Bleaching Agents: A Review of Their Utilization and Management
Abstract
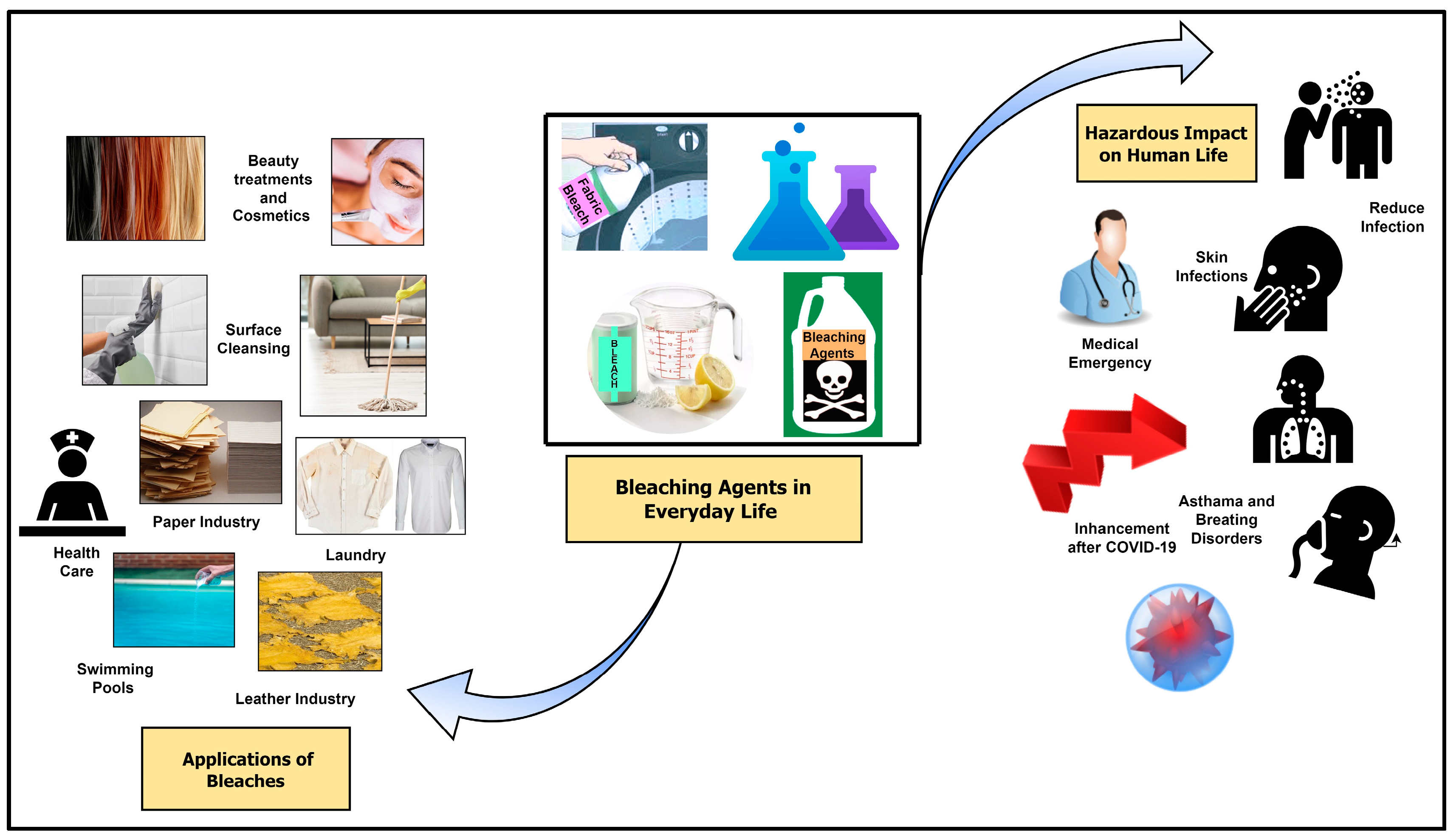
1. Introduction
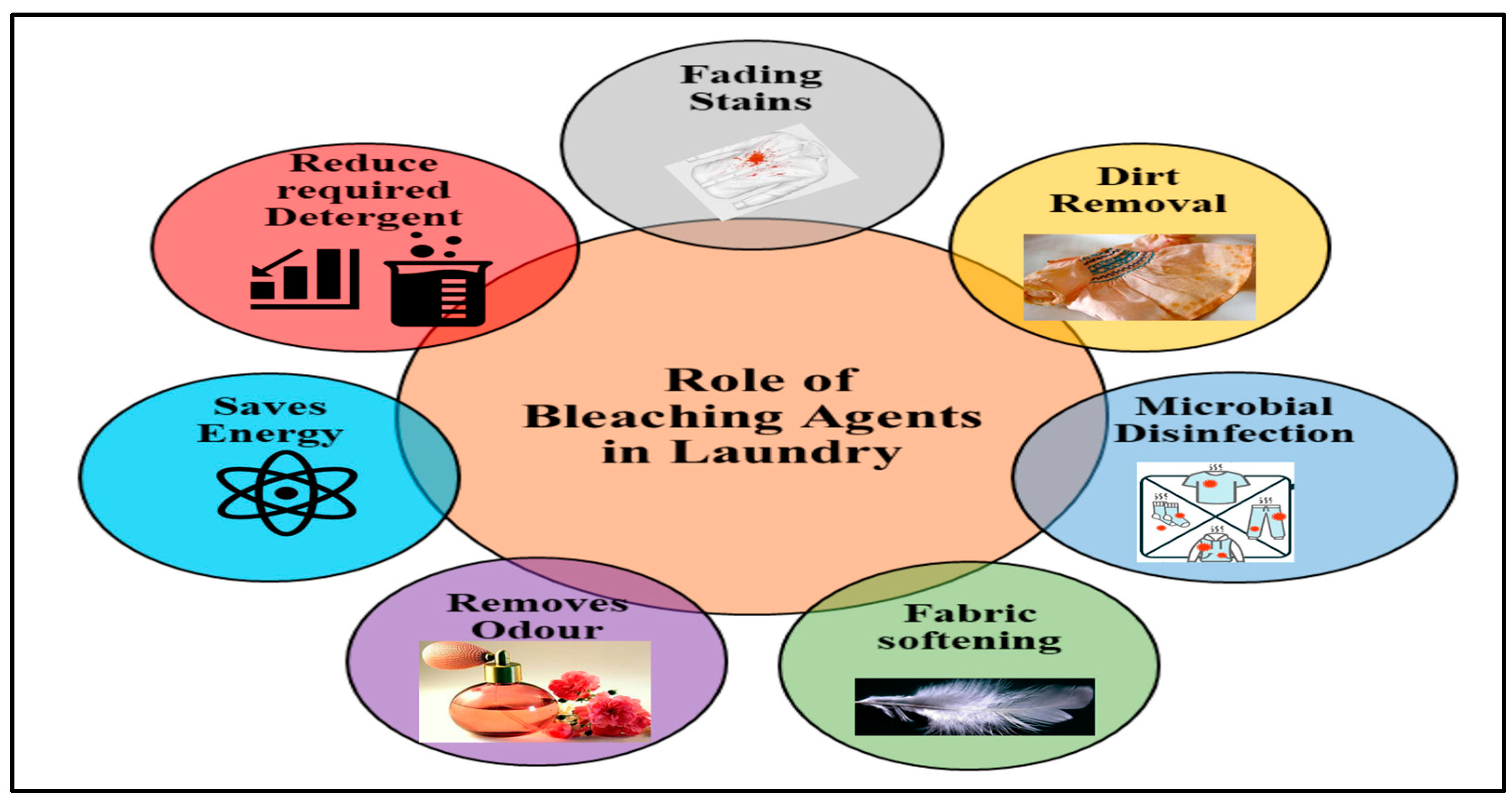
2. Bleaching Agents in the Laundry Industry
3. Conventional or Pre-Pandemic Approach in Applications
4. Post-Pandemic Role as Disinfectants
5. Post-Utility Effects
6. Health Hazards and Other Impacts
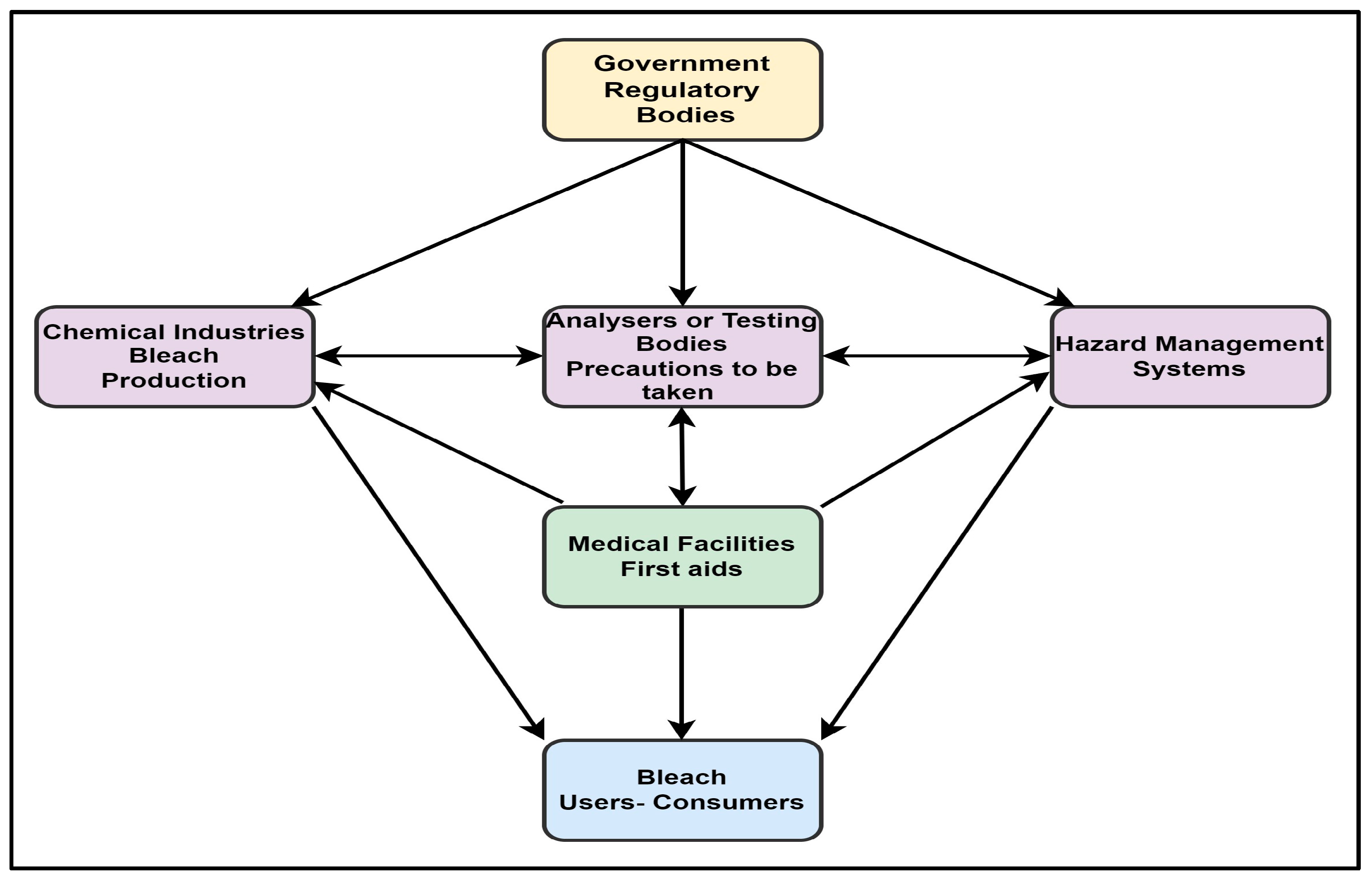
7. Existing Scenario and Future Management
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOB | Active oxygen bleach |
| BAC | Benzalkonium chloride |
| CDI | Clostridium difficile infection |
| DDAC | Dodecyl dimethyl ammonium chloride |
| DAS | Diamino stilbene types |
| DOBA | Decanoyl oxy-benzoic acid |
| DSBP | Di styryl biphenyl |
| FWA | Fluorescent whitening agents |
| LOBS | Lauryl oxy-benzenesulfonate sodium |
| NaPB | Sodium perborate |
| NIR | Near infrared |
| NOBS | Nonanoyl oxy-benzenesulfonate sodium |
| PC | Poison centres |
| PDC | Peroxy dicarbonate |
| RO | reverse osmosis |
| SARS | Severe acute respiratory syndrome |
| SOA | Secondary organic aerosol |
| TAED | Tetraacetylethylenediamine |
| VOC | Volatile organic compounds |
References
- Van Hoof, G.; Fan, M.; Lievens, A. Use of product and ingredient tools to assess the environmental profile of automatic dishwashing detergents. J. Clean. Prod. 2017, 142, 3536–3543. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M.; Maghsoodlu, M. Application of zeolites as non-phosphate detergent builders: A review. J. Environ. Chem. Eng. 2020, 8, 104287. [Google Scholar] [CrossRef]
- Hashemi, H.; Ghareghani, S.; Nasimi, N.; Shahbazi, M.; Derakhshan, Z.; Sarkodie, S.A. Health Consequences of Overexposure to Disinfectants and Self-Medication against SARS-CoV-2: A Cautionary Tale Review. Sustainability 2022, 14, 13614. [Google Scholar] [CrossRef]
- Soave, P.M.; Grassi, S.; Oliva, A.; Romano, B.; Di Stasio, E.; Dominici, L.; Pascali, V.; Antonelli, M. Household disinfectant exposure during the COVID-19 pandemic: A retrospective study of the data from an Italian poison control center. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1738–1742. [Google Scholar] [CrossRef]
