Abstract
Despite advances in recycling technologies and practices, the world still mostly operates on a once-through materials use cycle. The once-through approach to the materials supply chain cannot work into perpetuity. The vast majority of current recycling efforts focus on mechanical or chemical separation-based techniques and are often subsequently limited on the number of times a component can be recycled or upcycled. By looking at things from a particle physics and nuclear history perspective, we propose a thought experiment to determine the physical limit of recycling and propose subsequent limits and standards to evaluate all recycling efforts. This uncommon approach to analysis demonstrates that the current limits to recycling are not physical in nature but engineering.
1. Background
The availability of resources and their use and disposal have set limits on the growth of civilizations throughout human history [1]. The ability to recycle natural resources has been ubiquitous to the livelihoods of civilizations, especially in regions where necessary resources are sparse [2]. In current times, the amount of waste generated has increased over the past 50 years, contributing to the depletion of natural resources, the pollution of resources, and restricting the availability of future resources [3]. In fact, as Barles notes, the word “waste” derives from old French meaning “empty” or “desolate” [2]. However, through recycling efforts over the past few decades, we now recognize that the materials and products sent away as “waste” are clearly not “empty” and are actually full of potential for alternative use.
Despite the growth of product recycling, the world still generally operates on a once-through cycle, consuming products and disposing of them without recycling. Metallic components and plastics, commonly thought to be among the most recyclable products, are routinely only recycled about 10% of the time [4], with recycling complicated by the physical state of materials to be recycled, social behavior, and recycling technologies [5]. However, the recyclability and recycling rates of materials vary widely, not because of lack of need but because of the ease of recycling. As an example, we show the recycling of common materials in Figure 1 and Table 1. Aluminum (Al) is widely recycled, with current research noting that only up to 9% of Al is lost to the environment or discarded as scrap [6,7]. Aluminum is produced in quantities upwards of 45 million tonnes per year and used extensively in various industries, including the automotive industry [8]. Focusing on Al in the automotive industry, as shown in Figure 1, Al is shredded and separated in bulk, sorted, and remelted downstream, resulting in 80–98% of Al in automobiles being recycled [7].
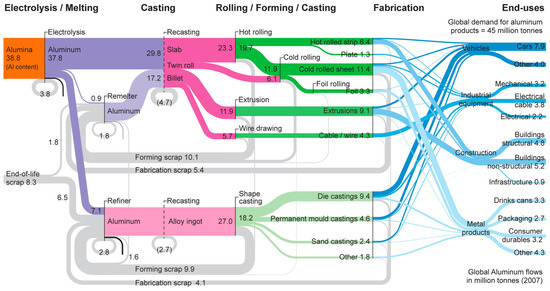
Figure 1.
A depiction of the current recycling pathway indicating the melting, casting, forming, fabrication, end-use, and final disposal of aluminum. The image was reprinted with permission from J.M. Cullen and J.M. Allwood. “Mapping the Global Flow of Aluminum: From Liquid Aluminum to End-Use Goods”, Environmental Science & Technology 47 (2013) 3057–3064 10.1021/es304256s. Copyright 2013 American Chemical Society [7].
In contrast, rare earth metals such as neodymium, scandium, yttrium, cerium, and lanthanum (among others) are crucial to modern society in advanced electronics and electromagnetic materials. Despite their supply chain issues and identification as critical minerals [9], they are among the least commonly recycled materials, with only ~1% of neodymium being recycled currently, as shown in Table 1 [10]. As an example, the recycling of neodymium in permanent magnets focuses on physical separation via demagnetization, followed by hydrometallurgical separation, leaching, or pyrometallurgical separation methods [11]. However, the amount of rare earth elements in each product and in an individual component is very small, making recycling difficult, and this may cause prices to rise [12]. Impacting recycling rates further is the lack of recycling programs, as Swain, et al. identified that there is not a well-organized rare earth waste collection and recycling system in Korea; hence, all 21.9 tons of waste are not recycled [13].
As a comparison, many other elements have end-of-life recycling rates falling in between those of Al and Nd. Table 1 illustrates the end-of-life recycling rates of common elements, highlighting the variability, dependent on the element, its application, its form following application, and available industrial recycling methods. While recycling rates are high for certain elements, there is certainly room for growth; if a universal recycling method existed, these recycling rates for all elements could increase.

