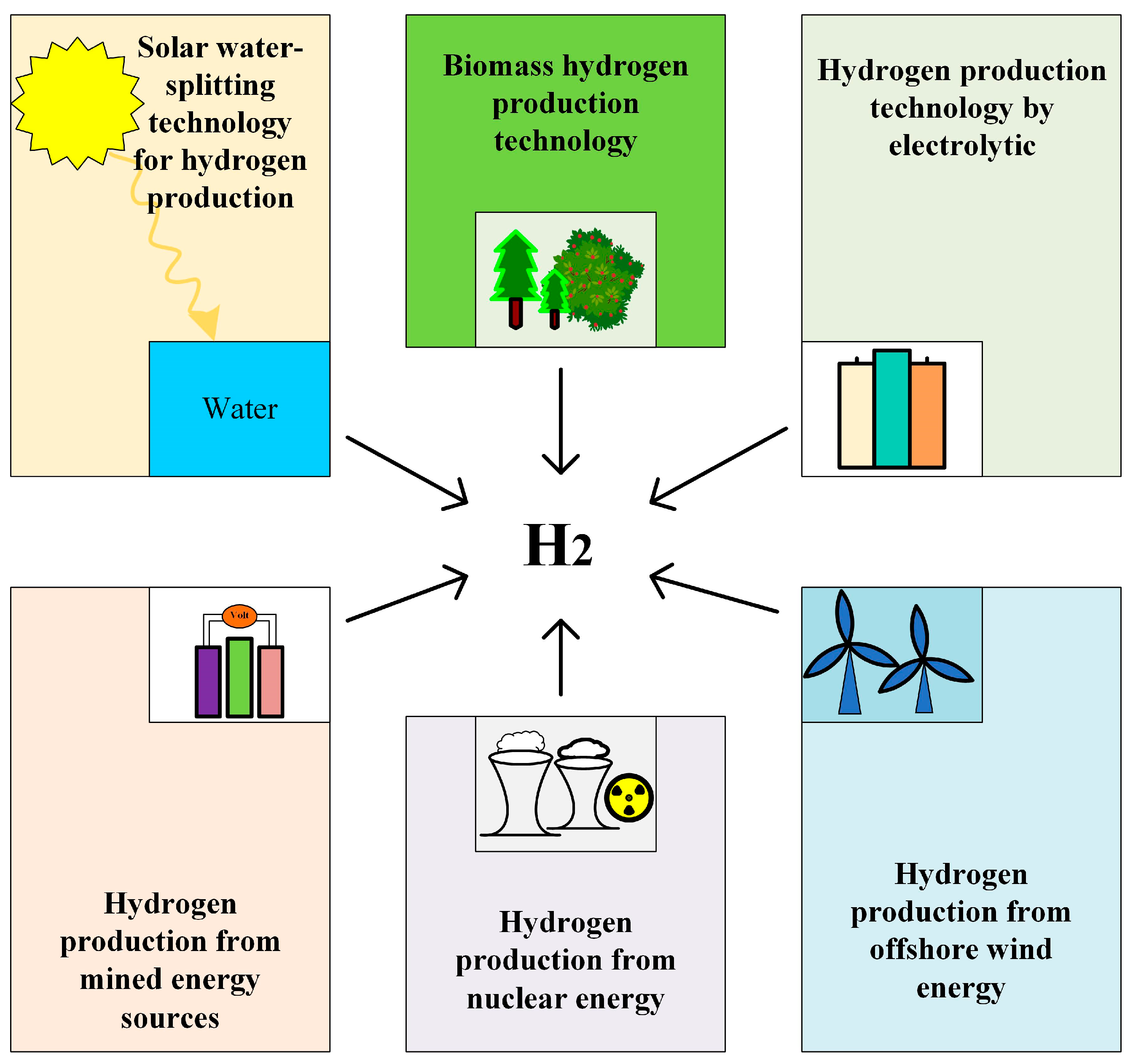
Hydrogen production technology is a critical component in realizing a hydrogen energy economy and advancing energy transformation, garnering significant global attention [22]. The primary methods of hydrogen production include fossil fuel reforming, water electrolysis, biological hydrogen production, and other techniques, as shown in Figure 3. Among these, natural gas steam reforming is the most widely employed due to its cost-effectiveness. However, this process generates substantial amounts of carbon dioxide, contradicting the goal of reducing greenhouse gas emissions. Biological hydrogen production, which involves using microorganisms such as algae and bacteria to produce hydrogen through the biodegradation of organic matter, is environmentally friendly but currently suffers from low efficiency. Hydrogen production via water electrolysis, on the other hand, can achieve zero emissions when powered by renewable energy sources. With ongoing advancements in electrolysis technology and the decreasing cost of renewable energy, the economic viability and feasibility of water electrolysis for hydrogen production are steadily improving. The development of hydrogen production technology is pivotal for the clean energy transition and the widespread adoption of hydrogen energy. In light of current technical challenges, it is essential to intensify research efforts and explore innovative solutions to enhance hydrogen production efficiency, reduce costs, and promote the commercialization and sustainable development of hydrogen technology. Specifically, leveraging renewable energy for water electrolysis hydrogen production can not only decrease reliance on fossil fuels but also significantly mitigate greenhouse gas emissions, making it a crucial strategy for addressing global energy and environmental challenges [23].
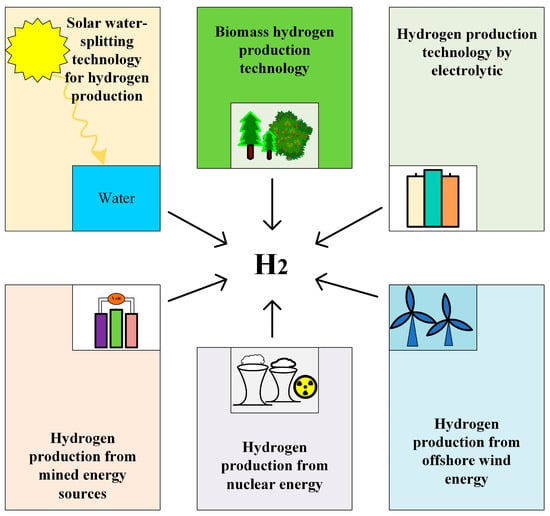
Figure 3.
Different green hydrogen production technologies.
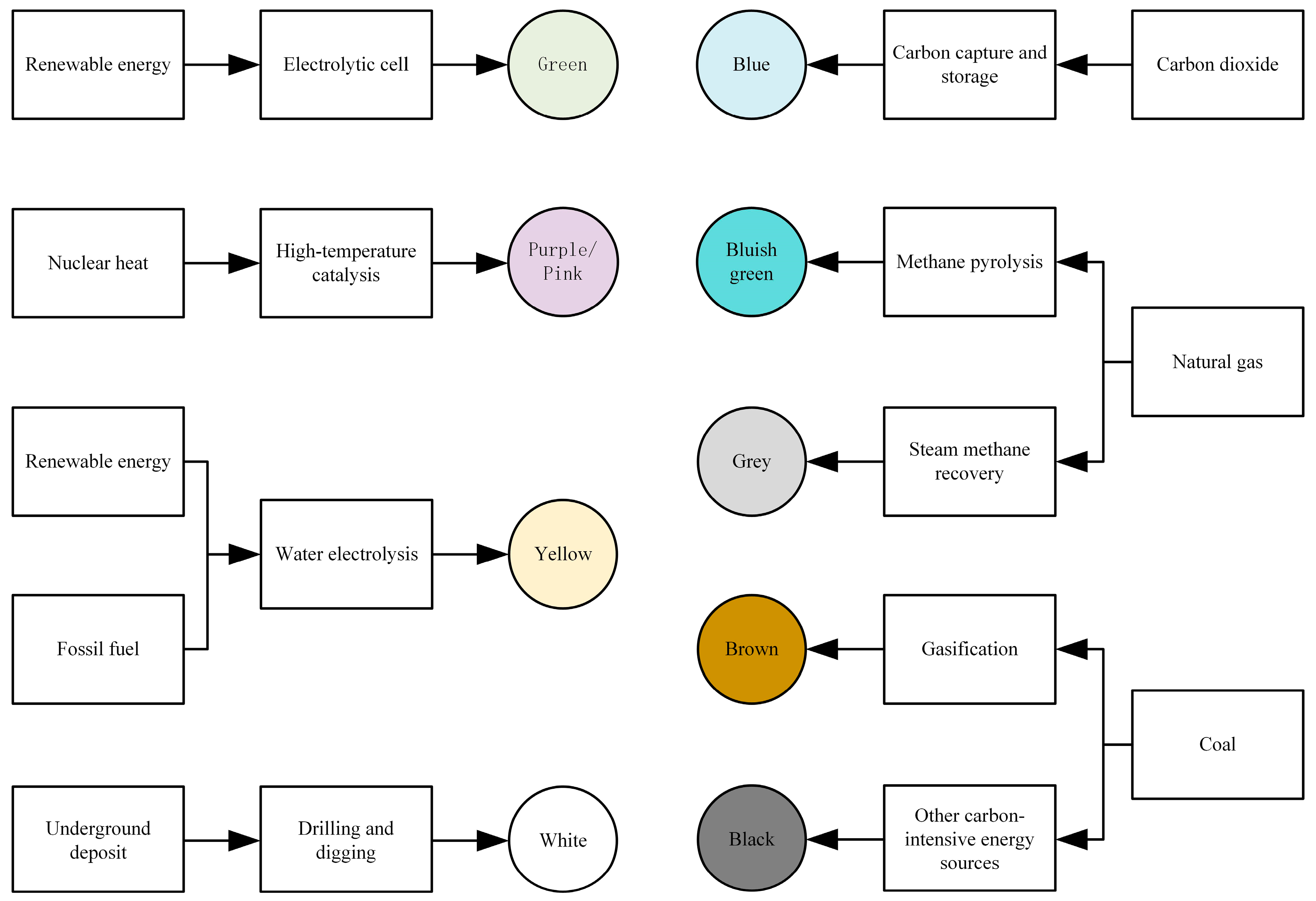
Hydrogen is generally categorized into nine different colors based on the production methods [24], namely green hydrogen, purple/pink hydrogen, yellow hydrogen, white hydrogen, blue hydrogen, blue-green hydrogen, gray hydrogen, brown hydrogen, and black hydrogen, as illustrated in Figure 4.
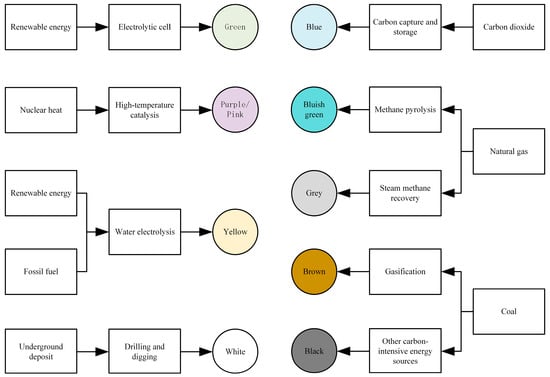
Figure 4.
Hydrogen color under different production methods.
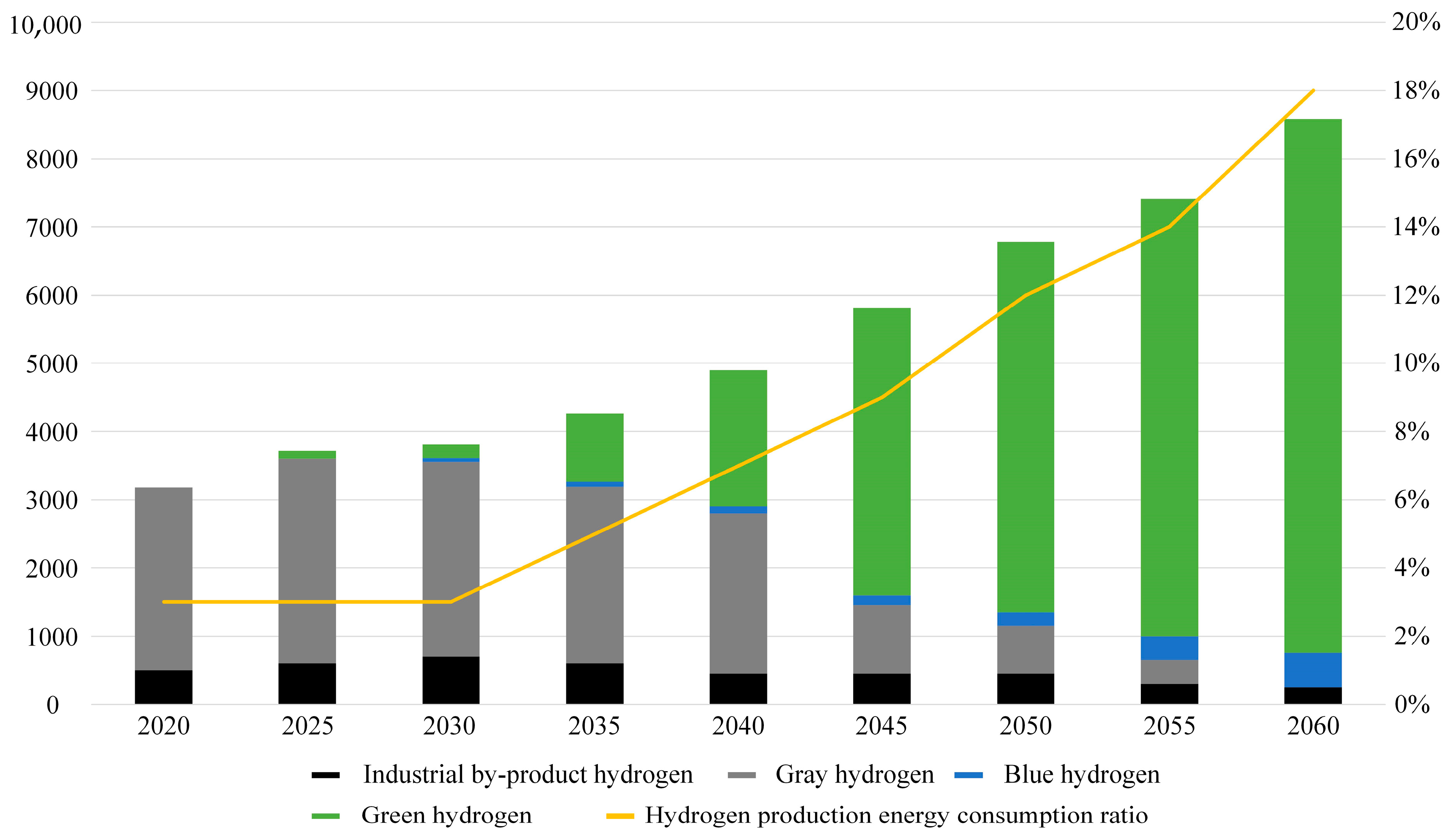
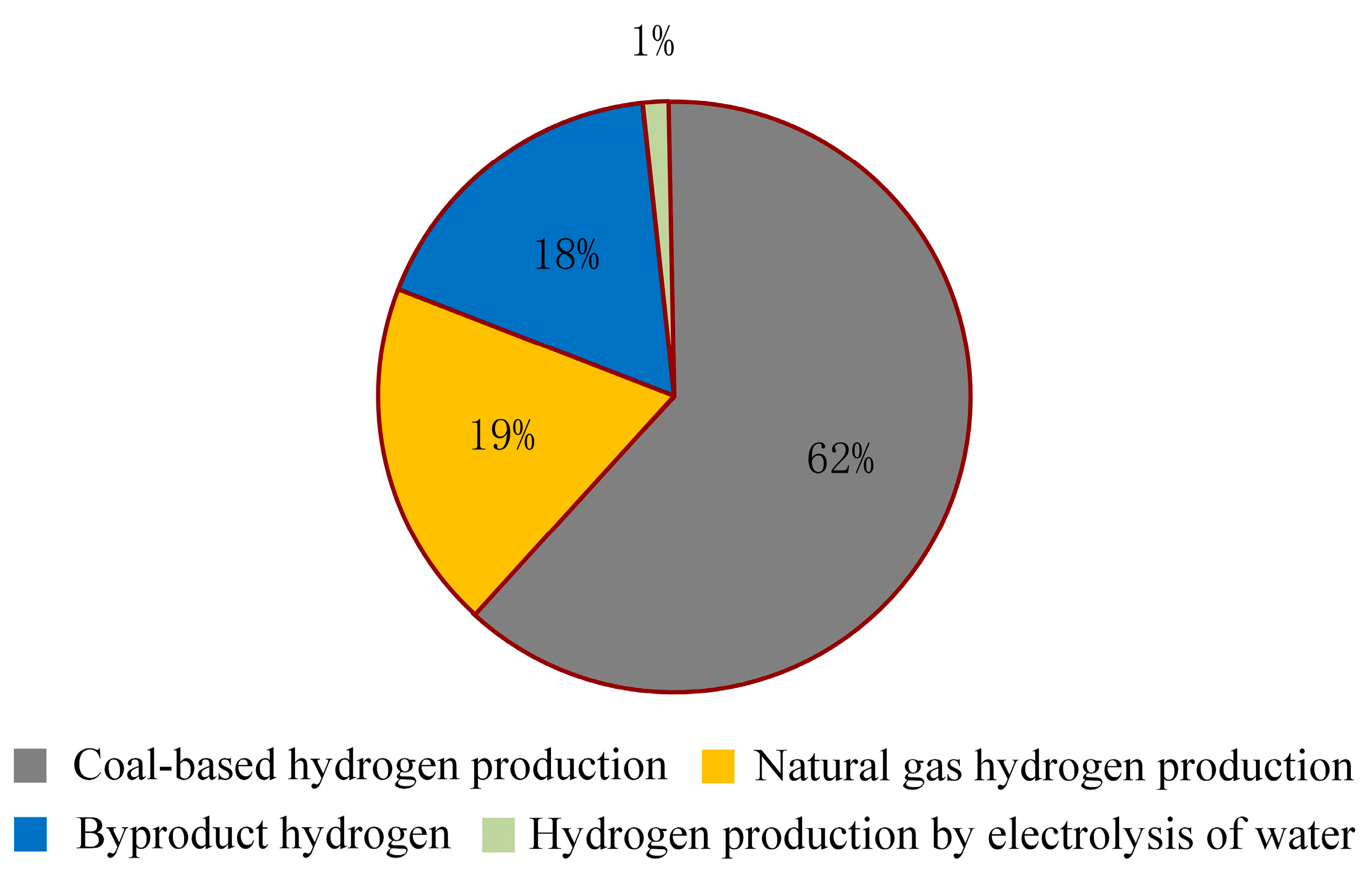
According to the China Energy Outlook 2060 (2024 edition) [25], as illustrated in Figure 5, China’s hydrogen supply in 2023 reached 35.41 million tons, with coal-based hydrogen production accounting for 64.6%, while hydrogen produced through water electrolysis constituted less than 0.5%.
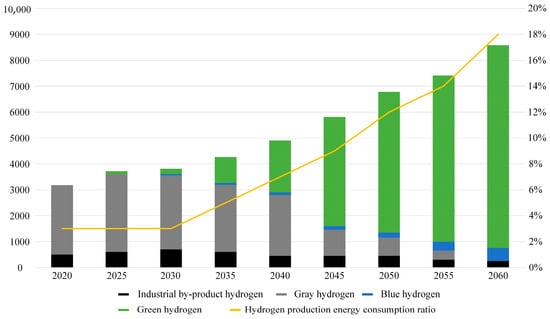
Figure 5.
Hydrogen supply scale and energy consumption in China.
With increasing carbon emission constraints and improvements in the economics of water electrolysis hydrogen production, hydrogen production from fossil energy in China is projected to peak and subsequently decline during the “15th Five-Year Plan” period. By around 2030, water electrolysis is expected to enter a phase of large-scale development. As shown in Table 3, it is anticipated that by 2060, China’s hydrogen supply will rise to 85.8 million tons, with coal and natural gas contributing 7.0%, water electrolysis 89.5%, and hydrogen production comprising 18% of China’s total primary energy consumption.

Table 3.
Total hydrogen supply and hydrogen production energy ratio.
The current hydrogen production methods in China are illustrated in Figure 6, where it is evident that coal dominates the production landscape, with water electrolysis playing a relatively minor role.
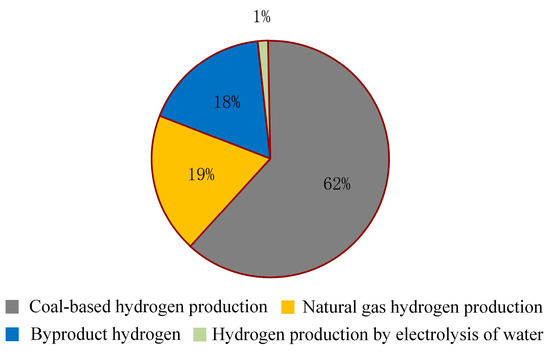
Figure 6.
Hydrogen production mode diagram.