- Le Roux, G.; Sinno-Tellier, S.; Puskarczyk, E.; Labadie, M.; von Fabeck, K.; Pélissier, F.; Nisse, P.; Paret, N.; French PCC Research Group; Descatha, A.; et al. Poisoning during the COVID-19 outbreak and lockdown: Retrospective analysis of exposures reported to French poison control centres. Clin. Toxicol. 2021, 59, 832–839. [Google Scholar] [CrossRef]
- Le Roux, G.; Sinno-Tellier, S.; Descatha, A. COVID-19: Home poisoning throughout the containment period. Lancet Public Health 2020, 5, e314. [Google Scholar] [CrossRef]
- Farr, J.P.; Smith, W.L.; Steichen, D.S. Bleaching agents. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 4, pp. 43–81. [Google Scholar]
- Sehulster, L.M. Healthcare Laundry and Textiles in the United States: Review and Commentary on Contemporary Infection Prevention Issues. Infect. Control Hosp. Epidemiol. 2015, 36, 1073–1088. [Google Scholar] [CrossRef]
- Manga, M.S.; Willis, D.; Ali, N.M.; York, D.W. The impact of raw material properties and process conditions on the color of a powdered formulated detergent product. Particuology 2019, 45, 35–41. [Google Scholar] [CrossRef]
- Ziogas, A.; Belda, J.; Kost, H.-J.; Magomajew, J.; Sperling, R.A.; Wernig, P. Peroxodicarbonate: Electrosynthesis and first directions to green industrial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100341. [Google Scholar] [CrossRef]
- Bockmühl, D. Laundry hygiene-how to get more than clean. J. Appl. Microbiol. 2017, 122, 1124–1133. [Google Scholar] [CrossRef]
- Schages, J.; Stamminger, R.; Bockmühl, D.P. A New Method to Evaluate the Antimicrobial Efficacy of Domestic Laundry Detergents. J. Surfactants Deterg. 2020, 23, 629–639. [Google Scholar] [CrossRef]
- Brands, B.; Brinkmann, A.; Bloomfield, S.; Bockmühl, D.P. Microbicidal Action of Heat, Detergents and Active Oxygen Bleach as Components of Laundry Hygiene. Tenside Surfactants Deterg. 2016, 53, 495–501. [Google Scholar] [CrossRef]
