The Management of Fungal Diseases in Organic Production Systems Through a Mixture of Durum Wheat Varieties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
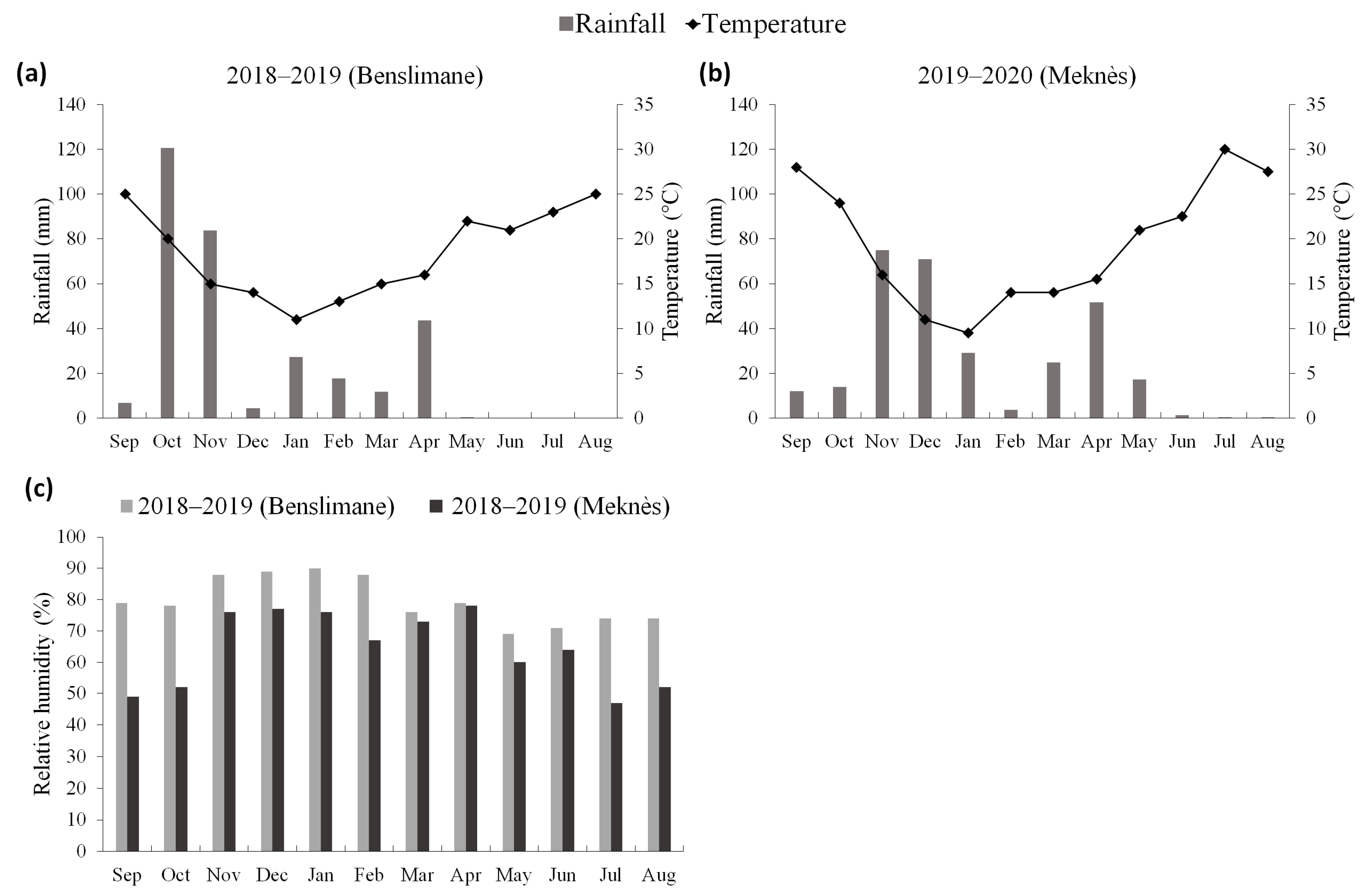
3.1. Climate Data
3.2. Severity of Leaf Rust, Leaf Blight (HLB), and Fusarium (FHB)
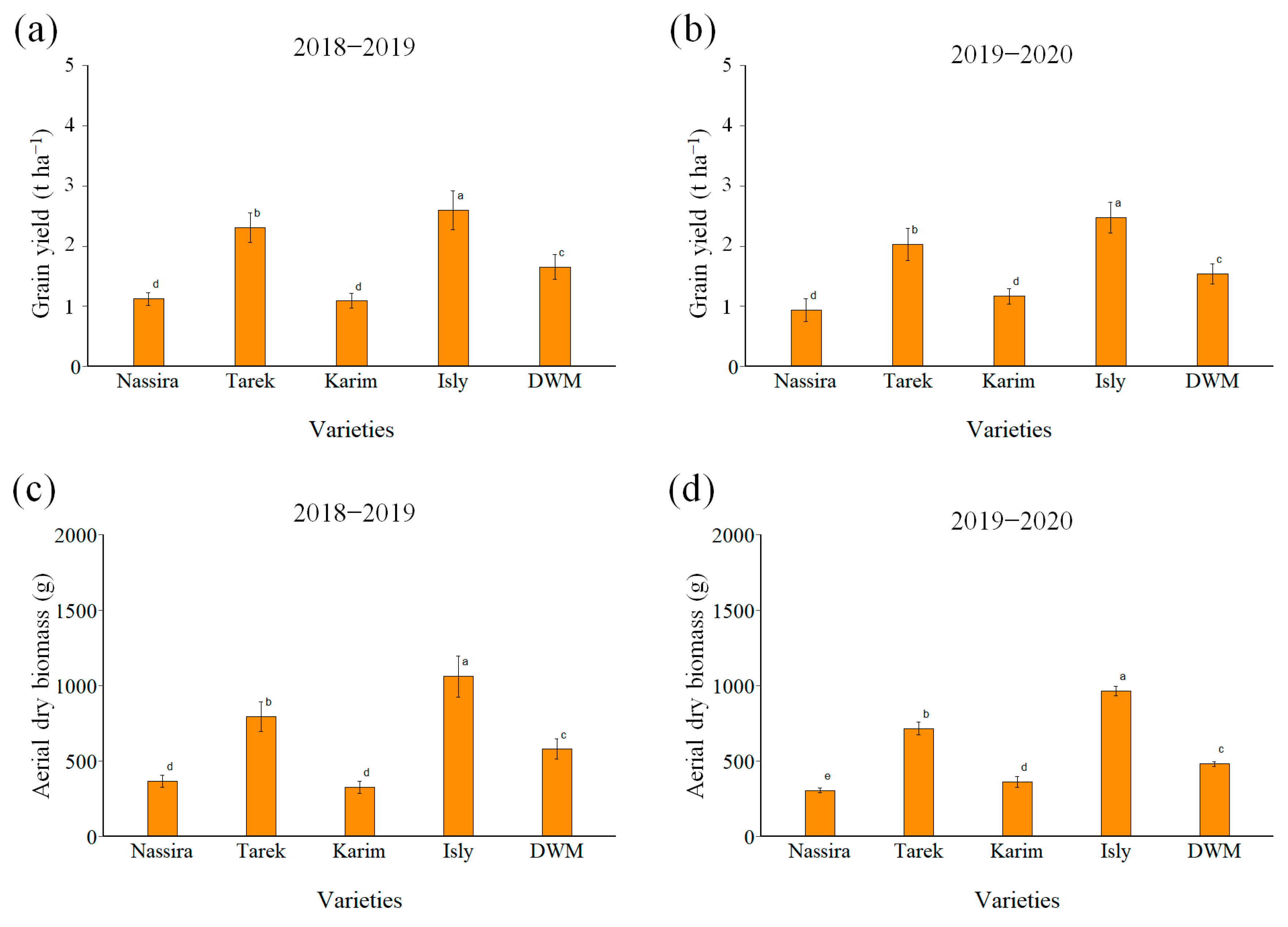
3.3. Biomass Production
3.4. Yield Components
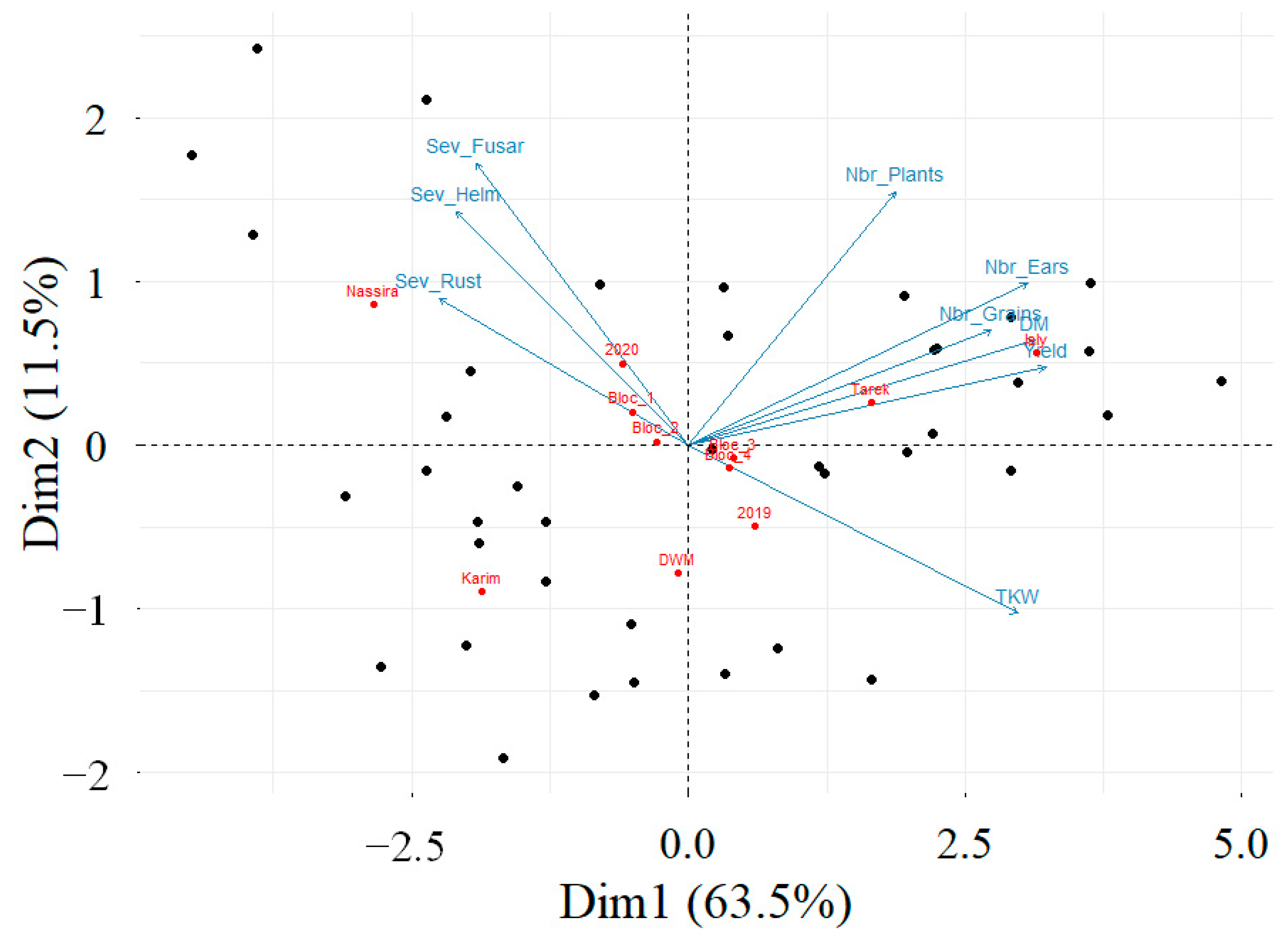
3.5. Correlation and PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ugur, S. Deciphering Genomic Regions and Putative Candidate Genes for Grain Size and Shape Traits in Durum Wheat through GWAS. Agronomy 2023, 13, 1882. [Google Scholar] [CrossRef]
- Bouras, E.; Jarlan, L.; Said Khabba, S.; Er-Raki, S.; Dezetter, A.; Sghir, F.; Tramblay, Y. Assessing the impact of global cli-382 mate changes on irrigated wheat yields and water requirements in a semi-arid environment of Morocco. Sci. Rep. 2019, 9, 19142. [Google Scholar] [CrossRef] [PubMed]
- Goutam, U.; Kukreja, S.; Yadav, R.; Salaria, N.; Thakur, K.; Goyal, A.K. Recent trends and perspectives of molecular markers against fungal diseases in wheat. Front. Microbiol. 2015, 6, 861. [Google Scholar] [CrossRef] [PubMed]
- Qostal, S.; Kribel, S.; Chliyeh, M.; Serghat, S.; Touhami, O.; Zaarati, H.; Benkirane, R.; Douira, A. Study of the fungal complex responsible for root rot of wheat and barley in the north-west of morocco. Plant Arch. 2019, 19, 2143–2157. [Google Scholar]