Table 1.
End-of-life recycling rates of select elements taken from [14].
Table 1.
End-of-life recycling rates of select elements taken from [14].
| Element | Recycling Rate |
|---|---|
| Al | 42–70% |
| Ni | 57–63% |
| Ru | 5–15% |
| Nd | <1% |
However, recycling limits have been historically lower than theoretically possible. The limits of recycling have been discussed in various contexts in the literature, from perspectives on social behaviors [5] to available technologies and processes [15] and considerations of the second law of thermodynamics [16]. While numerous factors clearly influence the current desire and ability to recycle materials and resources, ultimately, recycling is determined by the energy limits and energy required for separation [17]. However, the ultimate separation of materials can occur only on the atomistic level; the ultimate energy required for separation is the binding energy between atoms. Herein, then, we describe the potential for the separation of any and all materials, limited only by the availability of input energy, to the isotopic level, based on available technology that could enable the recycling of all materials, including complicated mixtures and alloys without harmful leachants or chemicals. The objective of this work is to provide a perspective on the ability to recycle any and all materials using isotope mass separation to encourage creative thought into the reprocessing and recycling of materials.
2. Thought Experiment
It is clear that materials supply chain challenges should be considered with food supply, water supply, and energy supply challenges as engineering problems with global impact. This is evident in the procurement of raw materials through end-of-life disposal. In this article, we take an unconventional perspective on recycling-based particle physics and nuclear history.
Such a thought experiment analyzing materials supply chain starts by first analyzing the materials balance around planet Earth. By drawing a black box system volume around the Earth and analyzing the elemental fluxes in and out, we notice there are minimal materials supply chain concerns, as can be seen in Figure 2a. Since the Paleocene period, the fluxes are generally small with an influx of small asteroids and cosmic rays and an out-flux of some spacecraft and gasses lighter than air; albeit, both fluxes are small relative to the mass of Earth. The small influx from iron-rich asteroids had a large impact on early metallurgy. Similarly, the relatively small loss of helium through the atmosphere via balloons and venting has contributed to the helium supply chain crisis, impacting many medical and scientific instruments [18]. Zooming in and considering a country-scale materials balance, as shown in Figure 2b with analysis of the amount of trade (in U.S. dollars) in and out of the United States, we observe the importance of global transport dictating materials fluxes in and out of a country on essential materials and less-common elements for advanced technological applications. The flow of elements and materials via international trade dates back to the Upper Paleolithic age and the transport of Obsidian up to 800 km [19], and it has recently had a geopolitical impact associated with the fluxes of Li and other rare earth metals’ impact on Li-ion batteries [20] and other renewable energy technologies [9]. If we continue this thought experiment down to the surface area of a large metropolitan region such as Los Angeles, as seen in Figure 2c, we can observe the itemized pathways contributing to the supply chain and the flux into and out of the region often by land, air, and sea. Any disruption of these metropolitan supply chains can have a large societal impact ranging from the rationing of toilet paper to radioactive elements (e.g., Tc-99) for medical imaging [21,22]. All of the fluxes of materials and raw elements in and out of a defined area on Earth require physical transportation on some scale (internationally, inter-communally, or intra-communally). As a result of this analysis from the Earth scale to the local scale, it is clear that for all but He and some predominantly man-made isotopes (H-3, Tc-99, etc.), the materials supply chain challenges at an elemental level are predominantly transport and separation/joining issues.
