Isolation and Identification of Multi-Traits PGPR for Sustainable Crop Productivity Under Salinity Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Rhizospheric Soil Samples
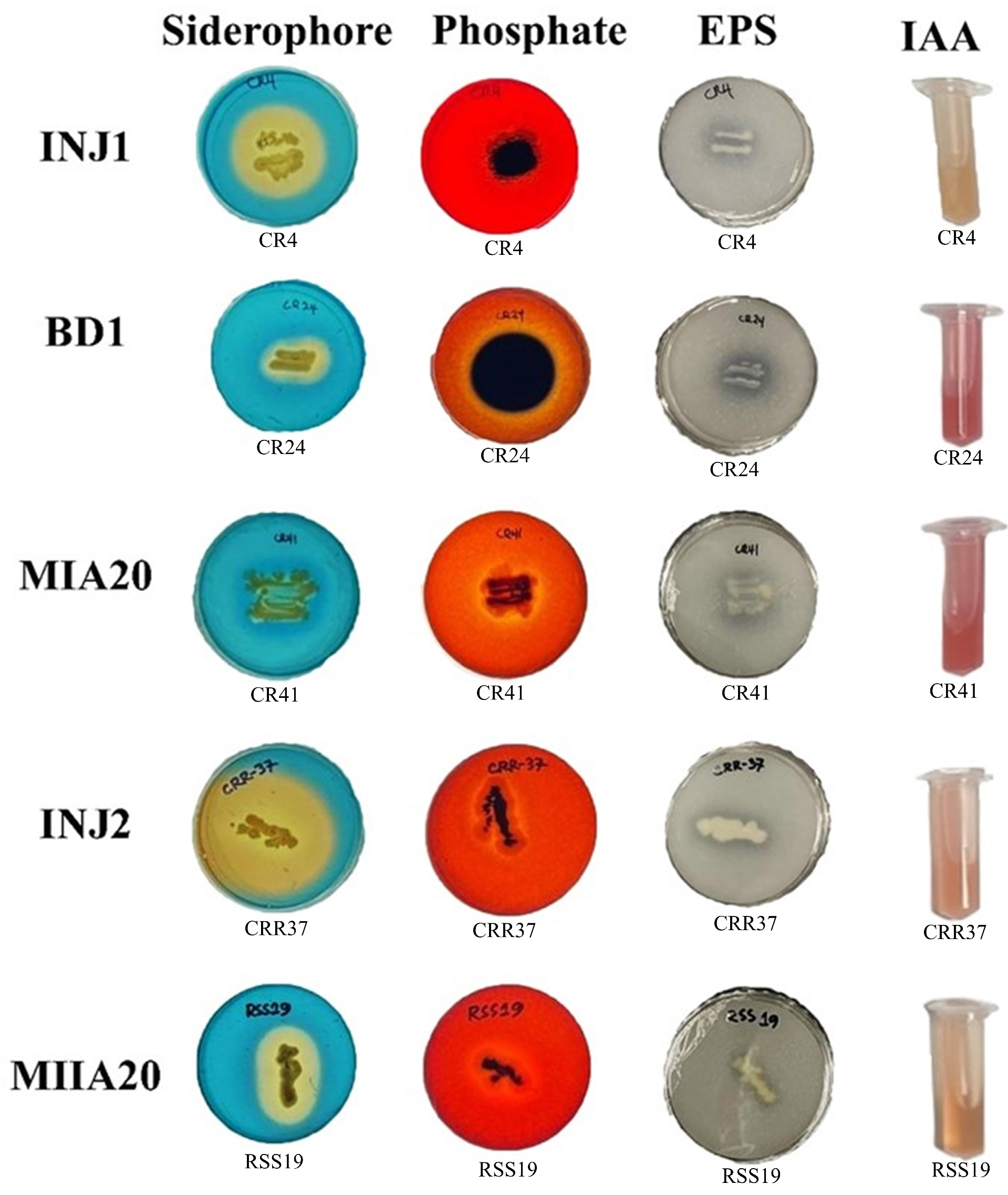
2.2. Qualitative Measurement of Exopolysaccharides, Siderophore, Indole-3-Acetic Acid (IAA), and Phosphate Solubilisation Produced in Isolates
2.3. Screening of Salt-Tolerant and Identification Using 16S rRNA Gene Sequence
2.4. In Vitro Quantification of Antioxidant Enzyme Activities in Bacterial Isolates
2.5. In Vitro Quantification of IAA and Salicylic Acid (SA) Produced by Isolates
2.6. In Vitro Quantification of Organic and Amino Acids Composition
2.7. Statistical Analysis
3. Results
3.1. Preliminary Recognition of PGPR by Means of PGP Characteristics
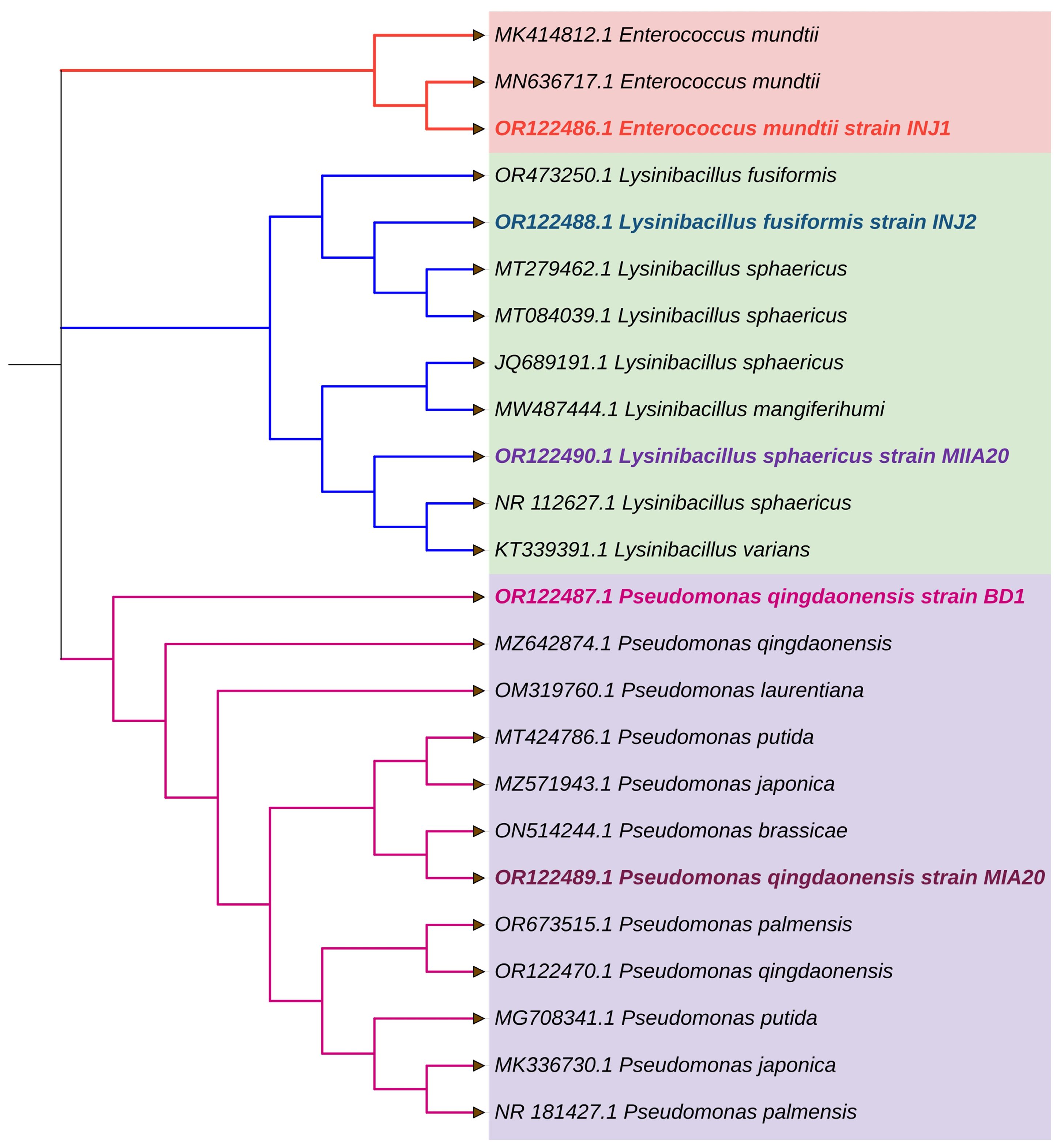
3.2. Molecular Identification of Bacterial Isolates
3.3. Measurement of Antioxidant Activity by Bacterial Isolates
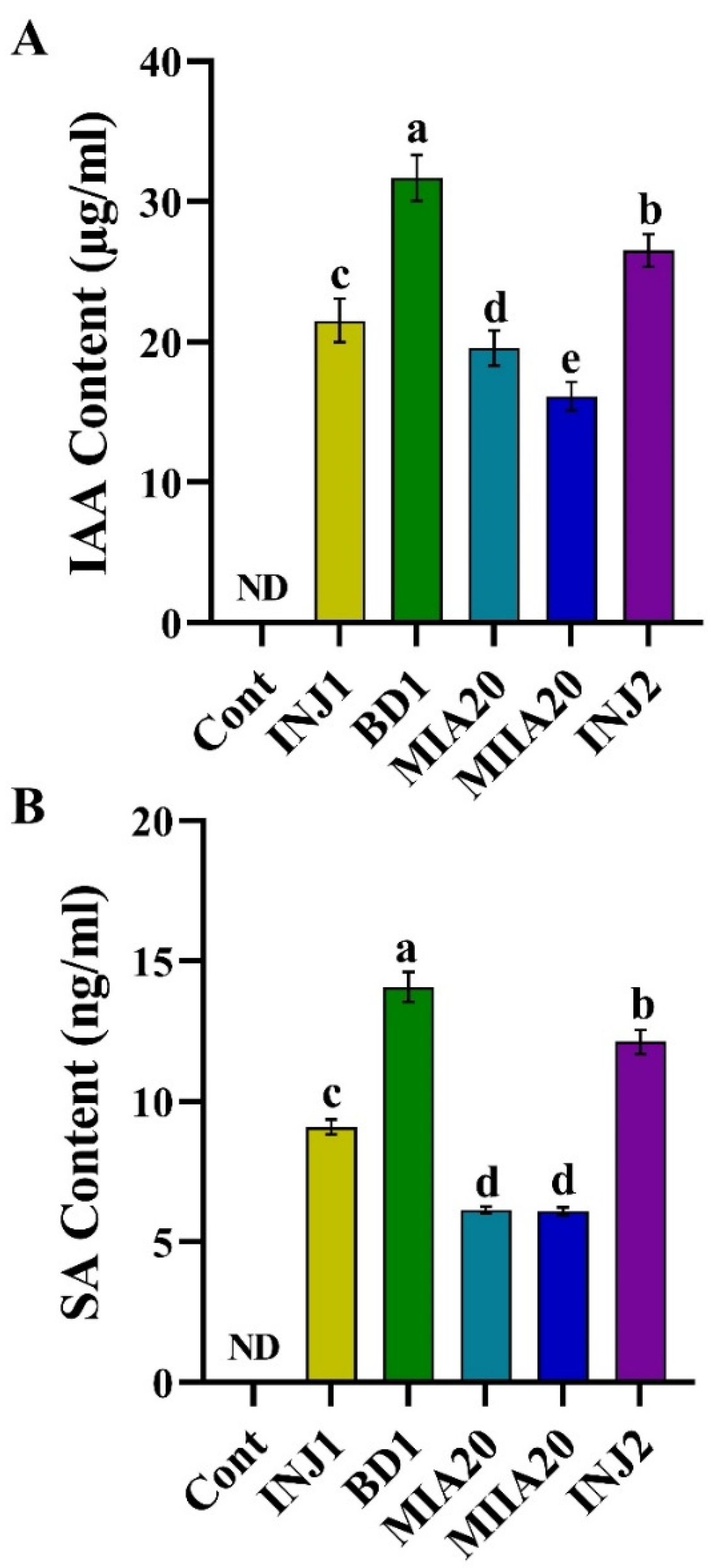
3.4. Determination of Phytohormone Produced by Bacterial Isolates
3.5. Quantification of Organic Acid Produced by Isolates
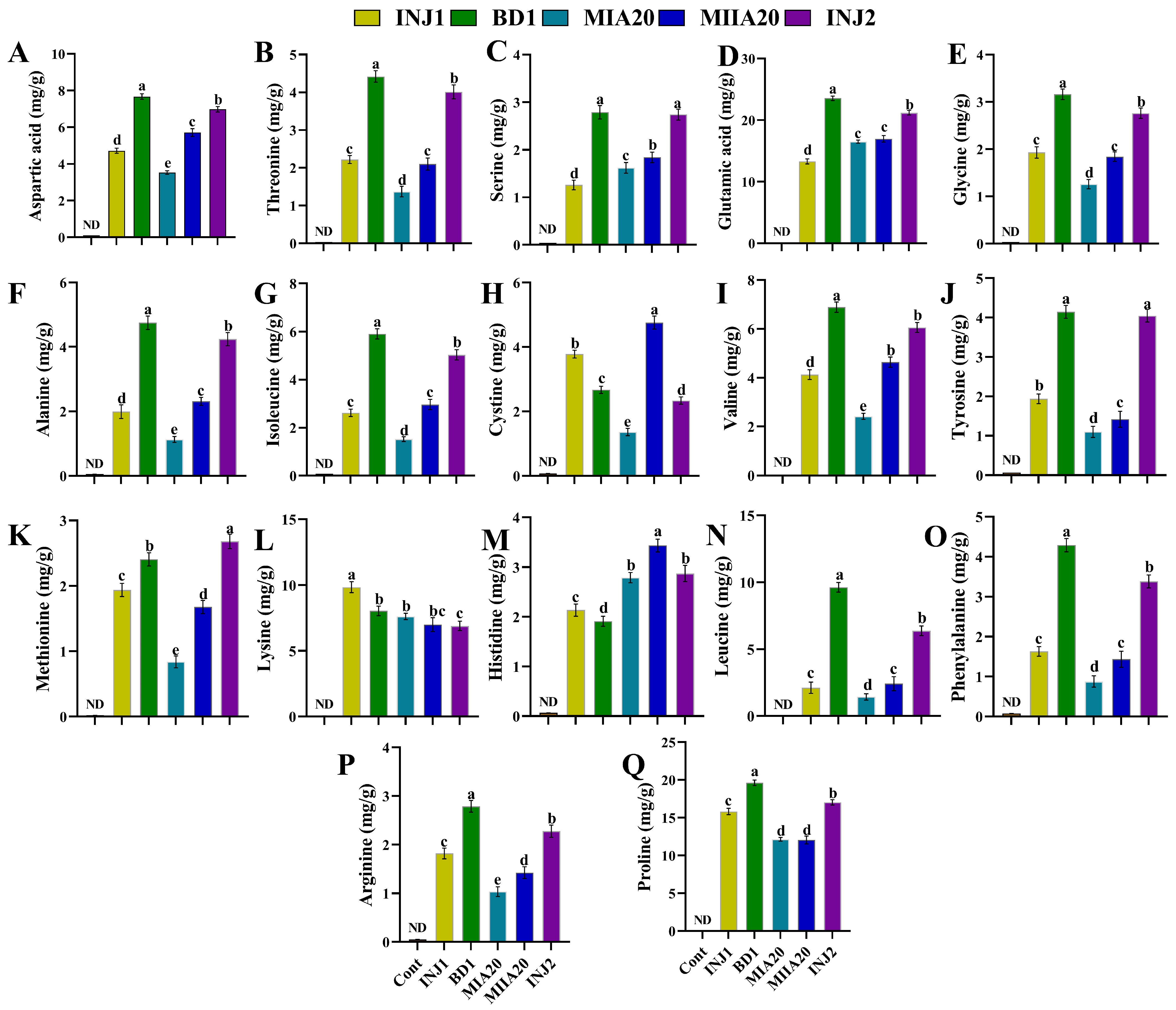
3.6. Determination of Amino Acid Produced by Bacterial Isolates
3.7. Screening of Salt Tolerance Assay
4. Discussion
5. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria With Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef]
- Kapadia, C.; Sayyed, R.Z.; El Enshasy, H.A.; Vaidya, H.; Sharma, D.; Patel, N.; Malek, R.A.; Syed, A.; Elgorban, A.M.; Ahmad, K.; et al. Halotolerant Microbial Consortia for Sustainable Mitigation of Salinity Stress, Growth Promotion, and Mineral Uptake in Tomato Plants and Soil Nutrient Enrichment. Sustainability 2021, 13, 8369. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Omer, A.M.; Badawy, A.A.; Osman, M.S.; Ragaey, M.M. Strategy of salt tolerance and interactive impact of Azotobacter chroococcum and/or Alcaligenes faecalis inoculation on canola (Brassica napus L.) plants grown in saline soil. Plants 2021, 10, 110. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt-Affected Soils; FAO: Rome, Italy, 2021. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Ji, Y.; Jiang, Z.; Wang, L. Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. S. Afr. J. Bot. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, V.; Prasad, S. Plant tolerance mechanism against salt stress: The nutrient management approach. Biochem. Pharmacol 2014, 3, e165. [Google Scholar] [CrossRef]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Pandey, S.; Kole, C. Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Front. Plant Sci. 2022, 12, 787292. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Bhat, J.A.; Devoto, A. The influence of endophytes on rice fitness under environmental stresses. Plant Mol. Biol. 2022, 109, 447–467. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Jalal, R.S. Use of plant growth-promoting bacteria to enhance salinity stress in soybean (Glycine max L.) plants. Saudi J. Biol. Sci. 2021, 28, 3823–3834. [Google Scholar] [CrossRef]
- Kang, S.-M.; Hoque, M.I.U.; Woo, J.-I.; Lee, I.-J. Mitigation of Salinity Stress on Soybean Seedlings Using Indole Acetic Acid-Producing Acinetobacter pittii YNA40. Agriculture 2023, 13, 1021. [Google Scholar] [CrossRef]
- Soldan, R.; Mapelli, F.; Crotti, E.; Schnell, S.; Daffonchio, D.; Marasco, R.; Fusi, M.; Borin, S.; Cardinale, M. Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol. Res. 2019, 223–225, 33–43. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Manasa, M.; Ravinder, P.; Gopalakrishnan, S.; Srinivas, V.; Sayyed, R.Z.; El Enshasy, H.A.; Yahayu, M.; Kee Zuan, A.T.; Kassem, H.S.; Hameeda, B. Co-Inoculation of Bacillus spp. for Growth Promotion and Iron Fortification in Sorghum. Sustainability 2021, 13, 12091. [Google Scholar] [CrossRef]