- Zahri, S.; Farih, A.; Douira, A. Statut des principales maladies cryptogamiques foliaires du blé au Maroc en 2013. J. Appl. Biosci. 2014, 77, 6543. [Google Scholar] [CrossRef]
- Gultyaeva, E.; Gannibal, P.; Shaydayuk, E. Long-term studies of wheat leaf rust in the north-western region of Russia. Agriculture 2023, 13, 255. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.A.; Haider, M.M.; Iqbal, Z.; Iftikhar, M.; Hussain, M. Comparison of yield loss in different wheat varieties/lines due to leaf rust disease. PAK. J. Phytopathol 2010, 22, 13–15. [Google Scholar]
- El-Orabey, W.M.; Elkot, A.F. Prediction of leaf rust severity and yield loss in wheat based on environmental factors. J. Plant Dis. Prot. 2020, 127, 507–519. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, A.K.; Bhattacharya, P.M.; Singh, G. Incidence of Helminthosporium leaf blight of wheat and biochemical back-ground of disease resistance in the Eastern Gangetic Plains. J. Wheat Res. 2011, 3, 26–28. [Google Scholar]
- Sharma, R.C.; Duveiller, E.; Gyawali, S.; Shrestha, S.M.; Chaudhary, N.K.; Bhatta, M.R. Resistance to Helminthosporium leaf blight and agronomic performance of spring wheat genotypes of diverse origins. Euphytica 2004, 139, 33–44. