A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags
Abstract
:1. Introduction
2. Research Section
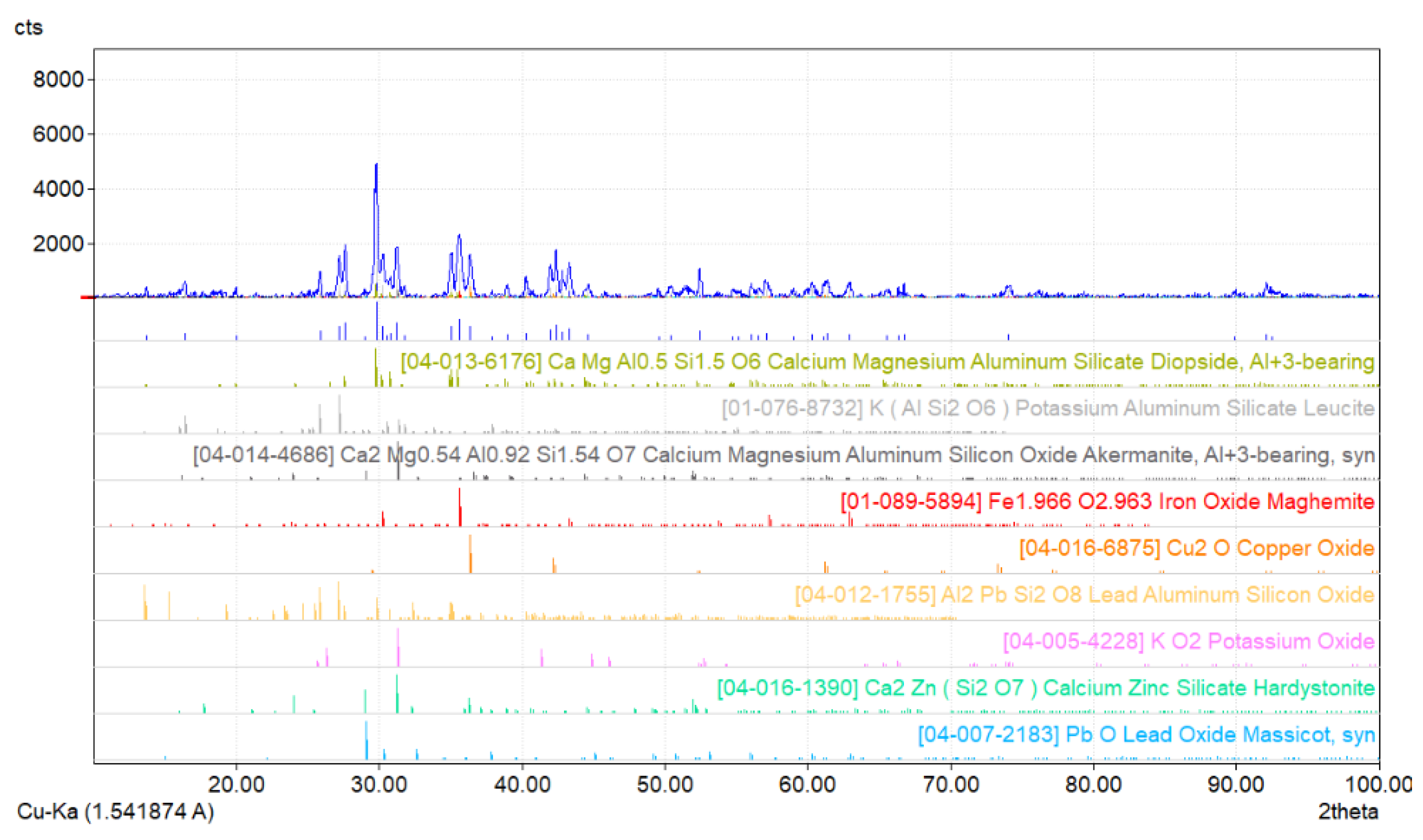
2.1. Research Materials
- Maghemite Fe1.966O2.963 (tetragonal structure).
- Cuprite Cu2O (regular structure).
- Al2PbSi2O8 (monoclinic structure).
- Massicot PbO (rhombic structure).