- Reinhardt, G. Fingerprints of bleach systems. J. Mol. Catal. A Chem. 2006, 251, 177–184. [Google Scholar] [CrossRef]
- Tavčer, P.F. Influence of Bleach Activators in Removing Different Soils from Cotton Fabric. Fibres Text. East. Eur. 2020, 28, 74–78. [Google Scholar] [CrossRef]
- Balpetek, F.G.; Gülümser, T. Shading of Polyamide 6.6 after multi Washing Process and Investigation of Chemical Changes on Fabric Surface. Text. Appar. 2018, 28, 125–134. [Google Scholar]
- Kramer, J.B.; Canonica, S.; Hoigné, J.; Kaschig, J. Degradation of Fluorescent Whitening Agents in Sunlit Natural Waters. Environ. Sci. Technol. 1996, 30, 2227–2234. [Google Scholar] [CrossRef]
- Poonsin, T.; Simpson, B.K.; Benjakul, S.; Visessanguan, W.; Yoshida, A.; Klomklao, S. Albacore tuna spleen trypsin: Potential application as laundry detergent additive and in carotenoprotein extraction from Pacific white shrimp shells. Biocatal. Agric. Biotechnol. 2019, 17, 638–646. [Google Scholar] [CrossRef]
- Vojcic, L.; Pitzler, C.; Körfer, G.; Jakob, F.; Martinez, R.; Maurer, K.-H.; Schwaneberg, U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef]
- Takenaka, S.; Umeda, M.; Senba, H.; Koyama, D.; Tanaka, K.; Yoshida, K.; Doi, M. Heterologous expression and characterisation of the Aspergillus aspartic protease involved in the hydrolysis and decolorisation of red-pigmented proteins. J. Sci. Food Agric. 2017, 97, 95–101. [Google Scholar] [CrossRef]
- Odabasi, M. Halogenated Volatile Organic Compounds from the Use of Chlorine-Bleach-Containing Household Products. Environ. Sci. Technol. 2008, 42, 1445–1451. [Google Scholar] [CrossRef]
- Zhang, L.; Fung, K.Y.; Zhang, X.; Fung, H.K.; Ng, K.M. An integrated framework for designing formulated products. Comput. Chem. Eng. 2017, 107, 61–76. [Google Scholar] [CrossRef]
- Hodes, J.; Sielaff, P.; Metz, H.; Kessler-Becker, D.; Gassenmeier, T.; Neubert, R.H. The role of chelating agents and amino acids in preventing free radical formation in bleaching systems. Free Radic. Biol. Med. 2018, 129, 194–201. [Google Scholar] [CrossRef]