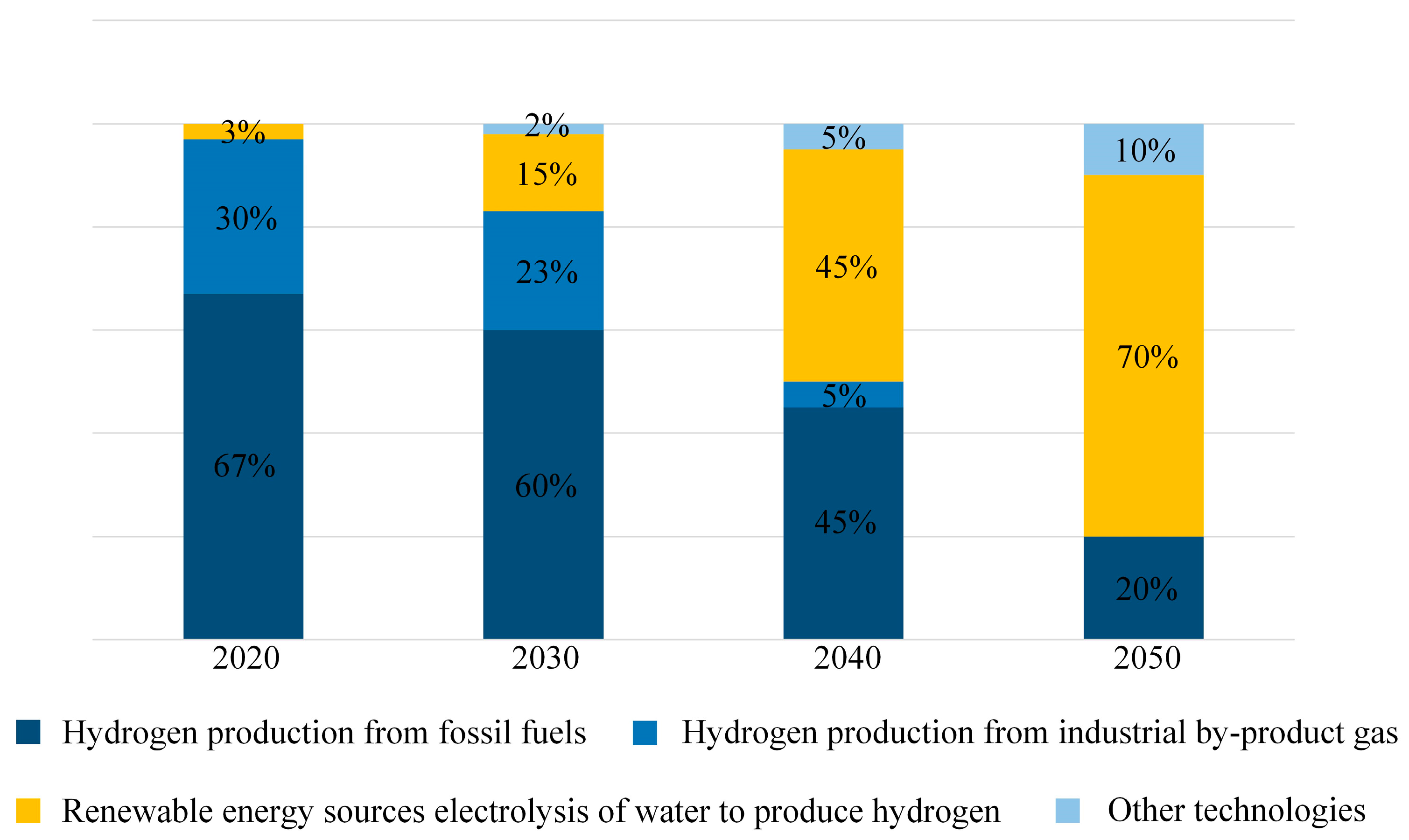
However, with advancements in hydrogen production technology and the pursuit of the “Carbon Peaking and Carbon Neutrality” goals [26], the future source of hydrogen energy is expected to increasingly shift toward water electrolysis. The projected trend of primary hydrogen energy sources is shown in Figure 7.
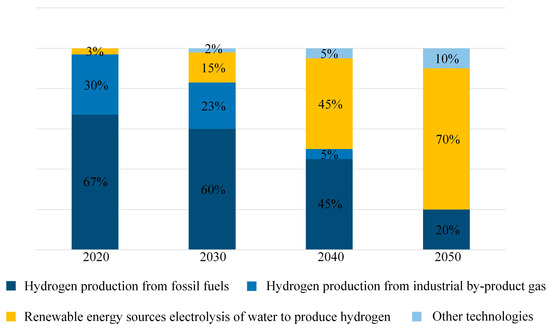
Figure 7.
Prediction diagram of hydrogen energy sources.
3. Solar Water Splitting Technology for Hydrogen Production
Solar water hydrogen production technology is primarily categorized into photocatalytic hydrogen production, solar thermochemical water splitting for hydrogen production, and photochemical hydrogen production, as outlined in Table 4. These processes generate no carbon emissions throughout, making them highly effective methods for producing green hydrogen [27].

Table 4.
Comparison of technical characteristics of hydrogen production by solar water splitting.
3.1. Photocatalytic Hydrogen Process
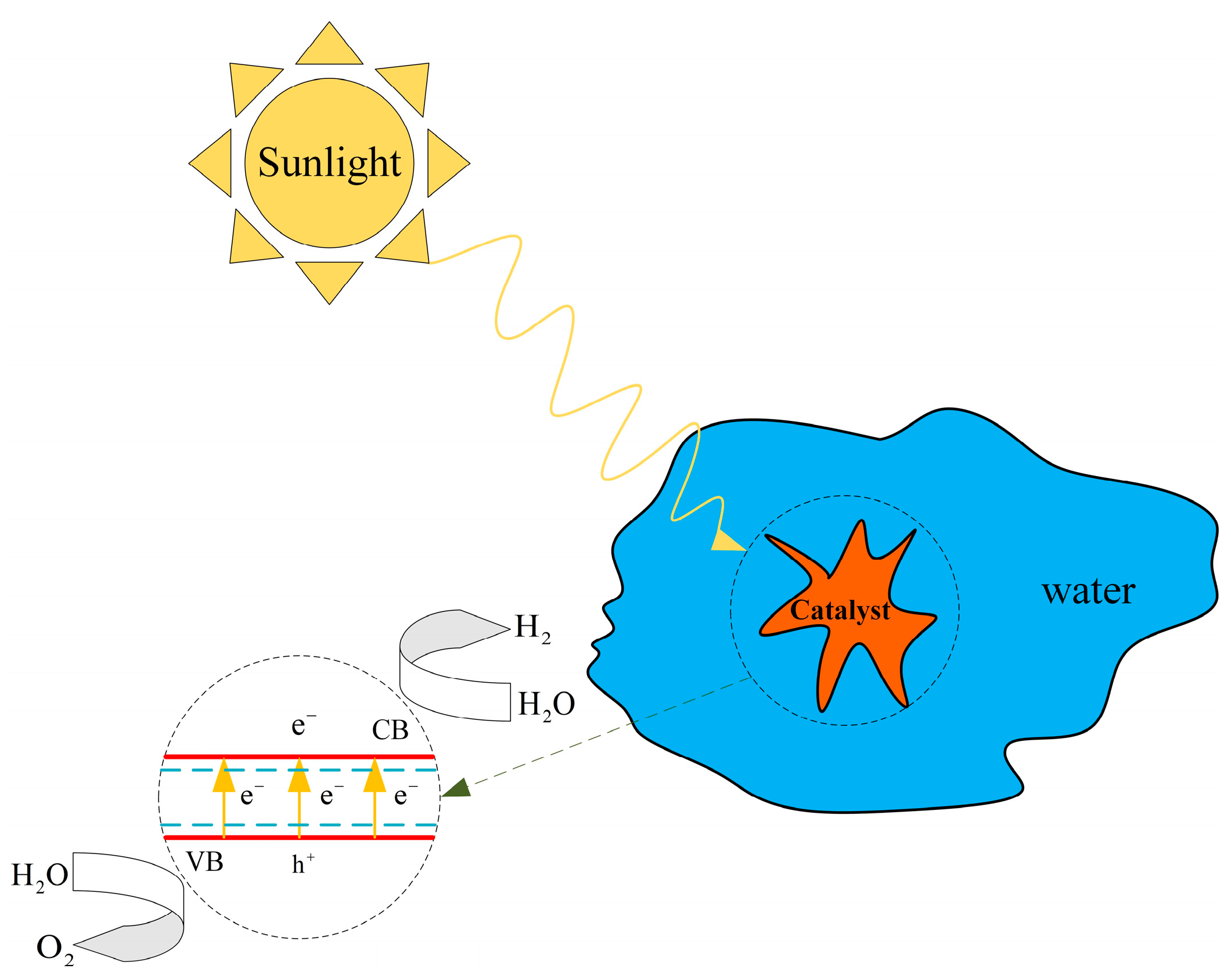
The photocatalytic hydrogen production process harnesses solar energy to directly decompose water, resulting in hydrogen generation. The principle behind photocatalytic hydrogen production is depicted in Figure 8 [28]. The fundamental mechanism involves the absorption of light by a photocatalyst, where the energy must reach or exceed the band gap energy of the material, thereby exciting electrons from the valence band (VB) to the conduction band (CB), leading to the formation of electron-hole pairs. Effective separation and rapid transfer of these electron-hole pairs to the catalyst surface are crucial to prevent their recombination within the material. The electrons in the conduction band and the holes in the valence band migrate to the catalyst surface to participate in reduction and oxidation reactions, respectively, as described by Equations (1)–(3), facilitating the decomposition of water.
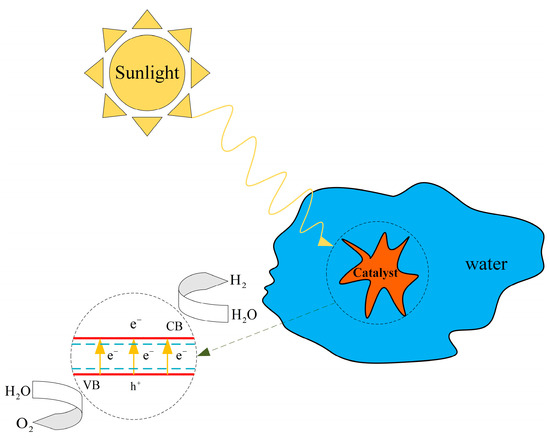
Figure 8.
Principle of photocatalytic hydrogen production.
Reduction reaction:
Oxidation reaction:
Overall response:
Current research in photocatalytic hydrogen production is focused on the development of novel high-efficiency photocatalysts, the construction of composite structures, and the exploration of new reaction systems to enhance the efficiency and stability of light energy conversion, thereby advancing the technology toward practical applications. An ideal photocatalyst should exhibit appropriate bandgap energy, high light absorption efficiency, effective charge separation and transport capabilities, and chemical stability. Titanium dioxide and cadmium sulfide are typical examples of such materials. Expanding light absorption into the visible spectrum is a key research direction, as visible light accounts for 43% of solar energy, making it critical to improving photocatalytic efficiency. Rapid recombination of charge carriers reduces overall efficiency, so research is concentrated on designing catalysts with specialized structures to enhance charge separation and migration. Additionally, considering the demands of prolonged illumination and reaction conditions on catalyst stability and safety, the search for non-toxic and stable catalyst alternatives is ongoing. For instance, the use of cadmium sulfide is limited by issues related to toxicity and stability. Thus, the development of efficient, environmentally friendly, and cost-effective photocatalysts has become a central focus in this field.
Nanomaterials have shown great potential for green hydrogen production due to their unique physicochemical properties, especially in catalysis and storage. Facing the limitations of electron-hole recombination in photocatalysts, Yan X et al. [29] propose a dual active site nickel catalyst rich in electron-holes to optimize the efficiency of water decomposition to produce hydrogen and oxygen without the need for sacrificial agents or noble metal co-catalysts. The catalyst is composed of nickel phosphide () combined with nickel sulfide (NiS) and polymerized carbon-oxygen semiconductor (PCOS). This setup enhances the separation of photogenerated charge carriers, reduces recombination, and significantly improves the photocatalytic efficiency of water decomposition. Catalysts are critical to improving the efficiency of hydrogen production. Thabet S M et al. [30] investigated the enhancement of the photocatalytic hydrogen production performance of TiO2 nanoparticles by introducing metals (e.g., Ru, Co, Ni) as co-catalysts. It was found that the type of co-catalyst, the loading amount, and the preparation method played an important role in enhancing the photocatalytic hydrogen production performance of TiO2. Transition metals such as Co and Ni also exhibited photocatalytic properties comparable to those of Ru, which implies that more economically advantageous materials can be utilized to replace the expensive metal Ru. Andreou E K et al. [31] designed 3D mesoporous networks of Co2P-modified CdIn2S4 nanoscale crystals (ca. 5–6 nm in size). The experimental results showed that this hybrid catalyst with 10 wt% Co2P content exhibited a hydrogen evolution rate of 20.9 mmol gcat−1 h−1 under visible light irradiation and an apparent quantum efficiency of up to 56.1% at 420 nm. This method provides a valuable idea for designing efficient and stable photocatalytic materials. Nguyen V C et al. [32] explore the effect of S- and N-doped graphene oxide dots with Pt and Ag as co-catalysts on the reaction pathway and hydrogen generation. Maulana F et al. [33] improve the photocatalytic hydrogen production from water decomposition of ZnO photocatalysts by doping with N and Ni. The results show that the synergistic doping of N and Ni can effectively reduce the band gap energy of ZnO, increase the surface area of the material, and inhibit the electron-hole complexation, which ultimately significantly improves the photocatalytic efficiency. Zhang K et al. [34] synthesized a three-dimensional S-scheme nanoscale heterojunction photocatalyst for application in hydrogen production by water decomposition. It was demonstrated that the hydrogen generation ability of the photocatalyst could be significantly enhanced by the synergistic effect of S-scheme nanohybrid heterojunction and oxygen vacancies. The study of photocatalysts is of great significance in promoting the development of photocatalytic hydrogen production technology.
3.2. Solar Thermochemical Water Splitting for Hydrogen Production
The solar thermochemical water splitting for hydrogen production utilizes solar-induced high-temperature pyrolysis of water or other chemicals to generate hydrogen, relying on solar thermal collection systems to convert solar radiation into heat energy, thereby achieving the elevated temperatures necessary to drive thermochemical reactions [35]. This approach concentrates sunlight to produce high temperatures using mirrors such as parabolic mirrors or tower heat collection systems, which then convert the solar energy into heat and store it in a medium, such as molten salt, ensuring a continuous reaction even in the absence of sunlight. At high temperatures, thermochemical reactions, facilitated by specific catalysts and reaction conditions, can decompose water or compounds such as sulfuric acid and iodide to produce hydrogen, which is subsequently separated, purified, and prepared for storage or transportation. The primary technical challenges include the development of efficient and cost-effective thermal collection systems, enabling effective thermal energy storage and recovery, optimizing appropriate thermochemical cycles, and ensuring the stability and durability of materials in high-temperature and corrosive environments. Additionally, given the high equipment and operational costs associated with photothermal decomposition, reducing costs and improving economies of scale are critical for its commercialization.
Pérez A et al. [36] describe the preparation and optimization of La0.8Al0.2NiO3-δ perovskite with a reticulated porous ceramic structure for the production of H2 by thermochemical hydrolysis. The experimental results show that the porous structured material is capable of producing 8.3 cm3 STP/g of hydrogen at an isothermal condition of 800 °C, and the hydrogen yield can be increased to 11.5 cm3 STP/g if the thermal reduction temperature is increased to 1000 °C. The material exhibits good stability and an almost constant hydrogen yield in multiple cycle experiments. Vidal A et al. [37] designed a 100 kW multi-tube cavity-receiver reactor that was integrated into a solar tower for hydrogen production. The two-step thermochemical cycle performance of the SolH2 cavity receiver was calculated to be about 2.5% based on the energy efficiency of solar-to-fuel conversion, confirming the feasibility of the multitube cavity reactor for hydrogen production, but the thermal efficiency needs to be improved. Qian X et al. [38] explored the thermodynamic properties and water splitting efficacy of the cubic perovskite SrTi0.5Mn0.5O3-δ. In the water splitting cycle, the hydrogen yield was 7.4 mL g−1, and the 2:1 yield ratio of H2:O2 was maintained over multiple cycles. Barcellos D R et al. [39] discuss the unique properties of BCM, which produces nearly three times more H2 than ceria at a thermal reduction temperature of 1350 °C. The BCM is also known to produce more hydrogen than cerium. And, compared to SrxLa1−xMnyAl1−yO3 (x, y = 0.4, 0.6), BCM not only performs better in terms of hydrogen yield but also shows good hydrogen generation capability with faster redox kinetics. Zhang D et al. [40] investigated the application of complex compositional oxides (La0.8Sr0.2)(Mn(1−x)/3Fe(1−x)/3CoxAl(1−x)/3)O3 in solar thermochemical water splitting, which produced a maximum H2 of 89.97 mmol moloxide−1 over a redox duration of 1 hr. Maintain relatively stable hydrogen production in 51 cycles.
As research progresses on enhancing solar energy conversion efficiency, reducing system costs, developing novel thermochemical cycles, and optimizing material processes, photothermal decomposition is poised to become a significant method for clean hydrogen production, addressing the increasing demand for green energy [41].
3.3. Photoelectrochemical Process of Hydrogen
Photoelectrochemical hydrogen production technology drives the decomposition of water into hydrogen and oxygen by directly converting solar energy into chemical energy using a photoelectrochemical cell [42]. The core of this technology lies in the material selection and design of the photoanode, which must absorb sunlight, generate electron-hole pairs, and then catalyze the decomposition of water into hydrogen and oxygen on the surface of the photoanode and electrode. Ideal photoanode materials should exhibit strong light absorption capabilities, efficient charge separation and transport characteristics, and chemical stability. Materials such as titanium dioxide and cadmium sulfide are typical examples. Improving photoelectric conversion efficiency while considering material cost and stability remains the focus of technological development.
Maragno A R A et al. [43] have investigated heat-integrated photoelectrochemical devices realized by perovskite/silicon tandem solar cells and designed a photoelectrochemical cell with an integrated heat exchanger for the direct decomposition of water to produce hydrogen. A 72-h outdoor stability test showed that the device had an STH efficiency of 6.3%, but during long-term operation, the interface between the perovskite layer and the p-layer becomes a key factor affecting the stability of the device. Präg R et al. [44] prepared high-purity CuFeO2 thin films with controllable morphology and crystal structure by pulsed laser deposition. The experiments show that the CuFeO2 films exhibit a positive photocurrent response during the water decomposition process, which is expected to be an efficient and stable photocathode material in the future. Lee H et al. [45] proposed a dual spin-controlled perovskite photoelectrode. Experiments show that this 2D/3D structure has a remarkable spin-polarization efficiency (up to 75%). The efficiency of photoelectrochemical water splitting is significantly improved. Shabbir S A et al. [46] designed a Co3O4/g-C3N4 heterostructure for photoelectrochemical water splitting. It is shown that in the oxygen precipitation reaction, the carbon-doped g-C3N4 heterostructure has an onset potential of 1.26 V and an overpotential of only 30 mV, whereas the Tafel slope is 112 mV/dec, which suggests that it has a significantly higher charge-transfer efficiency; and in the hydrogen precipitation reaction, the carbon-doped g-C3N4 heterostructure has an onset potential of 0.16 V, and the overpotential is reduced to 160 mV with a Tafel slope of 121 mV/dec, which exhibits excellent photoelectrocatalytic efficiency. Kim H et al. [47] present a new method based on a laser process to prepare flexible and highly efficient WO3 NRs thin film photoanodes by pulsed laser-induced graphene carbide substrate and femtosecond laser-induced crystalline phase transition. Experiments show that the photoanode can still maintain 86% photocurrent after 2 h of continuous operation, which proves its good long-term stability. The flexible photoanode also has excellent mechanical durability and can work properly under different curvature conditions. Flexible film photoanodes have excellent mechanical properties, and the effective area of the photoanode can be increased by changing its structure and reducing its size, thus increasing the production of hydrogen and oxygen.
In recent years, advancements in new semiconductor materials, surface modification technologies, and innovative structural designs of photoelectrochemical cells have significantly enhanced the light absorption range and stability of photoanodes, thereby reducing the cost of hydrogen production. Despite challenges related to efficiency and stability, photochemical hydrogen production technology has emerged as a critical area of future energy research due to its potential to harness renewable energy and produce clean hydrogen [48].
4. Biomass Hydrogen Production Technology
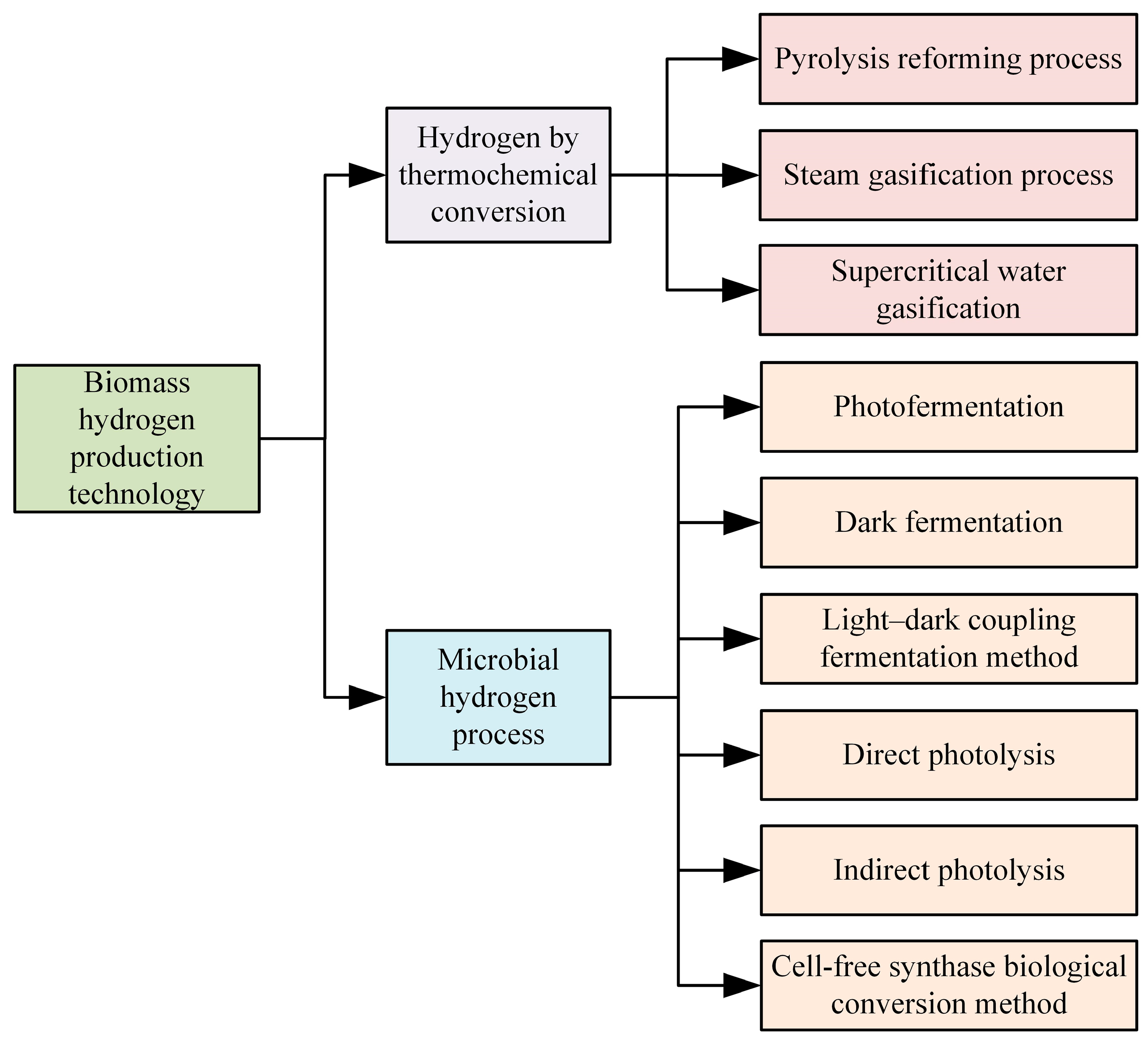
Biomass hydrogen production technology employs biomass resources to generate hydrogen. Biomass, including crop residues, forestry waste, urban organic waste, and other renewable resources, plays a significant role in reducing greenhouse gas emissions and dependence on fossil fuels. By recycling waste biomass, this technology further diversifies the energy structure, which is crucial for the development of energy, environmental protection, and the economy. Currently, biomass hydrogen production technology primarily encompasses two categories: thermochemical conversion and microbial processes, comprising nine specific methods [49], as illustrated in Figure 9.