- Diopside CaMgAl0.5Si1.5O6 (monoclinic structure).
- Leucite K(AlSi2O6) (tetragonal structure).
- Akermanite Ca2Mg0.54Al0.92Si1.54O7 (tetragonal structure).
- Potassium oxide KO2 (tetragonal structure).
- Hardystonite Ca2Zn(Si2O7) (tetragonal structure).
2.2. Research Equipment and Methods
3. Results
- (a)
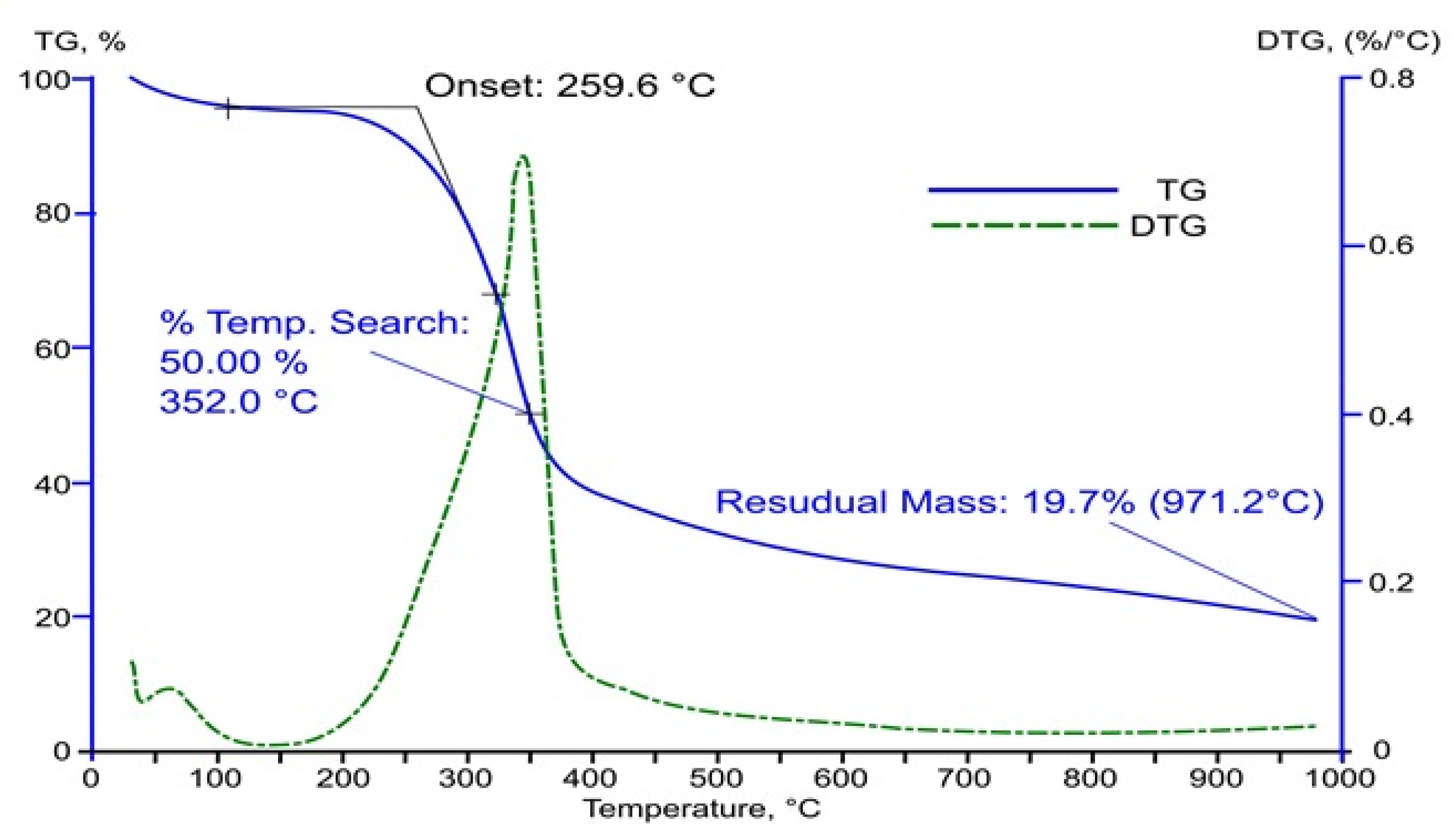
- The temperature of the theoretical start of thermal decomposition.