To further the theoretical thought experiment, the authors now postulate that energy concerns are fully ignored. Doing so largely illuminates the transport component of the materials supply chain concerns as additional redundancy and efficiencies could be engineered into the global distribution network. This still leaves questions such as ‘How many times can a material be recycled or even upcycled?’ This limit is often set by the quality and contamination type in the supplied materials for recycling, which can be difficult to track and subsequently make it difficult to recycle. Here, then, we reframe the question to ask ‘To what extent can materials be separated for recycling?’ From a particle physics perspective, the answer to the latter question is simple. Any gas, liquid, or solid can be turned into plasma and subsequently separated into individual mass-to-charge ratios (i.e., isotopic purity) with enough energy. The energy to create a plasma and then perform electromagnetic isotope separation can be immense but is ignored in this thought experiment. The technologies for plasma formation range from Radio Frequency (RF) through sputtering to laser-based approaches and have been extensively studied and are utilized in both industrial (e.g., sputtering deposition in the semiconductor industry) and household applications (e.g., fluorescent light bulbs) [23]. Subsequently, the physics behind the separation of isotopes has been known since 1878 and is governed by the Lorentz equation [24]:
where F denotes force, q denotes the electric charge, E denotes the external electric field, v denotes the particle velocity, and B denotes the applied magnetic field. This classical and elegant equation is routinely applied in ion beam accelerator applications and, when applied to the physical limits of recycling, suggests that all materials can be physically separated down to individual isotopes (mass-to-charge ratio). Thus, again, neglecting the electricity to transport, to build and operate the plasma source, to build and operate the high Tesla magnetic fields, to subsequently rejoin into isotopically pure feedstock, and to transport to the needed site, there is no physical limit to recycling/upcycling materials. Typically, landfills are viewed as a one-way path: waste enters and does not leave. However, with the isotopic recycling of waste, there is potential to transition landfills into sources of isotopically pure feedstock, as visualized in Figure 2d.
F = q(E + v × B),
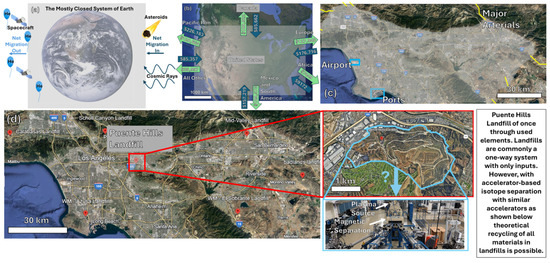
Figure 2.
The recycling thought experiment: (a) materials balance of planet Earth; (b) trade balance of the United States [25]; (c) materials/trade in/out flow routes of a large metropolitan center (e.g., Los Angeles); (d) the hypothetical potential of large-scale electromagnetic isotope separation to change any material from landfill to an isotopically pure resource.
By approaching the challenges of recycling and supply chain from a particle physics perspective over an economic or materials science perspective, we find that there is no physical limit to recycling. With enough energy, anything can be recycled! If we apply particle physics concepts and are willing to pay the price energetically to recycle at the nanoscale or atomic scale, we can apply the words of Professor Richard Feinman to recycling “There is plenty of room at the bottom” [26]. With this new perspective, what insight can be gained into the current methods for recycling?
3. Non-Dimensional Analysis
This thought experiment not only requires us to view recycling from a far different perspective than is commonly addressed, but it more importantly sets theoretical standards for both the mass and energy balances considered during any recycling process. Similar to how the Carnot cycle, Rankine cycle, and similar theoretical thermodynamic processes set theoretical standards that can be utilized by any power plant from the simplest steam boiler to the largest nuclear power plant [27,28], we propose analogous standards for any proposed recycling process. Taking a mass and energy balance approach, we can create a normalized mass standard to quantify recycling purity and an energy standard to evaluate recycling effectiveness.
Since we know from our thought experiment that using electromagnetic isotope separation permits recycling down to the individual isotope, we have a theoretical standard to compare all recycling processes. This can be quantified utilizing the simple non-dimensional recycling purity relationship:
Recycle Purity = Isotope concentration in recycled product/100% of only the isotope desired,
Based on this approach, the recycle purity can never be greater than 1. The closer it is to 1, the higher the isotopic purity of the recycled product. For most applications, the isotopic specificity is not required, and the limit can be relaxed to
Recycle Purity = Final element concentration in recycled product/100% of only the element desired,
Although this non-dimensional analysis is simple to understand and can be rapidly determined through one of many post-processing quality control compositional analysis techniques such as X-ray Fluorescence (XRF), Inductively Coupled Plasma Mass Spectroscopy (ICP-MS), etc. [29,30], it does have several limitations. It does not compare to the composition of the material prior to recycling; as such, it does not evaluate the efficiency of the recycling process. In contrast, it evaluates the effectiveness of the recycling process to produce complete segregation of the isotopes or elements. This analysis is also limited based on the isotopic/elemental nature of the approach. Most modern functional and structural materials utilize a combination of diverse composition and crystallographic phases to achieve the desired properties. By not considering either the initial provided or final desired type of chemical bonding or microstructure, optimizing for just this recycling purity standard might result in extremely wasteful over-upcycling during the recycling process. For example, one should never isotopically separate a 2″ × 4″ wood board into C, H, O, etc., to reassemble it into something akin to a particle board.