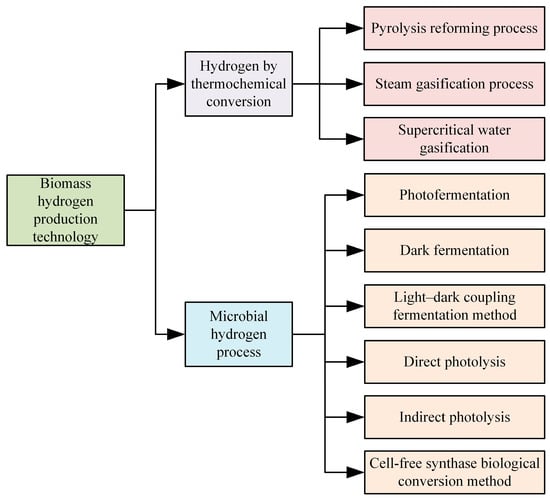
Figure 9.
Technical classification of hydrogen production from biomass.
4.1. Thermochemical Conversion of Hydrogen
The thermochemical conversion method transforms biomass and its derived intermediates (such as alcohols, phenols, and carboxylic acids) into hydrogen-rich gas through thermal reactions, followed by the purification of this gas to obtain pure hydrogen. This process is characterized by its high adaptability and rapid conversion speed, making it particularly suitable for large-scale applications. The primary components of this method include pyrolysis reforming, steam gasification, and supercritical water gasification.
4.1.1. Pyrolysis Reforming Method
Under hypoxic or anaerobic conditions, biomass is heated to high temperatures (typically 300–600 °C), leading to its decomposition into gaseous products (such as carbon monoxide, carbon dioxide, methane, and other organic gases), liquid products (bio-oil), and solid products (biochar). The gaseous components of the pyrolysis products undergo steam reforming reactions with water vapor to produce hydrogen and carbon dioxide. This process, known as the steam reforming reaction, is described by the following chemical equation:
Bio-oil, a product of biomass pyrolysis, is a potential feedstock for renewable hydrogen production. Lan p et al. [50] suggest that the catalytic steam reforming of bio-oil is an economical and feasible approach to hydrogen production. Bio-oil offers advantages in terms of ease of collection, storage, and transportation, effectively addressing challenges associated with solid biomass and reducing overall costs. The development of new catalysts to improve hydrogen production efficiency and reduce costs is one of the research priorities. Yao D et al. [51] use modified Ni-Al catalysts for catalytic reforming of the aqueous fraction of pyrolyzed bio-oils to hydrogen. In particular, the Ni-Al catalysts were modified by the addition of metals such as Ca, Ce, Mg, Mn, and zinc Zn. It was shown that the Ni-Mg-Al catalyst exhibited the best hydrogen yield (56.46%) while maintaining high stability over a long period of operation. Arandia A et al. [52] analyzed the APR of representative model compounds of bio-oil aqueous fraction, including acetic acid, ethanol, 1-hydroxypropan-2-one (acetol), and benzene-1, 2-diol (catechol), as well as a mixture of all of them. Experiments showed that ethanol had the highest hydrogen yield over Ni/CeO2-γAl2O3 catalysts, and Ni/La2O3-αAl2O3 catalysts had the highest hydrogen yields in the APRs of the mixtures, whereas NiAl2O4 catalysts showed good stability while avoiding the leaching of Ni. Bimbela F et al. [53] studied the performance of different Ni/Mg Al catalysts modified with Ce. It was found that the introduction of Ce significantly increased the carbon conversion and hydrogen yield of the catalysts. The catalyst containing 0.5% Ce prepared by the impregnation method showed the best overall performance with 78.7% carbon conversion, the highest hydrogen yield, and the lowest carbon accumulation. In addition, the catalyst with 0.5% cerium content was the most economically advantageous, which could reduce the production cost while maintaining the high performance.
As a renewable energy technology, pyrolytic reforming has a wide range of feedstock sources for hydrogen, which helps to reduce dependence on fossil fuels and promote sustainable development. However, during the pyrolysis reforming process, the catalyst may be deactivated due to problems such as sintering and carbon buildup, and the tar produced during the process may clog the pipeline and reactor, affecting the stable operation of the system.
4.1.2. Steam Gasification Method
Steam gasification directly converts biomass and water vapor into hydrogen-rich gas at high temperatures (700–1200 °C), achieving a hydrogen production rate of up to 52%, making it one of the most efficient and economical hydrogen production technologies currently available [54]. The key reactions involved include the following:
Water–gas shift reaction: carbon monoxide in syngas is further converted into hydrogen and carbon dioxide through its reaction with water vapor:
Biomass steam gasification is a thermochemical conversion of biomass into syngas (consisting mainly of hydrogen, methane, carbon monoxide, and carbon dioxide), which has the potential to reduce carbon dioxide emissions and increase fuel production efficiency. However, the tar produced during steam gasification is a major obstacle to industrial applications, leading to problems such as corrosion of equipment and clogging of pipelines. Cortazar M et al. [55] investigated the incorporation of a fountain flow limiting device in a conical spouted bed reactor to increase the gas–catalyst contact time during gasification, resulting in lower tar content, higher syngas and hydrogen yields, and improved carbon conversion efficiency. Kargbo H O et al. [56] apply artificial neural networks to biomass gasification for hydrogen production to predict and optimize the two-stage gasification process to reduce the time and cost of development and testing. The model predictions were experimentally verified to be in excellent agreement with the experimental data, with a correlation coefficient R2 of more than 0.99. Lysne A et al. [57] investigated steam reforming of tar at 700 °C. The performance of Ni and Ni-Co catalysts was compared through cyclic regeneration experiments. The experiments showed that the Ni-Co catalyst outperformed the single Ni catalyst in terms of balance between coke deposition and gasification rate. Under Switch-SRCG operation, the coke generation of the Ni-Co catalyst was significantly lower than that of the Ni catalyst, which was attributed to the enhanced carbon dioxide adsorption capacity of the Ni-Co catalyst, which facilitated coke gasification. Okoji A I et al. [58] utilize the Aspen Plus simulator and artificial intelligence techniques such as response surface methodology and adaptive neuro-fuzzy inference system (ANFIS) to optimize and predict the hydrogen production rate. The ANFIS model predicted a hydrogen molar fraction of 0.5045 under specific conditions, while the RSM optimized these conditions with a 19% increase in hydrogen production. This demonstrates that by optimizing the biomass steam gasification process and introducing an advanced AI model, hydrogen production efficiency can be significantly improved. Li T et al. [59] present a novel integrated biomass-to-energy system that combines gasification and pyrolysis technologies for efficient and sustainable energy production. The heat generated through the gasification process provides thermal energy for the pyrolysis process, thus increasing the yield of pyrolysis products and improving the efficiency of biomass energy utilization. The economic analysis shows that the system has good economic efficiency. The proposed system provides an efficient and sustainable way for biomass energy utilization.
4.1.3. Supercritical Water Gasification Method
Supercritical water gasification law biomass pyrolysis [60] is carried out under a supercritical water environment (temperature and pressure exceeding 374.15 °C and 22.12 MPa, respectively), and biomass molecules are rapidly degraded into small molecular gases (mainly hydrogen, carbon monoxide, carbon dioxide, and methane). The main reactions are as follows:
Supercritical aqueous biomass gasification for solar hydrogen production is one of the clean and renewable ways to utilize solar energy and high-water biomass, and so far most solar thermochemical hydrogen production has focused on utilizing solar thermal energy above 1000 °C. Liao B et al. [61] designed a novel solar concentrating reactor for hydrogen production from supercritical water gasification of biomass. The receiver/reactor temperature was gradually increased with increasing direct normal solar irradiation. The results showed an increase in gas production rate. This suggests that supercritical water gasification using concentrated solar energy is a promising method for hydrogen production. Kumar M et al. [62] compare the efficiency of two thermochemical methods, thermal gasification and supercritical water gasification, for the conversion of algal biomass to hydrogen. Supercritical water gasification has a lower cost of hydrogen production due to its higher hydrogen yield, although the capital investment is higher. In contrast, thermal gasification has a higher overall cost due to the need for drying treatment. Martins A H et al. [63] compare three different gasification technologies: conventional gasification, plasma gasification, and supercritical water gasification, with three biomass gasification process models developed in Aspen Plus® to estimate the maximum hydrogen yield through parametric studies. The results show that supercritical water gasification is the most suitable process for hydrogen production. Wu L et al. [64] focus on the optimized molecular dynamics of phenol gasification in supercritical water for hydrogen production and its optimization. In this paper, simulations were carried out using ReaxFF reaction force field and AMS software, and the results showed that the reaction temperature was positively correlated with the gasification efficiency, and the residence time and relative concentration also had an effect on the hydrogen production rate. This study provides a theoretical basis for future biomass gasification processes in supercritical water. Qiu Y et al. [65] focus on the treatment of oily sludge using supercritical water gasification technology and analyze its environmental impact through life cycle assessment. The results showed that the gasification process of oil-containing sludge could obtain high H2 production and low CO2 emission under the conditions of 500 °C, 15% sludge concentration, and 0.1 oxidation factor.
The advantages of supercritical water gasification are high conversion rate and hydrogen content without tar and coke by-products, showing its potential in green hydrogen energy production. However, it is demanding and costly in terms of equipment.
4.2. Microbial Process of Hydrogen
By harnessing the ability of microorganisms to decompose biomass, hydrogen production technology converts water molecules and organic matter in biomass into hydrogen through the catalytic actions of hydrogenase and nitrogenase [66]. This process is highly regarded for its environmental and economic benefits, as well as its operation under mild conditions and low energy consumption, making it an energy-saving and environmentally friendly technology. Based on the specific reaction environments and processes of microorganisms, microbial hydrogen production technology can be categorized into six main types: photo-fermentation, dark fermentation, light–dark coupled fermentation, direct photolysis, indirect photolysis, and cell-free synthase-based biological conversion.
4.2.1. Photo-Fermentation Method
Photo-fermentation is an efficient hydrogen production method primarily involving anaerobic photosynthetic microorganisms, such as photosynthetic purple non-sulfur bacteria (PNS), which utilize nitrogenase to convert organic matter into hydrogen [67]. However, the efficiency of this method is highly sensitive to ambient light intensity and oxygen levels in the reactor, both of which can inhibit nitrogenase activity and thereby affect hydrogen production efficiency.
Hu C et al. [68] investigated the use of fluorescent and incandescent lighting systems in batch photo-fermentation of four purple non-sulfur photosynthesizing bacteria for hydrogen production and found that incandescent lighting was more effective for bacterial cell growth and hydrogen production. Sahrin N T et al. [69] used palm kernel extract (PKE) as an organic nutrient source to fuel the photo-fermentation process, and it was shown that the hydrogen generation efficiency of microalgae was the highest at a light intensity of 200 μmol/m2s and a PKE concentration of 5 g/L. However, higher light intensities and substrate concentrations resulted in oxygen accumulation, which inhibited hydrogen production. Yue T et al. [70] convert agricultural wastes, such as corn stover, into biohydrogen by photo-fermentation and explore the role of titanium dioxide/activated carbon fiber (TiO2/ACF) catalysts in the photo-fermentation process. Results show the maximum cumulative hydrogen yield (CHY) obtained under the optimal conditions was 74.0 ± 1.3 mL/g TS with the TiO2/ACF addition of 100 mg/L, which was twice that without TiO2/ACF addition (36.9 ± 1.0 mL/g TS). Ren C et al. [71] investigated a novel 70-L composite tubular photobioreactor for outdoor photo-fermentative hydrogen production. The characterization of photo-fermentative hydrogen production was evaluated in an outdoor environment using glucose as substrate in both intermittent and continuous modes of operation. Increasing the light energy conversion efficiency by sunlight during the daytime and combining it with an artificial light source to make up for the lack of light intensity at night enabled the system to exhibit good performance in both intermittent and continuous fermentation processes, which effectively enhanced the efficiency of hydrogen production. Zhang Y et al. [72] engineered Rhodobacter sphaeroides to improve the hydrogen production by manipulating the light harvesting (LH) complexes, the electron transfer chain (ETC), the adenosine diphosphate (ADP) synthetic pathway, the F0F1- ATPase expression, and the nitrogenase expression. The experimental results showed that the modified strain achieved more efficient hydrogen production.
4.2.2. Dark Fermentation Method
As a result, significant research has shifted toward dark fermentation, which does not depend on light or nitrogenase but instead relies on hydrogenases found in microorganisms such as Clostridium and Enterobacter to produce hydrogen [73].
Unlike photo-fermentation, dark fermentation can occur in the absence of light, offering advantages such as lower costs, a faster hydrogen production rate, and ease of scaling up for large-scale production. Consequently, it has garnered widespread attention. However, dark fermentation may produce toxic by-products, such as volatile fatty acids, which can reduce the value of the fermentation environment, inhibit microbial activity, and complicate subsequent treatment processes. Pre-processing is one of the solutions. Garduño I R et al. [74] utilized white-rot fungi (WRF) for the pretreatment of brewery waste sludge (BWS) to enhance organic load degradation of wastewater and to increase the efficiency of dark fermentation hydrogen production. Fungal pretreatment significantly improved the degradation efficiency of the brewery waste stream compared to direct dark fermentation, and this process significantly increased the efficiency of dark fermentation hydrogen production while reducing pollutant emissions. Ramprakash B et al. [75] produced biohydrogen by pretreating garden waste and utilizing Escherichia coli (E. coli) under dark fermentation conditions. The experimental results showed that the combined hydrolysis of acid plus enzyme resulted in a hydrogen yield of 97 mL/g, which was 2.7 times higher than the hydrogen yield of untreated waste and had obvious advantages over other pretreatment methods. Rao R et al. [76] subjected pretreated cheese whey to dark fermentation, and the experimental volumetric hydrogen production rate was 24.7 mL L−1 h−1 at the optimal concentration, which effectively enhanced the hydrogen production efficiency of cheese whey as a substrate. Kovalev A A et al. [77] estimated the conversion factors of thermal and electrical energy consumed in dark fermentation of various organic wastes into hydrogen, and the experimental results showed that the conversion factor of energy into biohydrogen during dark fermentation is less than 1. However, the feedstock for hydrogen production in dark fermentation is organic waste, and in addition, the use of a vortex layer device for pretreatment of the dark fermentation substrate can result in a 40% higher conversion factor of electrical energy than that of water electrolysis. Eggers N et al. [78] combine dark fermentation and anaerobic digestion, and the results of the study show that combining dark fermentation with an existing biogas plant provides hydrogen in addition to biogas and improves substrate turnover by up to 50%.
4.2.3. Light–Dark Coupling Fermentation Method
Photo-fermentation faces challenges in processing macromolecular organic compounds like cellulose and starch, resulting in relatively low hydrogen production efficiency, which can lead to resource wastage. In contrast, dark fermentation can effectively degrade and utilize these macromolecular organic compounds for hydrogen production, offering broader application potential.
Combining light and dark fermentation is effective in increasing hydrogen production as well as reducing substrate waste. Zhao Y et al. [79] proposed an effective strategy to significantly increase the biological hydrogen production from sewage sludge, i.e., continuous dark fermentation and photo-fermentation. The experimental results showed that the total hydrogen production of continuous dark fermentation and photo-fermentation reached 30 mL H-2/g-COD. Zagrodnik R et al. [80] used synthetic lignocellulosic hydrolysate as substrate for dark and light fermentation and compared the hydrogen production efficiency of sequential and co-cultured dark–light fermentations. The experimental results showed that the combined dark photo-fermentation carried out in sequential mode increased the hydrogen production compared to the dark fermentation process alone. Combined systems of photo and dark fermentation have been studied to improve the efficiency of hydrogen production. Cai J et al. [81] investigated dark and photo-fermenting bacterial populations, where dark fermentation broth without pretreatment served as a control, and the addition of photo-fermenting bacteria after dark fermentation increased hydrogen production (134%) and substrate utilization (67%), which suggests that photosynthesizing bacteria can increase hydrogen production from the combination of dark and photo-fermentation. Li Y et al. [82] investigated the effect of hydraulic retention time and dilution ratio on continuous hydrogen production by dark and photo-fermentation in a baffled bioreactor by using corn stover enzymatic hydrolysate as the initial carbon source. The experimental results showed that the hydrogen production reached 12.73 L/d when the combined system was operated with an HRT of 12 h (dark fermentation) and a dilution ratio of 1:0.5 (dark fermentation effluents). Niño-Navarro C et al. [83] demonstrated the feasibility of increasing H2 production through a two-stage process of dark and light fermentation.
Therefore, integrating the advantages of both photo-fermentation and dark fermentation into a collaborative hydrogen production system can enhance hydrogen production efficiency and expand the range of usable raw materials. This complementary integration strategy is expected to drive further advancements in microbial hydrogen technology, achieving a more efficient and environmentally sustainable biological hydrogen production process [84].
4.2.4. Direct Photolysis Method
Photosynthetic organisms such as cyanobacteria and certain green algae are utilized to produce hydrogen through photosynthesis under light conditions. This process primarily relies on the synergistic action of photosynthetic reaction centers and hydrogenases to split water molecules into oxygen and hydrogen.
The direct use of solar energy simplifies the process, eliminating the need for external organic substrates or abundant resources. Tamburic B et al. [85] investigated the ability of the green unicellular alga Chlamydomonas reinhardtii to photosynthesize hydrogen production under anaerobic conditions, and the efficiency of hydrogen production can be significantly improved by optimizing light, agitation, and culture conditions. Li H et al. [86] provide a continuous hydrogen production method that does not require media changes and is more operational and cost-effective than the traditional sulfur deprivation method. The ability to achieve continuous hydrogen production by repeating the thermal induction treatment opens up the possibility of industrializing hydrogen production from microalgae. Bechara R et al. [87] used the sulfur deprivation method to enhance hydrogen production in microalgae, and the experimental results showed that the hydrogen production was increased by 54%. Chen J et al. [88] successfully demonstrated that the photobiological hydrogen production efficiency of algae can be significantly improved by adding an appropriate amount of glucose (50 mM) to the algal culture. This method has the advantages of low cost, simple operation, and no need to change the culture medium, and has a promising future in large-scale application. Chen J et al. [89] proposed the formation of algal aggregates directly in algal cultures by chemical flocculation, which reduces the cost and reduces the complexity of the treatment process, by utilizing cationic etherified starch as a flocculating agent to induce the formation of aggregates of Chlorella pyrenoidosa, which in turn creates an anaerobic environment within the aggregates in order to facilitate the generation of hydrogen gas.
However, hydrogen production efficiency remains low, as oxygen production inhibits hydrogenase activity, necessitating effective oxygen management.
4.2.5. Indirect Photolysis Method
Certain photosynthetic bacteria utilize photosynthesis to produce energy storage substances, such as sugars, which are subsequently decomposed under dark conditions to release hydrogen. This process, known as indirect photolysis, is typically divided into two stages: the photosynthetic stage and the fermentation stage. Delavar M A et al. [90] developed a platform based on the cellular automata coupled lattice Boltzmann method to study the effects of different light intensities and carbon dioxide concentrations on indirect photolysis in bioreactors. The experimental results showed that the biofilm concentration increased significantly with increasing light intensity. Increasing the inlet CO2 concentration could significantly improve the biofilm growth and hydrogen production efficiency. Ban S et al. [91] added Ca2+ to Chlamydomonas reinhardtii in pure culture or in co-culture with bacteria; Ca2+ promoted starch production, and starch degradation in turn promoted H2 production through an indirect photolytic pathway. The experimental results showed that the addition of Ca2⁺ could significantly enhance the photolytic hydrogen production of the algae through multiple mechanisms. Touloupakis E et al. [92] utilize Chlorella vulgaris strain G-120 to produce hydrogen by direct and indirect pathways of photosynthesis without imposing nutrient starvation. The experimental results showed that the microalgae Chlorella vulgaris strain G-120 was able to produce large amounts of biohydrogen without nutrient starvation and had high photoconversion efficiency and respiration rate. Ban S et al. [93] proposed an efficient and long-lasting algal-bacterial cooperative system for enhancing photolysis-mediated H2 production in green algae.
The separation of hydrogen generation from oxygen generation reduces the inhibitory effect of oxygen on hydrogenase. Co-culturing photosynthetic bacteria with other microorganisms can further enhance hydrogen production efficiency. However, the process is complex, requiring distinct light and dark phases, and the overall conversion efficiency is constrained by the production and decomposition rates of the energy storage material.