- (b)
- The temperature of the 50% sample weight loss.
- (c)
- The weight of the solid sample residue.
- (d)
- The temperatures at which the particular peaks of the derivative of the weight loss over time are observed.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.enerdata.net/publications/executive-briefing/carbon-price-projections-eu-ets.html (accessed on 1 July 2024).
- Radomiak, H.; Bala-Litwiniak, A.; Zajemska, M.; Musiał, D. Numerical Prediction of the Chemical Composition of Gas Products at Biomass Combustion and Co-Combustion in a Domestic Boiler. E3S Web Conf. 2017, 14, 02043. [Google Scholar] [CrossRef]
- Niedziolk, I.; Szpryngiel, M. Possibilities of Using Biomass for Energy Purposes. Agric. Eng. 2014, 1, 155–164. [Google Scholar] [CrossRef]
- Bala-Litwiniak, A.; Radomiak, H. Possibility of the Utilization of Waste Glycerol as an Addition to Wood Pellets. Waste Biomass Valor. 2019, 10, 2193–2199. [Google Scholar] [CrossRef]
- Roy, M.M.; Corscadden, K.W. An Experimental Study of Combustion and Emissions of Biomass Briquettes in a Domestic Wood Stove. Appl. Energy 2012, 99, 206–212. [Google Scholar] [CrossRef]
- Kowalik, P. Perspektywy Peletyzacji Biomasy w Polsce. Czysta Energ. 2002, 10, 14–15. [Google Scholar]
- Obernberger, I.; Thek, G. Physical Characterisation and Chemical Composition of Densified Biomass Fuels with Regard to Their Combustion Behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Benny, M.; Suraj, P.; Arun, P.; Muraleedharan, C. Agglomeration Behavior of Lignocellulosic Biomasses in Fluidized Bed Gasification: A Comprehensive Review. J. Therm. Anal. Calorim. 2023, 148, 9289–9308. [Google Scholar] [CrossRef]
- Miao, Z.; Jiang, E.; Hu, Z. Review of Agglomeration in Biomass Chemical Looping Technology. Fuel 2022, 309, 122199. [Google Scholar] [CrossRef]
- Tijjani Usman, I.M.; Ho, Y.-C.; Baloo, L.; Lam, M.-K.; Sujarwo, W. A Comprehensive Review on the Advances of Bioproducts from Biomass towards Meeting Net Zero Carbon Emissions (NZCE). Bioresour. Technol. 2022, 366, 128167. [Google Scholar] [CrossRef]
- Cherubini, F.; Peters, G.P.; Berntsen, T.; Strømman, A.H.; Hertwich, E. CO2 Emissions from Biomass Combustion for Bioenergy: Atmospheric Decay and Contribution to Global Warming: Global Warming potential of CO2 from bioenergy. GCB Bioenergy 2011, 3, 413–426. [Google Scholar] [CrossRef]
- Pulles, T.; Gillenwater, M.; Radunsky, K. CO2 Emissions from Biomass Combustion Accounting of CO2 Emissions from Biomass under the UNFCCC. Carbon Manag. 2022, 13, 181–189. [Google Scholar] [CrossRef]
- Łabaj, J.; Blacha, L.; Jodkowski, M.; Smalcerz, A.; Fröhlichová, M.; Findorak, R. The Use of Waste, Fine-Grained Carbonaceous Material in the Process of Copper Slag Reduction. J. Clean. Prod. 2021, 288, 125640. [Google Scholar] [CrossRef]
- Pomianek, T.; Adamkiewicz, L.; Bratek, S. Analiza Termodynamiczna Procesu Odmiedziowania Żużla Zawiesinowego. Prace Inst. Met. Nieżelaznych 1980, 9, 5–11. [Google Scholar]