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Houmairi, H.; Oubayoucef, A.; Idrissi, I.; Benchekroun, K.F.E. Haute prévalence de Fusarium spp. associés aux grains de céréales dans la région centrale du Maroc: Risques pathogénique et toxinogène. Rev. Mar. Sci. Agron 2018, 6, 355–361. [Google Scholar]
- Wegulo, S.N.; Baenziger, P.S.; Nopsa, J.H.; Bockus, W.W. Heather Hallen-Adams d. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Benbrook, C.; Kegley, S.; Baker, B. Organic Farming Lessens Reliance on Pesticides and Promotes Public Health by Lowering. Dietary Risks. Agronomy 2021, 11, 1266. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, S.; Gul, A.; Rehman Hakeem, K. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Springer: Cham, Switzerland, 2016; pp. 254–266. [Google Scholar] [CrossRef]
- Kolmer, J. Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 2013, 4, 70–84. [Google Scholar] [CrossRef]
- Liebman, M.; Dyck, E. Crop Rotation And Intercropping Strategies For Weed Management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef]
- Bolluyt, J.; Johnson, S.E.; Lowy, P.; McGrath, M.T.; Mohler, C.L.; Rangarajan, A.; Stoner, K.A.; Toensmeier, E.; van Es, H. Crop Rotation on Organic Farms a Planning Manual; Mohler, C.L., Johnson, S.E., Eds.; NRAES: Ithaca, NY, USA, 2009. [Google Scholar]
- Trenbath, B.R. Intercropping for the management of pests and diseases Field. Crops Res. 1993, 34, 381–405. [Google Scholar] [CrossRef]