- Rice, R.G. Applications of ozone for industrial wastewater treatment—A review. Ozone Sci. Eng. 1996, 18, 477–515. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Kanika; Kedawat, G.; Papanai, G.S.; Gupta, B.K. Single excitable dual emissive novel luminescent pigment to generate advanced security features for anti-counterfeiting applications. J. Mater. Chem. C 2019, 7, 13867–13877. [Google Scholar] [CrossRef]
- Affrald, R.J. Sodium Hypochlorite and its Environmental Impacts; Time to Switch for Herbal Alternatives. Toxicol. Int. 2022, 29, 215–226. [Google Scholar] [CrossRef]
- Doebley, C.E.; Lewin, S.Z.; Aronson, S. Detergent and Hypochlorites for the Cleaning of Travertine. APT Bull. J. Preserv. Technol. 1991, 23, 54. [Google Scholar] [CrossRef]
- Najid, N.; Hakizimana, J.N.; Kouzbour, S.; Gourich, B.; Ruiz-García, A.; Vial, C.; Stiriba, Y.; Semiat, R. Fouling control and modeling in reverse osmosis for seawater desalination: A review. Comput. Chem. Eng. 2022, 162, 107794. [Google Scholar] [CrossRef]
- McLaren, K.; McCauley, E.; O’Neill, B.; Tinker, S.; Jenkins, N.; Sehulster, L. The efficacy of a simulated tunnel washer process on removal and destruction of Clostridioides difficile spores from health care textiles. Am. J. Infect. Control 2019, 47, 1375–1381. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Wedebye, E.B.; Nikolov, N.G.; Carøe, T.K.; Sørli, J.B.; Frydendall, K.B.; Liguori, B.; Sejbaek, C.S.; Wolkoff, P.; et al. Asthma-inducing potential of 28 substances in spray cleaning products—Assessed by quantitative structure activity relationship (QSAR) testing and literature review. J. Appl. Toxicol. 2022, 42, 130–153. [Google Scholar] [CrossRef]
- Tsai, W.-T. An overview of health hazards of volatile organic compounds regulated as indoor air pollutants. Rev. Environ. Health 2019, 34, 81–89. [Google Scholar] [CrossRef]
- Hickman, W.S. Peracetic acid and its use in fibre bleaching. Color. Technol. 2002, 32, 13–27. [Google Scholar] [CrossRef]
