Abstract
Biofertilizers offer a sustainable method for improving rice growth and productivity, yet their effects on the interaction between plant growth, photosynthetic activity, and gene expression remain under-researched. This study examines how biofertilizer influences rice physiology, focusing on photosynthetic regulation and expression of chlorophyll-related genes. Eight fertilizer treatments were applied: control (CNT), biofertilizer (BF), deactivated biofertilizer (DABF), rice straw (RS), rice straw with biofertilizer (RS+BF), organic fertilizer (OF), organic fertilizer with biofertilizer (OF+BF), and inorganic fertilizer (IOF). Plant height, tiller number, SPAD, NDVI, chlorophyll content, and photosynthesis rates were measured, while gene expression analysis was conducted using RT-qPCR. The OF+BF treatment produced the most significant results, leading to a 31% increase in plant height, a 135% increase in tiller number, and a 42% increase in chlorophyll content (SPAD values) compared to the control. Additionally, OF+BF enhanced photosynthetic efficiency by 74%, with the highest net photosynthetic rate of 48.23 μmol CO2 m−2 s−1. Gene expression analysis revealed that OF+BF upregulated key photosynthesis-related genes, such as OsChlD and OsCHLM, showing a 70% and 90% increase in expression. These findings highlight the potential of integrating biofertilizers with organic fertilizers to sustainably boost rice growth and productivity, contributing to global food security and climate change mitigation.
1. Introduction
Global warming and climate change are challenges that directly affect agricultural productivity. Rising temperatures, erratic weather patterns, and increasing greenhouse gas emissions have led to a decrease in plant production, posing a threat to food security [1,2,3]. According to the Intergovernmental Panel on Climate Change (IPCC), the agricultural sector is both a contributor to and a victim of climate change, as unsustainable farming practices intensify environmental degradation [4,5]. Addressing these issues is critical to achieving the United Nations Sustainable Development Goals (SDGs) by 2030, particularly SDG 2 (Zero Hunger) and SDG 13 (Climate Action), which focus on ending hunger, promoting sustainable agriculture, and taking urgent action to combat climate change. The growing population, especially in Asia, where rice is a staple food, further exacerbates the pressure on food production. With the world population expected to reach 9.7 billion by 2050 [6,7,8,9], there is a need to increase rice production sustainably to meet the demands of a rapidly growing population and mitigate the adverse effects of climate change.
To increase rice production, genetically improving photosynthesis is a key strategy to boost crop yields and meet the rising demand for food. Photosynthesis is a highly regulated process, controlled by the expression of genes involved in producing chlorophyll, photosynthetic pigments, and key enzymes for energy conversion [10,11]. Studies by Li et al., 2021 and Ambavaram et al., 2014 demonstrate how genetic control of photoprotection pathways shields photosynthetic machinery from light stress, while Kromdijk et al., 2016 and Muhie et al., 2022, show that targeted gene modifications can enhance photosynthetic efficiency [11,12,13,14].
Improving photosynthetic efficiency through biofertilizers and organic fertilizers offers a sustainable means to increase crop productivity while lowering environmental impacts. Despite these advancements, there remains a significant gap in understanding how combining biofertilizers and organic fertilizers affects gene expression related to chlorophyll production and photosynthetic efficiency. Understanding these interactions is essential for developing sustainable agriculture strategies that improve crop yields while minimizing environmental damage. By addressing this knowledge gap, this research supports both increased productivity and climate resilience, advancing the objectives of SDG 2 and SDG 13.
Photosynthesis is important for plant growth and productivity, with chlorophyll playing a significant role in capturing light energy and converting it into chemical energy [14,15,16]. In this context, enhancing chlorophyll synthesis through sustainable fertilization strategies, such as biofertilizers combined with organic fertilizers, is critical for improving photosynthetic efficiency and supporting climate-smart agriculture. Studies by Vessey et al., 2003, and He et al., 2023 highlight the roles of genes like OsαCA1 and LHCB8 in optimizing photosynthesis by managing carbon flow and light energy within chloroplasts [17,18]. These genes are regulated by internal signals such as circadian rhythms and developmental stages, as well as external factors like light intensity, temperature, and nutrient availability. However, much remains unknown about how the integration of biofertilizers with organic fertilizers can influence the gene expression pathways regulating photosynthesis and chlorophyll synthesis, particularly under varying environmental conditions. Understanding this interaction is important for promoting sustainable, high-yield rice production in a changing climate.
Sustainable agricultural practices, particularly the integration of biofertilizers and organic fertilizers, offer a promising solution for enhancing crop yields while minimizing the environmental footprint of farming. Biofertilizers improve soil health and nutrient cycling, leading to a reduction in chemical fertilizers that contribute to greenhouse gas emissions [19,20,21,22]. This approach aligns with SDG 13 by mitigating climate change impacts through eco-friendly practices, and it supports SDG 2 by enhancing plant resilience to environmental stress, thus securing food security.
Historically, traditional farming relied on organic amendments like manure and compost, a practice dating back to ancient civilizations [23,24]. The Green Revolution introduced high-yield crops and chemical fertilizers, which significantly boosted food production but also caused soil degradation and environmental issues [25,26]. In response to the emergence of biostimulants and biofertilizers, such as microbial inoculants and seaweed extracts, which enhance plant growth and soil health [27,28]. Nitrogen-fixing bacteria like Rhizobium and Azospirillum provide essential nitrogen for chlorophyll production, while phosphate-solubilizing bacteria convert insoluble phosphorus into forms that plants can absorb [28]. Recent studies have demonstrated that biofertilizers not only enhance nutrient uptake but also lower the environmental impact of agriculture by reducing greenhouse gas emissions and improving soil biodiversity [29,30,31,32]. Thus, biofertilizers are important in sustainable agriculture, contributing to increased productivity and environmental conservation. In this study, the objective is to explore the effects of combining biofertilizers with organic fertilizers on the growth of rice plants. Biofertilizers enhance nutrient availability and promote plant growth through processes like nitrogen fixation and phosphorus solubilization. Organic fertilizers, derived from plant and animal residues, improve soil health by enhancing its structure and increasing microbial activity. This study hypothesizes that combining these fertilizers will produce a synergistic effect, leading to enhanced plant growth, improved photosynthetic efficiency, and upregulated gene expression.
2. Materials and Methods
2.1. Experimental Site Description and Soil Analysis
This study was conducted at the Kariwa Village Advanced Agro-Biotechnological Research Centre in Kashiwazaki, Japan. The experimental work was carried out in biotron facilities, which allowed for the precise control of environmental conditions essential for the growth of rice plants. The temperature inside the biotrons was regulated to maintain a day/night cycle of 28 °C/23 °C. The relative humidity was controlled at 70%, and the ambient CO2 concentration conducive to plant growth and development is around 400–450 ppm.
The soil used in the experiment was collected from a paddy field in Kashiwazaki, Japan (37°22′40.8″ N latitude and 138°36′53.5″ E longitude). Samples were taken from the top 0–20 cm of the soil profile using a soil auger at random locations to ensure representativeness. After collection, the soil was mixed thoroughly, air-dried, ground, and passed through a 2 mm sieve to prepare it for use. A physicochemical analysis of the prepared soil was conducted, revealing the following properties: nitrogen content of 27 mg/kg, phosphorus content of 37 mg/kg, potassium content of 74 mg/kg, electrical conductivity (EC) of 33.5 S/m, and a pH of 5.68. These baseline measurements were essential for understanding the initial soil conditions before the application of treatments.
2.2. Experimental Design and Treatments
The experiment followed a completely randomized design (CRD) in the biotrons, with each treatment replicated three times for reliable comparative and statistical analysis. The experimental units consisted of pots with a diameter of 21 cm and a height of 20 cm, each filled with 4 kg of the prepared soil. The pots were designed with drainage holes and were placed in a water pool to simulate paddy field conditions and to ensure that the water level remained consistently around 10 cm above the soil surface throughout the entire growth period. Rice seeds of the Oryza sativa ssp. japonica cv. Koshihikari, a widely cultivated and highly regarded rice in Japan, were used for this study. The seeds were sourced from the Laboratory of Biochemistry, Faculty of Agriculture, Niigata University. The seeds were soaked for two days. Soaked seeds were placed in a viol for 2 weeks under a controlled growth chamber. After 2 weeks, seedlings were transferred to the ambient condition in a greenhouse. Thirty-day-old seedlings were transplanted into the pots to ensure uniformity in plant age and initial growth conditions. One rice seedling was planted in each pot, and there were five pots for each treatment. A single seedling was planted per pot, with five pots assigned to each treatment, and three plants were selected for measurements. The experiment included eight distinct fertilizer treatments: Control (CNT), biofertilizer (BF), deactivated biofertilizer (DABF), rice straw (RS), rice straw with biofertilizer (RS+BF), organic fertilizer (OF), organic fertilizer with biofertilizer (OF+BF), and inorganic fertilizer (IOF). The biofertilizer used in this study, Tokyo8, known as a microbial activator with abundant pseudomonas species, was sourced from Taiyo-Yuka Co., Ltd. (Tokyo, Japan), while rice straw and organic fertilizer, a mixture of lactic acid, eggshells, linsan guano excrete, and rice flour, were obtained from local farmers. The application of fertilizers followed the local farmers’ practices, with precise amounts being weighed using an analytical balance to ensure consistency across treatments. However, the application of the biofertilizer followed the manufacturer’s instructions manual. 1 mL of the biofertilizer was dissolved in 100 mL of water and applied at a rate of 20 mL per pot. The fertilizers’ application method, timing, and quantities for each treatment are presented in Table S1 in the Supplementary Materials. The plants were maintained under continuous submersion with 10 cm of water above the soil surface, replicating typical conditions in paddy fields until the time of harvest. This experimental design was intended to evaluate the effects of combining biofertilizers with organic fertilizers on rice growth, photosynthetic efficiency, and gene expression, providing insights into the potential benefits of these sustainable agricultural practices.
2.3. Justifications for the Selected Treatments
In this pot experiment, eight treatments were evaluated to assess their impact on plant growth and photosynthesis. Biofertilizer was tested to determine its efficacy in enhancing nutrient uptake, promoting root development, and improving soil health through beneficial microorganisms, leading to increased plant growth and photosynthetic efficiency. Rice straw with biofertilizer was investigated for its synergistic effects, providing a more complete nutrient profile, improving soil structure, and enhancing microbial activity, which resulted in greater plant growth and photosynthetic efficiency. Similarly, organic fertilizer with biofertilizer was examined to see if the combination of these inputs would offer a more comprehensive nutrient profile, improve soil structure, and boost microbial activity, leading to enhanced plant growth and photosynthetic efficiency. The control treatment served as a baseline, validating that any observed changes were attributable solely to the experimental treatments and no other factors. Autoclaved biofertilizer was used to isolate the effects of organic matter and other components in biofertilizers, excluding biological activity, to determine if benefits were due to biological activity or other factors such as nutrient content or soil structure. Rice straw was assessed for its impact as an organic matter source, improving soil structure, increasing water retention, and providing nutrients as it decomposes, thus enhancing plant growth and photosynthetic efficiency. Organic fertilizer was evaluated for its effectiveness in releasing nutrients gradually as it decomposes, which supports sustained nutrient availability and improves plant health, growth, and photosynthetic efficiency. Finally, inorganic fertilizer served as a positive control to compare the effects of organic and biological inputs with a traditional chemical fertilizer, which provides a quick release of nutrients but can also lead to nutrient leaching and environmental pollution.
2.4. Growth Parameters
During the plant growth process, plant height and tiller number were measured at the tillering stage (30 days, 30DAG). Plant height was determined by measuring the distance from the ground to the apical growth point using a tape measure, while the tiller number was counted. For leaf selection, the longest leaves with better growth and full sunlight exposure were chosen from the upper part of each selected Koshihikari plant and tagged as samples for photosynthetic light response curves and leaf chlorophyll content measurements. The leaf chlorophyll content was measured using the SPAD (Soil Plant Analysis Development) chlorophyll analyzer (Minolta Co. Ltd., Osaka, Japan) on the flag leaf, with readings taken from the middle position of the leaf and three measurements recorded per plant for each treatment. Additionally, to assess the impact of fertilizer application on rice plant health and growth, this study utilized the Normalized Difference Vegetation Index (NDVI) as a quantitative measure of vegetation vigor, measured using a GreenSeeker handheld crop sensor (Trimble Inc., Sunnyvale, CA, USA). This active sensor measures canopy reflectance at specific wavelengths in the red (670 ± 10 nm) and near-infrared (780 ± 10 nm) regions of the electromagnetic spectrum, calculating NDVI with the formula (ρ780 nm − ρ670 nm)/(ρ780 nm + ρ670 nm). Measurements were taken by holding the GreenSeeker in the nadir position and scanning it over the biomass sampling area at a constant height of 60 cm above the crop canopy. For each plot or treatment, the final NDVI value represented the average of three NDVI readings.