- Khanal, U.; Stott, K.J.; Armstrong, R.; Nuttall, J.G.; Henry, F.; Christy, B.P.; Mitchell, M.; Riffkin, P.A.; Wallace, A.J.; McCaskill, M.; et al. Intercropping—Evaluating the Advantages to Broadacre Systems. Agriculture 2021, 11, 453. [Google Scholar] [CrossRef]
- Bale, J.S.; Lenteren, J.C.V.; Bigler, F. Biological control and sustainable food production. Phil. Trans. R. Soc. B 2008, 363, 761–776. [Google Scholar] [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Barot, S.; Allard, V.; Cantarel, A.; Enjalbert, J.; Gauffreteau, A.; Goldringer, I.; Lata, J.C.; Le Roux, X.; Niboyet, A.; Porcher, E. Designing mixtures of varieties for multifunctional agriculture with the help of ecology. A review. Agron. Sustain. Dev. 2017, 37, 13. [Google Scholar] [CrossRef]
- Snyder, L.D.; Gómez, M.I.; Power, A.G. Crop Varietal Mixtures as a Strategy to Support Insect Pest Control, Yield,Economic, and Nutritional Services. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Jeuffroy, M.-H.; Meynard, J.M.; de Vallavieille-Pope, C.; Fraj, M.B.; Saulas, P. Les associations de variétés de blé: Performances et maitrise des maladies. Le Sélectionneur Français 2010, 61, 75–84. [Google Scholar]
- Les Mélanges Variétaux en blé: Un Levier Intéressant Pour Préserver les Résistances Génétiques et Réduire l’usage des Fongicides 2013. Available online: http://www.agroperspectives.fr (accessed on 20 October 2024).