- Smieszek, Z.; Czernecki, J.; Botor, J.; Sobierajski, S. Analiza Procesu Redukcji Tlenku Miedziawego z Żużla Zawiesinowego. Pr. Inst. Met. Nieżelaznych 1982, 10, 5–12. [Google Scholar]
- Kucharski, M. Effect of Thermodynamic and Physical Properties of Flash Smelting Slags on Copper Losses during Slags Cleaning in an Electric Furnace. Arch. Hut. 1987, 32, 307–323. [Google Scholar]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Perea-Moreno, M.-A.; Manzano-Agugliaro, F.; Hernandez-Escobedo, Q.; Perea-Moreno, A.-J. Peanut Shell for Energy: Properties and Its Potential to Respect the Environment. Sustainability 2018, 10, 3254. [Google Scholar] [CrossRef]
- Duc, P.A.; Dharanipriya, P.; Velmurugan, B.K.; Shanmugavadivu, M. Groundnut Shell -a Beneficial Bio-Waste. Biocatal. Agric. Biotechnol. 2019, 20, 101206. [Google Scholar] [CrossRef]
- Omidi, J. Application of Peanut Shells Composts in Replacement with Peat on Growth Indices and Physical and Chemical Properties of Violet Growth Media (Viola spp.) in Outdoor. Front. Environ. Microbiol. 2017, 3, 68. [Google Scholar] [CrossRef]
- Hill, G.M. Peanut By-Products Fed to Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 295–315. [Google Scholar] [CrossRef]
- Collins, J.L.; Kalantari, S.M.; Post, A.R. Peanut Hull Flour as Dietary Fiber in Wheat Bread. J. Food Sci. 1982, 47, 1899–1902. [Google Scholar] [CrossRef]
- Gajula, C.; Chandel, A.K.; Konakalla, R.; Rudravaram, R.; Pogaku, R.; Mangamoori, L.N. Fermentation of Groundnut Shell Enzymatic Hydrolysate for Fuel Ethanol Production by Free and Sorghum Stalks Immobilized Cells of Pichia Stipitis NCIM 3498. Int. J. Chem. React. Eng. 2011, 9. [Google Scholar] [CrossRef]
- Rabah, A.B.; Oyeleke, S.B.; Manga, S.B.; Hassan, L.G. Microbial Pretreatment of Rice Husk and Groundnut Shell for Bioethanol Production. Int. Res. J. Microbiol. 2011, 2, 253–258. [Google Scholar]
- Sheelendra, M.B. Shilpa Bioethanol Production from Economical Agro Waste (Groundnut Shell) in SSF Mode. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 1210–1218. [Google Scholar]
- Akpan, U.G.; Kovo, A.S.; Abdullahi, M.; Ijah, J.J. The Production of Ethanol from Maize Cobs and Groundnut Shells. AU J.T. 2005, 9, 106–110. [Google Scholar]
- de Guzman, N.M.; Gabriel, M.J.J.; Pillo, E.V. Extraction of Ethyl Alcohol from the Shells of Arachis Hypogaea Linn. Asia Pacific High. Educ. Res. J. 2015, 2. [Google Scholar] [CrossRef]
- Udeh, B. Bio-Waste Transesterification Alternative for Biodiesel Production: A Combined Manipulation of Lipase Enzyme Action and Lignocellulosic Fermented Ethanol. Asian J. Biotechnol. Bioresour. Technol. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Nyachaka, C.J.; Yawas, D.S.; Pam, G.Y. Production and Performance Evaluation of Bioethanol Fuel from Groundnuts Shell Waste. Am. J. Eng. Res. 2013, 2, 303–312. [Google Scholar]
