Unleashing Nature’s Pesticide: A Systematic Review of Schinus molle Essential Oil’s Biopesticidal Potential
Abstract
:1. Introduction
2. Research Strategy
3. Results
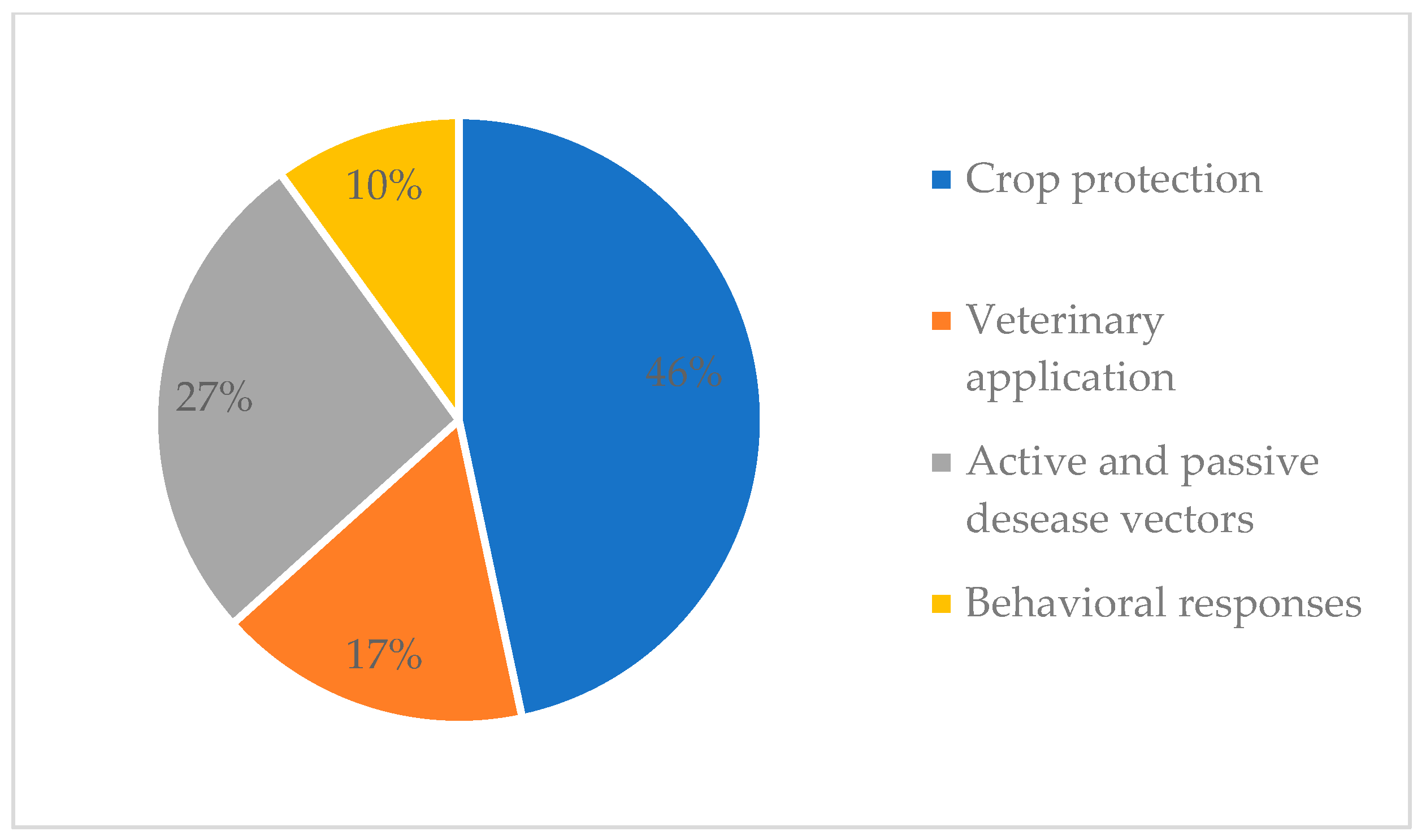
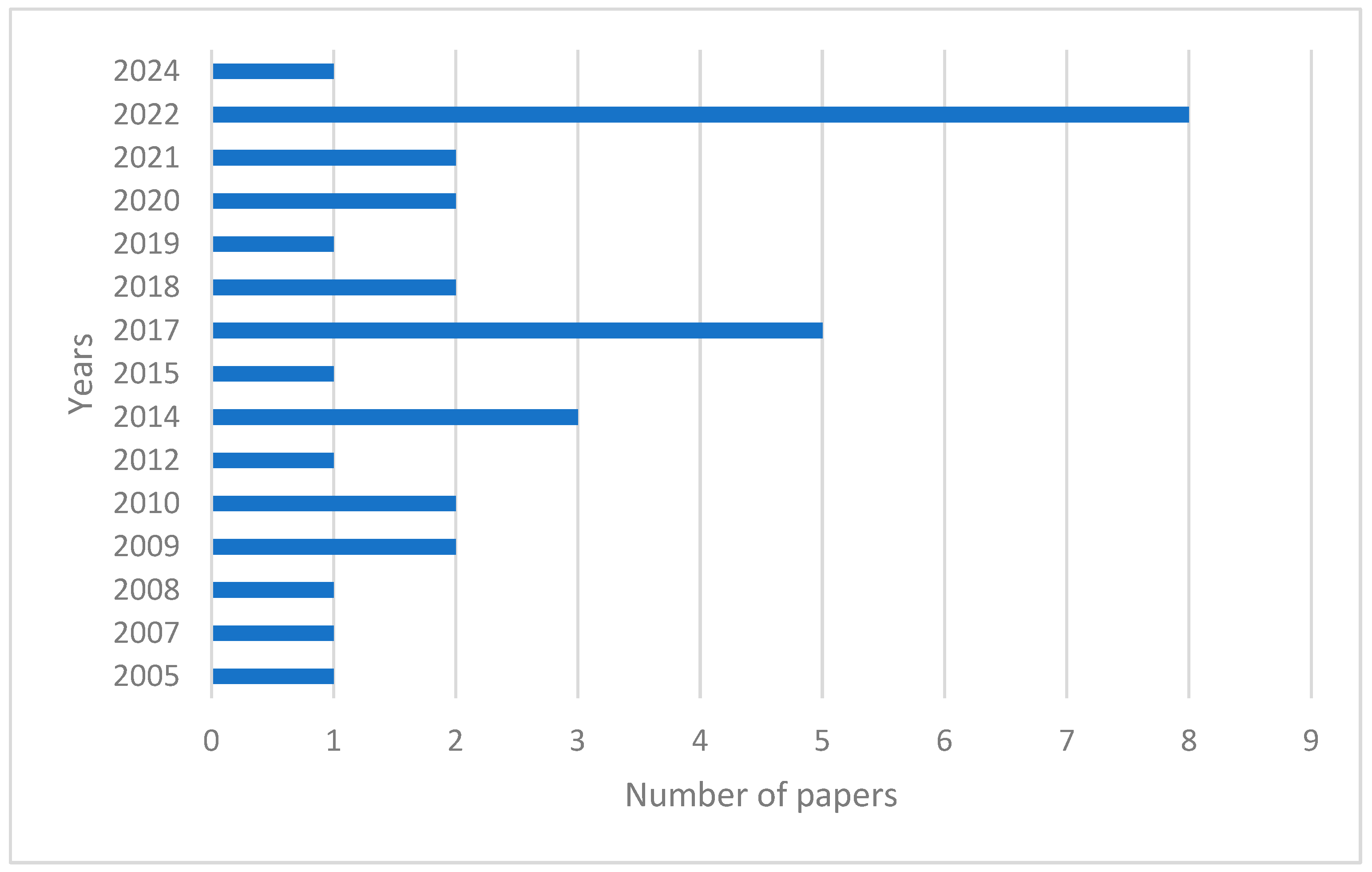
3.1. Aim of Selected Studies and Geographical Distribution
3.2. Biopesticide Activity of S. molle EO in Crop Protection
3.3. Biopesticide Activity of S. molle EO for Veterinary Application
3.4. Biopesticide Activity of S. molle EO as a Tool Against Active and Passive Vectors of Diseases
3.5. Development of Nanobiopesticides
3.6. Behavioral and Antennal Responses of Pest Insects to Volatile Compounds from S. molle EO
| Country | Part | Extraction | Main Compound | Application | Pest (Target) | Biological Activity | Ref |
|---|---|---|---|---|---|---|---|
| Algeria | AP | HD | β-phellandrene (25.1%), α-phellandrene (16.0%), and p-cymene (10.9%) | Fumigation and Repellency/Attractivity assay | Sitophilus zeamais (Col.: Curculionidae) | Adulticide (LC50 = 170.1 μL/L). Repellency (at 17.9 and 23.9 μL/L). Attractivity (at 1.19 and 2.39 μL/L). | [98] |
| Chile | L | HD | Limonene (17.6%), α-phellandrene (14.3%), and δ-cadinene (9.4%) | Olfactometer bioassay | Lobesia botrana (Lep.: Tortricidae) | Positive behavioral response at 1 × 103 µg/mL (female) and 1 × 104 µg/mL (male). | [96] |
| Tunisia | F | HD | 6-epi-shyobunol (16.2%), Spathulenol (8.2%), and 4-epi-Cubebol (7.8%) | Olfactometer bioassay | Acyrthosiphon pisum (Hem.: Aphididae) | Positive response (significant). | [99] |
| L | β-eudesmol (14.8%), Elemol (13.7%), and α-Eudesmol (12.8%) | Negative response (not significant). |
4. Discussion
4.1. Mode of Action of EOs in Biopesticide Activity: Preliminary Findings Concerning S. molle EO
4.2. Product Development, Feasibility, Scalability, and Technology Readiness Level (TRL) Trends
4.3. Critical Assessments of Papers Reviewed
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- de Castro Oliveira, J.A.; Ferreira, L.S.; Garcia, I.P.; de Lima Santos, H.; Ferreira, G.S.; Rocha, J.P.M.; Nunes, S.A.; de Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Eugenia uniflora, Melaleuca armillaris, and Schinus molle essential oils to manage larvae of the filarial vector Culex quinquefasciatus (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2022, 29, 34749–34758. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Spivak, M.; Phinney, R.; Montrose, L. Synthetic Pesticides and Health in Vulnerable Populations: Agricultural Workers. Curr. Environ. Health Rep. 2020, 7, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Bass, C.; Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 2023, 156, 103937. [Google Scholar] [CrossRef]