2.5. Leaf Gas Exchange and Photosynthetic Pigment Measurement
Leaf gas exchange measurements were conducted at the flowering stage using a portable photosynthesis system (LI-6400XL, LI-6400-20, LiCor Biosciences, Lincoln, NE, USA). For each treatment, three plants were selected, and their fully expanded functional leaves were placed in the leaf chamber. The chamber was secured to prevent air leakage for accurate measurements. The CO2 concentration in the chamber was maintained at 400 μmol mol−1 using a small CO2 cylinder, while the photosynthetic photon flux density (PPFD) was set to 1200 μmol m−2 s−1. Leaf temperature was controlled at 26 °C with a flow rate of 400 μmol s−1. Measurements were taken on sunny, cloudless days between 9:00 and 13:00 to ensure maximum photosynthetic activity, with relative humidity maintained between 45% and 50%. The net photosynthetic rate (An) (μmol CO2 m−2 s−1), stomatal conductance (gs) (mmol m−2 s−1), transpiration rate (E) (mmol m−2 s−1), intercellular CO2 concentration (Ci), and the ratio of intercellular to ambient CO2 concentration (Ci/Ca) were measured. The ratio of An/E was used to compute the instantaneous water use efficiency (WUE), indicating the amount of CO2 fixed per unit of water lost through transpiration.
After gas exchange measurements, all assimilating branches enclosed in the leaf chamber were carefully cut off for chlorophyll content determination. Chlorophyll content was determined using the methanol extraction method. Leaf tissue samples were extracted with 95% methanol at room temperature for over 24 h under dark conditions. After extraction, the supernatant was collected, and absorbance was measured at wavelengths of 655, 645, 495, and 480 nm using a UV-2550 UV-vis spectrometer (SHIMADZU Co., Ltd., Kyoto, Japan). The concentrations of chlorophyll a, b, the total chlorophyll, β-carotene, and Lutein were calculated according to 33, using Equations (1)–(6).
where A is absorption of the solution at 655, 645, 495, and 480 nm, Ca is the concentration of chlorophyll a, Cb is the concentration of chlorophyll b, and Ca+b is the sum of concentrations for chlorophyll a and b (mg/kg). Clut is the total concentration of lutein, Cβ-car is the total concentration of β-carotenoid, and Clut+β-car is the total concentration of xanthophylls (β-carotenoid and lutein) (mg/kg).
Ca = 19.00A655 − 7.61A645
Cb = 21.45A645 − 5.92A655
Ca+b = 13.08A655 + 13.84A645
Cβ-car = 17.16A495 − 3.96A480
CLut = 11.51A480 − 20.61A495
CLut+β-car = 7.55A480 − 3.45A495
2.6. Real-Time Quantitative PCR Analysis
High-quality total RNA was extracted from leaf samples of Koshihikari rice plants subjected to eight different treatments during the tillering stage using the TRIzol method [33]. 100 mg of leaf tissue was ground into a fine powder in liquid nitrogen using a pre-cooled mortar and pestle. The powdered tissue was immediately transferred to a 2 mL microcentrifuge tube containing 1 mL of TRIzol reagent and vortexed briefly. The samples were incubated at room temperature for 5 min to allow complete dissociation of nucleoprotein complexes. Chloroform (0.2 mL per 1 mL TRIzol) was added, and the tubes were shaken vigorously for 15 s and then incubated at room temperature for 2–3 min. The mixture was centrifuged at 12,000× g for 15 min at 4 °C, and the aqueous phase was carefully transferred to a fresh tube. An equal volume of isopropanol was added to the aqueous phase to precipitate the RNA. After incubation at room temperature for 10 min, the samples were centrifuged at 12,000× g for 10 min at 4 °C. The resulting RNA pellet was washed with 1 mL of 75% ethanol, briefly vortexed, and centrifuged at 7500× g for 5 min at 4 °C. The RNA pellet was air-dried and dissolved in RNase-free water. RNA concentration and purity were assessed using the NanoDropTM One/OneC Microvolume UV−Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA templates were produced from the total RNA samples through reverse transcription, utilizing the ReverTra Ace® qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan).
The real−time polymerase chain reaction (RT−PCR) analysis was conducted using the CFX96TM Real−Time PCR Detection System (Bio−Rad Laboratories GmbH, Hercules, CA, USA). The RT−PCR amplifications were conducted using a reaction volume of 10 μL. The reaction mixture consisted of 5 μL of SsoFastTM EvaGreen® Supermix (Bio−Rad Laboratories GmbH, Hercules, CA, USA), 3.6 μL of ddH2O, 0.2 μL of 10 pmol sense (forward) primer, 0.2 μL of 10 pmol antisense (reverse) primer, and 1 μL of cDNA. The PCR technique was followed to perform triplicate reactions for each gene. The melting curve analysis for the experiment involved 40 cycles of denaturation, annealing, and extension. Denaturation was performed at 98 °C for 2 min, followed by a brief denaturation at 98 °C for 2 s. Annealing was conducted at 60 °C for 5 s, and extension was performed at a gradient temperature from 75 °C to 95 °C for 10 s. The gene expression of the target gene (Table S2) was normalized by using the 2−ΔΔCT method as described by Livak and Schmittgen [34] employing reference gene OsUBQ5 (Os01g0328400).
2.7. Statistical Analysis
The obtained data were used to analyze variance (ANOVA) using RStudio software (Version 2024.04.0+735) to evaluate the significant variations across treatments. The method of determining separation was conducted by applying Tukey’s honestly significant difference (HSD) test at a significance level of p < 0.05, using RStudio software, explicitly utilizing the ‘glht’ function [35]. The principal component analysis (PCA) was performed on the correlation matrix of treatments. Index values for each treatment were first deliberated by assessing treatments. All the traits under each treatment were combined and used as index values for PCA analysis. These index values were used to identify the correlation of response variable vectors and comparisons across the ordination space. Two-way heatmap clustering analysis (HCA) was performed on the same dataset as PCA analysis. Pearson correlation was used as a correlation-based distance method. The ‘Euclidean algorithm’ was used to compute the dissimilarity matrix. PCA and HCA were created using the RStudio software, including the ‘prcomp’ function in the ‘factoextra’ package [36]. Data were hierarchically clustered using the heatmap function in the ‘pheatmap’ package with RStudio software [37].
3. Results
3.1. Plant Growth, Tiller Number, NDVI, and SPAD
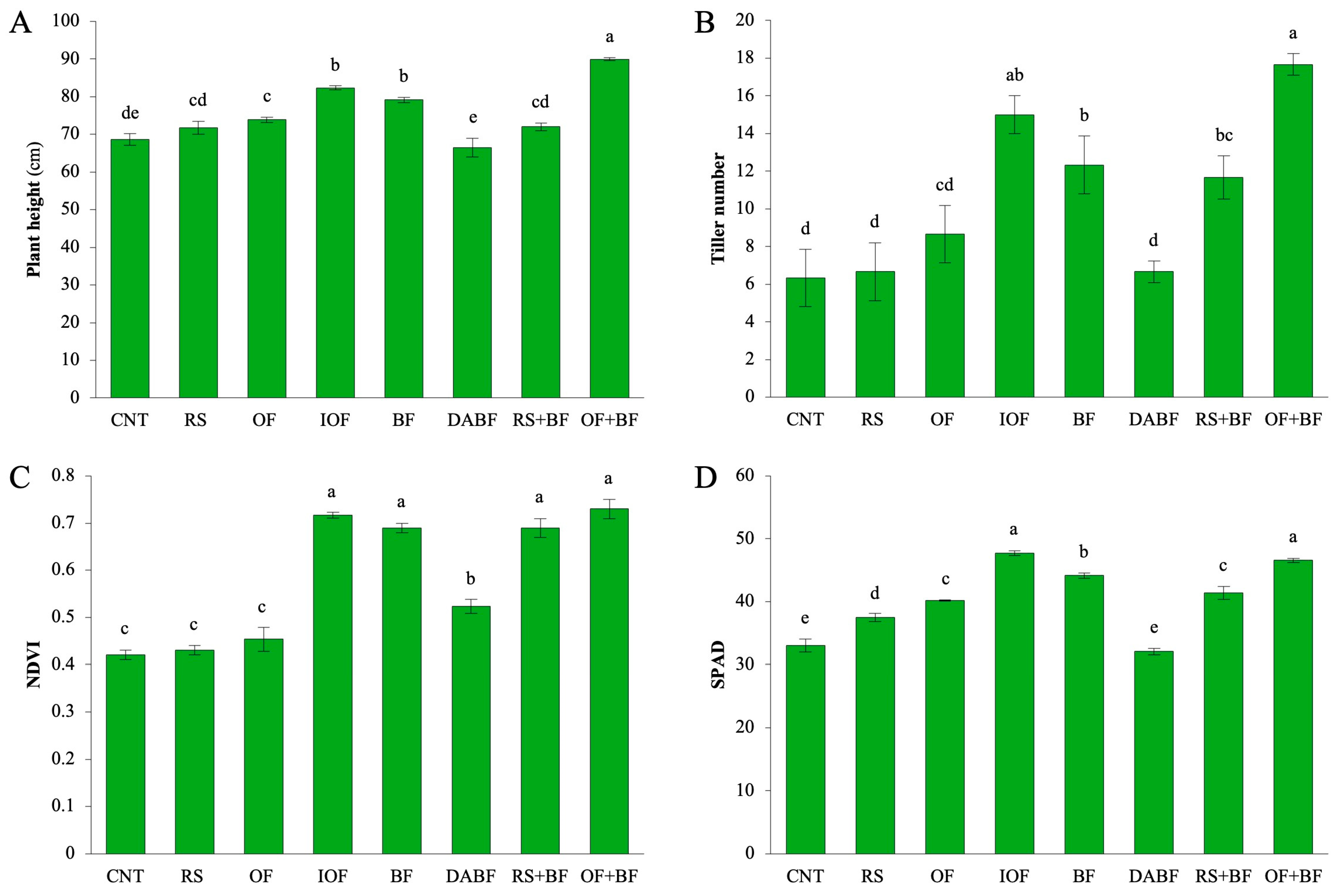
In the context of plant height, the OF+BF treatment resulted in the most significant increase, with plants reaching an average height of approximately 90 cm—31% higher than the control (CNT), which had a mean height of 68 cm. This treatment outperformed all others, indicating a positive synergistic effect between the organic and biofertilizer components. In comparison, CNT and rice straw (RS) produced shorter plants, averaging around 70 cm and 65 cm, respectively. The deactivated biofertilizer (DABF) treatment also showed reduced height, suggesting that the sterilization process diminished its effectiveness (Figure 1A). Other treatments, such as inorganic fertilizer (IOF) and biofertilizer (BF), demonstrated statistically significant increases in height, with IOF achieving about a 20% increase and BF a 15% increase over the control. In contrast, RS and DABF did not show significant differences from CNT, indicating limited effectiveness. For the tiller number, OF+BF produced an average of 18 tillers per plant, a significant increase compared to the CNT’s average of 8. IOF and BF also led to higher tiller counts, averaging 15 and 14, respectively. The OF+BF treatment yielded a remarkable 135% increase in tiller production compared to the control. IOF and BF showed substantial improvements as well, with increases of 90% and approximately 55%, respectively, underscoring the enhanced reproductive capacity associated with the combined use of organic and biofertilizers (Figure 1B). NDVI values were highest in IOF, BF, and OF+BF, each around 0.75, indicating effective maintenance of chlorophyll and plant vigor. CNT, RS, and OF exhibited lower NDVI values of around 0.6. The high NDVI in OF+BF (0.73) represents a 74% increase in plant health compared to the control (0.42). IOF and BF also showed significant increases in NDVI, at 71% and 65%, respectively, while RS and DABF did not exhibit significant improvements (Figure 1C). For chlorophyll content, as measured by SPAD, OF+BF resulted in a significant 42% increase over the control, raising the mean SPAD value from 33 to nearly 47. IOF also led to a 37% increase, while BF showed a 33% increase. SPAD values were highest in OF+BF, significantly surpassing those of CNT and RS, which averaged around 35 (Figure 1D). Statistical analysis highlights that the combination of organic fertilizer and biofertilizer (OF+BF) consistently produces the most substantial improvements in plant height, tiller number, NDVI, and SPAD, with increases ranging from 31% to 135% compared to the control. While inorganic fertilizer (IOF) and biofertilizer (BF) also show positive effects, the combined treatment consistently outperforms these individual applications, enhancing both growth and photosynthetic capacity.
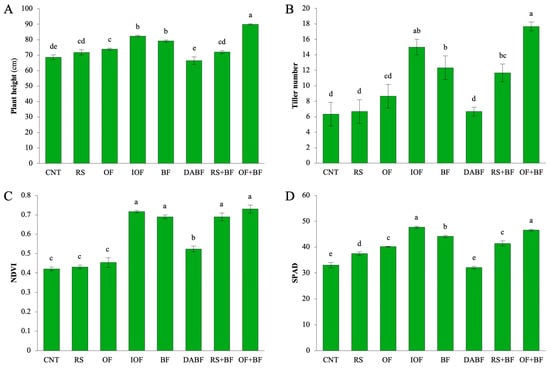
Figure 1.