- William, J.A.; Vinoth, A.; Xavier, J.; Chozhavendhan, S. Simultaneous Saccharification and Fermentation of Rice Bran and Ground Nut Shell for the Production of Ethanol. J. Chem. Pharm. Sci. 2016, 9, 202–205. [Google Scholar]
- Fei Ling, P.; Tan, C.-Y.; Dang, W.-H.; Kumaran, P. Peanut Shells Derived Solid Acid Catalyst for Biodiesel Production. Asian Res. Publ. Netw. 2015, 10, 7704–7706. [Google Scholar]
- Dai, Y.-M.; Chen, K.-T.; Wang, Y.-J.; Chen, C.-C. Application of Peanut Husk Ash as a Low-Cost Solid Catalyst for Biodiesel Production. Int. J. Chem. Eng. Appl. 2014, 5, 276–280. [Google Scholar] [CrossRef]
- Venkatachalam, S.G.; Jayamanoharan, J.; Krishnaswamy, S. Biological Pretreatment of Six Lignocellulosic Wastes for Bioethanol Production. Int. J. Sci. Res. 2014, 3, 1860–1864. [Google Scholar]
- Gupta, A.D. Extraction of cellulose and biofuel production from groundnut shells and its application to increase crop yield. World J. Pharm. Pharm. Sci. 2017, 6, 1820–1831. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the Volarization of Lignocellulosic Materials by Biotecnnology: An Overview. Bioresources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Jones, G.; Gan, Y.; Aglan, H.; McConnell, R.; Smith, R.; Trotman, A.; Lu, J. Development and Characterization of Paper Products from Dried Sweetpotato Stems, Peanut Shells and Soybean Pods. SAE Tech. Pap. 1998, 981563. [Google Scholar] [CrossRef]
- Upendra, K.; Akshay, T.; Vedika, H.; Dhanashree, K.; Prathamesh, S.; Vivek, N. Production of Paper from Groundnuts Shell. Int. J. Adv. Res. Sci. Eng. 2018, 7, 288–293. [Google Scholar]
- Zajemska, M.; Musial, D. Energy Use of Biomass from Agricultural Production in Co-Combustion Process. Probl. Agric. Eng. 2013, 4, 107–118. [Google Scholar]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A Review on Biomass as a Fuel for Boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Adeosun, S.; Taiwo, O.; Akpan, E.; Gbenebor, O.; Gbagba, S.; Olaleye, S. Mechanical Characteristics of Groundnut Shell Particle Reinforced Polylactide Nano Fibre. Matéria 2016, 21, 482–491. [Google Scholar] [CrossRef]
- Bobet, O.; Nassio, S.; Seynou, M.; Remy, B.; Zerbo, L.; Sanou, I.; Sawadogo, M.; Millogo, Y.; Gilles, E. Characterization of Peanut Shells for Their Valorization in Earth Brick. J. Miner. Mater. Charact. Eng. 2020, 8, 301–315. [Google Scholar] [CrossRef]
- Batalla, L.; Nuñez, A.J.; Marcovich, N.E. Particleboards from Peanut-shell Flour. J. Appl. Polym. Sci. 2005, 97, 916–923. [Google Scholar] [CrossRef]
- Mahmoud, H.; Belel, Z.A.; Nwakaire, C. Groundnut Shell Ash as a Partial Replacement of Cement in Sandcrete Blocks Production. Int. J. Dev. Sustain. 2012, 1, 1026–1032. [Google Scholar]
- Nwofor, T.C.; Sule, S. Stability of Groundnut Shell Ash (GSA)/Ordinary Portland Cement (OPC) Concrete in Nigeria. Adv. Appl. Sci. Res. 2012, 3, 2283–2287. [Google Scholar]
- EN ISO 1716:2018; Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). International Organization for Standarization: Geneva, Switzerland, 2018.