- Akanda, S.I.; Mundt, C.C. Effects of two-component wheat cultivar mixtures on stripe rust severity. Ecol. Epidemiol. 1996, 86, 347–353. [Google Scholar] [CrossRef]
- Mundt, C.C.; Brophy, L.S.; Kolar, S.C. Effect of genotype unit number and spatial arrangement on severity of yellow rust in wheat cultivar mixtures. Plant Pathol. 1996, 45, 215–222. [Google Scholar] [CrossRef]
- Cox, C.M.; Garrett, K.A.; Bowden, R.L.; Fritz, A.K.; Dendy, S.P.; Heer, W.F. Cultivar mixtures for the simultaneous management of multiple diseases: Tan Spot and Leaf Rust of Wheat. Ecol. Epidemiol. 2004, 94, 961–969. [Google Scholar] [CrossRef]
- Lazzaro, M.; Costanzo, A.; Bàrberi, P. Single vs multiple agroecosystem services provided by common wheat cultivar mixtures: Weed suppression, grain yield and quality. Field Crops Res. 2018, 221, 277–297. [Google Scholar] [CrossRef]
- Fletcher, A.; Ogden, G.; Sharma, D. Mixing it up–wheat cultivar mixtures can increase yield and buffer the risk of flowering too early or too late. Eur. J. Agron. 2019, 103, 90–97. [Google Scholar] [CrossRef]
- Dileone, J.A.; Mundt, C.C. Effect of wheat cultivar mixtures on populations of Puccinia striiformis races. Plant Pathol. 1994, 43, 917–930. [Google Scholar] [CrossRef]
- Mille, B.; Fraj, M.B.; Monod, H.; De Vallavieille-Pope, C. Assessing four-way mixtures of winter wheat cultivars from the Performances of their two-way and individual components. Eur. J. Plant Pathol. 2006, 114, 163–173. [Google Scholar] [CrossRef]
- Mehta, Y.R. Management in Wheat Diseases and Their Management; Springer: New York, NY, USA, 2014; pp. 249–252. [Google Scholar]
- Ghimire, B.; Sapkota, S.; Bahri, B.A.; Martinez-Espinoza, A.D.; Buck, J.W.; Mergoum, M. Fusarium Head Blight and Rust Diseases in Soft Red Winter Wheat in the Southeast United States: State of the Art, Challenges and Future Perspective for Breeding. Front. Plant Sci. 2020, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Lê, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017; ISBN 978-0-429-22543-7. [Google Scholar]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat; Hettel, G.P., Ed.; CIMMYT: El Batán, Mexico, 1992; pp. 7–14. [Google Scholar]
- Bataille, C.; Duvivier, M.; Heens, B.; Mahieu, O.; Meza, R.; Monfort, B. Chapitre 5: Lutte intégrée contre les maladies. Livre Blanc « Céréales », Février. 2018. [Google Scholar]
- Moussa, E.J.; Louis, K.; Mustapha, E.J.; Jürgen, J.; Clive, B.; Abdoul, A.D.; Philippe, D. Improving fungal disease forecasts in winter wheat: A critical role of intraday variations of meteorological conditions in the development of Septoria leaf blotch. Field Crops Res. 2017, 213, 12–20. [Google Scholar] [CrossRef]
- Xiangming, X. Effects of environmental conditions on the development of Fusarium ear blight. Eur. J. Plant Pathol. 2003, 109, 683–689. [Google Scholar]
- Mazzola, M. Mechanisms of natural soil suppressiveness to soilborne diseases. Kluwer Acad. Publ. 2002, 8, 557–564. [Google Scholar]
- Broers, L.H.M.; Parlevliet, J.E. Environmental stability of partial resistance in spring wheat to wheat leaf rust. Euphytica 1989, 44, 241–245. [Google Scholar] [CrossRef]
- Alvarez, C.E.; Garcia, V.; Robles, J.; Diaz, A. Influence des caractéristiques du sol sur l’incidence de ta Maladie de Panama. Fruits 1981, 36, 71–81. [Google Scholar]