4.2.6. Cell-Free Synthase Biological Conversion Method
The cell-free synthase bioconversion method utilizes hydrogenase and other related enzymes extracted from microorganisms to catalyze the generation of hydrogen from organic substrates and water in a cell-free environment [94]. The advantages are simple operation and the engineering and optimization of enzymes to enhance their catalytic efficiency and stability; the disadvantages are high costs. The basic reaction formula is shown in Equation (10):
Researchers have proposed a method for extracting hydrogen from renewable biomass (e.g., starch) by aiming to lower the cost of hydrogen production and reduce dependence on fossil fuels while achieving zero carbon emissions. Zhang Y H P et al. [95] describe a synthetic enzymatic reaction pathway consisting of 13 enzymes that generate hydrogen and carbon dioxide from starch and water through a series of reactions. The entire reaction process was carried out at 30 °C and atmospheric pressure, and the production of hydrogen was unidirectional, the reaction proceeded spontaneously, and the free energy of the reaction was negative, indicating that the process was thermodynamically favorable. Experimental results showed that the hydrogen production from starch using this synthetic enzymatic pathway was much higher than that of conventional fermentation methods.
In summary, the characteristics of biomass hydrogen production technology are compared, as shown in Table 5.

Table 5.
Comparison of the technical characteristics of hydrogen production from biomass.
5. Hydrogen Production Technology by Electrolytic Water
Electrolytic water hydrogen production technology is one of the earliest and most typical green hydrogen production methods, using renewable energy to generate electricity that splits water molecules into hydrogen and oxygen through electrolysis [96]. This process generates hydrogen at the cathode and oxygen at the anode without emitting any carbon compounds, making it clean, efficient, and environmentally friendly. Hydrogen production via water electrolysis is a promising method for fully harnessing excess renewable energy. Among the various hydrogen production technologies, water electrolysis powered by renewable energy shows significant potential. The current state of this technology primarily includes four main types: alkaline water electrolysis (AWE), proton exchange membrane electrolysis (PEM), anion exchange membrane electrolysis (AEM), and solid oxide electrolysis (SOE). The characteristics of these four electrolytic water production technologies are detailed in Table 6.

Table 6.
Comparison of characteristics of hydrogen production technology by electrolytic water.
5.1. Hydrogen Production by Alkaline Water Electrolysis
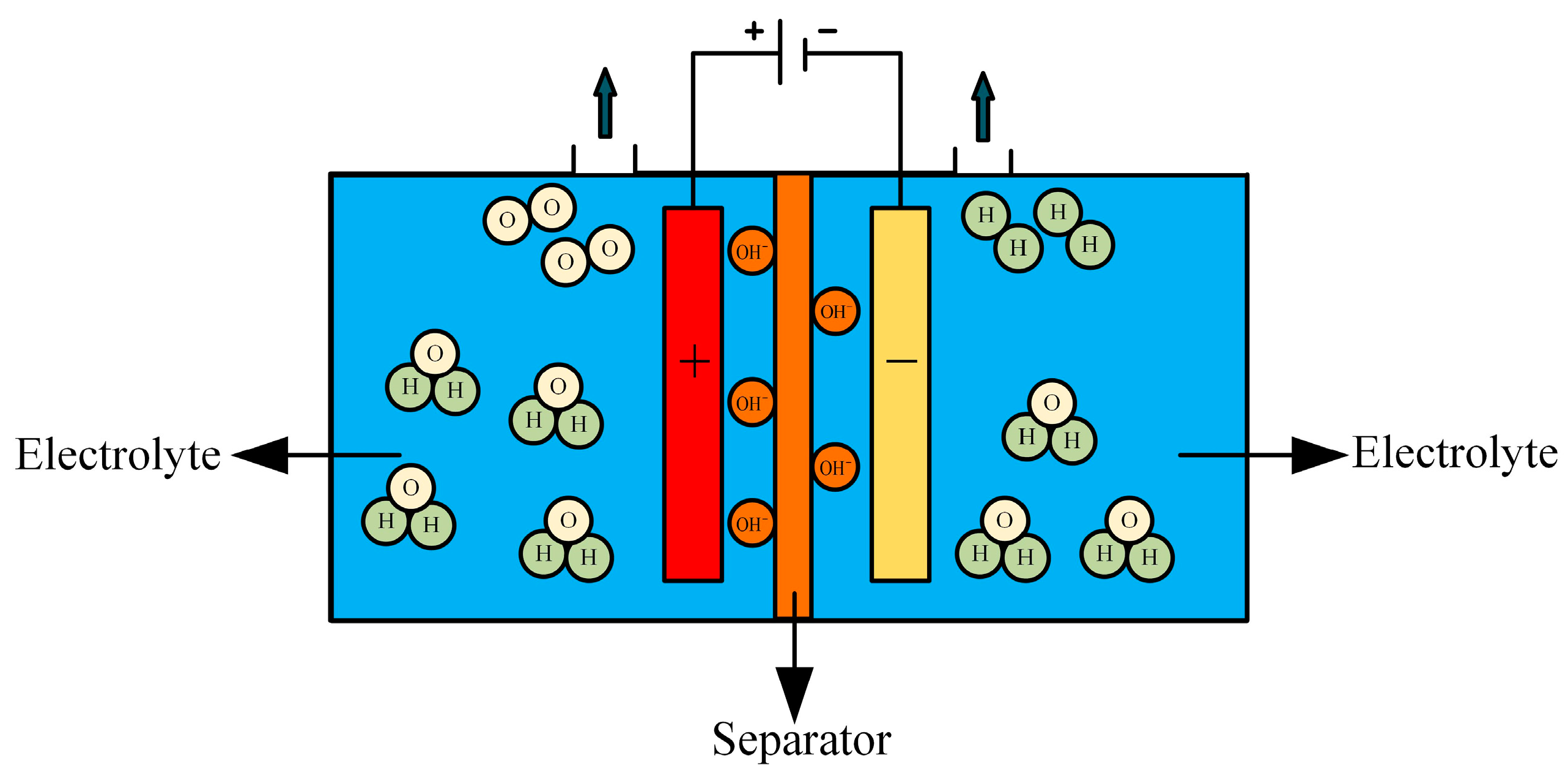
AWE hydrogen production technology is one of the most mature and widely adopted methods and has been fully industrialized both domestically and internationally [97]. The electrolytic cell structure is illustrated in Figure 10. The working principle involves the reduction of water molecules at the cathode under the influence of direct current, producing hydrogen and hydroxide ions. These hydroxide ions migrate through the diaphragm to the anode, driven by the electric field and the concentration gradient between the hydrogen and oxygen sides, where an oxygen evolution reaction occurs, generating oxygen and water. This process is represented by Equation (11).
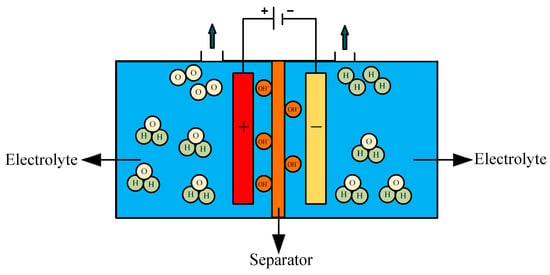
Figure 10.
Structure of the AWE electrolytic cell.
Anode reaction formula:
The cathodic reaction equation is shown in Equation (1).
In AWE electrolytic cells, the electrolyte typically consists of a 30% (by mass) KOH solution, with an operating temperature range of 70–90 °C. The diaphragm material may include asbestos or polyphenylene sulfide, and the anode and cathode catalysts are generally nickel-plated perforated stainless steel. AWE technology is known for its simplicity, maturity, and reliability, and it operates effectively at ambient temperature and pressure. However, it has certain drawbacks, including a long start-up and shutdown response time, low current density, corrosive electrolyte, alkali infiltration, environmental pollution concerns, and the need for complex maintenance of the alkaline fluid. Demnitz M et al. [98] investigated the effect of iron addition to the electrolyte on the enhancement of electrolysis efficiency. The experimental results show that moderate iron addition can significantly improve the efficiency of alkaline water electrolysis, but iron may be deposited on the electrode surface or re-dissolved back into the electrolyte during the electrolysis process, and the long-term effects need to be further investigated. There have been studies in terms of temperature to improve the efficiency of alkaline water electrolysis. Zhu Q et al. [99] proposed an asymmetric temperature regulation strategy in which the hydrogen precipitation reaction and oxygen precipitation reaction were carried out at different temperatures during the electrolysis process. Experimental results show that the efficiency of alkaline water electrolysis can be significantly improved by asymmetric temperature regulation. This strategy originates from the intrinsic thermodynamic properties of the electrolysis process and is independent of the catalyst, thus it has a wide application potential. Considering the prevalence of low-grade heat sources in industrial processes, this strategy offers new possibilities to enhance the energy utilization efficiency of industrial alkaline water electrolysis. Pressurizing the alkaline water electrolyzer is also one of the important technologies for large-scale hydrogen production. Brauns J et al. [100] proposed a dynamic process model containing four submodels to describe the system behavior in terms of gas contamination, electrolyte concentration, cell potential, and temperature in a pressurized alkaline water electrolyzer system. Experimental results show that a reasonable operation strategy and system design can effectively reduce gas contamination, optimize the electrolyte cycle, and improve the energy efficiency of the overall system. Utilizing photovoltaic (PV) power to provide electricity for hydrogen production from water electrolysis not only makes full use of renewable energy but also achieves zero pollution. However, due to the fluctuating nature of PV power, the direct use of PV power to drive alkaline water electrolysis can lead to a reduction in system efficiency and may even damage the electrolyzer. Therefore, Cao X et al. [101] proposed an energy management strategy that can adapt to the rapid fluctuation of PV power that predicts the PV power forecast for the next hour and adjusts the power of the electrolyzer accordingly. The system performs well in spite of the errors in the PV power prediction. Shaarawy H H et al. [102] developed an electrodeposition-based Ni-Co-nano graphene thin film cathode, which achieved an electrolysis efficiency of 95.6% in a 25% KOH solution for water electrolysis using Ni-Co-nano graphene cathode. Additionally, the hydrogen produced requires purification before collection, and the output pressure is low, necessitating additional pressurization for storage and transportation, which increases costs. Recent studies indicate that optimizing the system-level control of AWE, along with improving wave adaptability by enhancing the reactor’s material and structure, can significantly improve the rapid regulation of auxiliary systems, such as pressure and temperature control.
5.2. Proton Exchange Membrane Electrolysis of Water to Produce Hydrogen
PEM (proton exchange membrane) electrolysis water hydrogen production technology is a method of generating hydrogen and oxygen by electrolyzing water molecules. This technique utilizes a specialized proton exchange membrane as the electrolyte, which efficiently dissociates water molecules into hydrogen and oxygen. PEM electrolysis offers high conversion efficiency, enabling more effective conversion of electrical energy into chemical energy in the form of high-purity hydrogen. Compared to traditional alkaline water electrolysis technology, PEM electrolysis operates at lower temperatures, reducing energy loss. Additionally, PEM systems are capable of responding quickly to load changes, making them well-suited for integration with intermittent renewable energy sources, such as wind and solar, thereby enhancing overall energy efficiency [103].
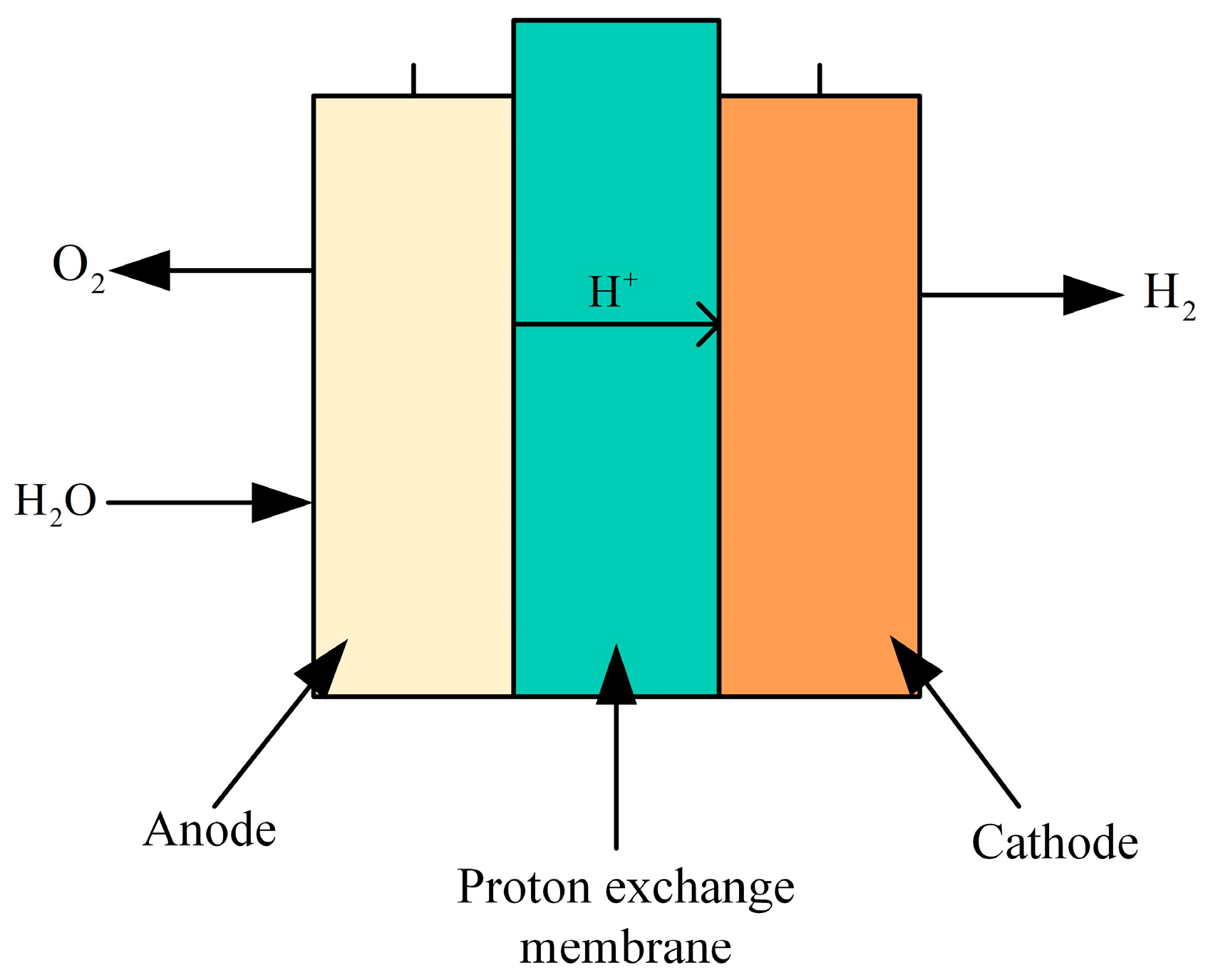
A PEM electrolyzer is a device used to electrolyze water to produce hydrogen, utilizing a special proton exchange membrane as the electrolyte, as depicted in Figure 11. The core of the electrolyzer is a thin film that selectively allows protons (hydrogen ions) to pass through while blocking electrons. This creates a voltage difference between the electrodes, facilitating the dissociation of water molecules into hydrogen and oxygen. During electrolysis, water molecules are oxidized at the anode (positive electrode), producing oxygen, protons, and electrons. Protons migrate through the proton exchange membrane to the cathode (negative electrode), where they combine with electrons to form hydrogen gas via a reduction reaction. The electrons flow from the anode to the cathode through an external circuit, supplying the necessary power for the process.
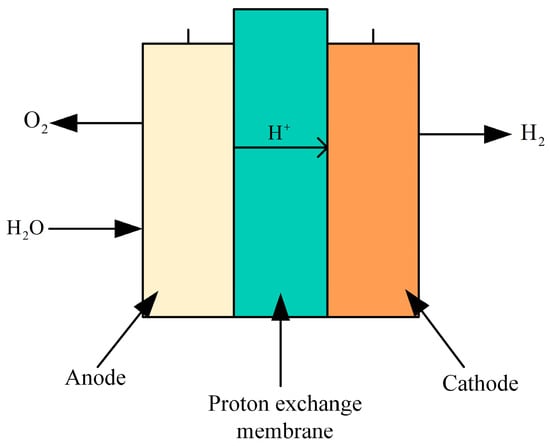
Figure 11.
Structure diagram of the PEM electrolyzer.
The anode reaction equation is shown in Equation (2).
Cathode (reduction reaction):
The total reaction formula of the entire electrolytic process:
PEM electrolyzers are designed to be more compact than conventional electrolyzers and are generally more energy efficient than conventional alkaline electrolyzers because they can operate at higher current densities without causing excessive energy loss. Due to the selectivity of the membrane, the resulting hydrogen is very pure and does not require a complex post-processing process.
With the increasing global demand for clean energy, PEM electrolysis of water for hydrogen production is recognized as a promising method of hydrogen production due to its high efficiency and environmental friendliness. Especially when used in combination with renewable energy sources, it can provide an efficient way to store and transport energy and is expected to play an important role in future energy systems. However, further breakthroughs in cost reduction, system stability, and durability are needed to realize this. Ma Z et al. [104] describe a CFD model based on ANSYS/Fluent software version 19.2 that enables detailed simulation of complex physical and chemical processes inside PEMEC. The model analysis shows that the electrolysis efficiency can be improved by designing an optimized flow field and efficient gas venting strategy, reducing the interfacial resistance and increasing the proton conductivity. Höglinger M et al. [105] present a systematic test methodology for PEMEC stacks, which provides valuable insights for evaluating the design, material suitability, and operational performance by comparing the test results of different stacks, thus advancing the development and application of proton exchange membrane water electrolysis technology. Padgett E et al. [106] investigated two different structures of anodic catalyst layers: an IrO2 nanoparticle catalyst layer and a dispersed nanostructured thin film (NSTF) Ir catalyst layer. It was found that high electronic resistance leads to an increase in the local overpotential of the catalyst layer, which triggers inhomogeneous catalyst degradation. In this regard, effective strategies to enhance the performance of PEMWE were proposed in the paper to reduce the catalyst layer resistance, enhance catalyst utilization, and optimize the porous transport layer design. Zhang X L et al. [107] investigated a novel sulfur-doped orthosulfide (marcasite)-type electrocatalyst to enhance the hydrogen precipitation performance and stability of the catalyst in acidic media. The noble metal-free hydrogen precipitation catalyst reduces the cost. Calnan S et al. [108] also mention the direct electrical coupling of PV modules to a proton exchange membrane electrolyzer, where the solar-hydrogen conversion efficiency remained above 10% during the test period.
5.3. Anion Exchange Membrane Electrolysis of Water to Produce Hydrogen
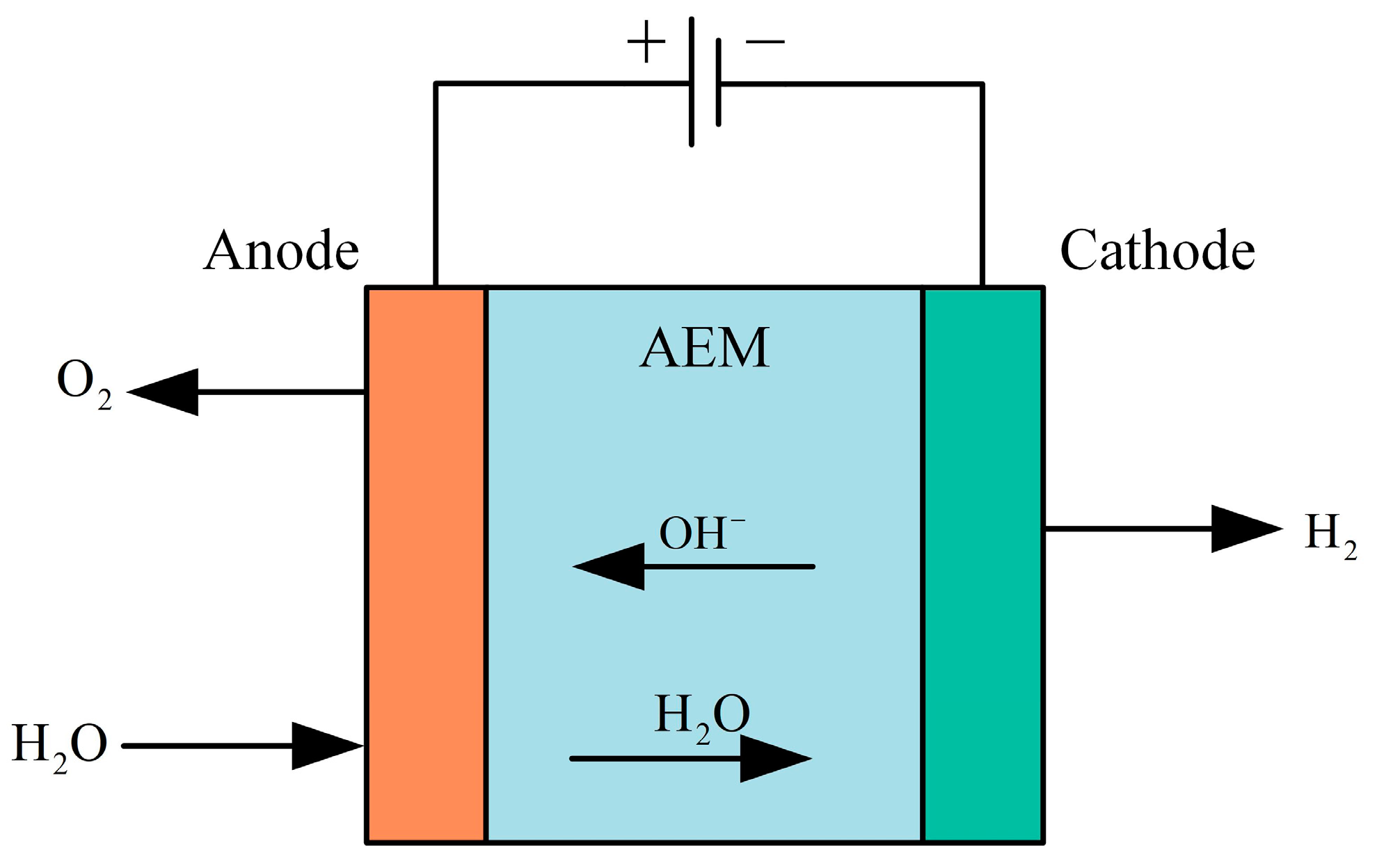
AEM (anion exchange membrane) hydrogen production technology is one of the more advanced water electrolysis technologies currently in the early stages of research. The structure of its electrolytic cell is shown in Figure 12. It utilizes an anion exchange membrane instead of a PEM or proton exchange membrane. This anion exchange membrane offers high air tightness and ion conductivity, effectively reducing pressure-related issues and gas venting during hydrogen production in AWE systems. During operation, raw water enters from the cathode side of the AEM equipment, where water molecules undergo a reduction reaction at the cathode, gaining electrons and forming hydroxide ions and hydrogen. The hydroxide ions then migrate through the polymer anion exchange membrane to the anode, where they participate in an oxidation reaction, losing electrons to form water and oxygen. Occasionally, a potassium hydroxide or sodium bicarbonate solution is added to the raw water as an auxiliary electrolyte to enhance the efficiency of the AEM electrolyzer [109].