Biofertilizer combined with organic fertilizer increased rice growth. (A) Plant height, (B) Tiller number, (C) NDVI, and (D) SPAD values for control (CNT), rice straw (RS), organic fertilizer (OF), inorganic fertilizer (IOF), biofertilizer (BF), autoclave biofertilizer (DA BF), rice straw combined with biofertilizer (RS+BF), and organic fertilizer combined with biofertilizer (OF+BF). Error bars represent ±SD (n = 3). Values with the same letter are not statistically different among all treatments (Tukey HSD test, p < 0.05).
3.2. Photosynthetic Pigments
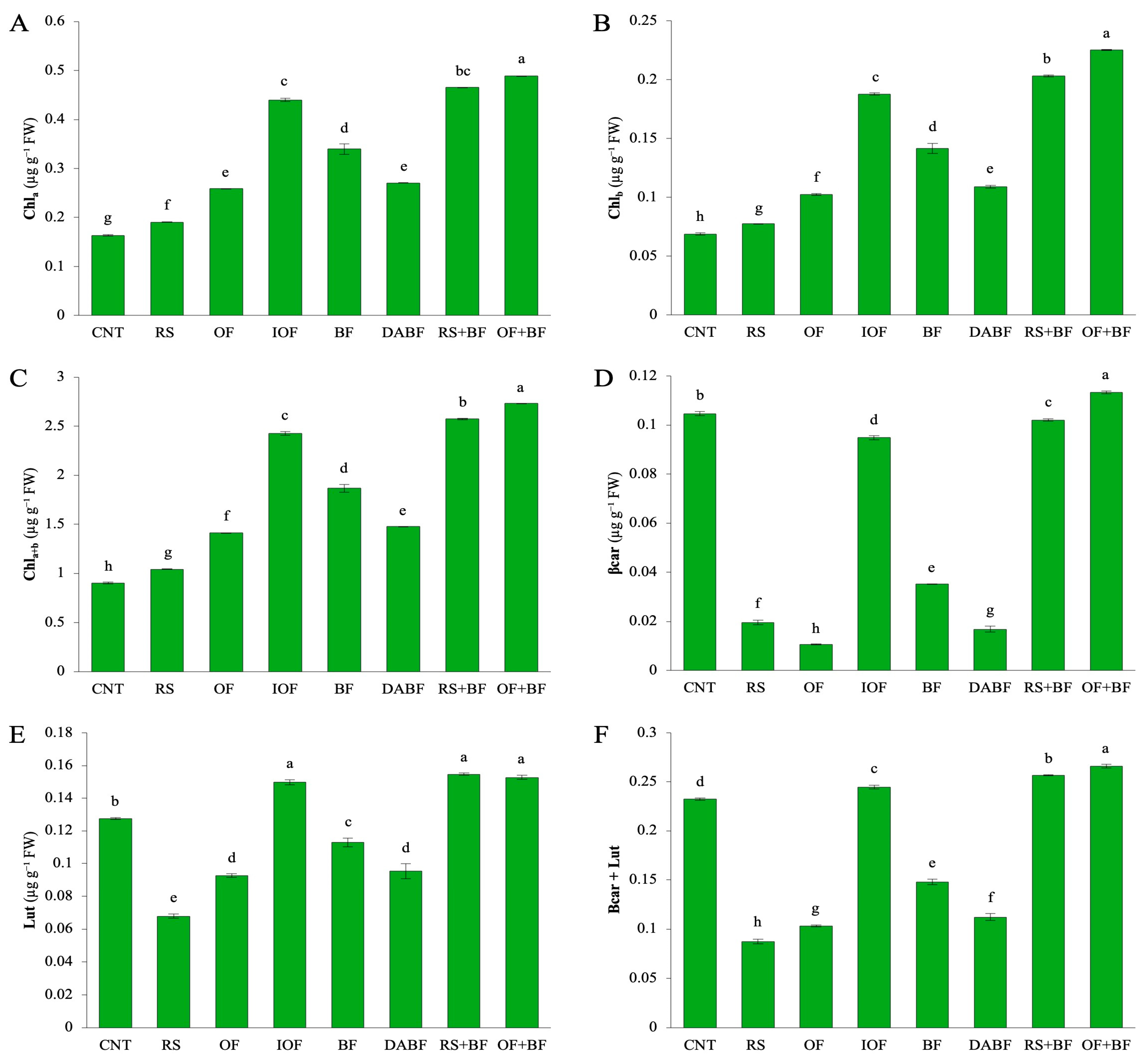
Chlorophyll a (Chl a) content (Figure 2A) was highest in the OF+BF treatment, reaching approximately 0.45 mg/g fresh weight, a significant increase of 66% compared to the control (CNT), which had a mean value of 0.165 mg/g. In contrast, CNT and rice straw (RS) treatments exhibited the lowest Chl levels, around 0.15 mg/g and 0.20 mg/g, respectively, indicating suboptimal conditions for chlorophyll production. Biofertilizer (BF) alone showed a moderate increase, with levels at about 0.35 mg/g, but still fell short of the OF+BF combination. For chlorophyll b (Chl b) (Figure 2B), OF+BF also produced the highest levels, approximately 0.22 mg/g fresh weight, reflecting a 72% increase over the control, which had a mean of 0.069 mg/g. CNT and RS treatments resulted in lower Chl b contents, around 0.08 mg/g and 0.10 mg/g, respectively. Inorganic fertilizer (IOF) and BF contributed to increased Chl b levels, but not as significantly as the OF+BF treatment. Total chlorophyll content (Chl a+b) (Figure 2C) was highest in OF+BF, reaching approximately 2.6 mg/g fresh weight, representing a 67% increase compared to the control (1.0 mg/g). RS+BF also showed high total chlorophyll levels, close to 2.5 mg/g, indicating the effectiveness of biofertilizer in enhancing chlorophyll content. IOF and BF were effective as well, though not to the same extent as the OF+BF combination. β-carotene (β-car) levels (Figure 2D) peaked at around 0.11 mg/g fresh weight in the OF+BF treatment, marking a 50% increase over the control, which had 0.076 mg/g. BF alone reached about 0.07 mg/g, while CNT and DA BF recorded the lowest β-car levels at around 0.03 mg/g. The increase in β-carotene indicates enhanced photoprotection and stress mitigation in plants treated with combined fertilizers. Lutein (Lut) content (Figure 2E) mirrored this trend, with the highest levels in OF+BF and RS+BF treatments, each reaching approximately 0.14 mg/g fresh weight, significantly higher than CNT (0.08 mg/g) and RS (0.09 mg/g). The combined levels of β-carotene and lutein (β-car + Lut) (Figure 2F) were highest in OF+BF, at about 0.25 mg/g fresh weight, reflecting a 58% increase over the control (0.12 mg/g). RS+BF also resulted in high levels, close to 0.22 mg/g, further emphasizing the benefits of integrating biofertilizers with organic fertilizers. The descriptive statistics for chlorophyll-related parameters highlight the superior performance of the OF+BF treatment across all measured pigments. Significant increases in Chl a, Chl b, total chlorophyll, β-carotene, and lutein content illustrate enhanced light capture and environmental stress resilience in plants treated with the OF+BF combination. In contrast, treatments like rice straw and autoclaved biofertilizer showed minimal improvements, underscoring the effectiveness of combined fertilization strategies in optimizing plant growth and photosynthetic pigment production.
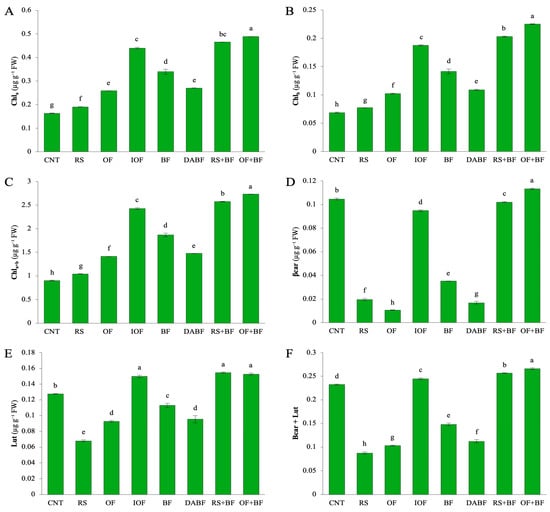
Figure 2.
Biofertilizer combined with organic fertilizer increased key pigments involved in photosynthesis, including chlorophyll (A) a (Chla), (B) chlorophyll b (Chlb), (C) total chlorophyll (Chla+b), (D) β-carotene (β-car), (E) lutein (Lut), and (F) the combined levels of β-carotene and lutein (β-car + Lut). Error bars represent ±SD (n = 3). Values with the same letter are not statistically different among all treatments (Tukey HSD test, p < 0.05).
3.3. Photosynthesis
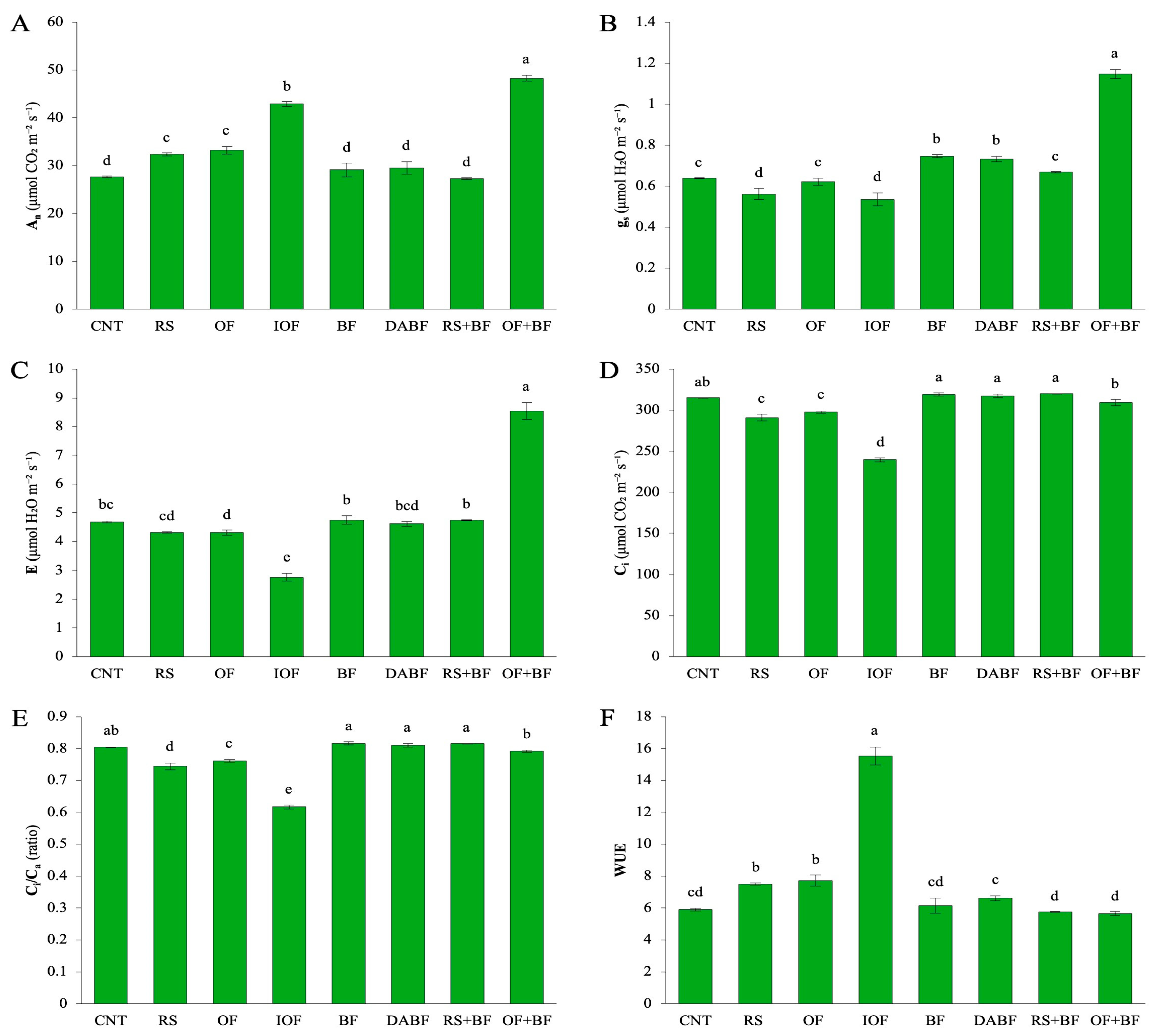
For the net photosynthetic rate (An) in Figure 3A, the OF+BF treatment had the highest mean value of 48.23 μmol CO2 m−2 s−1, reflecting a 74% increase compared to the control’s mean of 27.68 μmol CO2 m−2 s−1. Inorganic fertilizer (IOF) also showed notable improvement, with a mean An value of 42.86 μmol CO2 m−2 s−1, representing a 55% increase over the control. The biofertilizer (BF) treatment showed more moderate improvements, with a mean of 29.10 μmol CO2 m−2 s−1.