- Nsarellah, N.; Amri, A.; Nachit, M. Amélioration Génétique du blé dur. In la Création Variétale à L’INRA Méthodologie, Acquis et Perspectives; Abbad Andaloussi, F., Chahbar, A., Eds.; INRA: Rabat, Maroc, 2005; pp. 7–55. [Google Scholar]
- Radzikowski, P.; Nczyk, K.J.; Feledyn-Szewczyk, B.; Jóźwicki, T. Assessment of Resistance of Different Varieties of Winter Wheat to Leaf Fungal Diseases in Organic Farming. Agriculture 2023, 13, 875. [Google Scholar] [CrossRef]
- Otten, W.; Gilligan, C.A. Soil structure and soil-borne diseases: Using epidemiological concepts to scale fromfungal spread to plant epidemics. Eur. J. Soil Sci. 2006, 57, 26–37. [Google Scholar] [CrossRef]
- Sturz, A.V.; Carter, M.R.; Johnston, H.W. A review of plant disease, pathogen interactions and microbial antagonism under conservation tillage in temperate humid agriculture. Soil Tillage Res. 1997, 41, 169–189. [Google Scholar] [CrossRef]
- Conway, K.E. An overview of the influence of sustainable agricultural systems on plant diseases. Crop Prot. 1996, 15, 223–228. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C. Plant Disease Severity in High-Input Compared to Reduced-Input and Organic Farming Systems. Plant Dis. 1995, 79. [Google Scholar]
- Krupinsky, J.M.; Bailey, K.L.; McMullen, M.P.; Gossen, B.D.; Turkington, T.K. Managing Plant Disease Risk in Diversified Cropping Systems. Agron. J. 2002, 94, 198–209. [Google Scholar] [CrossRef]
- Yan Fang, Y.; Xu, B.; Liu, L.; Gu, Y.; Liu, Q.; Turner, N.C.; Li, F.M. Does a mixture of old and modern winter wheat cultivars increase yield and water use efficiency in water-limited environments? Field Crops Res. 2014, 156, 12–21. [Google Scholar] [CrossRef]
- Villaréal, L.M.M.A.; Lannou, C. Selection for increased spore efficacy by host genetic background in a wheat powdery mildew population. Ecol. Popul. Biol. 2000, 90, 1300–1306. [Google Scholar] [CrossRef]
- Roohallah, S.R.; Vazvani, M.G. Unveiling Methods to Stimulate Plant Resistance against Pathogens. Front. Biosci. 2024, 29, 188. [Google Scholar] [CrossRef]
- Mapuranga, J.; Chang, J.; Zhao, J.; Liang, M.; Li, R.; Wu, Y.; Zhang, N.; Zhang, L.; Yang, W. The Underexplored Mechanisms of Wheat Resistance to Leaf Rust. Plants 2023, 12, 3996. [Google Scholar] [CrossRef]
- Gärtner, B.H.; Munich, M.; Kleijer, G.; Mascher, F. Characterisation of kernel resistance against Fusarium infection in spring wheat by baking quality and mycotoxin assessments. Eur. J. Plant Pathol. 2008, 120, 61–68. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; He, Q.; Zhuangbo, T.; Weihao, H.; Xinyu, S.; Jie, L.; Bronwyn, J.B.; Chuanxi, M.; Hongqi, S. Comparative transcriptomic analysis of wheat cultivars differing in their resistance to Fusarium head blight infection during grain-filling stages reveals unique defense mechanisms at play. Chen Al. BMC Plant Biol. 2023, 23, 433. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Wang, Q.; Guo, Y.; Zhang, P.; Xie, H.; Liu, J.; Li, L.; Zhang, C.; Qin, P. Mechanisms of Resistance to Spot Blotch in Yunnan Iron Shell Wheat Based on Metabolome and Transcriptomics. Int. J. Mol. Sci. 2022, 23, 5184. [Google Scholar] [CrossRef]
- Kristoffersen, R.; Jørgensen, L.N.; Eriksen, L.B.; Nielsen, G.C.; Kiær, L.P. Control of Septoria tritici blotch by winter wheat cultivar mixtures: Meta-analysis of 19 years of cultivar trials. Field Crops Res. 2020, 249, 107696. [Google Scholar] [CrossRef]