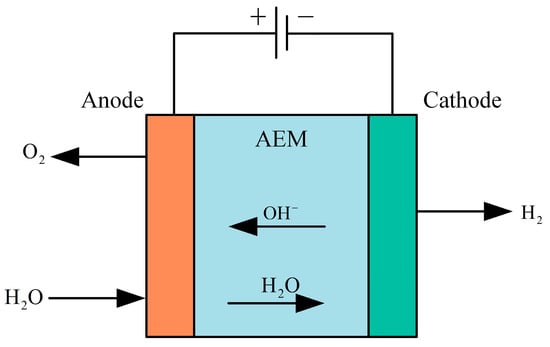
Figure 12.
Structure of the AEM electrolyzer.
Anode reaction formula:
The cathodic reaction equation is shown in Equation (1).
Anion exchange membrane water electrolysis combines the advantages of both alkaline water electrolysis and PEM electrolysis, offering higher current density, faster response, and greater energy conversion efficiency. AEM electrolysis can operate under near-neutral conditions, which are milder than the high pH of alkaline water electrolysis and the low pH of PEM electrolysis. These neutral or near-neutral operating conditions reduce corrosion issues, allowing for the use of less expensive materials. The electrolyte does not require high concentrations of acids or bases, reducing the need to handle and store highly corrosive chemicals, thereby lowering operating costs and safety risks and significantly reducing unit manufacturing costs. Although anion exchange membrane technology mitigates corrosion and material selection challenges, it still faces issues related to membrane stability and ion transport efficiency. Ongoing research and development efforts are focused on improving membrane materials and enhancing the long-term stability and efficiency of the system. Ha J S et al. [110] developed a novel NiFeCo-OOH catalyst and integrated it with silicon-based solar cells to achieve a solar hydrogen efficiency of 12.44%. Khataee A et al. [111] investigated Aemion™ anion exchange membranes tested in a flow-through electrolyzer, and the experimental results showed that the Aemion™ anion exchange membranes were able to maintain good electrochemical stability in both mid-term (>100 h) and long-term (>700 h) experiments. Li K et al. [112] utilize micro- and nanofabrication techniques to develop an ultrathin, pore-structured liquid/gas diffusion layer with tunable pore structure to enhance the performance of anion-exchange membrane electrolyzers with efficiencies as high as 81.9% at 60 °C. The results are summarized as follows. Kang X et al. [113] investigated a corrosion-resistant RuMoNi electrocatalyst, where the RuMoNi electrocatalyst was applied to an anion exchange membrane electrolyzer, and a complete AEM electrolyzer system was assembled with an electrolysis efficiency of up to 77.9% using RuMoNi as the cathode and anode catalysts.
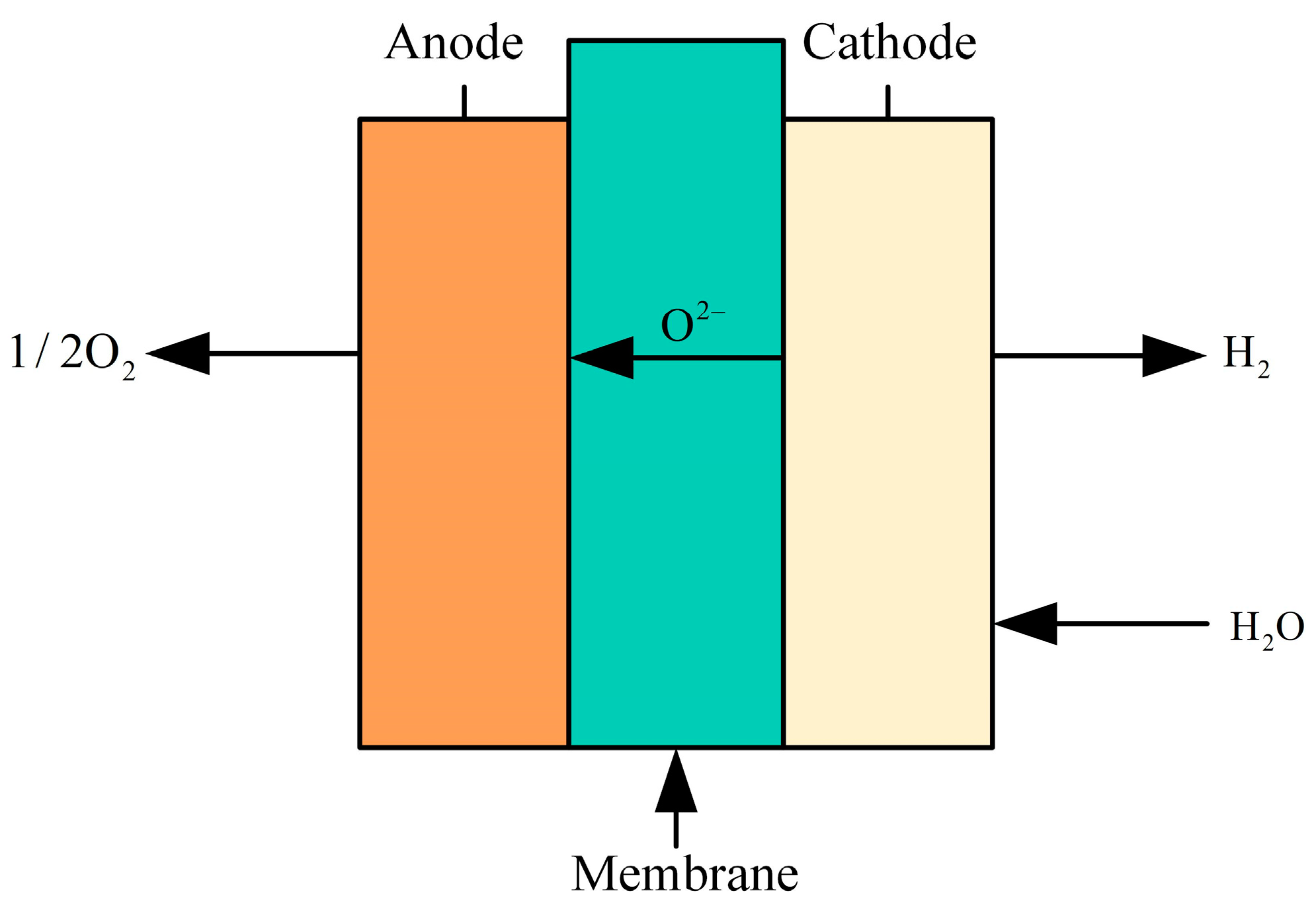
5.4. Solid Oxide Electrolysis of Water to Produce Hydrogen
SOE (solid oxide electrolysis) hydrogen production technology involves the high-temperature electrolysis of water to produce hydrogen. This method utilizes a solid oxide electrolyte to dissociate water into hydrogen and oxygen by applying a direct current voltage at elevated temperatures (typically between 600 and 1000 °C), as shown in Figure 13. The solid oxide electrolytic cell consists of a dense electrolyte layer in the center and porous electrodes at both ends. SOE requires materials that can withstand high temperatures, exhibit good chemical stability, and possess strong electrical conductivity, such as stabilized zirconia. These materials facilitate the separation of hydrogen and oxygen through the electrolyte and the conduction of oxygen ions or protons, while porous electrodes aid in the diffusion and transmission of gases. SOE technology offers advantages such as high efficiency, simple electrode reactions, stable system operation, high flexibility, long lifespan, low cost, and the ability to utilize industrial waste heat. However, SOE technology is limited to specific high-temperature applications, where the demand for electrical energy decreases and the demand for thermal energy increases. High-temperature conditions may lead to heat loss and overutilization of water resources [114].
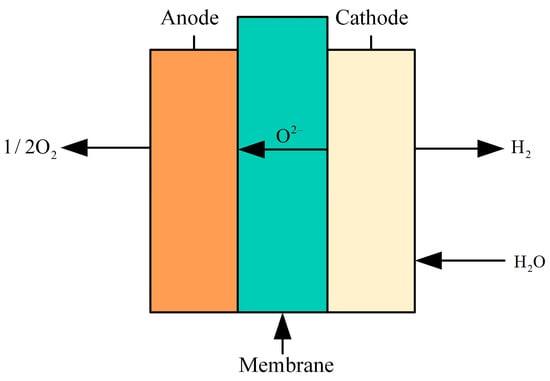
Figure 13.
Structure of the SOE electrolyzer.
Most current studies have focused on low-power SOEC systems, while relatively few economic analyses have been conducted on high-power SOEC systems. Therefore, Bui T et al. [115] developed and simulated a 20 kW scale high power SOEC system, and the results show that the high power SOEC system is economically superior to the conventional low power system. In Case 1, the cost of hydrogen production can be reduced to USD 3.65/kg by integrating the high-power SOEC system into a nuclear power plant. In Case 2, the hydrogen production cost is reduced by 24% when the system size is scaled up from 20 kW to 2 MW. Jolaoso L A et al. [116] present a novel efficient and low-cost SOEC system for hydrogen production using steam generated from flue gas as feedstock, which reduces the consumption of fresh water. Experimental results show that the SOEC system can achieve a total heat-hydrogen efficiency of up to 56.3% and an efficiency of 97.4% at an operating temperature of 830 °C. Liu H et al. [117] simulated the heat balance and degradation processes at the system level and compared the levelized cost of hydrogen through three scenarios: thermal integration, supergrid connection, and SOEC technology. By 2035, the cost of hydrogen is expected to drop to USD 1.40/kg, surpassing that of gray hydrogen, as the SOEC system is commercialized on a large scale and the production process matures. Freshwater resources are becoming increasingly strained, and some scholars have begun to explore the use of non-traditional water sources (e.g., wastewater and seawater) for electrolysis to reduce dependence on freshwater. Maddaloni M et al. [118] selected different treated wastewater treatment streams from four municipal wastewater treatment plants in Northern Italy to model the SOEC system using Aspen Plus software. The results showed that Stream A could produce 26.2 kg of hydrogen per cubic meter of wastewater at 27% evaporation rate, while Stream C could generate 9.7 kg of hydrogen per cubic meter of wastewater at 10% evaporation rate. It was confirmed that the treated wastewater can be used as a source of hydrogen production for SOEC.
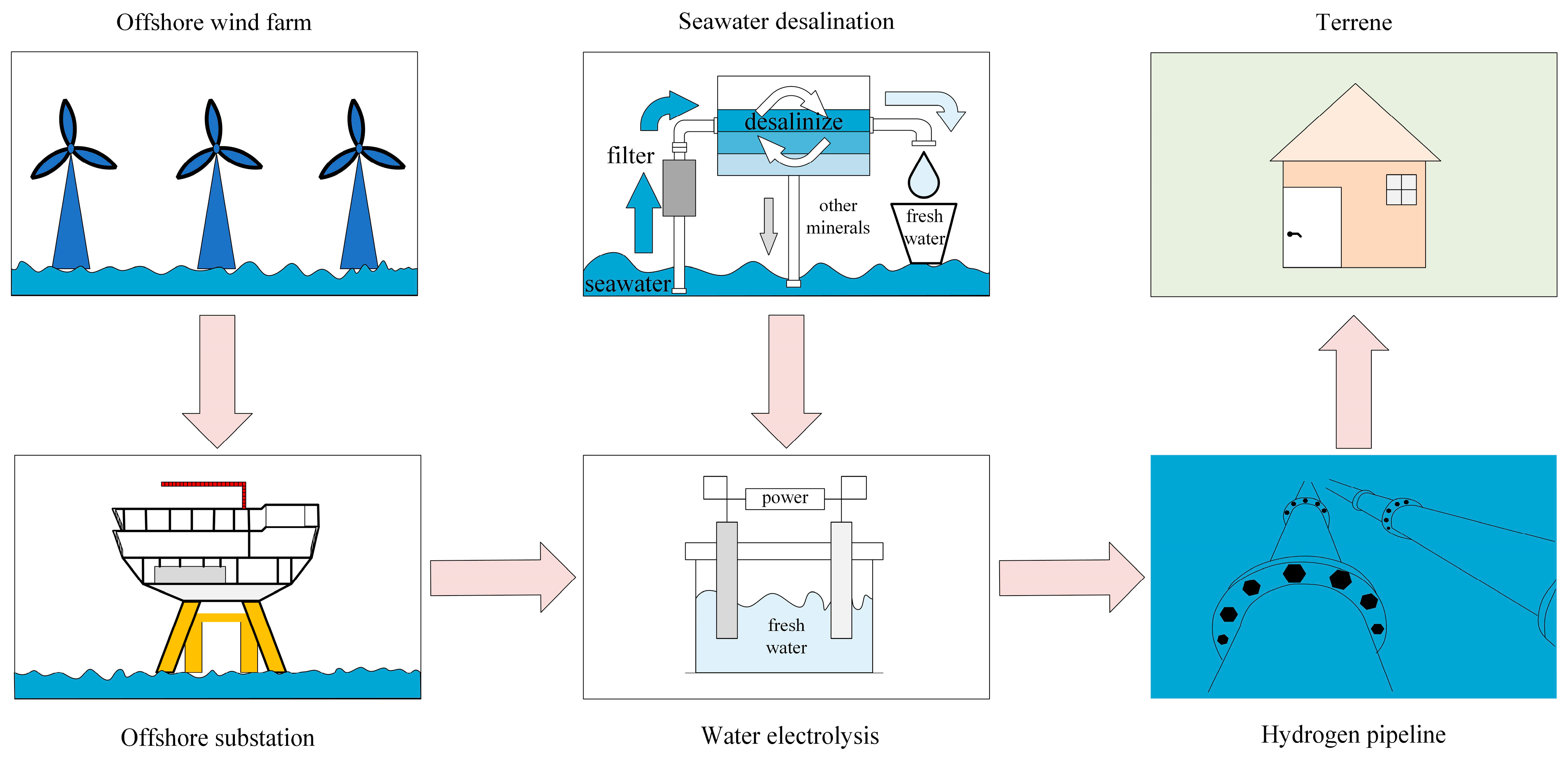
6. Hydrogen Production from Offshore Wind Energy
As illustrated in Figure 14, the rapid advancement of offshore wind power generation has significantly enhanced the potential for hydrogen production using offshore wind energy [119]. This development not only provides a novel approach to utilizing offshore wind power and constructing power transmission systems but also presents new opportunities for the growth of China’s offshore wind power sector, hydrogen energy utilization, and marine resource development. China should actively promote the integration of offshore wind power with hydrogen production technology and explore the potential of marine resources to further advance the hydrogen energy industry. Such efforts will contribute to the pursuit of sustainable “Green hydrogen” energy sources, supporting the long-term goal of achieving carbon neutrality [120].
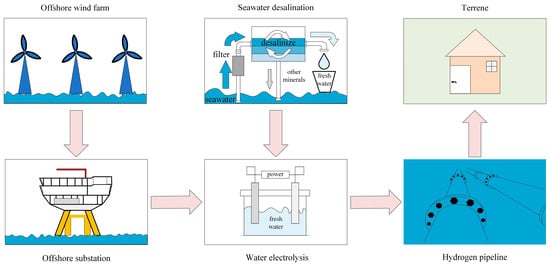
Figure 14.
Schematic diagram of hydrogen production from offshore wind power.
The economic viability of hydrogen production systems for offshore wind is important. Leahy P et al. [121] developed an integrated and analyzable model to assess the feasibility of hydrogen production from dedicated offshore wind farms. A hypothetical offshore wind farm located in the Irish Sea was studied. The wind farm consists of 16 wind turbines (with a total capacity of 101.3 MW) with proton exchange membrane electrolyzers, producing hydrogen that is stored underground and periodically transported. The analysis shows that the model performs well in terms of accuracy and feasibility under different hydrogen storage scenarios. The short-term hydrogen storage scheme is more economically feasible. Durakovic G et al. [122] investigated the impact of large-scale deployment of offshore wind farms and hydrogen production in the North Sea region on the European electricity system and electricity prices, and the results of the study show that the total electricity-generating capacity in Europe increases by about 50 percent, taking into account the demand for hydrogen production. Large-scale hydrogen production significantly increases electricity demand, but electricity prices do not necessarily increase significantly. Hill S J P et al. [123] present a new techno-economic model of offshore electrolysis production costs that utilizes geologic salt caverns to store hydrogen. The economics of hydrogen production from offshore wind power in Europe (especially in the UK) is verified through a detailed techno-economic analysis. The results show that offshore wind-driven hydrogen production can be realized at a cost comparable to conventional fossil fuel hydrogen production when wind farm and electrolyzer costs are reduced to a certain level. Sorrenti I et al. [124] investigate the techno-economic feasibility of building energy islands in the North Sea region to produce green hydrogen. The study shows that under the current conditions, purchasing green electricity from the grid is more economically competitive than supplying it exclusively from offshore wind farms. Rogeau A et al. [125] have developed harmonized detailed cost models to assess the cost of hydrogen production under different configurations, including onshore electrolysis, centralized offshore electrolysis, and decentralized offshore electrolysis. The study suggests that in 2020, centralized offshore electrolysis is more economical than onshore electrolysis only in areas far from the coast and in the deep sea. However, over time, by 2030, the cost of offshore centralized electrolysis falls significantly, providing a cost advantage even in areas up to 100 km from the coast. By 2050, onshore electrolysis is the optimal configuration only when close to the coast (within 100 km) and in shallow water (<55 m), while in all other regions, both offshore centralized and decentralized electrolysis have better LCOH than onshore electrolysis. Groenemans H et al. [126] analyze the techno-economic feasibility of using offshore wind power to drive a proton exchange membrane electrolyzer for hydrogen production. In the conventional mode, the cost of hydrogen production is USD 3.86/kg, while in the direct-coupled mode, the cost of hydrogen production is only USD 2.09/kg, which is significantly lower than that in the conventional mode.
7. Hydrogen Production from Nuclear Energy
As a kind of clean energy, nuclear reactors can not only produce a lot of electricity but also generate a lot of waste heat. The nuclear reactor and hydrogen production technology are combined, and the nuclear reactor power generation is directly electrolyzed to produce hydrogen, and the whole hydrogen production process does not produce carbon dioxide and no greenhouse gas emissions. The waste heat of nuclear reactors is fully utilized to decompose water into hydrogen and oxygen through methane steam reforming, high-temperature steam electrolysis, or thermochemical cycling [127]. Hydrogen production using nuclear energy is a clean and efficient technology, with nuclear reactor heating for hydrogen production showing particular promise for future applications. The principle of this hydrogen production method is depicted in Figure 15.
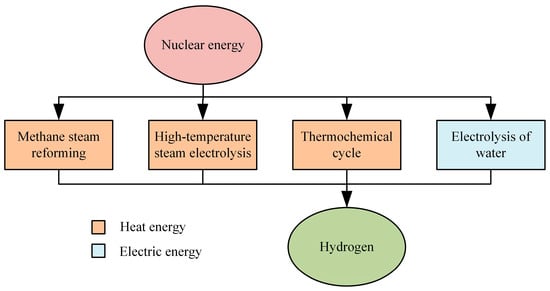
Figure 15.
Technology roadmap for hydrogen production from nuclear energy.
7.1. Methane Steam Reforming
Methane steam reforming is a traditional hydrogen production method that produces hydrogen and carbon dioxide by reacting methane with water vapor at high temperatures and pressure [128]. The main chemical equations of the reaction are as follows:
Carbon monoxide (CO) then reacts further with water vapor to produce more hydrogen.
Leveraging the high-temperature heat energy provided by nuclear reactors can reduce dependence on fossil fuels and enhance hydrogen production efficiency. The high-temperature heat source from nuclear energy decreases the amount of electrical energy required for the reaction, thereby improving economic efficiency. However, the methane steam reforming process generates carbon dioxide, necessitating the use of carbon capture and storage technology to mitigate carbon emissions. The process itself is complex, requiring high-temperature and high-pressure conditions, which increases equipment costs. Pruvost F et al. [129] investigated different pathways for the production of low-carbon hydrogen using methane vapor reforming and carbon dioxide capture technologies. The results of the study show that CO2 capture before combustion can avoid 80% of CO2 emissions, but the remaining 20% capture is more costly. Therefore, the use of post-combustion CO2 capture provides higher economics than pre-combustion CO2 capture for scenarios where the CO2 capture rate exceeds 90%. Garcia-Villalva R et al. [130] compare three methane reforming routes: methane reforming, dry methane reforming, and steam methane reforming. The results showed that steam methane reforming performed the best among the three routes, with a methane conversion of 24% and a hydrogen yield of up to 80%. This is because steam methane reforming uses a Ni/Al2O3 catalyst to improve the methane conversion. Lee S et al. [131] study designed six different scenarios, adjusting the conditions of reactor pressure, number of water gas shift reactors, temperature, and CO2 emission and capture. The results showed that the scenario that included CO2 capture and sale (Sc6) was the most economical, with a hydrogen production cost of USD 1.80 per kilogram of hydrogen. Ingale G U et al. [132] compare two hydrogen production methods, and while carbon capture and storage (CCS) technology can reduce carbon emissions by 47% to 53%, blue and turquoise hydrogen have higher methane escape emissions than gray hydrogen. The study also explored the possibility of using biogas as a feedstock to replace natural gas, and by integrating the Boudouard reaction, it is also possible to further utilize the solid carbon produced during methane cracking and the carbon dioxide from biogas, converting it to carbon monoxide, which can effectively improve carbon utilization and reduce overall carbon emissions. Fatigati F et al. [133] developed a comprehensive energy model for Sorption-enhanced steam–methane reforming (SESMR) to simulate the adsorption/regeneration process with multiple cycles. The experimental results showed that SESMR exhibited higher hydrogen concentration and improved energy efficiency by about 10% compared to the conventional steam–methane reforming reaction. Agnolin S et al. [134] utilized Pd-Ag membranes for the enhancement of hydrogen production in membrane-assisted steam methane reforming processes. Experimental results showed that 99.3% H2 purity was achieved at 500 °C and 4 bar pressure.