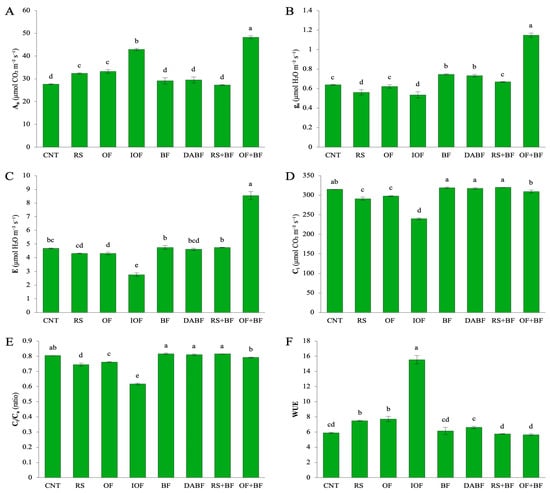
Figure 3.
(A) The net photosynthetic rate (An), (B) stomatal conductance (gs), (C) transpiration rate (E), (D) the intercellular CO2 concentration (Ci), (E) the ratio of intercellular to ambient CO2 concentration (Ci/Ca), and (F) water use efficiency (WUE). Error bars represent ±SD (n = 3). Values with the same letter are not statistically different among all treatments (Tukey HSD test, p < 0.05).
The control and rice straw (RS) treatments had the lowest rates, resulting in significantly lower carbon assimilation. For stomatal conductance (gs) in (Figure 3B), the OF+BF treatment led with a mean value of 1.14 mmol H2O m−2 s−1, an 81% increase over the control (0.63 mmol H2O m−2 s−1). IOF also demonstrated a significant increase, with a mean gs of 0.53 mmol H2O m−2 s−1, representing a 45% increase compared to the control. Conversely, RS and deactivated biofertilizer (DA-BF) treatments had much lower GS values, indicating poorer performance. Regarding intercellular CO2 concentration (Ci), the highest values were recorded under the control treatment (315.26 µmol CO2 m−2 s−1), which did not effectively promote CO2 fixation. The OF+BF treatment exhibited a slight 3% reduction in Ci, with a mean of 309.52 µmol CO2 m−2 s−1, suggesting improved CO2 fixation efficiency. IOF showed a more pronounced reduction in Ci to 239.59 µmol CO2 m−2 s−1, indicating strong CO2 assimilation. The biofertilizer treatment displayed intermediate values, while DA BF and RS had higher Ci values, reflecting their lower effectiveness. The transpiration rate (E) was highest under the OF+BF treatment in (Figure 3C), with a mean of 6.53 µmol H2O m−2 s−1, marking a 39% increase over the control (4.66 µmol H2O m−2 s−1). This suggests that plants under OF+BF were using water more effectively for growth. IOF also demonstrated an increase in transpiration, with a mean E of 5.53 µmol H2O m−2 s−1, while control and DA BF treatments yielded significantly lower rates. For the Ci/Ca ratio in (Figure 3E), the OF+BF treatment had a mean value of 0.79, representing a 32% increase compared to the control (0.60). This indicates improved CO2 assimilation efficiency relative to external availability. IOF also showed a significant improvement with a mean value of 0.61, while the control, DA BF, and RS treatments had much lower ratios, reflecting a limited impact on CO2 fixation efficiency. Finally, water use efficiency (WUE) in (Figure 3F) was significantly enhanced under the OF+BF treatment, with a mean value of 7.72 µmol CO2 mmol−1 H2O, reflecting a 30% increase compared to the control (5.91 µmol CO2 mmol−1 H2O). This indicates that plants under OF+BF were more efficient in using water for biomass production. IOF showed moderate improvements in WUE, while treatments like DA BF and RS exhibited significantly lower values, indicating less effective water usage. Among all the treatments, the OF+BF treatment consistently resulted in the highest values across all physiological parameters, with significant increases in photosynthesis, stomatal conductance, and water use efficiency. The percentage increases confirm the effectiveness of combining organic fertilizer with biofertilizer. Inorganic fertilizer (IOF) also showed substantial improvements but was generally less effective than the combined treatment. Treatments like rice straw (RS) and DA BF showed minimal improvements, highlighting the importance of enriched or combined fertilization strategies for optimizing plant growth and physiological function (Figure 3).
3.4. Principal Component Analysis (PCA) Highlights Differences in Fertilizer Treatments
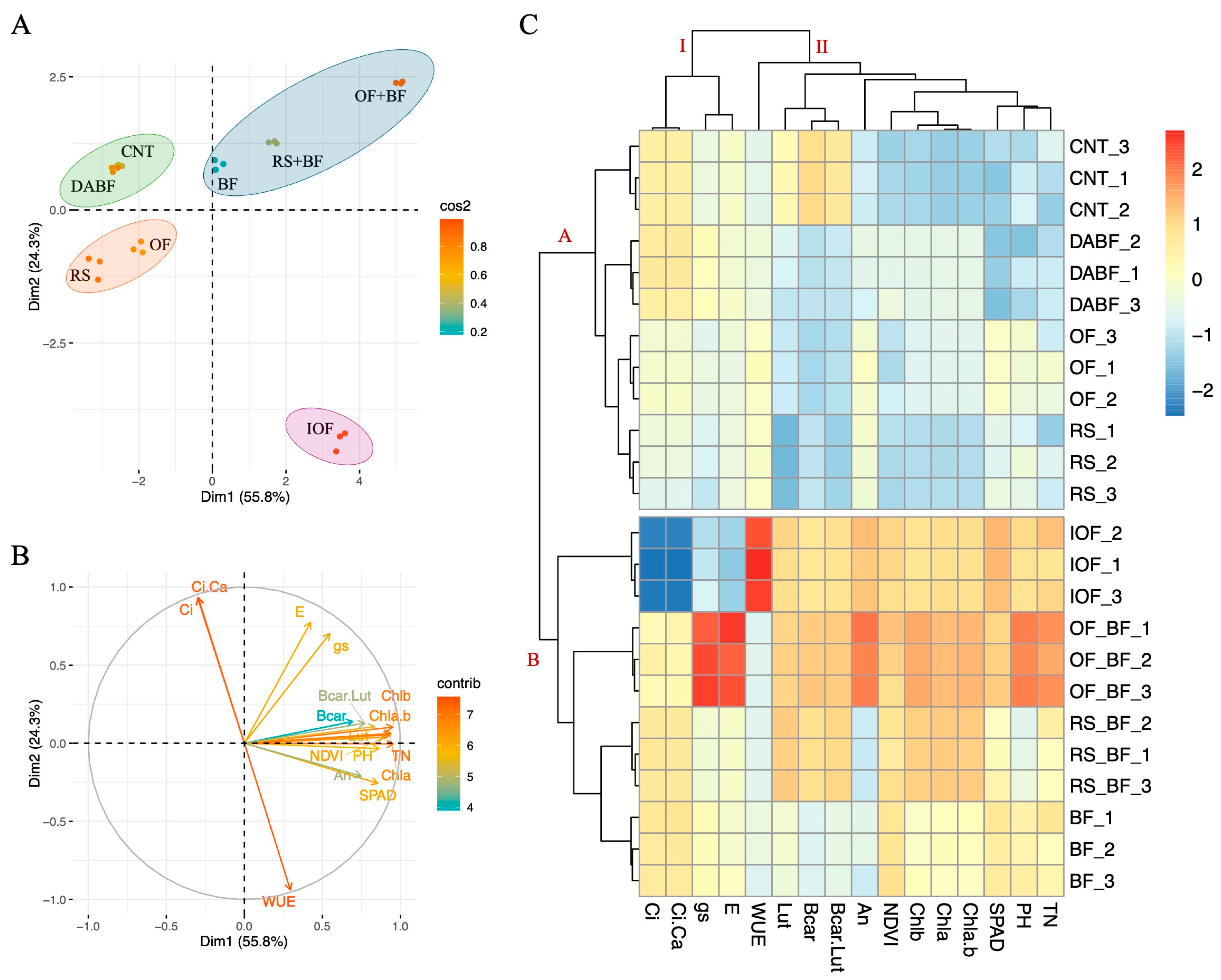
Panel A of Figure 4 presents the PCA, where the first principal component (Dim1) accounts for 55.8% of the total variance, while the second principal component (Dim2) explains 26.3%. Together, Dim1 and Dim2 capture 82.1% of the variability in the data, making them highly informative for distinguishing the effects of the treatments.
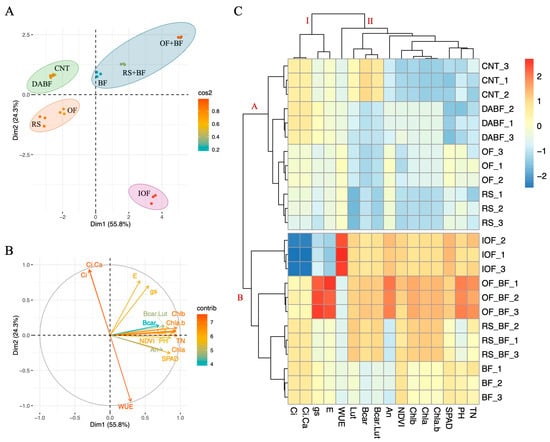
Figure 4.
Principal component analysis (PCA) and hierarchical clustering analysis (HCA) highlight differences in rice growth, photosynthesis, SPAD, and NDVI in fertilizer treatments. (A) principal component analysis (PCA), (B) PCA of the studied traits, (C) hierarchical clustering analysis of studied traits in Koshihikari under control (CNT), rice straw (RS), organic fertilizer (OF), inorganic fertilizer, biofertilizer (BF), deactivated biofertilizer (DABF), rice straw + biofertilizer (RS+BF), organic fertilizer + biofertilizer (OF+BF), plant height (PH), tiller number (TN), the Normalized Difference Vegetation Index (NDVI) and SPAD chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (ChlT) content, β-carotene, (β-car) Lutein (Lut) and β-carotene + Lutein (β-car + Lut). The net photosynthetic rate (An), stomatal conductance (gs), transpiration rate (E), intercellular CO2 concentration (Ci), the ratio of intercellular to ambient CO2 concentration (Ci/Ca), and Water Use Efficiency (WUE).
OF+BF is located between 2.5 and 3.5 units on the Dim1 axis and around 0.5 to 1.5 units on the Dim2 axis, indicating its strong influence on the studied traits. This treatment forms a distinct cluster, indicating a significant impact on rice growth and photosynthesis. RS+BF, clustered close to OF+BF, is positioned around 1.5 to 2.5 units on the Dim1 axis and 1.5 to 2.5 units on the Dim2 axis, suggesting some overlap in the effects of these treatments due to the use of biofertilizers. On the opposite side, IOF is isolated, with 3.5 units on the Dim1 axis and −1.0 units on the Dim2 axis. This isolation reflects a distinct response pattern likely due to the different modes of action of inorganic fertilizers. CNT, RS, and DA BF cluster together at −2.0 to −3.5 units on the Dim1 axis and 0.5 to 1.5 units on the Dim2 axis, indicating similar and less pronounced effects on the measured traits.
Panel B shows the PCA loading plot, where the vectors representing the different traits provide insights into their contributions to the principal components. An, Chla, Chlb, Chla+b, and β-car are closely aligned with the OF+BF cluster. These vectors extend from the origin towards the upper right quadrant, with angles between 15° and 45° relative to Dim1. The lengths of these vectors range from approximately 0.7 to 0.9 units, indicating a strong positive contribution to Dim1, which distinguishes the OF+BF and RS+BF treatments from the others. The vectors for WUE and Ci are directed towards the lower right quadrant, with angles between −15° and −30° relative to Dim1, and have shorter lengths, around 0.5 to 0.7 units. This directionality and length suggest that these traits are more associated with IOF.
Panel C presents the hierarchical clustering heatmap divided into two main clusters, I and II. Cluster I include the CNT, DA BF, and RS treatments, which show relatively lower values for most traits. CNT is characterized by low values across the board, particularly in An, Chla, Chlb, Chla+b, and β-car, with values ranging from −1.5 to −2.0 standardized units. In contrast, Cluster II encompasses the OF+BF, RS+BF, and BF treatments that exhibit higher trait values. OF+BF shows increased An, Chla, Chlb, Chla+b, and β-car levels, with standardized values ranging from 1.5 to 2.0 units, indicating a strong positive impact on these physiological and biochemical parameters.
Within Cluster II, the IOF forms a distinct subgroup, particularly influencing WUE and Ci. The WUE for IOF is higher, with standardized values around 1.5 units, compared to other treatments in Cluster II. This indicates that the IOF treatment, while enhancing water use efficiency, operates through different physiological pathways than the biofertilizer-based treatments. The clustering pattern observed in the heatmap reinforces the unique impacts of the OF+BF, which consistently shows the highest positive values across multiple traits, distinguishing it from both CNT and IOF.