- Rzychoń, T.; Matuła, T.; Chmiela, B.; Łabaj, J.; Rogóż, K. Modifications of the Chemical Composition and Microstructure of Flash Smelting Copper Slags in the Process of Their Reduction. Metalurgija 2015, 54, 151–153. [Google Scholar]
- Siwiec, G.; Sozańska, M.; Blacha, L.; Smalcerz, A. Behaviour of Iron during Reduction of Slag Obtained from Copper Flash Smelting. Metalurgija 2015, 54, 113–115. [Google Scholar]
- Matula, T.; Labaj, J.; Nowacki, K.; Blacha, L.; Kortyka, L.; Mycka, L.; Madej, P.; Jaworek, L.; Wojtal, T. Application of Spent Coffee Grounds (SCGs) as a Fuel and Alternative Reducer of Slags from the Copper Industry. Energies 2023, 16, 2415. [Google Scholar] [CrossRef]
- Kumar, M.; Rai, D.; Bhardwaj, G.; Upadhyay, S.N.; Mishra, P.K. Pyrolysis of Peanut Shell: Kinetic Analysis and Optimization of Thermal Degradation Process. Ind. Crops Prod. 2021, 174, 114128. [Google Scholar] [CrossRef]
- Varma, A.K.; Singh, S.; Rathore, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Investigation of Kinetic and Thermodynamic Parameters for Pyrolysis of Peanut Shell Using Thermogravimetric Analysis. Biomass Conv. Bioref. 2022, 12, 4877–4888. [Google Scholar] [CrossRef]
- Ptak, S.; Matuła, T.; Blacha, L.; Łabaj, J.; Smalcerz, A.; Półka, M. Reduction of Metallurgical Slags Using Sunflower Pellets. Metall Mater. Trans. B 2024, 55, 1690–1699. [Google Scholar] [CrossRef]