7.2. High-Temperature Steam Electrolysis
High-temperature steam electrolysis is a method of producing hydrogen by electrolysis of high-temperature water vapor [135]. The electrolysis process is carried out at high temperatures (usually 700–1000 °C), using the heat and electricity generated by a nuclear reactor to split water into hydrogen and oxygen:
Under high-temperature conditions, the power consumption of electrolytic water is significantly reduced and the energy efficiency is improved. The high-temperature thermal energy provided by nuclear reactors significantly reduces the demand for electric energy and is suitable for large-scale hydrogen production. It produces no greenhouse gases and is an environmentally friendly method of hydrogen production. However, the technology of high-temperature electrolysis is not yet mature and needs to be further developed to improve efficiency and stability. The high-temperature operation has high requirements for materials and equipment, which increases the cost and maintenance difficulty. Frick K et al. [136] focus on the techno-economic evaluation of hydrogen co-production through high-temperature steam electrolysis combined with light water reactors. It was concluded that the system is capable of producing hydrogen at a low cost. The levelized cost of hydrogen analysis showed that the cost of hydrogen production could potentially be as low as USD 1.20/kg, which is lower than that of conventional steam methane reforming. Yildiz B et al. [137] compare various technologies for hydrogen production using nuclear energy, focusing on analyzing hydrogen production routes combining high-temperature nuclear reactors with electrochemical and thermochemical processes. It is shown that high-temperature steam electrolysis combined with supercritical carbon dioxide cycles has the potential to provide higher energy efficiencies than other alternative technologies in a lower temperature range. Loreti G et al. [138] present an innovative multi-energy system that combines membrane-integrated steam reforming of biofuels with renewable electricity electrolysis to achieve carbon-negative hydrogen production. Experimental results show that under optimal operating conditions, the system achieves an energy storage efficiency of 70% and a carbon capture rate of 14.1 g of CO2 per MJ of hydrogen stored. Norman E A et al. [139] analyze two main vapor electrolysis technologies: solid oxide electrolysis cells and proton exchange membrane vapor electrolysis. PEM vapor electrolysis is suitable for low-temperature applications compared to SOEC, whereas at high temperatures the degradation of proton-conducting membranes needs to be overcome. Kim T et al. [140] analyze the energy, fire analysis, and thermal economy of hydrogen production systems for high-temperature gas-cooled reactors and high-temperature water-cooled reactors. A thermo-economic method called “modified production structure analysis” was used to calculate a reasonable unit cost of hydrogen production. The results show that the unit cost of hydrogen produced in a high-temperature gas-cooled reactor (HTGR) system is about USD 35.6/GJ, while the unit cost of hydrogen produced in a high-temperature water-cooled reactor (HWCR) system is about USD 13.92/GJ. The thermal efficiency of the HWCR system is higher than that of the HTGR system. Hydrogen production from high-temperature, water-cooled reactors has better economics.
7.3. Thermochemical Cycle
Thermochemical recycling uses a series of chemical reactions to split water at high temperatures to produce hydrogen [141]. Common thermochemical cycles include the sulfur iodine cycle (S-I cycle) and the calcium bromine cycle (Ca-Br cycle).
7.3.1. Sulfur Iodine Cycle (S-I Cycle)
First, sulfur dioxide, iodine, and water react to form hydrogen iodide and sulfuric acid, then sulfuric acid decomposes to produce oxygen, and finally, hydrogen iodide decomposes at high temperatures:
The S-I thermochemical cycle is an efficient method for hydrogen production. Juárez-Martínez L C et al. [142] used a heuristic approach to thermally optimize a S-I nuclear hydrogen cycle, where four different heat transfer networks were designed and analyzed by setting different minimum temperature differentials (5 K, 10 K, 15 K, and 20 K) in order to evaluate their effects on energy consumption and efficiency. The experimental results show that the optimization significantly reduces the energy input of the S-I cycle and increases the thermal efficiency of the S-I cycle by about 10% on average. And hydrogen iodide decomposition is a key step in determining the efficiency of cyclic hydrogen production. Kong R et al. [143] investigated the problem of minimizing the entropy generation rate in a hydriodic acid decomposition reactor heated by high-temperature helium. A one-dimensional plug flow model of the hydrogen iodide decomposition reactor is established by the finite-time thermodynamic method, and the reactor with optimal performance is designed with the minimization of entropy generation rate as the optimization objective. The results show that the optimized reactor reduces the entropy generation rate by 13.3% compared with the reference reactor at the same hydrogen production rate, which improves hydrogen production efficiency. Qian X et al. [144] propose the use of high-temperature waste heat by combining a sulfuric acid decomposer with a solid oxide fuel cell to sustain the sulfuric acid decomposition reaction in the S-I cycle. The utilization of waste heat significantly improves the thermal efficiency of sulfuric acid decomposition while providing additional power output. Wang Q et al. [145] present an internal heat exchange network based on the I-S cycle for hydrogen production using Aspen Plus. The internal heat exchange network is designed based on the principle of energy ladder utilization, and the study introduces heat exchange constraints in different temperature zones during the design process. Simulation results show that for the proposed I-S system, the thermal efficiency of the system is estimated to be between 15.8% and 49.8% under different operating conditions. With the introduction of common waste heat recovery measures, the thermal efficiency of the system can reach 36.7%, demonstrating much room for optimization. Zhang J et al. [146] investigated the experimental characterization of HI distillation in the I-S thermochemical cycle, where the distillation of the HIx (HI-I2-H2O) solution in the I-S cycle is a necessary step to achieve high concentrations of HI. The results showed that the best distillation effect was achieved when the feed position was at 1/3 of the tower height, the feed temperature was close to the boiling point, and the reflux ratio was 1:4. The optimized system can effectively reduce energy consumption and increase hydrogen production.
7.3.2. Calcium Bromide Cycle (Ca-Br Cycle)
Calcium oxide is first regenerated from calcium bromide at high temperatures by directly utilizing the high-temperature thermal energy of a nuclear reactor, and then calcium oxide (CaO) reacts with hydrogen bromide (HBr) to produce hydrogen. The whole process does not consume electricity or produce carbon dioxide, but the reaction process is complex and costly, and the technology needs further development. The reaction formula is shown in Equation (18):
The use of molten CaBr2 containing CaO as a reaction medium was proposed by earlier authors. Simpson M F et al. [147] present a hydrogen production method based on the Ca-Br cycle, optimizing the reaction process by means of a molten salt reactor. Preliminary experimental results show that the solubility of calcium oxide in calcium bromide at 800 °C is at least 1.2 wt%, the solubility of CaO in molten CaBr2 is sufficient to support the process, and the hydrolysis reaction is capable of generating HBr, which is decomposed by electrolysis or plasma to produce hydrogen and bromine gas. Lee M S et al. [148] developed a novel calcium oxide immobilization method by dispersing and immobilizing nanoscale calcium carbonate on yttrium fiber substrates. The nanoscale calcium carbonate was converted to calcium oxide through a sintering process. The results showed that the conversion of CaBr2 to hydrolysis reaction increased significantly with increasing reaction temperature but was limited by the melting point of CaBr2 (1000 K). Excess steam helped to increase the conversion of the hydrolysis reaction, especially at high temperatures, where 100-fold excess steam led to a conversion of about 9.6%. The conversion of the reaction can be further improved by continuous removal of product gases.
Through the three methods of methane steam reforming, high-temperature steam electrolysis, and thermochemical cycling, the thermal and electrical energy generated by nuclear reactors can be employed to achieve efficient and clean hydrogen production. Each method presents unique advantages and challenges, necessitating careful selection and optimization based on specific needs and the maturity of the technology. As technology continues to evolve and improve, hydrogen production from nuclear energy is expected to play a crucial role in the future energy landscape. A comparison of the characteristics of nuclear hydrogen production technologies is shown in Table 7.

Table 7.
Comparison of nuclear hydrogen production technology characteristics.
8. Hydrogen Production from Mixed Energy Sources
The key to hydrogen production from mixed energy sources lies in the effective integration and management of energy sources, as well as the continuous optimization of related technologies, to improve the economic and environmental sustainability of hydrogen production [149]. Solar and wind, for example, are highly volatile, and by combining them with other forms of energy, the overall stability and efficiency of the system can be improved. In addition, energy storage and conversion technologies during hydrogen production, such as the application of batteries and fuel cells, are also important research directions. With technological advances and cost reductions, hydrogen production from hybrid energy sources is expected to play a greater role in the future energy system [150]. The hybrid energy system is shown in Figure 16.
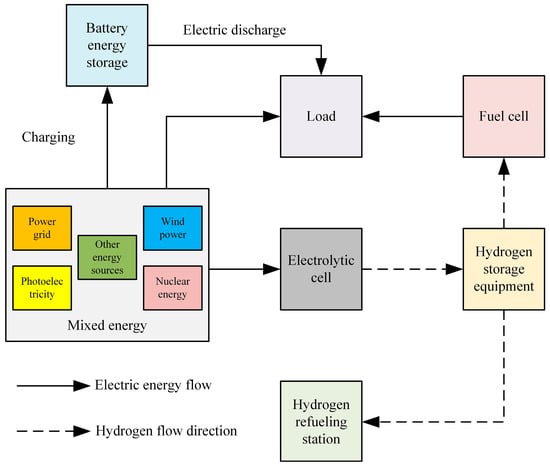
Figure 16.
Hybrid energy system.
By rationally designing and optimizing the hybrid energy hydrogen production system, the economic and technical performance of the system can be effectively enhanced. Song Y et al. [151] propose a hybrid energy system that utilizes surplus power to produce hydrogen. It combines a solar energy and a hydrogen production system with combined cooling, heating, and power generation, and hydrogen production with a combined cooling, heating, and power generation system. The proposed hybrid energy system not only effectively utilizes surplus photovoltaic power but also meets the energy demand of public buildings in small areas and provides hydrogen for hydrogen fuel cell vehicles, which has high economic and environmental benefits. Abdolmaleki L et al. [152] present a solar-hydrogen-based hybrid energy system for net-zero energy management and a multi-objective optimization analysis for sustainable power supply, heating, cooling, and hydrogen production. The system integrates solar photovoltaic cells, a hydrogen electrolyzer, a hydrogen storage tank, and a fuel cell. The power generated by the PV cells is prioritized to meet the building’s needs, and the excess power is used to produce hydrogen through the electrolyzer, which is stored in the storage tank. When needed, the fuel cell converts hydrogen into electricity to meet the demand. Experimental results show that a hybrid energy system based on solar and hydrogen can achieve the goal of net-zero energy consumption and significantly reduce carbon emissions. The volatility and instability of wind and solar energy pose a challenge to the efficient utilization of energy. Su W et al. [153] developed a multi-state scheduling strategy based on an alkaline electrolyzer to improve the flexibility of the system through cooperative operation with batteries. It is shown that the energy efficiency and economy of the wind-photovoltaic hybrid energy hydrogen production system can be effectively improved by optimizing the capacity allocation and daily scheduling strategy. Baral S et al. [154] provide a comprehensive analysis of green hydrogen production technologies in eight different scenarios, and the authors compare these scenarios by using economic parameters such as net present value, minimum cost of production, payback period, and sensitivity analysis and explore the possibility of combining a solar thermal power with an organic Rankine cycle system for waste heat recovery in the future. The results show that the most economically efficient scenario is the combination of a solar photovoltaic system with an energy storage technology and an onshore wind turbine, with a minimum cost of USD 3.01 for the production of 1 kg of hydrogen, an internal rate of return of 5.04%, and a payback period of 8 years. Yang T et al. [155] investigate the parametric study and optimization of a hybrid renewable energy hydrogen production system based on solar and wind energy, using the Kucha region as a case study. For grid-connected systems, the study recommends optimization by reducing the dependence of the system on the grid, especially the capacity allocation of photovoltaic and wind turbines. For the off-grid system, it is recommended to optimize by reducing the excess power rate and increasing the energy consumption rate of the system. Ultimately, the optimal grid-connected system is the PV/WT system, and the optimal off-grid system is the PV/WT/FC system.
9. Comparison of Characteristics of Different Green Hydrogen Production Technologies
In recent years, nations across the globe have conducted extensive research on technologies such as solar water splitting for hydrogen production, biomass-derived hydrogen production, offshore wind energy hydrogen production, and hybrid energy hydrogen production [156]. The field of green hydrogen production is evolving towards diversification, with various emerging technologies rapidly gaining traction. These innovations are playing a critical role in enhancing the efficient utilization of global hydrogen energy resources. Each green hydrogen production technology exhibits unique characteristics in terms of technical principles, environmental adaptability, efficiency, and economic cost. As outlined in Table 8, these technologies are tailored to different application scenarios and requirements, offering diverse solutions for the realization of green and low-carbon energy systems [157].

Table 8.
Comparison of the characteristics of various green hydrogen production technologies.
10. Discussion
As hydrogen production technology continues to advance, green hydrogen is expected to progressively become a vital strategic energy source, supporting China’s economic development. However, the development of green hydrogen production presents both challenges and opportunities, and the future application of green hydrogen energy is anticipated to diversify [158].
10.1. Challenges in Developing Green Hydrogen Production Technology
Whether considering solar water decomposition, biomass hydrogen production, water electrolysis, or offshore wind energy hydrogen production, relying solely on a single hydrogen production method is insufficient to meet the growing societal demand for hydrogen. Currently, hydrogen production in China remains predominantly coal-based, making it crucial to enhance the efficiency of fossil fuels in the hydrogen production process and reduce reliance on these resources. As a clean and efficient energy source, green hydrogen fundamentally drives the transformation of the energy structure. Ultimately, a diversified hydrogen supply model will emerge, centered on renewable energy-based water electrolysis and supplemented by solar water decomposition, biomass hydrogen production, and offshore wind energy hydrogen production, to build a green, sustainable, and low-carbon energy system. Although the early stages of green hydrogen development are constrained by low conversion efficiency and high costs, advancements in power electronic converters and control strategies for water electrolysis are essential. As hydrogen production technology evolves and the goals of “Carbon Peaking and Carbon Neutrality” are realized, renewable energy-based water electrolysis will play a pivotal role in the technological trajectory of China’s hydrogen production industry [159].
10.2. Application Prospect
Green hydrogen is a clean and sustainable energy source, as illustrated in Figure 17, spanning multiple sectors, including energy storage, transportation, industrial production, and thermal energy applications. Green hydrogen is expected to play a significant role in energy transformation and in addressing climate change. However, realizing its full potential will require technological innovation, cost reduction, policy support, and the enhancement of the industrial chain to facilitate the widespread adoption of green hydrogen across various fields [160].
Figure 17.
Prospect of the application of green hydrogen energy.
10.2.1. Energy Storage
Green hydrogen is a good medium for energy storage. Since renewable energy sources such as solar and wind power are greatly affected by the weather when generating electricity, to a certain extent, it results in waste such as abandoned wind and light. The electricity generated from these renewable energy sources is converted into hydrogen energy by electrolyzing water to produce hydrogen and other technologies, and then converted into electricity by fuel cells when energy is needed. After this series of conversions, the problem of energy storage can effectively be solved.
Diversified hydrogen storage is one of the keys to achieving green hydrogen energy applications [161]. The diversification of hydrogen storage technologies encompasses a range of methods and approaches tailored to different application needs and environmental conditions. The primary hydrogen storage methods can be categorized into physical storage and chemical storage. Physical storage includes compressed hydrogen storage, liquefied hydrogen storage, and underground hydrogen storage, while chemical storage includes metal hydride storage and liquid ammonia storage. Butt F K et al. [162] used a template-free method to synthesize ZnV2O4 spherical nanomicrospheres, which were shown to have a maximum hydrogen storage capacity of 2.165 wt% at 623 K and rapid hydrogen adsorption and release kinetics. It is suggested that the layered structure of the nanospheres may provide a larger storage space for hydrogen. Tarasov B P et al. [163] studied intermetallic compounds of types AB5 and AB2. These materials have tunable hydrogen adsorption and desorption properties that can be optimized for pressure and temperature conditions for different applications. The research team developed two integrated energy storage systems combining a metal hydride hydrogen storage unit and a compression unit. The systems can use grid, solar, or wind power as the primary energy source and utilize low-pressure hydrogen produced in an electrolyzer and stored in high-pressure cylinders via a metal hydride compression system. These systems can also supply hydrogen to fuel cells for efficient energy utilization. The high surface area and unique adsorption properties of nanomaterials make them excellent hydrogen storage materials. Jayaprabakar J et al. [164] analyze the role of nanomaterials in hydrogen production and storage, exploring the selection of nanomaterials, their production routes, and their prospects for commercial applications. For example, metal nanoparticles are capable of storing large amounts of hydrogen through hydrogenation reactions and can release hydrogen at relatively low temperatures and pressures. The article evaluates the production costs of green hydrogen and points out that high production costs are currently a major obstacle to the large-scale application of green hydrogen. By using low-cost, sustainable nanomaterials, the cost of hydrogen production and storage can be reduced. Recycling and reuse of nanomaterials is also an important means of reducing overall costs. Ledwaba K et al. [165] analyze the potential of boronene, an emerging two-dimensional material, for hydrogen storage. The unique geometry and electronic properties of boronene allow for a higher hydrogen adsorption capacity compared to metal-based complex hydrides, exceeding the goals set by the U.S. Department of Energy. Boronene possesses reversibility and moderate binding energy as a hydrogen storage material, and while some progress has been made at the laboratory stage, it has not yet been generalized for commercial applications.
The integrated use of these diverse hydrogen storage technologies enables effective storage and flexible utilization of green hydrogen to meet the demands of various application scenarios. As hydrogen storage technologies continue to innovate and improve, the application prospects for green hydrogen will be further expanded and strengthened.
10.2.2. Transportation
Green hydrogen energy has great potential in the field of transportation, especially in fuel cell vehicles. These vehicles use hydrogen to react with oxygen to produce electricity that drives an electric motor. Compared to traditional internal combustion engine vehicles, fuel cell vehicles have the advantages of zero emissions and long driving range, which can bring cleaner and more sustainable solutions to the transportation sector [166].
Green hydrogen is also viewed as a promising future energy option for the aerospace sector. The application of fuel cell technology in aircraft and spacecraft is anticipated to reduce emissions and enhance efficiency, steering the aerospace industry towards a cleaner and more sustainable future [167].
10.2.3. Industrial Application
Hydrogen plays a critical role in various industrial production processes. For example, in the production of ammonia, hydrogen is one of the key raw materials of ammonia, and the use of green hydrogen energy can make the ammonia production process cleaner and more sustainable. In addition, hydrogen can also be used in metal processing, glass production, food processing, and other processes. By using green hydrogen to replace traditional fossil fuels, carbon emissions and environmental pollution in industrial processes can be reduced, and production costs can be reduced.
By promoting the use of green hydrogen energy in these industrial applications, the clean, sustainable development of industrial production processes and the improvement of resource utilization efficiency can be achieved. However, some challenges, such as technology maturity, cost competitiveness, and market promotion, need to be overcome to achieve the wide application of green hydrogen energy in the industrial field [168].
10.2.4. Thermal Energy Application
In green hydrogen production, waste heat recovery technologies can effectively enhance energy efficiency, reduce production costs, and minimize environmental impact. Key applications include the utilization of waste heat in electrolytic hydrogen production, cogeneration systems, and thermal energy circulation systems [169]. During electrolytic hydrogen production, capturing and utilizing the waste heat generated can heat water or steam, thereby reducing external energy consumption. Additionally, cogeneration and thermal energy recycling systems can further optimize waste heat utilization and improve overall energy efficiency. Green hydrogen can also serve as a clean thermal energy source. The heat generated from the reaction of hydrogen with oxygen can be employed in heating systems, industrial heating processes, and domestic hot water supply, reducing reliance on traditional combustion fuels and lowering carbon emissions. The comprehensive utilization of waste heat not only reduces production costs but also decreases carbon emissions, providing crucial support for green hydrogen production [170].
10.3. Economic Feasibility of Green Hydrogen Technology
The production cost of green hydrogen production technology is first analyzed. Electricity cost is the main cost source of green hydrogen production. For example, when electricity is supplied to the electrolyzer through photovoltaic or wind power generation, the fluctuation of the electricity price directly affects the production cost of hydrogen. Moreover, the service life and maintenance costs of the equipment will also affect the overall economics of the project. Currently, the efficiency of electrolyzers ranges from 60% to 80%, which can be further enhanced by heat recovery technologies (e.g., Organic Rankine Cycle, ORC) up to 98% [171], and key materials required for the production of electrolyzers include precious metals such as platinum and ruthenium, which are used to improve the efficiency of hydrogen production by electrolysis of water. Global reserves of these resources, however, are limited and concentrated in a few countries (e.g., China), which could lead to resource bottlenecks if hydrogen energy is promoted on a large scale. The manufacture and extraction of these materials can also have a negative impact on the environment, particularly in terms of carbon emissions during the production process, damage to ecosystems from mineral extraction, and disposal of waste at the end of the life cycle of the equipment.
The cost of hydrogen production is then measured in terms of levelized hydrogen costs, with solar PV-based electrolytic hydrogen production costing USD 9.31/kg [172], and levelized costs of hydrogen from gasification and fermentation of waste-to-hydrogen ranging from GBP 2.02 to GBP 2.29/kg [173], and when production configurations are optimized for wind-energy hydrogen production, the minimum selling price of hydrogen would need to be in the range of EUR 4.5 to EUR 6.5/kg in order to be profitable [174].
Finally, improving the economics of green hydrogen production can be improved through power purchase agreements, which can ensure a stable supply of low-cost renewable electricity for hydrogen production facilities [175], or co-production of hydrogen with other energy products (e.g., compressed natural gas, biofuels, etc.) can improve the overall return on investment of a project. For example, the return on investment is significantly higher when waste is utilized for conversion into multiple products (e.g., hydrogen, fuel, and electricity) [176]. On the other hand, the storage and transportation of green hydrogen requires large-scale infrastructure investments, especially for large-scale hydrogen production and storage systems [177], and with the improvement of electrolyzer technology, especially in the field of water electrolysis with proton exchange membrane and water electrolysis with solid oxides, the cost of equipment is gradually decreasing, which will further reduce the production cost of green hydrogen production.