3.5. Gene Expression
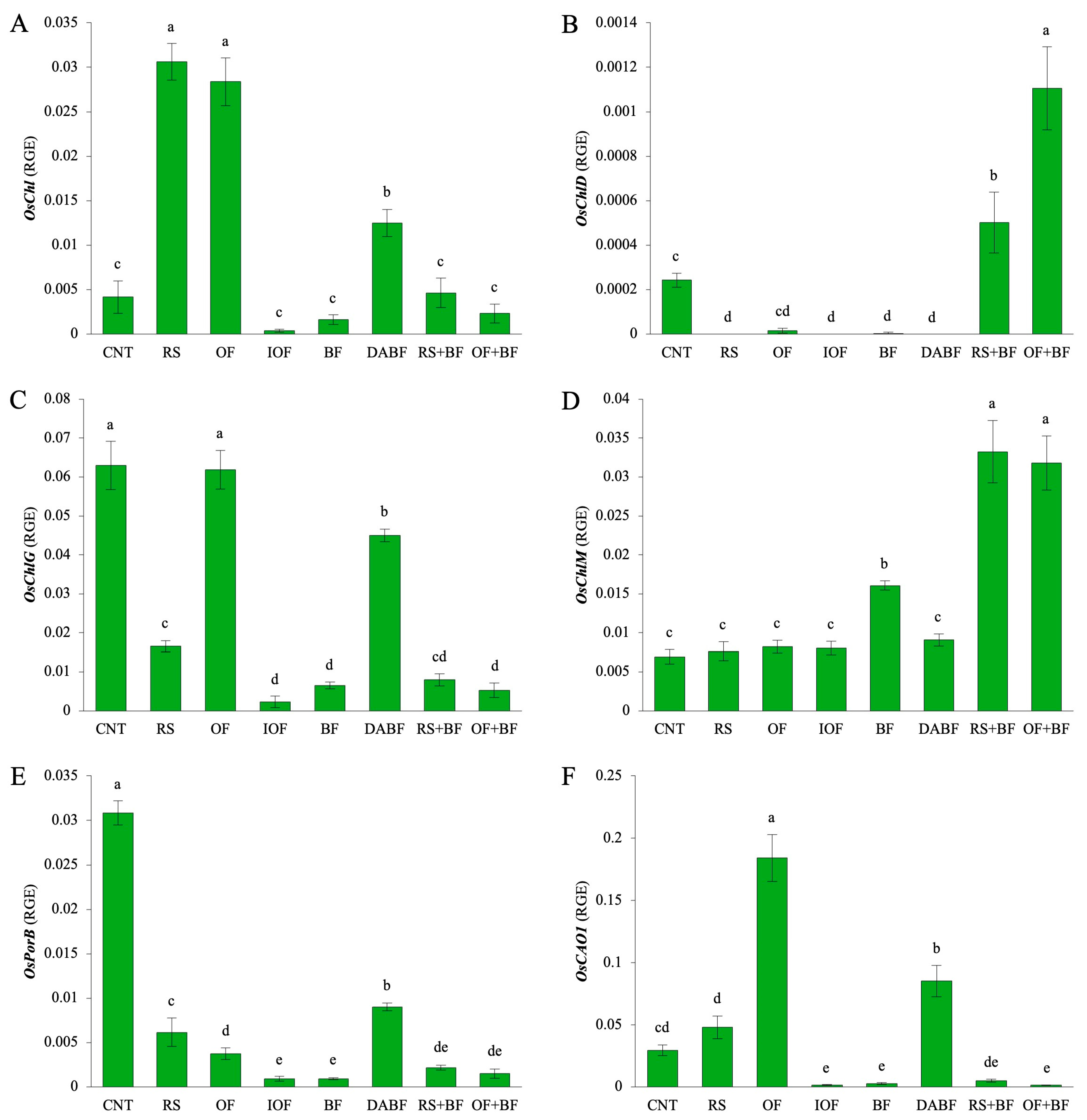
Figure 5A displays the expression of OsChl, a gene involved in chlorophyll synthesis. The rice straw (RS) treatment exhibited the highest expression level, with a mean value of 0.031 units, representing a 7.8-fold increase compared to the control (CNT, mean value 0.004 units). The organic fertilizer (OF) treatment also increased, showing 7.1-fold rise over the control. In contrast, the inorganic fertilizer (IOF) and biofertilizer (BF) treatments led to lower expression levels, with reductions to 11% and 42%, respectively, compared to the control. Figure 5B illustrates the expression of OsChlD, a gene involved in chlorophyll metabolism. The highest expression level was observed in the organic fertilizer combined with biofertilizer (OF+BF) treatment, with a mean value of 0.0011 units, showing a 4.4-fold increase over the control (0.00025 units). This indicates that OF+BF significantly enhances chlorophyll formation via OsChlD. RS+BF also showed increases, although to a lesser extent, while RS, OF, and BF alone exhibited much lower expression levels, suggesting a reduced impact on this gene. Figure 5C highlights the expression of OsChlG, a gene that plays a role in forming chlorophyll–protein complexes. The CNT condition resulted in the highest expression, and the OF and deactivated biofertilizer (DA BF) treatments also resulted in a significant OsChlG expression. The OF+BF treatment exhibited a weak expression, with a mean value of 0.005 units, indicating a level that reached only 8% of the control (0.063 units). Thus, the formation of chlorophyll–protein complexes via OsChlG is not involved in the accumulation of chlorophyll resulting from the OF+BF treatment. Figure 5D presents the expression of OsCHLM, a gene associated with the methylation of chlorophyll. The RS+BF treatment showed the highest expression level, with a mean of 0.033 units, representing a 4.8-fold increase over the control. OF+BF similarly increased the OsCHLM expression, underscoring its positive influence on the chlorophyll biosynthesis pathway. However, RS, OF, and DA BF treatments exhibited lower expression levels, indicating a lesser capacity to enhance the methylation process. Figure 5E focuses on the expression of OsPorB, a gene involved in chlorophyll biosynthesis under low-light conditions. The CNT condition yielded the highest expression level, with a mean value of 0.033 units. DA BF also showed a moderate expression of OsPorB (0.009 units). At the same time, the BF, RS+BF, and OF+BF treatments exhibited much lower levels, indicating that the BF treatments do not affect chlorophyll biosynthesis through OsPorB. Figure 5F shows the expression of OsCAO1, a gene responsible for converting chlorophyll a to chlorophyll b. The OF treatment demonstrated the highest expression, with a mean value of 0.184 units, representing a 6.1-fold increase compared to the control (0.030 units). The RS and DA BF treatments exhibited moderate increases, while BF, RS+BF, and OF+BF showed significantly lower expression levels, highlighting their limited role in enhancing the conversion of chlorophyll types required for efficient light absorption.
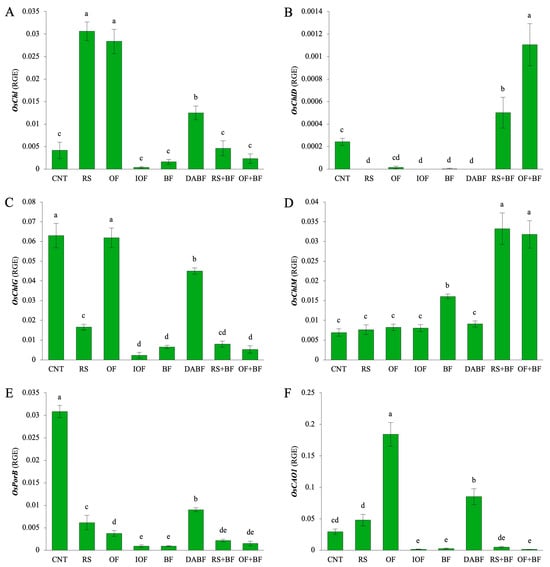
Figure 5.
Biofertilizer combined with organic fertilizer increased the expression levels of key chlorophyll-related genes in rice: gene expression levels of (A) OsChl, (B) OsChlD, (C) OsCHLG, (D) OsCHLM, (E) OsPorB, and (F) OsCAO1 under control (CNT), rice straw (RS), organic fertilizer (OF), inorganic fertilizer, biofertilizer (BF), deactivated biofertilizer (DA BF), rice straw + biofertilizer (RS+BF), and organic fertilizer + biofertilizer (OF+BF) conditions. RGE: Relative Gene Expression for the expression levels of a target gene relative to a housekeeping gene. Error bars represent ±SD (n = 3). Values indicated by the same letter are not statistically different among all treatments (Tukey’s HSD test, p < 0.05).
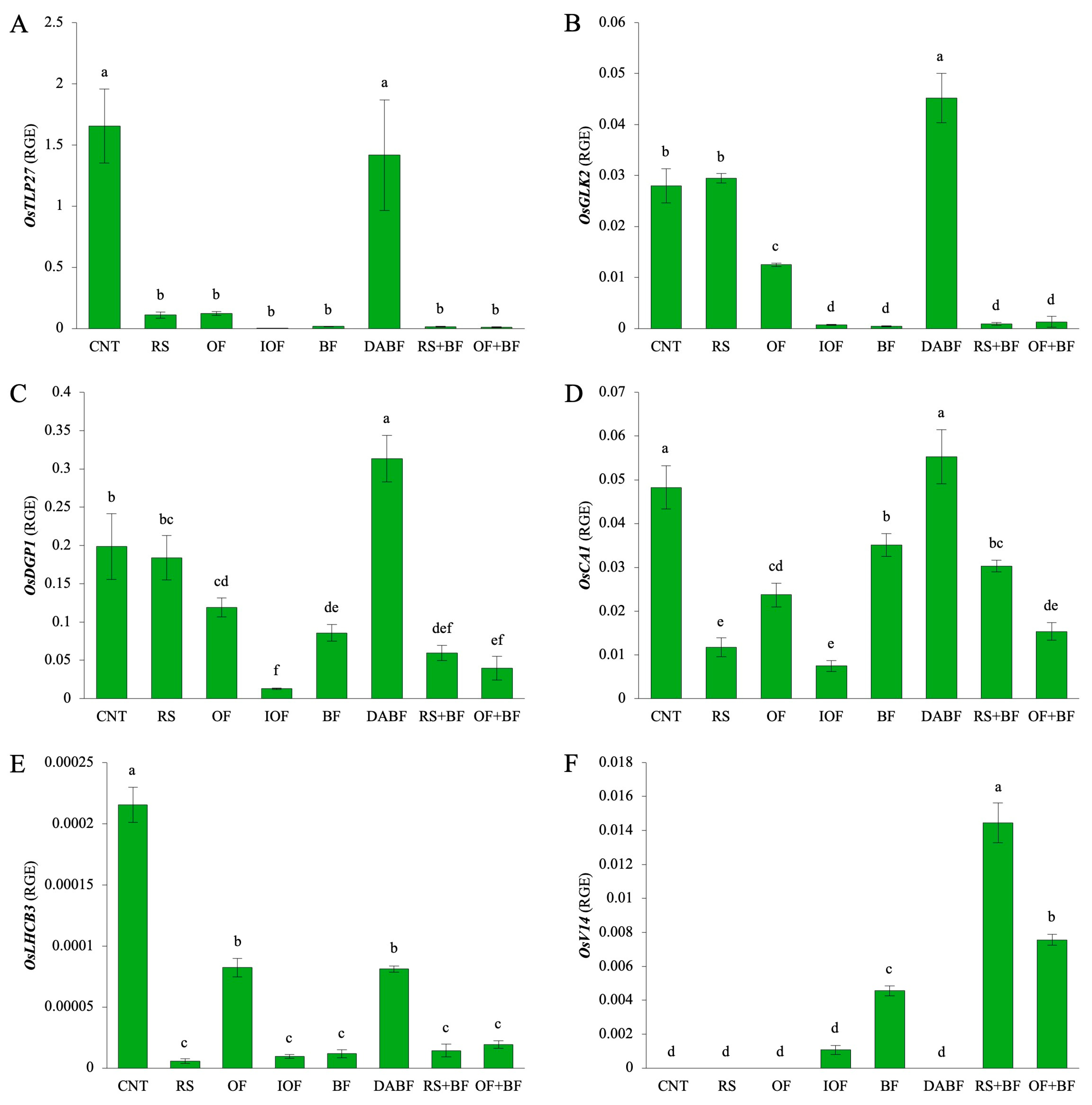
Figure 6A shows the expression of OsTLP27, a gene involved in leaf expansion and photosynthesis. The CNT condition exhibited the highest expression level, with values of around 1.66 relative expression units, which is significantly higher than all other treatments. DA BF also showed a relatively high expression level of approximately 1.42 units, which is similar to that of the control. In contrast, treatments like RS, OF, IOF, BF, RS+BF, and OF+BF showed significantly lower expression levels, all of which were below 0.02 units, indicating minimal impact on OsTLP27 expression. Figure 6B displays the expression of OsGLK2, a gene regulating chloroplast development. The highest expression was recorded for DA BF, with 0.0452 units, while RS showed a slight increase of about 0.0295 units. This was 45% lower than that of DA BF but significantly higher than those of the other treatments. OF, IOF, BF, RS+BF, and OF+BF exhibited lower expression levels compared to the control (0.0280 units), ranging from 0.0007 to 0.0125 units, indicating that these treatments had a lesser effect on OsGLK2 expression. Figure 6C highlights the expression of OsDGP1, a gene linked to photosynthetic protein accumulation. The highest expression was seen for DA BF, with a value of around 0.313 units, which was significantly higher than those of all other treatments. The different treatments, including RS, OF, IOF, BF, and RS+BF, exhibited lower expression levels compared to the control (0.0199 units), ranging from 0.013 to 0.184 units. IOF exhibited the lowest expression level, reflecting a 96% decrease compared to the highest value. Figure 6D presents the expression of OsαCA1, a gene involved in carbon fixation. DA BF exhibited the highest expression level of approximately 0.0553 units, which was significantly higher than those of all other treatments. The remaining treatments, including RS, OF, IOF, BF, RS+BF, and OF+BF, exhibited lower expression levels, reflecting a 28% to 85% decrease compared to the control (0.0482 units). Figure 6E shows the expression of OsLHCB3, a gene involved in light-harvesting complex formation. The CNT condition led to the highest expression level of approximately 0.00022 units, which was significantly higher than those of all other treatments. The treatments of RS, OF, IOF, BF, RS+BF, and OF+BF exhibited lower expression levels, reflecting a 73% to 97% decrease compared to the control. Figure 6F presents the expression of OsV14, a gene involved in the stress response and chloroplast development. RS+BF showed the highest expression level of about 0.0145 units, and OF+BF also resulted in a high expression level of 0.0076 units. BF exhibited a moderate expression level of 0.0046 units, which was about 68% lower than the highest expression. CNT, RS, OF, and DABF exhibited very low expression levels, which were below the detection limit.