- Busolic, D.; Parada, F.; Parra, R.; Sanchez, M.; Palacios, J.; Hino, M. Recovery of Iron from Copper Flash Smelting Slags. Miner. Process. Extr. Metall. 2011, 120, 32–36. [Google Scholar] [CrossRef]


| Component | Carbon | Oxygen | Hydrogen | Nitrogen | Sulphur | Ash |
|---|---|---|---|---|---|---|
| Fraction, wt% | 46.41–46.43 | 39.32–44.22 | 6.59–6.62 | 0.49–0.51 | 0.53–0.55 | 4.11–4.41 |
| Component | Carbon | Oxygen | Hydrogen | Nitrogen | Heat of Combustion, kJ/kg |
|---|---|---|---|---|---|
| Component content, wt% | 47.2 | 40.0 | 6.17 | 3.11 | 18,005 |
| Slag Component | Cu | Pb | Fe | SiO2 | CaO | Al2O3 |
|---|---|---|---|---|---|---|
| Component content, wt% | 10.3 | 2.25 | 11.1 | 34.5 | 14.1 | 8.3 |
| No | Atmosphere | Initial Weight Loss Temperature, °C | 50% Weight Loss Temperature, °C | Sample Residue, % |
|---|---|---|---|---|
| 1 | Nitrogen | 269.46 | 352 | 19.67 |
| 2 | Air | 259.6 | 313.4 | 1.20 |
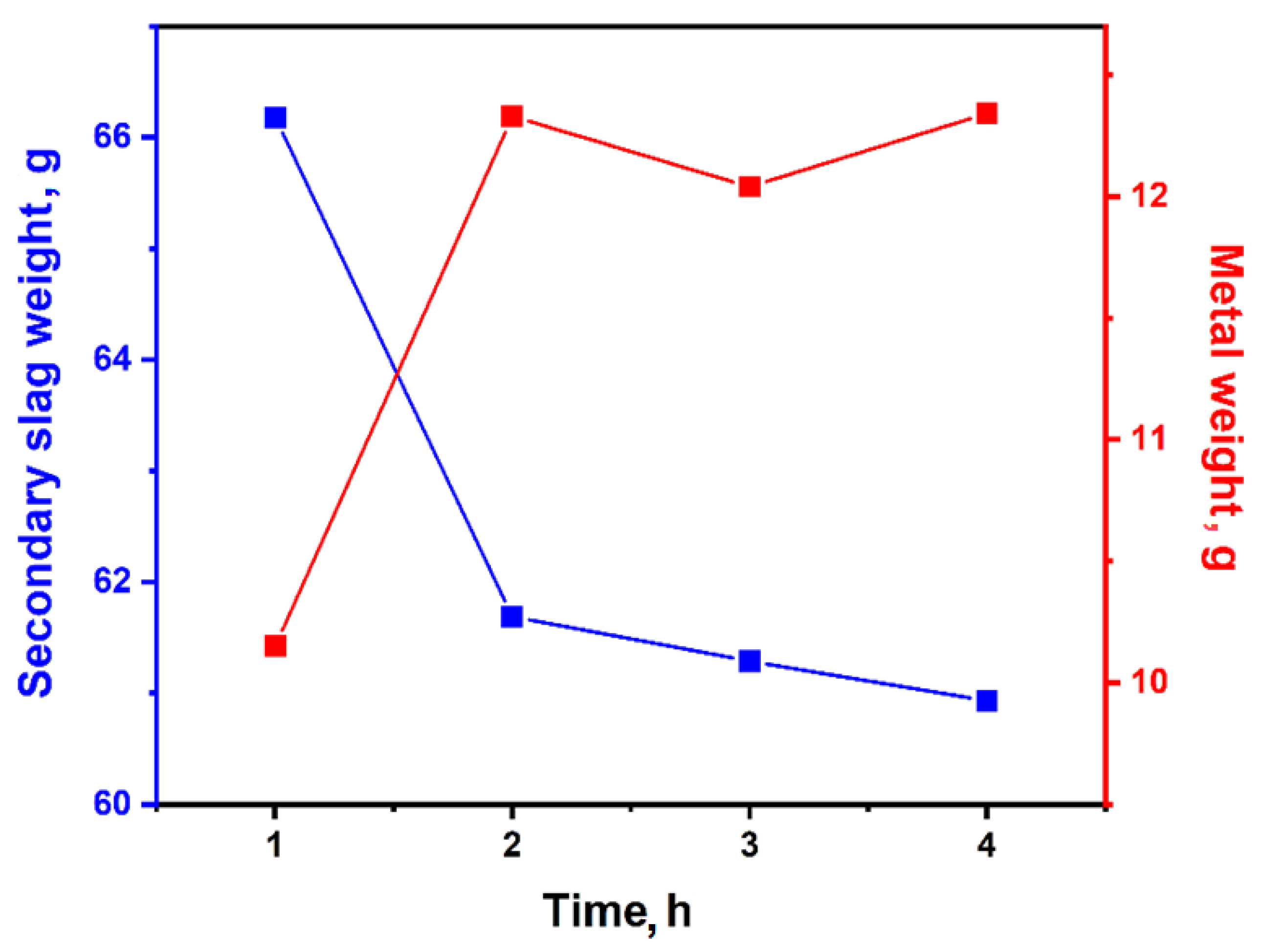
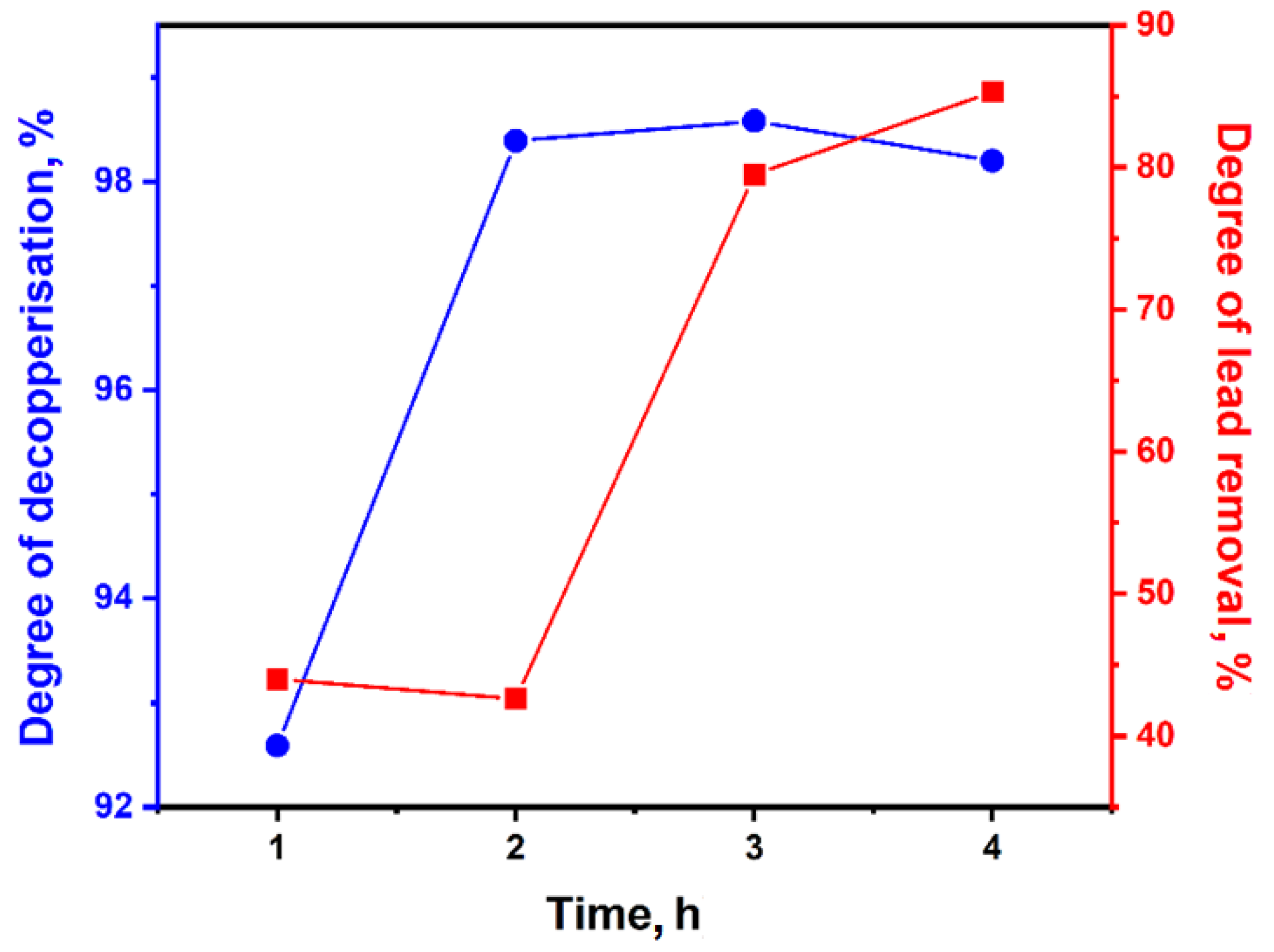
| No | Temp., °C | Initial Slag Weight, g | Time, h | Reducer Weight, g | Secondary Slag Weight, g | Metal Weight, g | Degree of Decopperisation, % | Degree of Lead Removal, % |
|---|---|---|---|---|---|---|---|---|
| 1 | 1300 | 80.0 | 1 | 18.0 | 66.18 | 10.15 | 92.59 | 44.0 |
| 2 | 2 | 61.69 | 12.33 | 98.39 | 42.6 | |||
| 3 | 3 | 61.29 | 12.04 | 98.58 | 79.5 | |||