- Kathare, M.; Julander, A.; Erfani, B.; Schenk, L. An Overview of Cleaning Agents’ Health Hazards and Occupational Injuries and Diseases Attributed to Them in Sweden. Ann. Work. Expo. Health 2022, 66, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Schnall, A.H.; Law, R.; Bronstein, A.C.; Marraffa, J.M.; Spiller, H.A.; Hays, H.L.; Funk, A.R.; Mercurio-Zappala, M.; Calello, D.P.; et al. Cleaning and Disinfectant Chemical Exposures and Temporal Associations with COVID-19—National Poison Data System, United States, January 1, 2020–March 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Merettig, N.; Bockmühl, D.P. Virucidal Efficacy of Laundering. Pathogens 2022, 11, 993. [Google Scholar] [CrossRef]
- Gharpure, R.; Hunter, C.M.; Schnall, A.H.; Barrett, C.E.; Kirby, A.E.; Kunz, J.; Berling, K.; Mercante, J.W.; Murphy, J.L.; Garcia-Williams, A.G. Knowledge and Practices Regarding Safe Household Cleaning and Disinfection for COVID-19 Prevention—United States, May 2020. MMWR-Morb. Mortal. Wkly. Rep. 2020, 69, 705–709. [Google Scholar] [CrossRef]
- Lemire, P.; Dumas, O.; Chanoine, S.; Temam, S.; Severi, G.; Boutron-Ruault, M.-C.; Zock, J.-P.; Siroux, V.; Varraso, R.; Le Moual, N. Domestic exposure to irritant cleaning agents and asthma in women. Environ. Int. 2020, 144, 106017. [Google Scholar] [CrossRef]
- Oudejans, L.; See, D.; Dodds, C.; Corlew, M.; Magnuson, M. Decontamination options for indoor surfaces contaminated with realistic fentanyl preparations. J. Environ. Manag. 2021, 297, 113327. [Google Scholar] [CrossRef]
- Nickmilder, M.; Carbonnelle, S.; Bernard, A. House cleaning with chlorine bleach and the risks of allergic and respiratory diseases in children. Pediatr. Allergy Immunol. 2007, 18, 27–35. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Immich, A.P.S. Textile Re-Engineering: Eco-responsible solutions for a more sustainable industry. Sustain. Prod. Consum. 2021, 28, 1232–1248. [Google Scholar] [CrossRef]
- Sarayu, K.; Sandhya, S. Current Technologies for Biological Treatment of Textile Wastewater—A Review. Appl. Biochem. Biotechnol. 2012, 167, 645–661. [Google Scholar] [CrossRef]