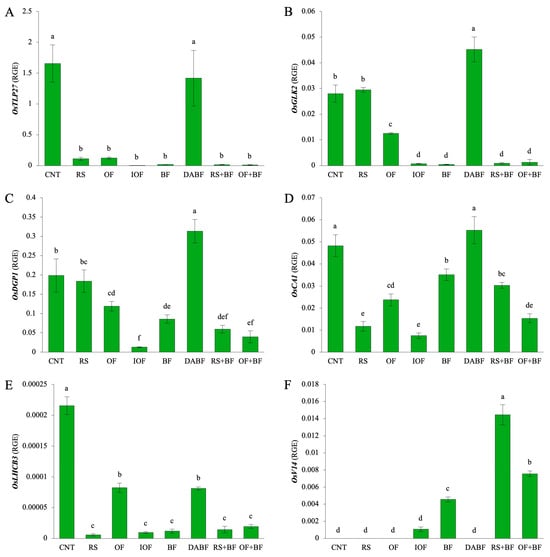
Figure 6.
Biofertilizer combined with organic fertilizer increased the expression levels of six key genes related to photosynthesis and chloroplast development in rice: gene expression levels of (A) OsTLP27, (B) OsGLK2, (C) OsDGP1, (D) OsαCA1, (E) OsLHCB3, and (F) OsV14 under control (CNT), rice straw (RS), organic fertilizer (OF), inorganic fertilizer (IOF), biofertilizer (BF), deactivated biofertilizer (DA BF), rice straw + biofertilizer (RS+BF), and organic fertilizer + biofertilizer (OF+BF) conditions. RGE: Relative Gene Expression for the expression levels of a target gene relative to a housekeeping gene. Error bars represent ±SD (n = 3). Values indicated by the same letter are not statistically different (Tukey’s HSD test, p < 0.05).
4. Discussion
4.1. Rice Growth Parameters
The results of this study indicate that the combination of biofertilizers with organic fertilizers enhances rice growth parameters, including plant height and tiller number. The OF+BF treatment demonstrated superior performance, resulting in a 31% increase in plant height and a 135% increase in tiller number. The results of this study indicate that the combination of biofertilizers with organic fertilizers enhances rice growth parameters, including plant height, tiller number, and vegetative vigor. This finding aligns with research showing that biofertilizers, when combined with organic inputs, optimize nutrient availability and support more vigorous growth than when either is applied alone [38,39,40]. The 135% increase in tiller number with the OF+BF treatment (18 tillers per plant) over the CNT (8 tillers per plant) indicates that this combination is highly effective in promoting vegetative growth, and it was demonstrated where biofertilizer treatments promoted better root development and nutrient absorption, leading to enhanced above-ground growth [40]. The synergistic between these inputs creates a favorable environment for rice growth, as the complementary action of biofertilizers and organic fertilizers improves nutrient use efficiency. The observed increase in plant height and tiller number under the OF+BF treatment can be attributed to the presence of beneficial microorganisms in biofertilizers, such as nitrogen-fixing bacteria with Rhizobium, Azospirillum, and phosphorus-solubilizing bacteria with Pseudomonas, Bacillus, which improve nutrient uptake by making soil nutrients more accessible to plants [40,41,42]. The individual treatments showed varied effectiveness. The inorganic fertilizer (IOF) treatment increased plant height by 20% and tiller number by 90% compared to the control, providing a significant but short-term growth boost. Similarly, the biofertilizer (BF) treatment increased plant height by 15% and tiller number by 55%. However, CNT, BF, DABF, RS, OF, and RS+BF were less effective than the combined organic fertilizer and biofertilizer (OF+BF) treatment, highlighting the importance of nutrient synergy for sustained growth. When biofertilizers are combined with organic fertilizers, they supply nutrients leading to vigorous plant growth, as shown in studies on rice yield improvement using integrated organic and bio-based inputs [42]. This demonstrates the potential of integrating biofertilizers with organic fertilizers, which is important for sustainable agriculture. For farmers, this combination reduces their reliance on synthetic fertilizers, which can lead to long-term soil degradation, water contamination, and greenhouse gas emissions. Organic fertilizers release nutrients, while biofertilizers improve nutrient uptake through beneficial microbial activity, creating a synergistic effect that enhances soil fertility and plant resilience [43,44,45,46,47]. These results demonstrate the potential of integrated nutrient management in enhancing rice production, particularly in systems that rely on sustainable agricultural practices. Notably, incorporating biofertilizers enriches the soil microbiome, sustaining a dynamic ecosystem that can better support plant health and resilience against environmental stresses [48,49]. The rice straw (RS) and deactivated biofertilizer (DABF) treatments showed limited effectiveness. RS produced plants averaging 65 cm in height with minimal improvement in tiller number, likely due to the slow nutrient release from straw decomposition. Similarly, DABF resulted in reduced plant height and tiller number, comparable to the control, suggesting that sterilization diminished the biofertilizer’s effectiveness. These results align with studies emphasizing the role of active microbes in biofertilizers for improving nutrient uptake [50,51]. A similar result was observed in a study in which combined biofertilizers with organic fertilizers enhanced plant growth more than single treatments [51,52,53]. While inorganic fertilizers provide immediate nutrient availability, their effects are often short-lived and can lead to soil degradation over time. Inorganic fertilizers do not enhance soil microbial activity or structure, both of which are essential for sustained plant growth and soil health [53,54,55]. The combined use of organic and biofertilizers improves soil structure, leading to sustained growth and higher crop yields over the long term [50,56]. These findings support the growing advocacy for integrated nutrient management (INM) as a sustainable strategy to boost agricultural productivity. INM emphasizes the balanced use of organic inputs, biofertilizers, and minimal chemical fertilizers, maximizing nutrient use efficiency. The ability of the OF+BF treatment to maintain soil fertility, promote plant health, and enhance nutrient availability highlights its potential to be a key tool in sustainable rice production systems. The OF+BF treatment not only demonstrates its efficacy in improving rice growth but also aligns with the broader objectives of SDG 2. By enhancing productivity, integrating biofertilizers with organic fertilizers can help address the challenges posed by climate change, declining soil fertility, and the need to feed a growing global population.
4.2. Photosynthetic Rate
The OF+BF treatment demonstrated a significant impact on photosynthetic efficiency, achieving a 74% increase in the net photosynthetic rate (An) compared to the control, reaching 48.23 μmol CO2 m−2 s−1 Figure 3A). This substantial improvement in photosynthetic capacity is linked to a 42% increase in chlorophyll content, as observed through SPAD measurements, which averaged 44.67 compared to 31.52 in the control (Figure 2C). This substantial improvement in photosynthetic capacity is linked to the 42% increase in chlorophyll content observed through SPAD measurements. The OF+BF treatment resulted in the highest net photosynthetic rate (An) among all treatments, indicating an improvement in the plant’s ability to convert light energy into chemical energy. This improvement can be attributed to nutrient availability, increased chlorophyll content, and enhanced stomatal conductance [55,56,57]. The environmental benefits of this practice are notable, as the increased efficiency in photosynthesis and nutrient uptake reduces the need for chemical fertilizers, thereby lowering the environmental burden associated with their excessive use. By reducing the reliance on synthetic fertilizers, biofertilizers help to minimize nutrient leaching and runoff into water systems. This can lead to eutrophication and other harmful environmental effects [58,59,60]. Excessive chemical fertilizer use has been linked to the contamination of freshwater through the accumulation of nitrates and phosphates. These environmental issues promote algal blooms and deplete oxygen levels in aquatic ecosystems. The use of biofertilizers mitigates these risks by providing sustainable nutritional sources. Moreover, biofertilizers contribute to reducing soil degradation caused by the long-term use of chemical fertilizers. Continuous application of synthetic fertilizers can alter soil pH, reduce organic matter, and negatively impact beneficial microbial communities. Inversely, the integration of biofertilizers improves soil fertility by promoting microbial diversity and organic matter content [44,61,62].
Biofertilizers contribute to the enhancement of soil microbial activity, which leads to improved nutrient cycling. Microbial communities, particularly nitrogen-fixing bacteria like Rhizobium and Azospirillum, convert atmospheric nitrogen into a form that plants can readily absorb. Phosphorus-solubilizing bacteria break down insoluble forms of phosphorus in the soil, increasing its availability to plants. This increased nitrogen and phosphorus availability not only boosts chlorophyll production but also strengthens the plant’s metabolic processes, enabling crop growth [63,64,65]. The improved microbial interactions in the rhizosphere also enhance iron and zinc bioavailability. Biofertilizers improve both chlorophyll content and photosynthetic efficiency by enhancing the uptake of these micronutrients [66,67,68]. They also play an essential role in enhancing chlorophyll content because they improve the bioavailability of essential nutrients like nitrogen, magnesium, and iron, key elements required for chlorophyll biosynthesis. By enhancing the availability of these nutrients, biofertilizers directly support chlorophyll synthesis, thus improving the overall photosynthetic capacity of the plant [69,70]. Consequently, the improved soil structure resulting from organic matter decomposition by microorganisms creates an ideal environment for root expansion and efficient water uptake, which are necessary for sustaining photosynthesis under different environmental conditions [71,72]. Organic fertilizers provide a steady source of carbon for microbial populations, which helps improve soil aeration and water retention, allowing plants to maintain a stable supply of water and nutrients. This, in turn, supports the plant’s ability to regulate stomatal conductance (gs) and gas exchange, leading to more efficient CO2 uptake during photosynthesis (Figure 3A). The combination of biofertilizers with organic fertilizers likely stimulates microbial activity in the soil, leading to better root development and nutrient absorption, which further supports photosynthetic efficiency. These findings align with previous research that highlights the positive effects of biofertilizers on photosynthetic efficiency, particularly in promoting beneficial microorganisms in the rhizosphere and improving nutrient uptake, especially nitrogen and phosphorus, which are key elements in the photosynthetic process [72]. These synergistic effects illustrate the potential of integrating biofertilizers with organic fertilizers as a sustainable approach to improving rice production while minimizing chemical inputs [43]. Moreover, the increase in photosynthetic rate in the OF+BF treatment can be linked to higher chlorophyll content, as chlorophyll is essential for capturing light energy during photosynthesis. Chlorophyll absorbs light primarily in the blue and red wavelengths, driving the light-dependent reactions of photosynthesis that produce ATP and NADPH, essential energy molecules used in carbon fixation. By enhancing chlorophyll content, biofertilizers ensure that rice plants can capture more light energy, resulting in higher rates of photosynthesis and energy conversion [63]. Additionally, the improvement in stomatal conductance (gs) observed in the OF+BF treatment likely contributed to the enhanced photosynthetic rate. Stomatal conductance is fundamental for regulating gas exchange, including the uptake of carbon dioxide (CO2) required for photosynthesis. The higher gs values in the OF+BF treatment suggest that these plants were better able to regulate their stomata to optimize CO2 uptake, thereby supporting a higher photosynthetic rate [73]. In contrast, the lower photosynthetic rates observed in the control (CNT), rice straw (RS), and deactivated biofertilizer (DABF) treatments suggest that these treatments were less effective in promoting the physiological conditions necessary for optimal photosynthesis. Single-component or less-active fertilization strategies may not provide the comprehensive nutrient support needed to sustain high levels of photosynthetic activity [74]. This study reinforces the importance of integrated nutrient management, particularly the combination of biofertilizers and organic fertilizers, in achieving sustainable agricultural practices that support enhanced photosynthetic efficiency and overall crop productivity.