11. Concluding Remarks
As a clean and efficient energy solution, green hydrogen production technology has been widely used by the international community. The research progress of green hydrogen production technology is reviewed in this paper. This paper first introduces the development status of green hydrogen at home and abroad and then describes the green hydrogen production technology, including solar water decomposition hydrogen production, biomass hydrogen production, and electrolytic water hydrogen production. Then, comparing the advantages and disadvantages of different green hydrogen production technologies, the future source of green hydrogen energy tends to be electrolytic water hydrogen production. Finally, the challenges in the development of green hydrogen production technology and its future application prospects are discussed. Although green hydrogen production technology faces the challenges of low conversion efficiency and high cost, with the continuous development of technology and policy support, green hydrogen energy is expected to play an important role in energy storage, transportation, industrial production, and thermal energy applications. Green hydrogen energy has great potential to achieve sustainable global energy development and cope with climate change. Through continuous technological innovation and policy support, green hydrogen energy will become an important part of achieving sustainable global energy development.
Author Contributions
Conceptualization, Q.C.; methodology, Q.C.; software, Q.C.; validation, Q.C.; formal analysis, Q.C., Z.X. and T.S.; investigation, Q.C.; writing—original draft preparation, Q.C.; writing—review and editing, A.T., Q.C. and L.X.; supervision, A.T. and L.X.; project administration, A.T. and L.X.; funding acquisition, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tianchi Talent Program of Xinjiang Uygur Autonomous, Basic Scientific Research Project Funded by the General Research and Development Expenditure of Universities in the Xinjiang Uygur Autonomous Region, grant number XJEDU2023P027, Science and Technology Project of Xinjiang Uygur Autonomous Region—Major Science and Technology Project, grant number 2022A01007-4, Research on Integrated Energy System of Hydrogen Energy Storage Coupled with Coal Chemical Industry in Xinjiang, grant number 52266018, and Research on the Integrated Energy System with Multiple Types of Energy Storage for Advanced Power Systems with the “Source-Grid-Load-Storage” Integration Approach, grant number 2022TSYCCX0051.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent advancement and assessment of green hydrogen production technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xu, Z.; Yin, K.; Zhou, Y.; Zhang, J.; Cui, P.; Ma, S.; Wang, Y.; Zhu, Z. A review on renewable energy-based chemical engineering design and optimization. Renew. Sustain. Energy Rev. 2024, 189, 114015. [Google Scholar] [CrossRef]
- Renewables 2021. Available online: https://www.iea.org/reports/renewables-2021 (accessed on 20 June 2024).
- Statistical Yearbook of World Energy 2023. Available online: https://kpmg.com/cn/zh/home/campaigns/2023/10/statistical-review-of-world-energy-2023.html (accessed on 20 June 2024).
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of renewable-energy-based green hydrogen into the energy future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. Climate Change 2022: Impacts, Adaptation and Vulnerability. 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 20 June 2024).
- Qiu, A.; Sun, Y.; Wang, Y.; Fei, T. Analysis on the development trend of international green technology under the background of carbon neutrality. World Sci-Tech RD 2021, 43, 385. [Google Scholar]
- Olabi, A.G.; Abdelghafar, A.A.; Baroutaji, A.; Sayed, E.T.; Alami, A.H.; Rezk, H.; Abdelkareem, M.A. Large-vscale hydrogen production and storage technologies: Current status and future directions. Int. J. Hydrogen Energy 2021, 46, 23498–23528. [Google Scholar] [CrossRef]
- The National Development and Reform Commission and the National Energy Administration Jointly Issued the “Medium and Long-Term Plan for the Development of Hydrogen Energy Industry (2021–2035)”. Available online: https://www.nea.gov.cn/2022-03/23/c_1310525755.htm (accessed on 20 June 2024).
- Notice on the Issuance of the 14th Five-Year Plan for the Development of Renewable Energy. Available online: http://zfxxgk.nea.gov.cn/2021-10/21/c_1310611148.htm (accessed on 20 June 2024).
- Notice of the National Energy Administration on Issuing the Guiding Opinions on Energy Work in 2024. Available online: http://zfxxgk.nea.gov.cn/2024-03/18/c_1310768578.htm (accessed on 20 June 2024).
- World Class Green Hydrogen Ecological Innovation Zone “Hydrogen Island” Project Released. Available online: https://www.nea.gov.cn/2024-04/01/c_1310769703.htm (accessed on 20 June 2024).
- The National Energy Administration Organized the National Renewable Energy Development and Construction Situation Analysis Meeting in January 2024. Available online: https://www.nea.gov.cn/2024-02/07/c_1310763838.htm (accessed on 20 June 2024).
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the green hydrogen market—Current state and outlook on green hydrogen demand and electrolyzer manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2022. Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 20 June 2024).
- Ren, Y.; Li, G.; Wang, H.; Bie, Z. China’s Zero-Coal Power System Future. Int. J. Hydrogen Energy 2024, 156, 109748. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Fernández-Arias, P.; Antón-Sancho, Á.; Lampropoulos, G.; Vergara, D. On Green Hydrogen Generation Technologies: A Bibliometric Review. Appl. Sci. 2024, 14, 2524. [Google Scholar] [CrossRef]
- Dorel, S.; Lucian, M.; Gheorghe, L.; Cristian, L.G. Green Hydrogen, a Solution for Replacing Fossil Fuels to Reduce CO2 Emissions. Processes 2024, 12, 1651. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, B.; Huang, W.; Li, Y.; Hou, L.; Xiao, J.; Mao, Z.; Li, X. A review of hydrogen generation, storage, and applications in power system. J. Energy Storage 2024, 75, 109307. [Google Scholar] [CrossRef]
- Ham, K.; Bae, S.; Lee, J. Classification and technical target of water electrolysis hydrogen production. J. Energy Chem. 2024, 95, 554–576. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M. The hydrogen color spectrum: Techno-economic analysis of the available technologies for hydrogen production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Sinopec Releases China Energy Outlook 2060 (2024 Edition). Available online: http://edri.sinopec.com/edri/news/com_news/20240109/news_20240109_325732006670.shtml (accessed on 20 June 2024).
- Cheng, G.; Luo, E.; Zhao, Y.; Yang, Y.; Chen, B.; Cai, Y.; Wang, X.; Dong, C. Analysis and prediction of green hydrogen production potential by photovoltaic-powered water electrolysis using machine learning in China. Energy 2023, 284, 129302. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Liu, H.; Zhang, H.; Baeyens, J. Recent Research in Solar-Driven Hydrogen Production. Sustainability 2024, 16, 2883. [Google Scholar] [CrossRef]
- Yu, Q.; Fuxiang, Z. Photocatalytic Water Splitting for Hydrogen Production. Acta Chim. Sin. 2022, 80, 827. [Google Scholar]
- Yan, X.; Xia, M.; Liu, H.; Zhang, B.; Chang, C.; Wang, L.; Yang, G. An electron-hole rich dual-site nickel catalyst for efficient photocatalytic overall water splitting. Nat. Commun. 2023, 14, 1741. [Google Scholar] [CrossRef]
- Thabet, S.M.; Abdelhamid, H.N.; Ibrahim, S.A.; El-Bery, H.M. Boosting photocatalytic water splitting of TiO2 using metal (Ru, Co, or Ni) co-catalysts for hydrogen generation. Sci. Rep. 2024, 14, 10115. [Google Scholar] [CrossRef]
- Andreou, E.K.; Vamvasakis, I.; Armatas, G.S. Efficient Visible Light Photocatalytic Hydrogen Evolution by Boosting the Interfacial Electron Transfer in Mesoporous Mott–Schottky Heterojunctions of CO2P-Modified CdIn2S4 Nanocrystals. ACS Appl. Energy Mater. 2024, 7, 4891–4903. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Sanoe, M.; Putri, N.P.; Lee, Y.L.; Teng, H. Co-catalyst design to control charge transfer and product composition for photocatalytic H 2 production and biomass reforming. Sustain. Energy Fuels 2024, 8, 1412–1423. [Google Scholar] [CrossRef]
- Maulana, F.; Engge, Y.; Nurhuda, M.; Hakim, L.; Juwono, A.M. Boosting Hydrogen Production through Water Splitting: N, Ni, and N-Ni Doped ZnO Photocatalysts. J. Electrochem. Soc. 2024, 171, 046505. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R.; Zhong, X.; Jiang, F. Preparation and Application of a Novel S-Scheme Nanoheterojunction Photocatalyst (LaNi0.6Fe0.4O3/g-C3N4). ACS Omega 2024, 9, 28422–28436. [Google Scholar] [CrossRef]
- Ma, Z.; Davenport, P.; Saur, G. System and technoeconomic analysis of solar thermochemical hydrogen production. Renew. Energy 2022, 190, 294–308. [Google Scholar] [CrossRef]
- Pérez, A.; Orfila, M.; Díaz, E.; Linares, M.; Sanz, R.; Marugán, J.; Molina, R.; Botas, J.A. Reticulated porous structures of La0.8Al0.2NiO3-δ perovskite for enhanced green hydrogen production by thermochemical water splitting. Catal. Today 2024, 442, 114919. [Google Scholar] [CrossRef]
- Vidal, A.; Gonzalez, A.; Denk, T. A 100 kW cavity-receiver reactor with an integrated two-step thermochemical cycle: Thermal performance under solar transients. Renew. Energy 2020, 153, 270–279. [Google Scholar] [CrossRef]
- Qian, X.; He, J.; Mastronardo, E.; Baldassarri, B.; Wolverton, C.; Haile, S.M. Favorable redox thermodynamics of SrTi0.5Mn0.5O3-δ in solar thermochemical water splitting. Chem. Mat. 2020, 32, 9335–9346. [Google Scholar] [CrossRef]
- Barcellos, D.R.; Sanders, M.D.; Tong, J.; McDaniel, A.H.; O’Hayre, R.P. BaCe0.25Mn0.75O3-δ—A promising perovskite-type oxide for solar thermochemical hydrogen production. Energy Environ. Sci. 2018, 11, 3256–3265. [Google Scholar] [CrossRef]
- Zhang, D.; De Santiago, H.A.; Xu, B.; Liu, C.; Trindell, J.A.; Li, W.; Park, J.; Rodriguez, M.A.; Coker, E.N.; Luo, J. Compositionally complex perovskite oxides for solar thermochemical water splitting. Chem. Mat. 2023, 35, 1901–1915. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Song, Q.; Yang, Z.; Wang, H.; Duan, Y. A critical review on integrated system design of solar thermochemical water-splitting cycle for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 33619–33642. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Zheng, Z.; Ishaq, M.; Liang, G.; Fan, P.; Tang, J. Recent progress and perspectives on Sb2Se3-based photocathodes for solar hydrogen production via photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 508–523. [Google Scholar] [CrossRef]
- Maragno, A.R.A.; Morozan, A.; Fize, J.; Pellat, M.; Artero, V.; Charton, S.; Matheron, M. Thermally integrated photoelectrochemical devices with perovskite/silicon tandem solar cells: A modular approach for scalable direct water splitting. Sustain. Energy Fuels 2024, 8, 3726–3739. [Google Scholar] [CrossRef]
- Präg, R.; Kölbach, M.; Abdi, F.F.; Ahmet, I.Y.; Schleuning, M.; Friedrich, D.; van de Krol, R. Photoelectrochemical Properties of CuFeO2 Photocathodes Prepared by Pulsed Laser Deposition. Chem. Mat. 2024, 36, 7764–7780. [Google Scholar] [CrossRef]
- Lee, H.; Lee, C.U.; Yun, J.; Jeong, C.S.; Jeong, W.; Son, J.; Park, Y.S.; Moon, S.; Lee, S.; Kim, J.H.; et al. A dual spin-controlled chiral two-/three-dimensional perovskite artificial leaf for efficient overall photoelectrochemical water splitting. Nat. Commun. 2024, 15, 4672. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, S.A.; Ali, I.; Haris, M.; Latif, H.; Sabah, A.; Alshomrany, A.S.; Bakkour, Y. Bifunctional Co3O4/g-C3N4 Hetrostructures for Photoelectrochemical Water Splitting. ACS Omega 2024, 9, 21450–21458. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Kong, H.; Park, T.; Kim, T.S.; Yang, H.; Yeo, J. Laser-Printed Photoanode: Femtosecond Laser-Induced Crystalline Phase Transformation of WO3 Nanorods for Space-Efficient and Flexible Thin-Film Solar Water-Splitting Cells. Small 2024, 20, 2402051. [Google Scholar] [CrossRef]
- Zhang, X.; Schwarze, M.; Schomäcker, R.; van de Krol, R.; Abdi, F.F. Life cycle net energy assessment of sustainable H2 production and hydrogenation of chemicals in a coupled photoelectrochemical device. Nat. Commun. 2023, 14, 991. [Google Scholar] [CrossRef]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefin. 2020, 13, 8383–8401. [Google Scholar] [CrossRef]
- Lan, P.; Xu, Q.; Zhou, M.; Lan, L.; Zhang, S.; Yan, Y. Catalytic steam reforming of fast pyrolysis bio-oil in fixed bed and fluidized bed reactors. Chem. Eng. Technol. 2010, 33, 2021–2028. [Google Scholar] [CrossRef]
- Yao, D.; Wu, C.; Yang, H.; Hu, Q.; Nahil, M.A.; Chen, H.; Williams, P.T. Hydrogen production from catalytic reforming of the aqueous fraction of pyrolysis bio-oil with modified Ni–Al catalysts. Int. J. Hydrogen Energy 2014, 39, 14642–14652. [Google Scholar] [CrossRef]
- Arandia, A.; Coronado, I.; Remiro, A.; Gayubo, A.G.; Reinikainen, M. Aqueous-phase reforming of bio-oil aqueous fraction over nickel-based catalysts. Int. J. Hydrogen Energy 2019, 44, 13157–13168. [Google Scholar] [CrossRef]
- Bimbela, F.; Ábrego, J.; Puerta, R.; García, L.; Arauzo, J. Catalytic steam reforming of the aqueous fraction of bio-oil using Ni-Ce/Mg-Al catalysts. Appl. Catal. B Environ. 2017, 209, 346–357. [Google Scholar] [CrossRef]
- Brito, J.; Pinto, F.; Ferreira, A.; Soria, M.A.; Madeira, L.M. Steam reforming of biomass gasification gas for hydrogen production: From thermodynamic analysis to experimental validation. Fuel Process. Technol. 2023, 250, 107859. [Google Scholar] [CrossRef]
- Cortazar, M.; Lopez, G.; Alvarez, J.; Amutio, M.; Bilbao, J.; Olazar, M. Advantages of confining the fountain in a conical spouted bed reactor for biomass steam gasification. Energy 2018, 153, 455–463. [Google Scholar] [CrossRef]
- Kargbo, H.O.; Zhang, J.; Phan, A.N. Optimisation of two-stage biomass gasification for hydrogen production via artificial neural network. Appl. Energy 2021, 302, 117567. [Google Scholar] [CrossRef]
- Lysne, A.; Blekkan, E.A. Continuous coke management with Ni and Nico catalysts for bio-syngas tar steam reforming–The switch-SRCG unit. Appl. Catal. O Open 2024, 190, 206943. [Google Scholar] [CrossRef]
- Okoji, A.I.; Taiwo, A.E.; Adeoye, J.B.; Musonge, P.; Makinde, D.O.; Okoji, C.N. Process Integration of Hydrogen Production Using Steam Gasification and Water-Gas Shift Reactions: A Case of Response Surface Method and Machine Learning Techniques. Int. J. Energy Res. 2024, 2024, 6843951. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Chen, H.; Li, W.; Pan, P.; Wu, L.; Xu, G.; Chen, H. Performance analysis of an integrated biomass-to-energy system based on gasification and pyrolysis. Energy Convers. Manag. 2023, 287, 117085. [Google Scholar] [CrossRef]
- Kumar, G.; Eswari, A.P.; Kavitha, S.; Kumar, M.D.; Kannah, R.Y.; How, L.C.; Muthukaruppan, G.; Banu, J.R. Thermochemical conversion routes of hydrogen production from organic biomass: Processes, challenges and limitations. Biomass Convers. Biorefin. 2020, 13, 8509–8534. [Google Scholar] [CrossRef]
- Liao, B.; Guo, L.; Lu, Y.; Zhang, X. Solar receiver/reactor for hydrogen production with biomass gasification in supercritical water. Int. J. Hydrogen Energy 2013, 38, 13038–13044. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A comparative analysis of hydrogen production from the thermochemical conversion of algal biomass. Int. J. Hydrogen Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Martins, A.H.; Rouboa, A.; Monteiro, E. On the green hydrogen production through gasification processes: A techno-economic approach. J. Clean Prod. 2023, 383, 135476. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, Y.; Liu, F.; Liu, Z.; Chen, B.; Chen, J.; Yi, L.; Chen, B. Optimization of molecular dynamics in hydrogen production through phenol gasification in supercritical water. J. Energy Inst. 2024, 116, 101757. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Y.; Fang, C.; Ju, M.; Xia, R.; Wang, Y. Supercritical Water Gasification of Oily Sludge and Its Life Cycle Assessment. BioResources 2024, 19, 2327–2341. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of sustainable approaches for converting the organic waste to bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Ananthakrishnan, K.; Nandakumar, J.; Kannaiyan, S. Fermentative biohydrogen fuel production utilizing wastewater: A review. CLEAN–Soil Air Water 2023, 51, 2300112. [Google Scholar] [CrossRef]
- Hu, C.; Choy, S.Y.; Giannis, A. Evaluation of lighting systems, carbon sources, and bacteria cultures on photofermentative hydrogen production. Appl. Biochem. Biotechnol. 2018, 185, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Sahrin, N.T.; Khoo, K.S.; Masri, A.N.; Rawindran, H.; Tong, W.Y.; Altaf, M.; Sin, J.C.; Lam, S.M.; Bashir, M.J.K.; Lim, J.W. Enhancing microalgal hydrogen production via photo-fermentative modelling with alimentation derived from palm kernel expeller. Int. J. Hydrogen Energy 2024, 72, 388–394. [Google Scholar] [CrossRef]
- Yue, T.; Sun, Y.; Zhang, Q.; Jiang, D.; Zhang, Z.; Zhang, H.; Li, Y.; Zhang, Y.; Zhang, T. Enhancement of biohydrogen production by photo-fermentation of corn stover via visible light catalyzed titanium dioxide/activated carbon fiber. Bioresour. Technol. 2024, 399, 130459. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, S.; Li, Q.; Jiang, Q.; Li, Y.; Gao, Z.; Cao, W.; Guo, L. Pilot composite tubular bioreactor for outdoor photo-fermentation hydrogen production: From batch to continuous operation. Bioresour. Technol. 2024, 401, 130705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Yuan, J.; Guo, L. Multidimensional engineering of Rhodobacter sphaeroides for enhanced photo-fermentative hydrogen production. Chem. Eng. J. 2024, 488, 150852. [Google Scholar] [CrossRef]
- Chai, Y.; Lyu, Z.; Du, H.; Li, P.; Ding, S.; Jiang, Y.; Wang, H.; Min, Q.; Du, D.; Lin, Y.; et al. Recent progress on rational design of catalysts for fermentative hydrogen production. SusMat 2022, 2, 392–410. [Google Scholar] [CrossRef]
- Garduño, I.R.; Smith, M.; Baranova, E.; Kinsley, C. Hydrogen production in dark fermentation of a brewery sludge pre-treated with white-rot fungi in submerged culture. Bioresour. Technol. Rep. 2024, 25, 101773. [Google Scholar] [CrossRef]
- Ramprakash, B.; Incharoensakdi, A. Dark fermentative hydrogen production from pretreated garden wastes by Escherichia coli. Fuel. 2022, 310, 122217. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrogen Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Kovalev, А.A.; Kovalev, D.A.; Panchenko, V.A.; Zhuravleva, E.A.; Laikova, A.A.; Shekhurdina, S.V.; Ivanenko, A.A.; Litty, Y.V. Energy efficiency of hydrogen production during dark fermentation. Int. J. Hydrogen Energy 2024, 87, 171–178. [Google Scholar] [CrossRef]
- Eggers, N.; Giebner, F.; Heinemann, D.; Wagner, M.; Birth-Reichert, T. Improvement of substrate turnover through integrating dark fermentation into existing biogas plants. Biofuels Bioprod. Biorefin. 2024, 18, 855–864. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Mu, H.; Zhang, X. Biohydrogen production from sewage sludge by sequential dark and photo fermentation. J. Biobased Mater. Bioenergy 2015, 9, 95–100. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Duber, A. Continuous dark-photo fermentative H2 production from synthetic lignocellulose hydrolysate with different photoheterotrophic cultures: Sequential vs. co-culture processes. Energy 2024, 290, 130105. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Fan, J.; Li, F.; Feng, C.; Guan, Y.; Wang, R.; Tang, N. Photosynthetic bacteria improved hydrogen yield of combined dark-and photo-fermentation. J. Biotechnol. 2019, 302, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Jiang, D.; Jing, Y.; Lu, C.; Zhang, H.; Zhang, Q. Continuous dark and photo biohydrogen production in a baffled bioreactor and electrons distribution analysis. Bioresour. Technol. 2021, 337, 125440. [Google Scholar] [CrossRef] [PubMed]