As such, it is clear that the energetics of the recycling process must be reintroduced into the thought experiment and considered in any evaluation of the recycling process. Here, we can take a similar approach of producing a non-dimensional term based on the electromagnetic isotope separation thought experiment. To do so, we must expand our previous thought experiment. Take any proposed recycling process and the simplest necessary ion accelerator facility to separate the desired isotope and place them in a black box with only the energy needed to construct, operate, and decommission the facilities measured. Although very much a theoretical value, we feel a good estimate of such values could be determined. Based on this, a non-dimensional term for recycling effectiveness can be stated as:
Recycling Effectiveness = Total energy needed for proposed recycling process/total energy needed for magnetic isotope separation,
If the recycling effectiveness is greater than 1, then the proposed recycling process should not be performed or be completely reworked. Given the higher power demands to make and operate an ion accelerator and isotope separation magnet, we hypothesize that an economical recycling process would have a recycling effectiveness multiple orders of magnitude lower than electromagnetic isotope separation. As such, the closer to zero the recycling effectiveness, the more desirable the proposed recycling process. Also, note that the denominator is not a fixed number. It should continually decrease as electromagnetic isotope separation becomes more efficient, moving away from research-only applications. The denominator value also changes depending on the desired isotope being higher for heavier isotopes. For each isotope, this value can be calculated to determine the energy cost to operate per atom (kWh/recycled atom).
These two non-dimensional terms (recycling purity and effectiveness) provide a theoretical limit and standard, respectively, for evaluating any or all proposed and active recycling processes. The greatest benefit of this analysis is that all recycling processes can be directly compared and efforts to achieve a recycling purity of one and a recycling effectiveness of zero can be emphasized. Following this thought experiment and subsequent non-dimensional analysis, one might ask, is it even possible to perform electromagnetic isotope separation at an industrial scale? In the following section, we will show that it is and has been achieved for the heaviest, and thus hardest to isotopically separate, naturally forming element on earth, uranium.
4. Experimental Validation
Indeed, isotopic separation has a rich history dating to the development of the field of nuclear science around the turn of the 20th century with the discovery of the nucleus, the neutron, radioactive decay, and nuclear fission. On a fundamental level, elements are distinguished by their distinct numbers of protons, while isotopes are distinguished by their combined number of neutrons and protons. In sum, these numbers of protons and neutrons contribute to defining an element’s atomic number and atomic mass. Nuclear scientists, dating to the early 20th century, know the importance of isotopes in determining the decay of elements and the ability to fission. The field of nuclear engineering, ranging from energy to medicine, depends on the intrinsic, unique characteristics of isotopes. For example, the discovery of nuclear fission hinged on insights into the fissionability of uranium (U) and its isotopes [31,32].
The fissile nature of the U-235 isotope compared to other U isotopes garnered considerable attention during the Manhattan Project, prompting significant investment in isotope separation technologies and processes. The physical separation of U isotopes during the Manhattan Project pursued multiple routes, including centrifuge separation, electromagnetic separation, gaseous diffusion, and thermal diffusion techniques for uranium enrichment. Electromagnetic separation hinged on the research of E.L. Lawrence and the patenting of the calutron in 1932, a cyclotron that uses a magnetic field to separate charged particles based on their mass-to-charge ratio [33]. During the Manhattan Project, calutrons and electromagnetic isotope separation were widely used at the Y-12 plant at the Alpha I racetrack to enrich U, shown in Figure 3, ultimately producing 43 kg of >85 at.% enriched U-235 [34]. Since the Manhattan Project, calutrons have been used to separate about 250 isotopes of 50 elements, ranging from lithium to calcium and rhodium [35]. More recently, electromagnetic isotope separation for the production of enriched U was used in Iraq in the 1980s and 1990s [35]. Thus, electromagnetic separation of isotopes has a rich history and has been successful on a number of isotopes, primarily for nuclear applications. However, we propose that the same physics and methodologies can be applied to civilian uses, in particular the separation of elements and isotopes for the recycling of materials, including currently difficult-to-recycle materials.