- Chen, Y.H.; Cohen, Z.P.; Bueno, E.M.; Christensen, B.M.; Schoville, S.D. Rapid evolution of insecticide resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Curr. Opin. Insect Sci. 2023, 55, 101000. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Dubey, A.; Malla, M.A.; Kumar, A. Pesticide pestilence: Global scenario and recent advances in detection and degradation methods. J. Environ. Manag. 2023, 338, 117680. [Google Scholar] [CrossRef] [PubMed]
- Shahmohamadloo, R.S.; Febria, C.M.; Fraser, E.D.G.; Sibley, P.K. The sustainable agriculture imperative: A perspective on the need for an agrosystem approach to meet the United Nations Sustainable Development Goals by 2030. Integr. Environ. Assess. Manag. 2022, 18, 1199–1205. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Loy, A.C.M.; How, B.S.; Tamothran, A.M.; Yip, A.J.K.; Liew, R.K.; Peng, W.; Alstrup, A.K.; Lam, S.S.; et al. The nexus between biofuels and pesticides in agroforestry: Pathways toward United Nations sustainable development goals. Environ. Res. 2022, 214, 113751. [Google Scholar] [CrossRef] [PubMed]
- Wesseler, J. The EU’s farm-to-fork strategy: An assessment from the perspective of agricultural economics. Appl. Econ. Perspect. Policy 2022, 44, 1826–1843. [Google Scholar] [CrossRef]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper aduncum essential oil: A promising insecticide, acaricide and antiparasitic. A review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, B.C.F.; Barrozo, M.M.; Coutinho, A.L.; Pereira e Sousa, L.J.M.; Vale, F.L.; Marreto, L.; Marchesini, P.; de Castro Rodrigues, D.; de Souza, E.D.F.; Sabatini, G.A.; et al. Essential oils and isolated compounds for tick control: Advances beyond the laboratory. Parasites Vectors 2023, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Meersmans, J.; Lins, L.; Bouquillon, S.; Fauconnier, M.L. Essential oil-based bioherbicides: Human health risks analysis. Int. J. Mol. Sci. 2021, 22, 9396. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Rants’o, T.A.; Koekemoer, L.L.; Panayides, J.L.; van Zyl, R.L. Potential of Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors: A Review. Molecules 2022, 27, 7026. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.d.A.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Sources of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press & Taylor and Francis Groups: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2015; pp. 39–40. [Google Scholar]