- Niño-Navarro, C.; Chairez, I.; Christen, P.; Canul-Chan, M.; García-Peña, E.I. Enhanced hydrogen production by a sequential dark and photo fermentation process: Effects of initial feedstock composition, dilution and microbial population. Renew. Energy 2020, 147, 924–936. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. Parameters affecting the growth and hydrogen production of the green alga Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2011, 36, 7872–7876. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Shu, L.; Zhuang, X.; Liu, Y.; Chen, J.; Hu, Z. Sustainable photosynthetic H2-production mediated by artificial miRNA silencing of OEE2 gene in green alga Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2015, 40, 5609–5616. [Google Scholar] [CrossRef]
- Bechara, R.; Azizi, F.; Boyadjian, C. Process simulation and optimization for enhanced biophotolytic hydrogen production from green algae using the sulfur deprivation method. Int. J. Hydrogen Energy 2021, 46, 14096–14108. [Google Scholar] [CrossRef]
- Chen, J.; Liu, E.; Wang, J.; Liu, H. Mixotrophic cultivation of green algal aggregates boost photobiological hydrogen production. Int. J. Hydrogen Energy 2024, 76, 304–314. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, M.; Shi, J.; Wang, L.; Luo, S.; Liu, H. Chemical flocculation-based green algae materials for photobiological hydrogen production. ACS Appl. Bio Mater. 2022, 5, 897–903. [Google Scholar] [CrossRef]
- Delavar, M.A.; Wang, J. Advancing biohydrogen production through indirect photolysis: Insights from numerical simulation using the lattice boltzmann method. Fuel 2024, 376, 132707. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Luo, J. Ca2+ enhances algal photolysis hydrogen production by improving the direct and indirect pathways. Int. J. Hydrogen Energy 2019, 44, 1466–1473. [Google Scholar] [CrossRef]
- Touloupakis, E.; Faraloni, C.; Benavides, A.M.S.; Masojídek, J.; Torzillo, G. Sustained photobiological hydrogen production by Chlorella vulgaris without nutrient starvation. Int. J. Hydrogen Energy 2021, 46, 3684–3694. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Wu, F.; Luo, J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018, 251, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Hupp, B.; Pap, B.; Farkas, A.; Maróti, G. Development of a microalgae-based continuous starch-to-hydrogen conversion approach. Fermentation 2022, 8, 294. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Evans, B.R.; Mielenz, J.R.; Hopkins, R.C.; Adams, M.W.W. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway. PLoS ONE 2007, 2, e456. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Demnitz, M.; Lamas, Y.M.; Barros, R.L.G.; den Bouter, A.L.; van der Schaaf, J.; de Groot, M.T. Effect of iron addition to the electrolyte on alkaline water electrolysis performance. iScience 2024, 27, 108695. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, P.; Zhang, T.; Yu, Z.; Liu, K.; Fan, H.J. Boosting alkaline water electrolysis by asymmetric temperature modulation. Appl. Phys Lett. 2021, 119, 013901. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Model-based analysis and optimization of pressurized alkaline water electrolysis powered by renewable energy. J. Electrochem. Soc. 2023, 170, 064510. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Zhao, P.; Xia, H.; Li, Y.; Sun, L.; He, W. Hydrogen production system using alkaline water electrolysis adapting to fast fluctuating photovoltaic power. Energies 2023, 16, 3308. [Google Scholar] [CrossRef]
- Shaarawy, H.H.; Hussein, H.S.; Attia, A.; Hawash, S.I. Green hydrogen generation in alkaline solution using electrodeposited Ni-Co-nano-graphene thin film cathode. Environ. Sci. Pollut. Res. 2024, 31, 28719–28733. [Google Scholar] [CrossRef] [PubMed]
- Makhsoos, A.; Kandidayeni, M.; Pollet, B.G.; Boulon, L. A perspective on increasing the efficiency of proton exchange membrane water electrolyzers—A review. Int. J. Hydrogen Energy 2023, 48, 15341–15370. [Google Scholar] [CrossRef]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A comprehensive modeling method for proton exchange membrane electrolyzer development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- Höglinger, M.; Kartusch, S.; Eder, J.; Grabner, B.; Macherhammer, M.; Trattner, A. Advanced testing methods for proton exchange membrane electrolysis stacks. Int. J. Hydrogen Energy 2024, 77, 598–611. [Google Scholar] [CrossRef]
- Padgett, E.; Bender, G.; Haug, A.; Lewinski, K.; Sun, F.; Yu, H.; Cullen, D.A.; Steinbach, A.J.; Alia, S.M. Catalyst layer resistance and utilization in PEM electrolysis. J. Electrochem. Soc. 2023, 170, 084512. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yu, P.C.; Su, X.Z.; Hu, S.J.; Shi, L.; Wang, Y.H.; Yang, P.P.; Gao, F.Y.; Wu, Z.Z.; Chi, L.P.; et al. Efficient acidic hydrogen evolution in proton exchange membrane electrolyzers over a sulfur-doped marcasite-type electrocatalyst. Sci. Adv. 2023, 9, eadh2885. [Google Scholar] [CrossRef] [PubMed]
- Calnan, S.; Bagacki, R.; Bao, F.; Dorbandt, I.; Kemppainen, E.; Schary, C.; Schlatmann, R.; Pehlivan, I.B. Development of various photovoltaic-driven water electrolysis technologies for green solar hydrogen generation. Sol. RRL 2022, 6, 2100479. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Ha, J.S.; Park, Y.; Jeong, J.Y.; Lee, S.H.; Lee, S.J.; Kim, I.T.; Park, S.H.; Jin, H.; Kim, S.M.; Choi, S.; et al. Solar-Powered AEM Electrolyzer via PGM-Free (Oxy)hydroxide Anode with Solar to Hydrogen Conversion Efficiency of 12.44%. Adv. Sci. 2024, 11, 2401782. [Google Scholar] [CrossRef]
- Khataee, A.; Shirole, A.; Jannasch, P.; Krüger, A.; Cornell, A. Anion exchange membrane water electrolysis using Aemion™ membranes and nickel electrodes. J. Mater. Chem. A 2022, 10, 16061–16070. [Google Scholar] [CrossRef]
- Li, K.; Yu, S.; Li, D.; Ding, L.; Wang, W.; Xie, Z.; Park, E.J.; Fujimoto, C.; Cullen, D.A.; Kim, Y.S.; et al. Engineered thin diffusion layers for anion-exchange membrane electrolyzer cells with outstanding performance. ACS Appl. Mater. Interfaces 2021, 13, 50957–50964. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Yang, F.; Zhang, Z.; Liu, H.; Ge, S.; Hu, S.; Li, S.; Luo, Y.; Yu, Q.; Liu, Z.; et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat. Commun. 2023, 14, 3607. [Google Scholar] [CrossRef] [PubMed]
- Corigliano, O.; Pagnotta, L.; Fragiacomo, P. On the technology of solid oxide fuel cell (SOFC) energy systems for stationary power generation: A review. Sustainability 2022, 14, 15276. [Google Scholar] [CrossRef]
- Bui, T.; Lee, D.; Ahn, K.Y.; Kim, Y.S. Techno-economic analysis of high-power solid oxide electrolysis cell system. Energy Convers. Manag. 2023, 278, 116704. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Asadi, J.; Duan, C.; Kazempoor, P. A novel green hydrogen production using water-energy nexus framework. Energy Convers. Manag. 2023, 276, 116344. [Google Scholar] [CrossRef]
- Liu, H.; Clausen, L.R.; Wang, L.; Chen, M. Pathway toward cost-effective green hydrogen production by solid oxide electrolyzer. Energy Environ. Sci. 2023, 16, 2090–2111. [Google Scholar] [CrossRef]
- Maddaloni, M.; Marchionni, M.; Abbá, A.; Mascia, M.; Tola, V.; Carpanese, M.P.; Bertanza, G.; Artioli, N. Exploring the viability of utilizing treated wastewater as a sustainable water resource for green hydrogen generation using solid oxide electrolysis cells (SOECs). Water 2023, 15, 2569. [Google Scholar] [CrossRef]
- Kumar, S.; Arzaghi, E.; Baalisampang, T.; Garaniya, V.; Abbassi, R. Insights into decision-making for offshore green hydrogen infrastructure developments. Process Saf. Environ. Protect. 2023, 174, 805–817. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Delpisheh, M.; Convery, C.; Niblett, D.; Vinothkannan, M.; Mamlouk, M. Offshore green hydrogen production from wind energy: Critical review and perspective. Renew. Sustain. Energy Rev. 2024, 195, 114320. [Google Scholar] [CrossRef]
- Leahy, P.; McKeogh, E.; Murphy, J.; Cummins, V. Development of a viability assessment model for hydrogen production from dedicated offshore wind farms. Int. J. Hydrogen Energy 2021, 46, 24620–24631. [Google Scholar]
- Durakovic, G.; del Granado, P.C.; Tomasgard, A. Powering Europe with North Sea offshore wind: The impact of hydrogen investments on grid infrastructure and power prices. Energy 2023, 263, 125654. [Google Scholar] [CrossRef]
- Hill, S.J.P.; Bamisile, O.; Hatton, L.; Staffell, I.; Jansen, M. The cost of clean hydrogen from offshore wind and electrolysis. J. Clean. Prod. 2024, 445, 141162. [Google Scholar] [CrossRef]
- Sorrenti, I.; Rasmussen, T.B.H.; Xydis, G.; Enevoldsen, P.; You, S. Correlations between component size green hydrogen demand and breakeven price for energy islands. Renew. Sustain. Energy Rev. 2023, 183, 113439. [Google Scholar] [CrossRef]
- Rogeau, A.; Vieubled, J.; de Coatpont, M.; Nobrega, P.A.; Erbs, G.; Girard, R. Techno-economic evaluation and resource assessment of hydrogen production through offshore wind farms: A European perspective. Renew. Sustain. Energy Rev. 2023, 187, 113699. [Google Scholar] [CrossRef]
- Groenemans, H.; Saur, G.; Mittelsteadt, C.; Lattimer, J.; Xu, H. Techno-economic analysis of offshore wind PEM water electrolysis for H2 production. Curr. Opin. Chem. Eng. 2022, 37, 100828. [Google Scholar] [CrossRef]
- Kubo, S. The roles of nuclear energy in hydrogen production. Engineering 2021, 16, 16–20. [Google Scholar] [CrossRef]
- Mendrela, P.; Stanek, W.; Simla, T. Sustainability assessment of hydrogen production based on nuclear energy. Int. J. Hydrogen Energy 2024, 49, 729–744. [Google Scholar] [CrossRef]
- Pruvost, F.; Cloete, S.; del Pozo, C.A.; Zaabout, A. Blue, green, and turquoise pathways for minimizing hydrogen production costs from steam methane reforming with CO2 capture. Energy Convers. Manag. 2022, 274, 116458. [Google Scholar] [CrossRef]
- Garcia-Villalva, R.; Biset-Peiró, M.; Alarcón, A.; Bacariza, C.; Murcia-López, S.; Guilera, J. Comparison of methane reforming routes for hydrogen production using dielectric barrier discharge plasma-catalysis. Int. J. Hydrogen Energy 2024, 59, 1367–1375. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.H.; Lim, H.; Won, W. Scenario-based techno-economic analysis of steam methane reforming process for hydrogen production. Appl. Sci. 2021, 11, 6021. [Google Scholar] [CrossRef]
- Ingale, G.U.; Kwon, H.M.; Jeong, S.; Park, D.; Kim, W.; Bang, B.; Lim, Y.I.; Kim, S.W.; Kang, Y.B.; Mun, J. Assessment of Greenhouse Gas Emissions from Hydrogen Production Processes: Turquoise Hydrogen vs. Steam Methane Reforming. Energies 2022, 15, 8679. [Google Scholar] [CrossRef]
- Fatigati, F.; Di Giuliano, A.; Carapellucci, R.; Gallucci, K.; Cipollone, R. Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System. Processes 2021, 9, 1440. [Google Scholar] [CrossRef]
- Agnolin, S.; Di Felice, L.; Tanaka, A.P.; Tanco, M.L.; Ververs, W.J.; Gallucci, F. Intensification of hydrogen production: Pd–Ag membrane on tailored hastelloy-X filter for membrane-assisted steam methane reforming. Processes 2023, 12, 40. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Ozcan, H.; El-Emam, R.S.; Horri, B.A. Recent advances in high-temperature steam electrolysis with solid oxide electrolysers for green hydrogen production. Energies 2023, 16, 3327. [Google Scholar] [CrossRef]
- Frick, K.; Wendt, D.; Talbot, P.; Rabiti, C.; Boardman, R. Technoeconomic assessment of hydrogen cogeneration via high temperature steam electrolysis with a light-water reactor. Appl. Energy 2022, 306, 118044. [Google Scholar] [CrossRef]
- Yildiz, B.; Kazimi, M.S. Efficiency of hydrogen production systems using alternative nuclear energy technologies. Int. J. Hydrogen Energy 2006, 31, 77–92. [Google Scholar] [CrossRef]
- Loreti, G.; Facci, A.L.; Ubertini, S. Combining renewable sources towards negative carbon emission hydrogen. Int. J. Hydrogen Energy 2023, 48, 20875–20888. [Google Scholar] [CrossRef]
- Norman, E.A.; Maestre, V.M.; Ortiz, A.; Ortiz, I. Steam electrolysis for green hydrogen generation. State of the art and research perspective. Renew. Sustain. Energy Rev. 2024, 202, 114725. [Google Scholar] [CrossRef]
- Kim, T.; Lee, W.Y.; Seo, S.H.; Oh, S.D.; Kwak, H.Y. Energy, Exergy and Thermoeconomic Analyses on Hydrogen Production Systems Using High-Temperature Gas-Cooled and Water-Cooled Nuclear Reactors. Energies 2023, 16, 8090. [Google Scholar] [CrossRef]
- Özkaya, M.; Acır, A.; Yalçın, Ş. Investigation of the hydrogen production of the PACER fusion blanket integrated with Fe–Cl thermochemical water splitting cycle. Nucl. Eng. Technol. 2023, 55, 4287–4294. [Google Scholar] [CrossRef]
- Juárez-Martínez, L.C.; Espinosa-Paredes, G.; Vázquez-Rodríguez, A.; Romero-Paredes, H. Energy optimization of a Sulfur–Iodine thermochemical nuclear hydrogen production cycle. Nucl. Eng. Technol. 2021, 53, 2066–2073. [Google Scholar] [CrossRef]
- Kong, R.; Chen, L.; Xia, S.; Li, P.; Ge, Y. Minimization of entropy generation rate in hydrogen iodide decomposition reactor heated by high-temperature helium. Entropy 2021, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Jung, S. Computational design of H2SO4 decomposer combined with SOFC for thermochemical hydrogen production. Int. J. Energy Res. 2021, 45, 16576–16591. [Google Scholar] [CrossRef]
- Wang, Q.; Macián-Juan, R. Design and analysis of an iodine-sulfur thermochemical cycle-based hydrogen production system with an internal heat exchange network. Int. J. Energy Res. 2022, 46, 11849–11866. [Google Scholar] [CrossRef]
- Zhang, J.; Ling, B.; He, Y.; Zhu, Y.; Wang, Z. Experimental Study of the Characteristics of HI Distillation in the Thermochemical Iodine–Sulfur Cycle for Hydrogen Production. Processes 2024, 12, 1768. [Google Scholar] [CrossRef]
- Simpson, M.F.; Utgikar, V.; Sachdev, P.; McGrady, C. A novel method for producing hydrogen based on the Ca–Br cycle. Int. J. Hydrogen Energy 2007, 32, 505–509. [Google Scholar] [CrossRef]
- Lee, M.S.; Goswami, D.Y.; Stefanakos, E.K. Immobilization of calcium oxide solid reactant on a yttria fabric and thermodynamic analysis of UT-3 thermochemical hydrogen production cycle. Int. J. Hydrogen Energy 2009, 34, 745–752. [Google Scholar] [CrossRef]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of green hydrogen generation from hybrid renewable energy sources: A review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Rezazadeh, A. Hydrogen fuel and electricity generation from a new hybrid energy system based on wind and solar energies and alkaline fuel cell. Energy Rep. 2021, 7, 2594–2604. [Google Scholar] [CrossRef]
- Song, Y.; Mu, H.; Li, N.; Shi, X.; Zhao, X.; Chen, C.; Wang, H. Techno-economic analysis of a hybrid energy system for CCHP and hydrogen production based on solar energy. Int. J. Hydrogen Energy 2022, 47, 24533–24547. [Google Scholar] [CrossRef]
- Abdolmaleki, L.; Jahanbin, A.; Berardi, U. Net-zero energy management through multi-criteria optimizations of a hybrid solar-hydrogen energy system for a laboratory in Toronto, Canada. Energy Build. 2024, 312, 114186. [Google Scholar] [CrossRef]
- Su, W.; Li, Q.; Zheng, W.; Han, Y.; Yu, Z.; Bai, Z.; Han, Y. Enhancing wind-solar hybrid hydrogen production through multi-state electrolyzer management and complementary energy optimization. Energy Rep. 2024, 11, 1774–1786. [Google Scholar] [CrossRef]
- Baral, S.; Šebo, J. Techno-economic assessment of green hydrogen production integrated with hybrid and organic Rankine cycle (ORC) systems. Heliyon 2024, 10, e25742. [Google Scholar] [CrossRef]
- Yang, T.; Yan, X.; Cai, W.; Luo, H.; Xu, N.; Tong, L.; Yan, F.; Chahine, R.; Xiao, J. Parametric Study and Optimization of Hydrogen Production Systems Based on Solar/Wind Hybrid Renewable Energies: A Case Study in Kuqa, China. Sustainability 2024, 16, 896. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A brief review of hydrogen production methods and their challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Zhang, Z.; Lin, K.; Zheng, M. Current development status, policy support and promotion path of China’s green hydrogen industries under the target of carbon emission peaking and carbon neutrality. Sustainability 2023, 15, 10118. [Google Scholar] [CrossRef]
- Rey, J.; Segura, F.; Andújar, J.M. Green Hydrogen: Resources Consumption, Technological Maturity, and Regulatory Framework. Energies 2023, 16, 6222. [Google Scholar] [CrossRef]
- Morales-España, G.; Hernández-Serna, R.; Tejada-Arango, D.A.; Weeda, M. Impact of large-scale hydrogen electrification and retrofitting of natural gas infrastructure on the European power system. Int. J. Electr. Power Energy Syst. 2024, 155, 109686. [Google Scholar] [CrossRef]
- Du, Z.; Dai, Z.; Yang, Z.; Zhan, C.; Chen, W.; Cao, M.; Thanh, H.V.; Soltanian, M.R. Exploring hydrogen geologic storage in China for future energy: Opportunities and challenges. Renew. Sustain. Energy Rev. 2024, 196, 114366. [Google Scholar] [CrossRef]
- Butt, F.K.; Cao, C.; Wan, Q.; Li, P.; Idrees, F.; Tahir, M.; Khan, W.S.; Ali, Z.; Zapata, M.J.M.; Safdar, M.; et al. Synthesis, evolution and hydrogen storage properties of ZnV2O4 glomerulus nano/microspheres: A prospective material for energy storage. Int. J. Hydrogen Energy 2014, 39, 7842–7851. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrogen Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Jayaprabakar, J.; Hari, N.S.S.; Badreenath, M.; Anish, M.; Joy, N.; Prabhu, A.; Rajasimman, M.; Kumar, J.A. Nano materials for green hydrogen production: Technical insights on nano material selection, properties, production routes and commercial applications. Int. J. Hydrogen Energy 2024, 52, 674–686. [Google Scholar] [CrossRef]
- Ledwaba, K.; Karimzadeh, S.; Jen, T.C. Emerging borophene two-dimensional nanomaterials for hydrogen storage. Mater. Today Sustain. 2023, 22, 100412. [Google Scholar] [CrossRef]
- Durkin, K.; Khanafer, A.; Liseau, P.; Stjernström-Eriksson, A.; Svahn, A.; Tobiasson, L.; Andrade, T.S.; Ehnberg, J. Hydrogen-Powered Vehicles: Comparing the Powertrain Efficiency and Sustainability of Fuel Cell versus Internal Combustion Engine Cars. Energies 2024, 17, 1085. [Google Scholar] [CrossRef]
- Marksel, M.; Prapotnik Brdnik, A. Comparative Analysis of Direct Operating Costs: Conventional vs. Hydrogen Fuel Cell 19-Seat Aircraft. Sustainability 2023, 15, 11271. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Routes for Hydrogen Introduction in the Industrial Hard-to-Abate Sectors for Promoting Energy Transition. Energies 2023, 16, 6098. [Google Scholar] [CrossRef]
- Bedakhanian, A.; Maleki, A.; Haghighat, S. Multi-objective optimization of a cogeneration system based on solar energy for clean hydrogen, cooling, and electricity production. Mater. Today Sustain. 2024, 54, 103990. [Google Scholar] [CrossRef]
- Rad, H.N.; Ghasemi, A.; Marefati, M. Cost and environmental analysis and optimization of a new and green three-level waste heat recovery-based cogeneration cycle: A comparative study. Heliyon 2024, 10, e29087. [Google Scholar]
- Vives, A.M.V.; Wang, R.; Roy, S.; Smallbone, A. Techno-economic analysis of large-scale green hydrogen production and storage. Appl. Energy 2023, 346, 121333. [Google Scholar] [CrossRef]
- Frowijn, L.S.F.; van Sark, W.G. Analysis of photon-driven solar-to-hydrogen production methods in the Netherlands. Sustain. Energy Technol. Assess. 2021, 48, 101631. [Google Scholar] [CrossRef]
- Lui, J.; Paul, M.C.; Sloan, W.; You, S. Techno-economic feasibility of distributed waste-to-hydrogen systems to support green transport in Glasgow. Int. J. Hydrogen Energy 2022, 47, 13532–13551. [Google Scholar] [CrossRef]
- Liponi, A.; Baccioli, A.; Ferrari, L. Feasibility analysis of green hydrogen production from wind. Int. J. Hydrogen Energy 2023, 48, 37579–37593. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Naval, N. Techno-economic model and feasibility assessment of green hydrogen projects based on electrolysis supplied by photovoltaic PPAs. Int. J. Hydrogen Energy 2023, 48, 5053–5068. [Google Scholar] [CrossRef]
- Armoo, E.A.; Mohammed, M.; Narra, S.; Beguedou, E.; Agyenim, F.B.; Kemausuor, F. Achieving Techno-Economic Feasibility for Hybrid Renewable Energy Systems through the Production of Energy and Alternative Fuels. Energies 2024, 17, 735. [Google Scholar] [CrossRef]
- Hasan, T.; Emami, K.; Shah, R.; Hassan, N.M.S.; Anderson, J.; Thomas, D.; Louis, A. A study on green hydrogen-based isolated microgrid. Energy Rep. 2022, 8, 259–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).