Figure 3.
Picture of the Alpha Calutron track at the Y-12 Plant in Oak Ridge, TN, USA, that was used in part to perform U isotope separation for the Manhattan Project [35].
There are a few areas in civilian applications where electromagnetic isotope separation is utilized. One of the more mature applications of this technique is Accelerator Mass Spectroscopy (AMS). This characterization technique, a subset of which is sometimes called carbon dating, is applied to everything from archeological artifacts to works of art to determine the age of the sample [36]. Another civilian use of electromagnetic separation is the production of medical isotopes for a range of clinical applications [37]. Although both these applications have an important impact, they work at sample mass measured in the g or mg scale. To our knowledge, no electromagnetic isotope separation has been performed on the ton or even kg scale.
5. Future Direction
This perspective has two potential impacts on the direction of the recycling field. First, it could provide a rubric and methodology to evaluate and compare any two recycling processes independent of the mechanisms used for the process, rapidly comparing and contrasting the mass and energy efficiency of any recycling process through the non-dimensional terms of recycle purity and recycling effectiveness, respectively. The thought experiment created a boundary associated with physical recycling down to the atomistic scale, thus permitting such comparison. Energy efficiency and global economics will drive how close we get to the theoretical potential set here via the isotopic electromagnetic separation approach. This energy-intensive approach might find applications in smaller specialty fields with the potential for great profits and impact, extreme difficulty in other separation processes, or both. The following are a few potential examples of how isotopic electromagnetic separation could be incorporated. This separation technique could facilitate the recycling of stored nuclear waste for medical isotopes that are essential for several lifesaving applications. It could also be considered for increasing the isotopic purity of components ranging from Focused Ion Beam (FIB) sources through metal components for future fusion energy systems being developed. This would enhance the efficiency of FIB research and processing, as well as minimize the radioactive cooling period of nuclear fusion reactor materials at the end of the component lifetime [38]. This manuscript has focused on electromagnetic separation down to the isotopic level. This separation can be performed at the molecular scale using the same process which might be a last resort in the chemical industry for the separation of gasses or liquids. In addition, isotopic electromagnetic separation incorporated as part of a larger clean landfill mining effort would have the added benefit of decreasing the environmental and health impact of landfills throughout the world [39,40]. Even if implemented, this technique complements and does not replace any of the recycling techniques already operating at an industrial scale or those currently developed across the world. If electromagnetic isotope separation was implemented at scale, even as a last resort for separation, it would provide an excellent opportunity for a nearly, sans helium, closed-loop materials society.
6. Conclusions
As the resource needs of the world continue to grow, so too does the need to invest in the recycling and reuse of resources and materials. Earth is a planet with finite material resources, yet we predominately continue to operate on a once-through cycle of use of these materials, which is an unsustainable model. The recycling of materials has advanced over the past decades, although it is highly variable and influenced by material composition, form, and recycling processes. From this perspective, we propose a thought experiment for the recycling of materials (any form and any composition) into their constituent isotopes using electromagnetic isotope separation and discuss the subsequent insights gained from the perspective.
The current limit of recycling and upcycling is the economic cost of separation, not the actual physical separation of materials, which has been shown to be possible via electromagnetic isotope separation for decades. However, this technique has, to date, only been applied to nuclear isotopes, yet clearly offers the potential for the recycling of materials, limited only by the available energy supply. Despite the potential of electromagnetic isotope recycling, we must recognize that other cheaper, more scalable recycling methodologies should also be pursued for rapid deployment and maximal impact. Nonetheless, this perspective shows that electromagnetic isotope recycling could be used for isotopically pure up-cycling or recycling of difficult-to-recycle systems. Whatever the potential future applications of this method of recycling, its adoption and use would require, just as with its first use over eight decades ago in the Manhattan Project, no new science and just an infrastructure and monetary investment.