- Arias, J.; Silva, G.; Figueroa, I.; Fischer, S.; Robles-Bermúdez, A.; Rodríguez-Maciel, C.; Lagunes-Tejeda, A. Insecticidal, repellent and antifeeding activity of powder and essential oil of Schinus molle L. fruits against Sitophilus zeamais (Motschulsky). Chil. J. Agric. Sci. 2017, 33, 93–104. [Google Scholar]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; Bouyahya, A.; El Yadini, A.; Fozia, F.; Alotaibi, A.; Ullah, R.; Tabyaoui, M. Chemical composition, antioxidant, insecticidal activity, and comparative analysis of essential oils of leaves and fruits of Schinus molle and Schinus terebinthifolius. Evid. Based Complement. Altern. Med. 2022, 2022, 4288890. [Google Scholar] [CrossRef] [PubMed]
- de Batista, L.C.S.O.; Cid, Y.P.; De Almeida, A.P.; Prudêncio, E.R.; Riger, C.J.; De Souza, M.A.A.; Coumendouros, K.; Chaves, D.S.A. In vitro efficacy of essential oils and extracts of Schinus molle L. against Ctenocephalides felis felis. Parasitology 2016, 143, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Pereira, R.; Sousa, R.M. Nanobiopesticides: Are they the future of phytosanitary treatments in modern agriculture? Sci. Total Environ. 2023, 896, 166401. [Google Scholar] [CrossRef] [PubMed]
- Rey-Valeirón, C.; Pérez, K.; Guzmán, L.; López-Vargas, J.; Valarezo, E. Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae). Exp. Appl. Acarol. 2018, 76, 399–411. [Google Scholar] [CrossRef]
- World Flora Online WFO. Schinus molle L. Available online: https://www.worldfloraonline.org/taxon/wfo-0000435157 (accessed on 12 September 2024).
- Aboalhaija, A.; Amro, R.; Abaza, I.; Khalil, E.; Al-Aboudi, A.; Abu-Zarga, M.; Afifi, F.U. Schinus molle L. collected from Jordan and Turkey: Essential oil composition and anticholinesterase activity. J. Essent. Oil-Bear. Plants 2019, 22, 704–716. [Google Scholar] [CrossRef]
- Machado, C.; Raman, V.; Rehman, J.U.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Almeida, V.P.; Silva, R.Z.; Farago, P.V.; Khan, I.A.; Budel, J.M. Schinus molle: Anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Rev. Bras. Farmacogn. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Zaitoun, A.A.; Farag, M.A.; El Gayed, S.H.; Harraz, F.M.H. Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res. 2010, 24, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Mucha, W.; Witkowska, D. The applicability of essential oils in different stages of production of animal-based foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef]