- Rani, M.; Weadge, J.T.; Jabaji, S. Isolation and Characterization of Biosurfactant-Producing Bacteria From Oil Well Batteries With Antimicrobial Activities Against Food-Borne and Plant Pathogens. Front. Microbiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Glick, B.R.; Rathore, D. Biosurfactants as a biological tool to increase micronutrient availability in soil: A review. Pedosphere 2018, 28, 170–189. [Google Scholar] [CrossRef]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int. J. Mol. Sci. 2020, 21, 2152. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and characterization of halotolerant plant growth promoting rhizobacteria from durum wheat (Triticum turgidum subsp. durum) cultivated in saline areas of the dead sea region. Front. Microbiol. 2019, 10, 1639. [Google Scholar]
- Singh, R.P.; Jha, P.N. A Halotolerant Bacterium Bacillus licheniformis HSW-16 Augments Induced Systemic Tolerance to Salt Stress in Wheat Plant (Triticum aestivum). Front. Plant Sci. 2016, 7, 1890. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Sayyed, R.Z.; Mojahed, L.S.; Rahmani, A.F.; Ghafari, M.; Antonius, S.; Sukamto. Biofilm production: A strategic mechanism for survival of microbes under stress conditions. Biocatal. Agric. Biotechnol. 2022, 42, 102337. [Google Scholar] [CrossRef]
- Khairnar, M.; Hagir, A.; Parmar, K.; Sayyed, R.Z.; James, E.K.; Rahi, P. Phylogenetic diversity and plant growth-promoting activities of rhizobia nodulating fenugreek (Trigonella foenum-graecum Linn.) cultivated in different agroclimatic regions of India. FEMS Microbiol. Ecol. 2022, 98, fiac014. [Google Scholar] [CrossRef]
- Liu, X.; Chai, J.; Zhang, Y.; Zhang, C.; Lei, Y.; Li, Q.; Yao, T. Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environ. Exp. Bot. 2022, 204, 105098. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; Enshasy, H.A.E.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.; Patel, N.; Rana, A.; Vaidya, H.; Alfarraj, S.; Ansari, M.J.; Gafur, A.; Poczai, P.; Sayyed, R.Z. Evaluation of Plant Growth-Promoting and Salinity Ameliorating Potential of Halophilic Bacteria Isolated From Saline Soil. Front. Plant Sci. 2022, 13, 946217. [Google