Author Contributions
Conceptualization, K.H.; methodology, E.L. and K.H.; formal analysis, E.L. and K.H.; investigation, E.L. and KH.; data curation, E.L. and K.H.; writing—original draft preparation, E.L. and K.H.; writing—review and editing, E.L. and K.H.; visualization, E.L and K.H.; supervision, K.H.; project administration, E.L. and K.H.; funding acquisition, E.L. and K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed, in part, at the Center for Integrated Nanotechnologies, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Los Alamos National Laboratory (Contract 89233218CNA000001) and Sandia National Laboratories (Contract DE-NA-0003525).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to thank Dan Buller, Steven Frankowski, and Miguel L. Crespillo for useful discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Good, D.H.; Reuveny, R. On the Collapse of Historical Civilizations. Am. J. Agric. Econ. 2009, 91, 863–879. [Google Scholar] [CrossRef]
- Barles, S. History of Waste Management and the Social and Cultural Representations of Waste. In The Basic Environmental History; Springer International Publishing: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- “National Overview: Facts and Figures on Materials, Wastes and Recycling” United States Environmental Protection Agency, Data as of November 2023. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 12 August 2024).
- National Academies of Sciences, Engineering, and Medicine. Recycled Plastics in Infrastructure: Current Practices, Understanding, and Opportunities; The National Academies Press: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 377, 690–695. [Google Scholar] [CrossRef]
- Chen, W.-Q. Recycling Rates of Aluminum in the United States. J. Ind. Ecol. 2013, 17, 926–938. [Google Scholar] [CrossRef]
- Cullen, J.M.; Allwood, J.M. Mapping the Global Flow of Aluminum: From Liquid Aluminum to End-Use Goods. Environ. Sci. Technol. 2013, 47, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Apelian, D. Automotive Aluminum Recycling at End of Life: A Grave-to-Gate Analysis; Center for Resource Recovery and Recycling, Worcester Polytechnic Institute: Worcester, MA, USA, 2022. [Google Scholar]
- U.S. Geological Survey, Department of the Interior. 2022 Final List of Critical Minerals. Fed. Regist. 2022, 87, 10381–10382. [Google Scholar]
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, S.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Chapter 255—Recycling of Rare Earths from Scrap. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2013; Volume 43, pp. 159–211. [Google Scholar] [CrossRef]
- Ayres, R.U.; Mendez, G.V.; Peiro, L.T. Chapter 4—Recycling Rare Metals. In Handbook of Recycling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 27–38. [Google Scholar] [CrossRef]
- Swain, B.; Kang, L.; Mishra, C.; Ahn, J.W.; Hong, H.S. Materials flow analysis of neodymium, status of rare earth metal in the Republic of Korea. Waste Manag. 2015, 45, 351–360. [Google Scholar] [CrossRef]
- Graedel, T.E.; Allwood, J.; Birat, J.-P.; Buchert, M.; Hageluken, C.; Reck, B.K.; Sibley, S.F.; Sonnemann, G. What Do We Know About Metal Recycling Rates? J. Ind. Ecol. 2011, 15, 355–366. [Google Scholar] [CrossRef]
- Reuter, M.; van Schaik, A. Opportunities and limits of recycling: A dynamic-model-based analysis. MRS Bull. 2012, 37, 339–347. [Google Scholar] [CrossRef]
- Reuter, M.A. Limits of Design for Recycling and “Sustainability”: A Review. Waste Biomass Valorization 2011, 2, 183–208. [Google Scholar] [CrossRef]
- Craig, P.C. Energy limits on recycling. Ecol. Econ. 2001, 36, 373–384. [Google Scholar] [CrossRef]
- Siddhantakar, A.; Santillan-Saldivar, J.; Kippes, T.; Sonnemann, G.; Reller, A.; Young, S.B. Helium resource global supply and demand: Geopolitical supply risk analysis. Resour. Conserv. Recycl. 2023, 193, 106935. [Google Scholar] [CrossRef]
- Kuzmin, Y.V. Long-distance obsidian transport in prehistoric Northeast Asia. Bull. Indo-Pac. Prehistory Assoc. 2012, 32, 1–5. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-ion battery recycling—Overview of techniques and trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Seeverens, H.J.J. The economics of the molybdenum-99/technetium-99m supply chain. Dutch J. Nucl. Med. 2010, 32, 604–608. [Google Scholar]
- Paul, S.K.; Chowdhury, P. Strategies for managing the impacts of disruptions during COVID-19: An example of toilet paper. Glob. J. Flex. Syst. Manag. 2020, 21, 283–293. [Google Scholar] [CrossRef]
- Boulos, M.I.; Fauchais, P.L.; Pfender, E. The plasma state. In Handbook of Thermal Plasmas; Springer International Publishing: Cham, Switzerland, 2023; pp. 3–55. [Google Scholar]
- Lorentz, H.A. Electromagnetic Phenomena in a System Moving with any Velocity Smaller than That of Light. In Collected Papers; Springer: Dordrecht, The Netherlands, 1937; pp. 172–197. [Google Scholar] [CrossRef]
- “U.S. Trade in Goods by Selected Countries and Areas: 2024 in Millions of Dollars”. U.S. International Trade in Goods and Services (March 2024) U.S. Department of Commerce and U.S. Census Bureau Released May 2024, CB 24–70, BEA 24–18. Available online: https://www.bea.gov/news/2024/us-international-trade-goods-and-services-march-2024 (accessed on 25 August 2024).