4.3. Chlorophyll-Related Gene Expression
The expression analysis of chlorophyll-related genes reveals significant variations across different fertilizer treatments. The combination of organic fertilizer and biofertilizer (OF+BF) notably affected the key genes involved in chlorophyll biosynthesis and regulation. The OF+BF treatment led to an upregulation of OsChlD and OsCHLM, which are critical for chlorophyll synthesis and photoperiod-regulated production (Figure 5A,D). Specifically, the OF+BF treatment showed a 4.4-fold increase in OsChlD expression and a 4.6-fold increase in OsCHLM expression compared to the control. Chlorophyll biosynthesis involves the tetrapyrrole pathway, synthesizing chlorophyll from precursor molecules like protoporphyrin IX. The upregulation of OsChlD, encoding a subunit of magnesium chelatase, enhances the magnesium insertion step in this pathway, converting protoporphyrin IX into Mg-protoporphyrin IX, essential for photosynthesis [74,75,76]. This suggests that the OF+BF combination not only enhances chlorophyll production but also improves the overall nutritional status of the plants, contributing to better growth and photosynthetic efficiency. Studies have shown that the presence of specific nutrients in biofertilizers can directly influence the expression of chlorophyll-related genes, further supporting the observed enhancements [49,77]. The interactions between organic fertilizers and beneficial microbes can create a more favorable environment for nutrient uptake, which may explain the significant differences in gene expression between treatments. The presence of nitrogen-fixing bacteria in biofertilizers likely boosts nitrogen availability, which is important for chlorophyll biosynthesis and the expression of chlorophyll-related genes like OsChlD and OsCHLM. Interestingly, while OsChlD and OsCHLM were upregulated, the expression of OsChl and OsCHLG was lower in the OF+BF treatment compared to other treatments, such as rice straw (RS) and organic fertilizer (OF). OsChl expression decreased by 54%, and OsCHLG decreased by 92% in the OF+BF treatment relative to the control (Figure 5A,C). This differential expression could indicate that the OF+BF combination triggers regulatory feedback mechanisms that modulate the overall chlorophyll synthesis pathway, balancing production with the plant’s photosynthetic requirements [76,78,79,80]. Such feedback mechanisms are essential to prevent the overaccumulation of chlorophyll. By downregulating certain chlorophyll genes like OsChl and OsCHLG, the plant ensures that chlorophyll synthesis is tightly regulated in response to environmental light levels and nutrient availability. Additionally, the response of OsPorB, involved in chlorophyll synthesis, was also reduced in the OF+BF treatment compared to RS and OF, suggesting symbiotic relationship interactions where biofertilizers might influence specific chlorophyll synthesis genes differently from organic fertilizers alone [77,81,82]. By downregulating genes like OsChl and OsCHLG, the plant regulates chlorophyll synthesis in response to environmental light levels and nutrient availability. The expression of OsPorB, involved in chlorophyll synthesis, was reduced in the OF+BF treatment compared to RS and OF, suggesting that biofertilizers influence specific chlorophyll synthesis genes differently from organic fertilizers alone. OsPorB encodes protochlorophyllide oxidoreductase, an enzyme that converts protochlorophyllide to chlorophyllide. Its expression decreased by 5% under OF+BF treatment conditions compared to the control (Figure 5E). This reduction might indicate that increased chlorophyll levels suppress further gene expression, preventing oxidative stress [75]. Our study differs from earlier research by demonstrating the specific upregulation of chlorophyll-related genes like OsChlD and OsCHLM when biofertilizers are combined with organic fertilizers. This gene expression analysis adds a new dimension to understanding how biofertilizers enhance photosynthetic efficiency, less explored in previous studies focused on agronomic parameters. The lack of microbial activity in the DABF treatment likely impairs nutrient cycling, particularly nitrogen and magnesium, which are essential for chlorophyll biosynthesis, leading to reduced gene expression. OsChl expression in DABF decreased by 56% compared to that in OF, emphasizing the importance of maintaining a healthy soil microbiome. Beneficial microbes in biofertilizers enhance nutrient availability and interact with plant signaling pathways to upregulate genes involved in photosynthesis and chlorophyll production [56,79].
5. Limitations and Future Research
The experiment was conducted in a controlled climate chamber using potted plants. This limits the scalability and real-world applicability of the findings. The controlled environment does not fully replicate the complexity of field conditions, where factors such as soil variability and weather fluctuations can influence outcomes. Additionally, the short-term duration of one growing season may have been insufficient to observe the complete effect of biofertilizers and organic amendments, which often require longer periods to decompose. Therefore, extending the experiment over multiple growing seasons and in different agroecological zones is needed to capture the long-term effects on soil properties and plant productivity.
6. Conclusions
The integration of biofertilizers with organic fertilizers (OF+BF) significantly improves rice growth, photosynthetic efficiency, and expression of chlorophyll-related genes compared to other treatments. The OF+BF treatment consistently resulted in the tallest plants, highest tiller numbers, and highest photosynthetic activity, indicating a synergistic effect. It also upregulated genes involved in chlorophyll biosynthesis and photosynthetic regulation, such as OsChlD and OsCHLM, supporting the observed physiological benefits. Our data confirm that combining biofertilizers and organic fertilizers enhances plant height, tiller number, and photosynthetic rate by over 74% compared to all other treatments. These findings signify the potential of biofertilizers and organic fertilizers as sustainable alternatives to conventional farming practices, supporting global efforts to boost food security and combat climate change, particularly through the promotion of eco-friendly agricultural systems in alignment with SDGs 2 and 13.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su16219297/s1. Table S1: The fertilizers’ application method, timing, and quantities for each treatment; Table S2: Primer sequence of genes used in the experiment.
Author Contributions
Conceptualization, P.M., M.A. and T.M.; methodology, P.M., M.A. and T.M.; software, P.M. and M.A.; validation, P.M., M.A. and T.M.; formal analysis, P.M.; investigation, P.M.; resources, T.M.; data curation, P.M.; writing—original draft preparation, P.M.; writing—review and editing, M.A.; visualization, M.A.; supervision, T.M.; project administration, T.M.; funding acquisition, M.A. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant-in-Aid for JSPS Fellows (23KF0033) to M.A., a Grant-in-Aid for Scientific Research (24K08825) to T.M., and a Grant for promotion of the “KOME Co-creation Innovation” project from Niigata University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
We are grateful to K. Suzuki and N. Harada for valuable discussions at the early stage of this work. We thank the Japan International Cooperation Agency (JICA), DHET: UCDP Grant for the doctoral course scholarship provided to P.M. and Taio-Yuka Co., Ltd. for providing Tokyo8 biofertilizer and valuable advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashfaq, A.; Ahsan, R.; Muhammad, U.H.; Hesham, F.A.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; Ali, S.; et al. Impact of Climate Change on Agricultural Production; Issues, Challenges, and Opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Qin, M.; Zheng, E.; Hou, D.; Meng, X.; Meng, F.; Gao, Y.; Chen, P.; Qi, Z.; Xu, T. Response of Wheat, Maize, and Rice to Changes in Temperature, Precipitation, CO2 Concentration, and Uncertainty Based on Crop Simulation Approaches. Plants 2023, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Anwar, S.; Nawaz, T.; Fahad, S.; Saud, S.; Ur Rahman, T.; Khan, M.N.R.; Nawaz, T. Securing a Sustainable Future: The Climate Change Threat to Agriculture, Food Security, and Sustainable Development Goals. J. Umm Al-Qura Univ. Appl. Sci. 2024, 1–17. [Google Scholar] [CrossRef]
- Holerga, T.A.; Zemeleagă, C.G.; Chelaru Gaidargi, M.; Stoian, M. Adjustment of Agricultural Practices to Climate Change Effects. Proc. Int. Conf. Bus. Excell. 2024, 18, 1571–1581. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.; Kumari, A.; Jagadesh, M.; Singh, S.K.; Bhatt, R.; Singh, M.; Seth, C.S.; Li, Y. Climate Change Adaptation: Challenges for Agricultural Sustainability. Plant Cell Environ. 2024. [Google Scholar] [CrossRef]
- Siegel, F.R. Population Assessments: 2013–2050–2100: Growth, Stability, Contraction. In Countering 21st Century Social-Environmental Threats to Growing Global Populations; SpringerBriefs in Environmental Science; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–8. [Google Scholar] [CrossRef]
- Sahay, S.; Grzybowski, M.; Schnable, J.C.; Głowacka, K. Genetic control of photoprotection and photosystem II operating efficiency in plants. New Phytol. 2023, 239, 1068–1082. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Xiong, R.; Zeng, Y.; Zhang, J.; Tang, F.; Zeng, Y.; Huang, S. Effect of Climate Warming on the Grain Quality of Early Rice in a Double-Cropped Rice Field: A 3-Year Measurement. Front. Sustain. Food Syst. 2023, 7, 1133665. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cun, Z.; Chen, J.W. Photosynthetic performance and photosynthesis-related gene expression coordinated in a shade-tolerant species Panax notoginseng under nitrogen regimes. BMC Plant Biol. 2020, 20, 273. [Google Scholar] [CrossRef]
- Cutolo, E.A.; Guardini, Z.; Dall’Osto, L.; Bassi, R. A Paler Shade of Green: Engineering Cellular Chlorophyll Content to Enhance Photosynthesis in Crowded Environments. New Phytol. 2023, 239, 1567–1583. [Google Scholar] [CrossRef]
- Li, S.; Fleisher, D.H.; Wang, Z.; Barnaby, J.; Timlin, D.; Reddy, V.R. Application of a Coupled Model of Photosynthesis, Stomatal Conductance and Transpiration for Rice Leaves and Canopy. Comput. Electron. Agric. 2021, 182, 106047. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving Photosynthesis and Crop Productivity by Accelerating Recovery from Photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Muhie, S.H. Optimization of Photosynthesis for Sustainable Crop Production. CABI Agric. Biosci. 2022, 3, 50. [Google Scholar] [CrossRef]
- Ambavaram, M.M.R.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated Regulation of Photosynthesis in Rice Increases Yield and Tolerance to Environmental Stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Si, J.; Zhou, D.; Wang, C.; Zhao, C.; Jia, B.; Qin, J.; Zhu, X. Leaf Chlorophyll Parameters and Photosynthetic Characteristic Variations with Stand Age in a Typical Desert Species (Haloxylon Ammodendron). Front. Plant Sci. 2022, 13, 967849. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on Improving Photosynthesis to Increase Crop Yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef]
- He, Y.; Duan, W.; Xue, B.; Cong, X.; Sun, P.; Hou, X.; Liang, Y.-K. OsαCA1 Affects Photosynthesis, Yield Potential, and Water Use Efficiency in Rice. Int. J. Mol. Sci. 2023, 24, 5560. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and Challenges of Microbial Application for Crop Improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef]
- Ngearnpat, N.; Chunhachart, O.; Kotabin, N.; Issakul, K. Comparative Assessment of Gamma-Polyglutamic Acid and Bacillus Subtilis Cells as Biostimulants to Improve Rice Growth and Soil Quality. J. Ecol. Eng. 2023, 24, 46–59. [Google Scholar] [CrossRef]
- Matisic, M.; Dugan, I.; Bogunovic, I. Challenges in Sustainable Agriculture—The Role of Organic Amendments. Agriculture 2024, 14, 643. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of Organic Amendments for Improving Coastal Saline Soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Pingali, P.L. Green Revolution: Impacts, Limits, and the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, G.; Vishwakarma, S.; Dalin, C.; Komarek, A.M.; Kanter, D.R.; Davis, K.F.; Pfeifer, K.; Zhao, J.; Zou, T.; et al. Quantitative Assessment of Agricultural Sustainability Reveals Divergent Priorities among Nations. One Earth 2021, 4, 1262–1277. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant Growth-Promoting Rhizobacteria Allow Reduced Application Rates of Chemical Fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-Solubilizing Bacteria: Their Agroecological Function and Optimistic Application for Enhancing Agro-Productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Bulda, O.V.; Rassadina, V.V.; Alekseichuk, H.N.; Laman, N.A. Spectrophotometric Measurement of Carotenes, Xanthophylls, and Chlorophylls in Extracts from Plant Seeds. Russ. J. Plant Physiol. 2008, 55, 544–551. [Google Scholar] [CrossRef]
- Yin, C.; Ma, B.; Wang, W.; Xiong, Q.; Zhao, H.; Chen, S.; Zhang, J. RNA Extraction and Preparation in Rice (Oryza sativa). Curr. Protoc. Plant Biol. 2016, 1, 411–418. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, Version 1.0.7. 2017. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 14 September 2024).