| 4 | 4 | 60.93 | 12.34 | 98.20 | 85.3 |
| No | Time, h | Contents of Metals in the Secondary Slag, wt% | ||
|---|---|---|---|---|
| Cu | Pb | Fe | ||
| 1 | 1 | 0.78 | 1.25 | 14.38 |
| 2 | 2 | 0.17 | 1,29 | 15.07 |
| 3 | 3 | 0.15 | 0.46 | 16.36 |
| 4 | 4 | 0.19 | 0.33 | 17.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kortyka, L.; Labaj, J.; Ptak, S.; Smalcerz, A.; Blacha, L.; Mycka, L.; Matula, T.; Findorak, R. A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags. Sustainability 2024, 16, 9261. https://doi.org/10.3390/su16219261
Kortyka L, Labaj J, Ptak S, Smalcerz A, Blacha L, Mycka L, Matula T, Findorak R. A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags. Sustainability. 2024; 16(21):9261. https://doi.org/10.3390/su16219261
Chicago/Turabian StyleKortyka, Lukasz, Jerzy Labaj, Szymon Ptak, Albert Smalcerz, Leszek Blacha, Lukasz Mycka, Tomasz Matula, and Robert Findorak. 2024. "A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags" Sustainability 16, no. 21: 9261. https://doi.org/10.3390/su16219261
APA StyleKortyka, L., Labaj, J., Ptak, S., Smalcerz, A., Blacha, L., Mycka, L., Matula, T., & Findorak, R. (2024). A Study on the Potential for the Application of Peanut Shells as a Reducer in the Process of Metal Recovery from Metallurgical Slags. Sustainability, 16(21), 9261. https://doi.org/10.3390/su16219261