- Feynman, R. There’s plenty of room at the bottom. Reson. J. Sci. Educ. 2011, 16, 890–905. [Google Scholar] [CrossRef]
- Zohuri, B.; McDaniel, P. Thermodynamics in Nuclear Power Plant Systems; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Collins, M.W.; Stasiek, J.A.; Mikielewicz, J. Engineering Thermodynamics and the Carnot Cycle. WIT Trans. State-Art Sci. Eng. 2015, 89, 165–223. [Google Scholar]
- Rawat, K.; Sharma, N.; Singh, V.K. X-ray Fluorescence and Comparison with Other Analytical Methods (AAS, ICP-AES, LA-ICP-MS, IC, LIBS, SEM-EDS, and XRD). In X-Ray Fluorescence in Biological Sciences: Principles, Instrumentation, and Applications; Wiley: Abingdon, UK, 2022; pp. 1–20. [Google Scholar] [CrossRef]
- Smith, K.S.; Briggs, P.H.; Campbell, D.L.; Castle, C.J.; Desborough, G.A.; Eppinger, R.G., III; Fitterman, D.V.; Hageman, P.L.; Leinz, R.W.; Meeker, G.P.; et al. Tools for the rapid screening and characterization of historical metal-mining waste dumps. In Proceedings of the 2000 Billings Land Reclamation Symposium, Billings, MT, USA, 20–24 March 2000; pp. 20–24. [Google Scholar]
- Mietner, L.; Frisch, O.R. Disintegration of Uranium by Neutrons: A New Type of Nuclear Reaction. Nature 1939, 143, 239–240. [Google Scholar] [CrossRef]
- Nier, A.O.; Booth, E.T.; Dunning, J.R.; Grosse, A.V. Nuclear Fission of separated Uranium Isotopes. Phys. Rev. 1940, 57, 546. [Google Scholar] [CrossRef]
- Lawrence, E.O. Method and Apparatus for the Acceleration of Ions. U.S. Patent US1948384A, 20 February 1934. [Google Scholar]
- Yergey, A.L.; Yergey, A.K. Preparative Scale Mass Spectrometry: A Brief History of the Calutron. Am. Soc. Mass Spectrom. 1997, 8, 943–953. [Google Scholar] [CrossRef]
- Laughter, M.D. Profile of World Uranium Enrichment Programs—2009; ORNL/TM-2009/110; Global Nuclear Security Technology Division: Oak Ridge, TN, USA, 2009. [Google Scholar]
- Harris, D.R. The impact on archaeology of radiocarbon dating by accelerator mass spectrometry. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 323, 23–43. [Google Scholar] [CrossRef]
- Beyer, G.J.; Ruth, T.J. The role of electromagnetic separators in the production of radiotracers for bio-medical research and nuclear medical application. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 204, 694–700. [Google Scholar] [CrossRef]
- Anderton, M.D.; Lloyd, M.J.; Davis, T.P. Suppression of rhenium and osmium production in tungsten by selective isotopic enrichment. Fusion Eng. Des. 2023, 197, 114073. [Google Scholar] [CrossRef]
- Vrijheid, M. Health effects of residence near hazardous waste landfill sites: A review of epidemiologic literature. Environ. Health Perspect. 2000, 108, 101–112. [Google Scholar] [CrossRef]
- Vaverková, M.D. Landfill impacts on the environment. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).