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol activity of aromatic and medicinal plants and their bioactive components against soil-borne pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-chemical treatments for the pre-and post-harvest elicitation of defense mechanisms in the fungi–avocado pathosystem. Molecules 2021, 26, 6819. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, A.; Gomes, E.N.; Richardi, V.S.; Martins, C.E.N.; Brum, J.S.; Navarro-Silva, M.A.; Deschamps, C.; Molento, M.B. Data of insecticide effects of natural compounds against third instar larvae of Cochliomyia macellaria. Data Brief 2019, 25, 104181. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-phellandrene and alpha-phellandrene-rich essential oils: A systematic review of biological activities, pharmaceutical and food applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation optimization of D-limonene-loaded nanoemulsions as a natural and efficient biopesticide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124746. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Sanchez-Ballester, N.M.; Blázquez, M.A. Encapsulated limonene: A pleasant lemon-like aroma with promising application in the agri-food industry. A review. Molecules 2020, 25, 2598. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Benzi, V.; Murray, P.; Ferrero, A. Insecticidal and insect-repellent activities of essential oils from verbenaceae and anacardiaceae against Rhizopertha dominica. Nat. Prod. Commun. 2009, 4, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Benzi, V.; Stefanazzi, N.; Ferrero, A.A. Biological activity of essential oils from leaves and fruits of pepper tree (Schinus molle L.) to control rice weevil (Sitophilus oryzae L.). Chil. J. Agric. Res. 2009, 69, 154–159. [Google Scholar] [CrossRef]
- Vicenço, C.B.; Silvestre, W.P.; da Silva, V.T.; Menegol, I.V.; Hahn, R.C.; Lima, T.S.; Agostini, F.; Pauletti, G.F. Bioactivity of Schinus molle L. and Schinus terebinthifolia Raddi. Essential oils on Anticarsia gemmatalis (Hübner 1818). Braz. Arch. Biol. Technol. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- de Souza, L.; Cardoso, M.d.G.; Konig, I.F.M.; Ferreira, V.R.F.; Caetano, A.R.S.; Campolina, G.A.; Haddi, K. Toxicity, histopathological alterations and acetylcholinesterase inhibition of Illicium verum essential oil in Drosophila suzukii. Agriculture 2022, 12, 1667. [Google Scholar] [CrossRef]