- Hardie, A.G.; Madubela, N.; Clarke, C.E.; Lategan, E.L. Impact of powdered and liquid laundry detergent greywater on soil degradation. J. Hydrol. 2021, 595, 126059. [Google Scholar] [CrossRef]
- Mattila, J.M.; Arata, C.; Wang, C.; Katz, E.F.; Abeleira, A.; Zhou, Y.; Zhou, S.; Goldstein, A.H.; Abbatt, J.P.D.; DeCarlo, P.F.; et al. Dark Chemistry during Bleach Cleaning Enhances Oxidation of Organics and Secondary Organic Aerosol Production Indoors. Environ. Sci. Technol. Lett. 2020, 7, 795–801. [Google Scholar] [CrossRef]
- Kumar, M.R.; Kumar, T.S.; Prakash, C.; Jayakumari, M. Investigation on fastness properties of plated interlock knitted fabrics. Clean. Eng. Technol. 2022, 8, 100474. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S. Silver Recovery from Laundry Washwater: The Role of Detergent Chemistry. ACS Sustain. Chem. Eng. 2018, 6, 600–608. [Google Scholar] [CrossRef]
- Quirce, S.; Barranco, P. Cleaning agents and asthma. J. Investig. Allergol. Clin. Immunol. 2010, 20, 542. [Google Scholar]
- Elamin, M.E. Poisoning by household products. Medicine 2020, 48, 203–204. [Google Scholar] [CrossRef]
- Racioppi, F.; Daskaleros, P.; Besbelli, N.; Borges, A.; Deraemaeker, C.; Magalini, S.; Arrifta, R.M.; Pulce, C.; Ruggerone, M.; Vlachos, P. Household bleaches based on sodium hypochlorite: Review of acute toxicology and poison control center experience. Food Chem. Toxicol. 1994, 32, 845–861. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Williams, C.M. Science of Odor as a Potential Health Issue. J. Environ. Qual. 2005, 34, 129–138. [Google Scholar] [CrossRef]
- Medina-Ramón, M.; Zock, J.P.; Kogevinas, M.; Sunyer, J.; Torralba, Y.; Borrell, A.; Burgos, F.; Antó, J.M. Asthma, chronic bronchitis, and exposure to irritant agents in occupational domestic cleaning: A nested case-control study. Occup. Environ. Med. 2005, 62, 598–606. [Google Scholar] [CrossRef]
- McKenzie, L.B.; Ahir, N.; Stolz, U.; Nelson, N.G. Household Cleaning Product-Related Injuries Treated in US Emergency Departments in 1990–2006. Pediatrics 2010, 126, 509–516. [Google Scholar] [CrossRef]