Scholar] [CrossRef]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Portieles, R.; Du, L.; Gao, X.; Borras-Hidalgo, O. Endophytic bacterium Bacillus aryabhattai induces novel transcriptomic changes to stimulate plant growth. PLoS ONE 2022, 17, e0272500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-tolerant and plant-growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2022, 13, 1101862. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Neshat, M.; Chavan, D.D.; Shirmohammadi, E.; Pourbabaee, A.A.; Zamani, F.; Torkaman, Z. Canola inoculation with Pseudomonas baetica R27N3 under salt stress condition improved antioxidant defense and increased expression of salt resistance elements. Ind. Crop. Prod. 2023, 206, 117648. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, P.; Sirari, A.; Sharma, B.; Kumawat, G.; Nair, R.M.; H, B.; Kunal. Co-existence of halo-tolerant Pseudomonas fluorescens and Enterococcus hirae with multifunctional growth promoting traits to ameliorate salinity stress in Vigna radiata. Chemosphere 2024, 349, 140953. [Google Scholar] [CrossRef]
- Damodaran, T.; Jha, S.K.; Kumari, S.; Gupta, G.; Mishra, V.K.; Sharma, P.C.; Gopal, R.; Singh, A.; Jat, H.S. Development of Halotolerant Microbial Consortia for Salt Stress Mitigation and Sustainable Tomato Production in Sodic Soils: An Enzyme Mechanism Approach. Sustainability 2023, 15, 5186. [Google Scholar] [CrossRef]
- Mahmud, F.M.A.; Islam, M.A.; Rubel, M.H.; Mukharjee, S.K.; Kumar, M.; Bhattacharya, P.; Ahmed, F. Effects of halotolerant rhizobacteria on rice seedlings under salinity stress. Sci. Total Environ. 2023, 892, 163774. [Google Scholar] [CrossRef]
- Gandhi, A.D.; Vizhi, D.K.; Lavanya, K.; Kalpana, V.; Rajeswari, V.D.; Babujanarthanam, R. In vitro anti-biofilm and anti-bacterial activity of Sesbania grandiflora extract against Staphylococcus aureus. Biochem. Biophys. Rep. 2017, 12, 193–197. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Pseudomonas aeruginosa Oligoribonuclease Controls Tolerance to Polymyxin B by Regulating Pel Exopolysaccharide Production. Antimicrob. Agents Chemother. 2022, 66, e02072-21. [Google Scholar] [CrossRef]