- Raivo, K. Pheatmap: Pretty Heatmaps, Version 1.0.12. 2010. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 14 September 2024).
- Bhuiyan, M.; Islam, A.; Sarkar, M.; Mamun, M.; Salam, M.; Kabir, M. Agronomic Management and Interventions to Increase Rice Yield in Bangladesh. Bangladesh Rice J. 2021, 24, 161–181. [Google Scholar] [CrossRef]
- Pawar, N.B.; Suryawanshi, N.S. Impact of Biofertilizers on Paddy (Oryza Sativa L.) Cultivar Jaya. Int. J. Adv. Res. Sci. Commun. Technol. 2022, 2, 122–128. [Google Scholar] [CrossRef]
- Zahurul Islam, M. Improvement of Yield Potential of Rice through Combined Application of Biofertilizer and Chemical Nitrogen. Afr. J. Microbiol. Res. 2012, 6, 745–750. [Google Scholar] [CrossRef]
- Iniesta-Pallarés, M.; Álvarez, C.; Gordillo-Cantón, F.M.; Ramírez-Moncayo, C.; Alves-Martínez, P.; Molina-Heredia, F.P.; Mariscal, V. Sustaining Rice Production through Biofertilization with N2-Fixing Cyanobacteria. Appl. Sci. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Win, K.T.; Oo, A.Z.; Ohkama-Ohtsu, N.; Yokoyama, T. Bacillus Pumilus Strain TUAT-1 and Nitrogen Application in Nursery Phase Promote Growth of Rice Plants under Field Conditions. Agronomy 2018, 8, 216. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Elsayed, S.I.M.; Glala, A.A.; Abdalla, A.M.; El-Sayed, A.E.G.A.; Darwish, M.A. Effect of Biofertilizer and Organic Fertilization on Growth, Nutrient Contents and Fresh Yield of Dill (Anethum graveolens). Bull. Natl. Res. Cent. 2020, 44, 122. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential Use of Beneficial Microorganisms for Soil Amelioration, Phytopathogen Biocontrol, and Sustainable Crop Production in Smallholder Agroecosystems. Front. Sustain. Food Syst. 2021, 5, 606308. [Google Scholar] [CrossRef]
- Singh, S.P.; Pandey, S.; Mishra, N.; Giri, V.P.; Mahfooz, S.; Bhattacharya, A.; Kumari, M.; Chauhan, P.; Verma, P.; Nautiyal, C.S.; et al. Supplementation of Trichoderma Improves the Alteration of Nutrient Allocation and Transporter Genes Expression in Rice under Nutrient Deficiencies. Plant Physiol. Biochem. 2019, 143, 351–363. [Google Scholar] [CrossRef]
- Ammar, E.E.; Rady, H.A.; Khattab, A.M.; Amer, M.H.; Mohamed, S.A.; Elodamy, N.I.; AL-Farga, A.; Aioub, A.A.A. A Comprehensive Overview of Eco-Friendly Bio-Fertilizers Extracted from Living Organisms. Environ. Sci. Pollut. Res. 2023, 30, 113119–113137. [Google Scholar] [CrossRef]
- Paramesh, V.; Kumar, P.; Bhagat, T.; Nath, A.J.; Manohara, K.K.; Das, B.; Desai, B.F.; Jha, P.K.; Prasad, P.V.V. Integrated Nutrient Management Enhances Yield, Improves Soil Quality, and Conserves Energy under the Lowland Rice–Rice Cropping System. Agronomy 2023, 13, 1557. [Google Scholar] [CrossRef]
- Amrutha, K.K.; Santhy, P. Influence of Different Management Practices and Fertilizer Levels on the Growth and Yield of Rice in Sodic Soil. Madras Agric. J. 2018, 105, 44. [Google Scholar] [CrossRef]
- Du, T.-Y.; He, H.-Y.; Zhang, Q.; Lu, L.; Mao, W.-J.; Zhai, M.-Z. Positive Effects of Organic Fertilizers and Biofertilizers on Soil Microbial Community Composition and Walnut Yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Shirzad, H.; Siavash Moghaddam, S.; Rahimi, A.; Rezapour, S.; Xiao, J.; Popović-Djordjević, J. Combined Effect of Biological and Organic Fertilizers on Agrobiochemical Traits of Corn (Zea mays L.) under Wastewater Irrigation. Plants 2024, 13, 1331. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Carrascosa, A.; Pascual, J.A.; López-García, Á.; Romo-Vaquero, M.; De Santiago, A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.D.M. Effects of Inorganic and Compost Tea Fertilizers Application on the Taxonomic and Functional Microbial Diversity of the Purslane Rhizosphere. Front. Plant Sci. 2023, 14, 1159823. [Google Scholar] [CrossRef] [PubMed]
- Jeya Bharathi, M.; Anbarasu, M.; Raghu, R.; Subramanian, E. Assessment of Soil Microbial Diversity and Soil Enzyme Activities under Inorganic Input Sources on Maize and Rice Ecosystems. Saudi J. Biol. Sci. 2024, 31, 103978. [Google Scholar] [CrossRef] [PubMed]
- Ollio, I.; Santás-Miguel, V.; Gómez, D.S.; Lloret, E.; Sánchez-Navarro, V.; Martínez-Martínez, S.; Egea-Gilabert, C.; Fernández, J.A.; Calviño, D.F.; Zornoza, R. Effect of Biofertilizers on Broccoli Yield and Soil Quality Indicators. Horticulturae 2023, 10, 42. [Google Scholar] [CrossRef]
- Naher, U.A.; Biswas, J.C.; Maniruzzaman, M.; Khan, F.H.; Sarkar, M.I.U.; Jahan, A.; Hera, M.H.R.; Hossain, M.B.; Islam, A.; Islam, M.R.; et al. Bio-Organic Fertilizer: A Green Technology to Reduce Synthetic N and P Fertilizer for Rice Production. Front. Plant Sci. 2021, 12, 602052. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.M.; Olasehinde, D.A.; Abadunmi, T. The Role of Biofertilizers in Sustainable Agriculture: An Eco-Friendly Alternative to Conventional Chemical Fertilizers. Appl. Sci. Eng. Prog. 2024, 17, 6883. [Google Scholar] [CrossRef]
- Pourhosseini, S.H.; Azizi, A.; Sadat Seyedi, F.; Hadian, J. Bio-Fertilizer as a Pathway to Minimize Nitrate Leaching from Chemical Fertilizer in High Yield Peppermint Production. J. Clean. Prod. 2024, 468, 143100. [Google Scholar] [CrossRef]
- Just, B.S.; Marks, E.A.N.; Roquer-Beni, L.; Llenas, L.; Ponsà, S.; Vilaplana, R. Biofertilization increases soil organic carbon concentrations: Results of a meta-analysis. Int. J. Agric. Sustain. 2024, 22, 2361578. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.; Pei, Z.; Jiang, L.; Zhang, Y.; Wang, J.; Wang, J. Application of microbial organic fertilizers promotes the utilization of nutrients and restoration of microbial community structure and function in rhizosphere soils after dazomet fumigation. Front. Microbiol. 2023, 13, 1122611. [Google Scholar] [CrossRef]
- Nayak, M.; Swain, D.K.; Sen, R. Strategic Valorization of De-Oiled Microalgal Biomass Waste as Biofertilizer for Sustainable and Improved Agriculture of Rice (Oryza sativa L.) Crop. Sci. Total Environ. 2019, 682, 475–484. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Lu, D.; Cheng, L.; Li, J. Effects of Biofertilizers on the Growth, Leaf Physiological Indices and Chlorophyll Fluorescence Response of Spinach Seedling. PLoS ONE 2023, 18, e0294349. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Gu, C.; Zhong, P.; Song, N.; Li, M.; Dai, Z.; Fang, X.; Liu, Z.; Zhang, J.; et al. A Plant-Derived Natural Photosynthetic System for Improving Cell Anabolism. Nature 2022, 612, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.L.; Pandey, R.N.; Kumar, D.; Sharma, V.K.; Meena, M.D.; Karwal, M.; Dutta, D.; Meena, L.K.; Narwal, E.; Mishra, R.P.; et al. Impacts of Long-Term Rice-Based Organic Farming on Fractions and Forms of Soil Organic Carbon and Nitrogen in the Indo-Gangetic Plain. Soil Res. 2022, 61, 159–175. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; Mariscal, V.; Molina-Heredia, F.P.; Álvarez, C.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Plant Growth-Promoting Rhizobacteria Improve Rice Response to Climate Change Conditions. Plants 2023, 12, 2532. [Google Scholar] [CrossRef]
- Domingo, C.; San Segundo, B. Rice Thematic Special Issue: Beneficial Plant–Microbe Interactions in Rice. Rice 2023, 16, 50. [Google Scholar] [CrossRef]
- Islam, T.; Fatema; Hoque, M.N.; Gupta, D.R.; Mahmud, N.U.; Sakif, T.I.; Sharpe, A.G. Improvement of Growth, Yield and Associated Bacteriome of Rice by the Application of Probiotic Paraburkholderia and Delftia. Front. Microbiol. 2023, 14, 1212505. [Google Scholar] [CrossRef]
- Shan, S.; Wei, Z.; Cheng, W.; Du, D.; Zheng, D.; Ma, G. Biofertilizer Based on Halotolerant Microorganisms Promotes the Growth of Rice Plants and Alleviates the Effects of Saline Stress. Front. Microbiol. 2023, 14, 1165631. [Google Scholar] [CrossRef]
- Sarkodee-Addo, E.; Tokiwa, C.; Bonney, P.; Aboagye, D.A.; Yeboah, A.; Abebrese, S.O.; Bam, R.; Nartey, E.K.; Okazaki, S.; Yasuda, M. Biofertilizer Activity of Azospirillum Sp. B510 on the Rice Productivity in Ghana. Microorganisms 2021, 9, 2000. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.-B. Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient Soil Microorganisms: A New Dimension for Sustainable Agriculture and Environmental Development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Tan, G.; Fan, J.; Chen, Y.; Xiao, Y.; Wu, S.; Zhi, Q.; Liu, T.; Yin, H.; et al. Enhancing Plant Growth in Biofertilizer-Amended Soil through Nitrogen-Transforming Microbial Communities. Front. Plant Sci. 2023, 14, 1259853. [Google Scholar] [CrossRef]
- Javaid, A. Effects of Biofertilizers Combined with Different Soil Amendments on Potted Rice Plants. Chil. J. Agric. Res. 2011, 71, 157–163. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Kim, E.-Y.; Han, S.-H.; Piao, W.; An, G.; Todaka, D.; Yamaguchi-Shinozaki, K.; Paek, N.-C. Rice Phytochrome-Interacting Factor-Like1 (OsPIL1) Is Involved in the Promotion of Chlorophyll Biosynthesis through Feed-Forward Regulatory Loops. J. Exp. Bot. 2017, 68, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, H.; Guo, R.; Fan, J.; Liu, S.; Liao, J.; Huang, Y.; Wang, Z. Physiological, Cytological, and Transcriptomic Analysis of Magnesium Protoporphyrin IX Methyltransferase Mutant Reveal Complex Genetic Regulatory Network Linking Chlorophyll Synthesis and Chloroplast Development in Rice. Plants 2023, 12, 3785. [Google Scholar] [CrossRef]
- Cui, J.; Nishide, N.; Mashiguchi, K.; Kuroha, K.; Miya, M.; Sugimoto, K.; Itoh, J.-I.; Yamaguchi, S.; Izawa, T. Fertilization Controls Tiller Numbers via Transcriptional Regulation of a MAX1-like Gene in Rice Cultivation. Nat. Commun. 2023, 14, 3191. [Google Scholar] [CrossRef]
- Dawood, M.G.; Sadak, M.S.; Abdallah, M.M.S.; Bakry, B.A.; Darwish, O.M. Influence of Biofertilizers on Growth and Some Biochemical Aspects of Flax Cultivars Grown under Sandy Soil Conditions. Bull. Natl. Res. Cent. 2019, 43, 81. [Google Scholar] [CrossRef]
- Siswanti, D.U.; Riesty, O.S. Effects of Biofertilizer and Manure Application on Growth Rate and Chlorophyll Content of Spinach (Amaranthus tricolor L.) under Salinity Stress Condition. BIO Web Conf. 2021, 33, 05003. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Li, X.; Lu, C. The Role of Chloroplast Gene Expression in Plant Responses to Environmental Stress. Int. J. Mol. Sci. 2020, 21, 6082. [Google Scholar] [CrossRef]
- Jung, K.-H.; Hur, J.; Ryu, C.-H.; Choi, Y.; Chung, Y.-Y.; Miyao, A.; Hirochika, H.; An, G. Characterization of a Rice Chlorophyll-Deficient Mutant Using the T-DNA Gene-Trap System. Plant Cell Physiol. 2003, 44, 463–472. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, T.; Zhiguo, E.; Niu, B.; Chen, C. OsNF-YB7 inactivates OsGLK1 to inhibit chlorophyll biosynthesis in rice embryo. eLife 2024, 13, RP96553. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).