- Garrett, K.A.; Mundt, C.C. Effects of Planting Density and the Composition of Wheat Cultivar Mixtures on Stripe Rust: An Analysis Taking into Account Limits to the Replication of Controls. Epidemiology 2000, 90, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of leaf rust (Puccinia triticina) on photosynthesis and related processes of leaves in wheat crops grown at two contrasting sites and with different nitrogen levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Askegaard, M.; Thomsen, I.K.; Berntsen, J.; Hovmøller, M.S.; Kristensen, K. Performance of spring barley varieties and variety mixtures as affected by manure application and their order in an organic crop rotation. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 421–430. [Google Scholar] [CrossRef]
- El Ghmari, H.; Harbouze, R.; El Bilali, H. Pathways of Transition to Organic Agriculture in Morocco. World 2022, 3, 718–735. [Google Scholar] [CrossRef]



| Soil Properties | Benslimane Farm (2019) | The National School of Agriculture Farm (2020) |
|---|---|---|
| Clay (%) | 17.4 | 54.1 |
| Silt (%) | 28.6 | 16.9 |
| Sand (%) | 52.2 | 27.2 |
| Cation exchangeable capacity (dS m−1) | 19.7 | 30.7 |
| Organic matter (%) | 1.5 | 1.6 |
| pH | 8.2 | 7 |
| Total N (%) | 0.160 | 0.162 |
| P2O5 (mg kg−1) | 172 | 234 |
| K2O (mg kg−1) | 431 | 560 |
| Zn (mg kg−1) | 4.39 | 5.03 |
| Copper (mg kg−1) | 1.91 | 2.6 |
| Mn (mg kg−1) | 100 | 132.2 |
| Fe (mg kg−1) | 49.76 | 54.62 |
| Cultivar | Resistance to Leaf Rust | Resistance to Helminthosporiosis Leaf Blight (HLB) | Resistance to Fusarium Head Blight (FHB) |
|---|---|---|---|
| Karim | Moderately susceptible | Resistant | Unknown |
| Tarek | Moderately resistant | Moderately susceptible | Unknown |
| Isly | Resistant | Moderately resistant | Unknown |
| Nassira | Susceptible | Moderately susceptible to susceptible | Unknown |
| Year | Variety | CV |
|---|---|---|
| 2019 | Nassira | 10% |
| Tarek | 11% | |
| Karim | 11% | |
| Isly | 12% | |
| DWM | 12% | |
| Overall | 39% | |
| 2020 | Nassira | 21% |
| Tarek | 13% | |
| Karim | 11% | |
| Isly | 10% | |
| DWM | 11% | |
| Overall | 38% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozalmat, W.; Alaoui, S.B.; Sidikou, A.A.H.; Abouabdillah, A. The Management of Fungal Diseases in Organic Production Systems Through a Mixture of Durum Wheat Varieties. Sustainability 2024, 16, 9304. https://doi.org/10.3390/su16219304
Bozalmat W, Alaoui SB, Sidikou AAH, Abouabdillah A. The Management of Fungal Diseases in Organic Production Systems Through a Mixture of Durum Wheat Varieties. Sustainability. 2024; 16(21):9304. https://doi.org/10.3390/su16219304
Chicago/Turabian StyleBozalmat, Wissal, Si Bennasseur Alaoui, Abdel Aziz Hassane Sidikou, and Aziz Abouabdillah. 2024. "The Management of Fungal Diseases in Organic Production Systems Through a Mixture of Durum Wheat Varieties" Sustainability 16, no. 21: 9304. https://doi.org/10.3390/su16219304
APA StyleBozalmat, W., Alaoui, S. B., Sidikou, A. A. H., & Abouabdillah, A. (2024). The Management of Fungal Diseases in Organic Production Systems Through a Mixture of Durum Wheat Varieties. Sustainability, 16(21), 9304. https://doi.org/10.3390/su16219304