- Kang, D.; Kirienko, D.R.; Webster, P.; Fisher, A.L.; Kirienko, N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 2018, 9, 804–817. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kim, Y.-N.; Khan, M.A.; Kang, S.-M.; Hamayun, M.; Lee, I.-J. Enhancement of drought-stress tolerance of Brassica oleracea var. italica L. by newly isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500. [Google Scholar]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J. Microbiol. Methods 2015, 110, 7–14. [Google Scholar] [CrossRef]
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Quantification of indole-3-acetic acid from plant associated Bacillus spp. and their phytostimulatory effect on Vigna radiata (L.). World J. Microbiol. Biotechnol. 2009, 25, 519–526. [Google Scholar] [CrossRef]
- Saikia, R.; Kumar, R.; Arora, D.; Gogoi, D.; Azad, P. Pseudomonas aeruginosa inducing rice resistance against Rhizoctonia solani: Production of salicylic acid and peroxidases. Folia Microbiol. 2006, 51, 375–380. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.; Imran, M.; Khan, A.L.; Asaf, S.; Al-Rawahi, A.; Al-Azri, M.S.A.; Al-Harrasi, A.; Lee, I.-J. Silicon-and Boron-Induced Physio-Biochemical Alteration and Organic Acid Regulation Mitigates Aluminum Phytotoxicity in Date Palm Seedlings. Antioxidants 2022, 11, 1063. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Parveen, N.; Mahmood, S.; Haider, M.Z.; Mumtaz, S.; Nawaz, H.; Khan, S.A.; Hafeez, A.; Tipu, M.I. Herbicidal effectiveness of wild poisonous plant Rhazya stricta using different media by the sandwich method. Pak. J. Bot. 2023, 55, 749–754. [Google Scholar] [CrossRef]