- Kumar, M.S.; Goud, B.R.; Joseph, B. A study of occupational health and safety measures in the Laundry Department of a private tertiary care teaching hospital, Bengaluru. Indian J. Occup. Environ. Med. 2014, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Starke, K.R.; Friedrich, S.; Schubert, M.; Kämpf, D.; Girbig, M.; Pretzsch, A.; Nienhaus, A.; Seidler, A. Are Healthcare Workers at an Increased Risk for Obstructive Respiratory Diseases Due to Cleaning and Disinfection Agents? A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5159. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Ladbeck, R.; Kolla, P.; Karst, U. A field test for the detection of peroxide-based explosives. Analyst 2002, 127, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A.; Heumann, K.G.; Juraske, S.; Kashers, R. Boron isotope application for tracing sources of contamination in groundwater. Environ. Sci. Technol. 1994, 28, 1968. [Google Scholar] [CrossRef]
- Giordano, F.; Petrolini, V.M.; Spagnolo, D.; Fidente, R.M.; Lanciotti, L.; Baldassarri, L.; Moretti, F.L.; Brambilla, E.; Lonati, D.; Schicchi, A.; et al. Significant variations of dangerous exposures during COVID-19 pandemic in Italy: A possible association with the containment measures implemented to reduce the virus transmission. BMC Public Health 2022, 22, 441. [Google Scholar] [CrossRef]
- Peng, M.; Wu, S.; Du, J.; Sun, C.; Zhou, C.; Xu, C.; Hu, X. Establishing a Rapid Pad-Steam Process for Bleaching of Cotton Fabric with an Activated Peroxide System. ACS Sustain. Chem. Eng. 2018, 6, 8599–8603. [Google Scholar] [CrossRef]
- Tan, C.; Chen, H.; Lin, Z. Brand classification of detergent powder using near-infrared spectroscopy and extreme learning machines. Microchem. J. 2021, 160, 105691. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Update: May 2019. 2019. Available online: https://www.cdc.gov/infection-control/media/pdfs/guideline-disinfection-h.pdf (accessed on 28 August 2024).