- Ngom, M.; Oshone, R.; Diagne, N.; Cissoko, M.; Svistoonoff, S.; Tisa, L.S.; Laplaze, L.; Sy, M.O.; Champion, A. Tolerance to environmental stress by the nitrogen-fixing actinobacterium Frankia and its role in actinorhizal plants adaptation. Symbiosis 2016, 70, 17–29. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Jogaiah, S. Defense mechanism and diverse actions of fungal biological control agents against plant biotic stresses. In Plant Biotechnology: Progress in Genomic Era; Springer: Singapore, 2019; pp. 461–478. [Google Scholar]
- Ghosh, D.; Gupta, A.; Mohapatra, S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Kaushik, A.; Rani, N.; Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J. Environ. Biol. 2010, 31, 701–704. [Google Scholar] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef]
- Paul, C.S.; Mercl, F.; Száková, J.; Tejnecký, V.; Tlustoš, P. The role of low molecular weight organic acids in the release of phosphorus from sewage sludge-based biochar. All Life 2021, 14, 599–609. [Google Scholar] [CrossRef]
- Jan, M.; Shah, G.; Masood, S.; Iqbal Shinwari, K.; Hameed, R.; Rha, E.; Jamil, M. Bacillus cereus enhanced phytoremediation ability of rice seedlings under cadmium toxicity. BioMed Res. Int. 2019, 2019, 8134651. [Google Scholar] [CrossRef] [PubMed]
- Younis, T.; Rahman, S.; Rahman, L.; Iqrar, I.; Shinwari, Z.K. Exploring the impact of endophytic bacteria on mitigating salinity stress in Solanum lycopersicum L. Plant Stress 2024, 12, 100467. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mungbean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 2016, 22, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Paria, P.; Chakraborty, H.J.; Behera, B.K. Identification of novel salt tolerance-associated proteins from the secretome of Enterococcus faecalis. World J. Microbiol. Biotechnol. 2022, 38, 177. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Szymańska, S.; Lis, M.I.; Piernik, A.; Hrynkiewicz, K. Pseudomonas stutzeri and Kushneria marisflavi Alleviate Salinity Stress-Associated Damages in Barley, Lettuce, and Sunflower. Front. Microbiol. 2022, 13, 788893. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, C.; Ong, E.K.; Dalling, M.J.; Stevenson, T.W. Regulation of genes associated with auxin, ethylene and ABA pathways by 2, 4-dichlorophenoxyacetic acid in Arabidopsis. Funct. Integr. Genom. 2006, 6, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Manigundan, K.; Amaresan, N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J. Basic Microbiol. 2017, 57, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Z.; Xi, Y.; Wang, X.; Chen, S.; He, M.; Chen, Y.; Guo, Y. Improvement of salt tolerance of Arabidopsis thaliana seedlings inoculated with endophytic Bacillus cereus KP120. J. Plant Interact. 2022, 17, 884–893. [Google Scholar] [CrossRef]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kang, S.-M.; Baek, I.-Y.; Lee, I.-J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 2014, 9, 754–762. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Injamum-Ul-Hoque, M.; Imran, M.; Zainurin, N.; Shaffique, S.; Kang, S.-M.; Ahsan, S.M.; Odongkara, P.; Lee, I.-J. Isolation and Identification of Multi-Traits PGPR for Sustainable Crop Productivity Under Salinity Stress. Sustainability 2024, 16, 9263. https://doi.org/10.3390/su16219263
Injamum-Ul-Hoque M, Imran M, Zainurin N, Shaffique S, Kang S-M, Ahsan SM, Odongkara P, Lee I-J. Isolation and Identification of Multi-Traits PGPR for Sustainable Crop Productivity Under Salinity Stress. Sustainability. 2024; 16(21):9263. https://doi.org/10.3390/su16219263
Chicago/Turabian StyleInjamum-Ul-Hoque, Md., Muhammad Imran, Nazree Zainurin, Shifa Shaffique, Sang-Mo Kang, S. M. Ahsan, Peter Odongkara, and In-Jung Lee. 2024. "Isolation and Identification of Multi-Traits PGPR for Sustainable Crop Productivity Under Salinity Stress" Sustainability 16, no. 21: 9263. https://doi.org/10.3390/su16219263
APA StyleInjamum-Ul-Hoque, M., Imran, M., Zainurin, N., Shaffique, S., Kang, S.-M., Ahsan, S. M., Odongkara, P., & Lee, I.-J. (2024). Isolation and Identification of Multi-Traits PGPR for Sustainable Crop Productivity Under Salinity Stress. Sustainability, 16(21), 9263. https://doi.org/10.3390/su16219263