- Liu, Z.; Shen, Z.; Xiang, S.; Sun, Y.; Cui, J.; Jia, J. Evaluation of 1,4-naphthoquinone derivatives as antibacterial agents: Activity and mechanistic studies. Front. Environ. Sci. Eng. 2023, 17, 31. [Google Scholar] [CrossRef]
- Gerster, F.M.; Vernez, D.; Wild, P.P.; Hopf, N.B. Hazardous substances in frequently used professional cleaning products. Int. J. Occup. Environ. Health 2014, 20, 46–60. [Google Scholar] [CrossRef]
| Bleaching Agent | Formula | Preparation Reaction | Common Applications |
|---|---|---|---|
| Sodium Hypochlorite | NaOCl | Cl2 + 2NaOH → NaOCl + NaCl + H2O | swimming pool disinfection, domestic and institutional laundry |
| Calcium Hypochlorite | Ca (OCl)2 | Cl2 + 2Ca(OH)2 → Ca (OCl)2 + CaCl2 + 2H2O | water treatment, household disinfectants, cleaners, and mildewcides |
| Hypochlorous Acid | HOCl | Cl2 + H2O → HOCl + HCl | disinfection of pools, spas, and hot tubs, deodorizers |
| Chloramines N-Chloro Compounds | R2NCl | R2NH + NaOCl → R2NCl + NaOH | enhances the bleaching of peroxide bleaches in laundry |
| Chlorine Dioxide | ClO2 | 2NaClO2 + Cl2 → 2NaCl + 2ClO2 | bleaching wood pulp, flour, edible fats and oils along with textiles |
| Hydrogen Peroxide (via quinones) | H2O2 | H2 + O2 → H2O2 | bleaching agent for the textile industry, pulp, paper, home laundry |
| Sodium Perborates | Na2H4B2O8 | Na2B4O7(Borax) + 2NaOH → 4NaBO2 + H2O 4NaBO2 + 2H2O2 → Na2H4B2O8 | formulations of bleach and detergents, denture cleansers, tooth powders |
| Peracids | RCO3H | RCOOH + H2O2 → RCO3H + H2O | textile bleaching, detergent products |
| Sodium Sulphite | Na2SO3 | SO2 + 2NaOH → Na2SO3 + H2O | pulp and paper bleaching |
| Sodium Dithionite | Na2S2O4 | NaBH4 + 8SO2 + 8NaOH → 4Na2S2O4 + NaBO2 + 6H2O | textile industry for dyeing, printing, and stripping, industrial reducing agents, bleaching of mechanical pulp |
| Sr. No | Type of Fabric | Bleaching Agent Used | Concentration | pH | Duration | Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | Cotton and Cotton Polyester | Hydrogen peroxide | 0.3–0.6% | 10.5–11.5 | 1–3 h | 90–95 °C |
| Sodium hypochlorite | 0.1–0.5% | 10–11.5 | 15–30 min | 40–50 °C | ||
| Sodium chlorite | 0.1–3% | 3.8–4.2 | 1–6 h | 80–95 °C | ||
| 2 | Synthetic Fibres | Sodium chlorite | -- | 2.5–4.5 | 30–90 min | <80 °C |
| Peracetic acid | 0.10% | 6–7 | 1 h | 80–85 °C | ||
| 3 | Wool, Silk | Hydrogen peroxide | 1–5% | 5.5–8 | 20–60 min | 70–80 °C |
| 4 | Wool | Sodium dithionite | 0.2–0.5% | 5.5–7 | 1–2 h | 45–65 °C |
| Sr. No | Pre-Pandemic Utilization and Management | Pandemic/Post-Pandemic Utilization and Management |
|---|---|---|
| 1. | Chief utility—bleaching systems in various industrial sectors along with medical facility | Leading utility—disinfecting COVID-19 virus in medical, domestic, and commercial sectors, including traditional applications |
| 2. | Few cases of bleach mishaps were reported | Increase in the number of calls or cases reported to poison call centers |
| 3. | Management of bleaches did not receive much focus | Need for management of bleaches and their toxic effects has intensified after COVID-19 |
| 4. | Precautionary insights with safe functioning information by government and legal sectors were intermittent | Regularization of precautionary insights by government and legal sectors with necessary amendments is essential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, D.; Jaspal, D.; Itankar, N.; Petrounias, P.; Rogkala, A.; Lampropoulou, P. Bleaching Agents: A Review of Their Utilization and Management. Sustainability 2024, 16, 9084. https://doi.org/10.3390/su16209084
Kulkarni D, Jaspal D, Itankar N, Petrounias P, Rogkala A, Lampropoulou P. Bleaching Agents: A Review of Their Utilization and Management. Sustainability. 2024; 16(20):9084. https://doi.org/10.3390/su16209084
Chicago/Turabian StyleKulkarni, Deepali, Dipika Jaspal, Nilisha Itankar, Petros Petrounias, Aikaterini Rogkala, and Paraskevi Lampropoulou. 2024. "Bleaching Agents: A Review of Their Utilization and Management" Sustainability 16, no. 20: 9084. https://doi.org/10.3390/su16209084
APA StyleKulkarni, D., Jaspal, D., Itankar, N., Petrounias, P., Rogkala, A., & Lampropoulou, P. (2024). Bleaching Agents: A Review of Their Utilization and Management. Sustainability, 16(20), 9084. https://doi.org/10.3390/su16209084