- López, I.C.; Rivera, V.E.; Yánez, Á.W.; Artieda, J.R.; Villacres, G.E. Evaluación de la actividad insecticida de Schinus molle sobre Premnotrypes vorax en papa. Agron. Costarric. 2017, 41, 93–101. [Google Scholar] [CrossRef]
- Gad, H.A.; Hamza, A.F.; Abdelgaleil, S.A.M. Chemical composition and fumigant toxicity of essential oils from ten aromatic plants growing in Egypt against different stages of confused flour beetle, Tribolium confusum Jacquelin du Val. Int. J. Trop. Insect Sci. 2022, 42, 697–706. [Google Scholar] [CrossRef]
- Hussein, H.S.; Tawfeek, M.E.; Abdelgaleil, S.A.M. Chemical composition, aphicidal and antiacetylcholinesterase activities of essential oils against Aphis nerii Boyer de Fonscolombe (Hemiptera: Aphididae). J. Asia-Pac. Entomol. 2021, 24, 259–265. [Google Scholar] [CrossRef]
- Landero-Valenzuela, N.; Alonso-Hernández, N.; Lara-Viveros, F.; Gómez-Domínguez, N.S.; Juárez-Pelcastre, J.; Aguado-Rodríguez, J.; Luna-Cruz, A.; Lagunez-Rivera, L.; Aguilar-Pérez, L.A.; Hinojosa-Garro, D.; et al. Efficiency of Schinus molle essential oil against Bactericera cockerelli (Hemiptera: Triozidae) and Sitophilus zeamais (Coleoptera: Dryophthoridae). Agriculture 2022, 12, 554. [Google Scholar] [CrossRef]
- Chaaban, S.; Haouel-Hamdi, S.; Bachrouch, O.; Mahjoubi, K.; Mediouni Ben Jemâa, J. Fumigant toxicity of four essential oils against the carob moth Ectomyelois ceratoniae Zeller and the Mediterranean flour moth Ephestia kuehniella. Int. J. Environ. Health Res. 2022, 34, 419–431. [Google Scholar] [CrossRef]
- Topuz, E.; Madanlar, N.; Erler, F. Chemical composition, toxic and development- and reproduction-inhibiting effects of some essential oils against Tetranychus urticae Koch (Acarina: Tetranychidae) as fumigants. J. Plant Dis. Prot. 2018, 125, 377–387. [Google Scholar] [CrossRef]
- Rizwan-ul-Haq, M.; Aljabr, A.M. Rhynchophorus ferrugineus midgut cell line to evaluate insecticidal potency of different plant essential oils. Vitr. Cell. Dev. Biol.—Anim. 2015, 51, 281–286. [Google Scholar] [CrossRef]
- Salman, M.; Abbas, R.Z.; Israr, M.; Abbas, A.; Mehmood, K.; Khan, M.K.; Sindhu, Z.u.D.; Hussain, R.; Saleemi, M.K.; Shah, S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020, 283, 109178. [Google Scholar] [CrossRef] [PubMed]
- Ruffinengo, S.; Eguaras, M.; Floris, I.; Faverin, C.; Bailac, P.; Ponzi, M. LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J. Econ. Entomol. 2005, 98, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.M.C.; Miranda, L.F.C.; Salas, J.M.Z.; Quiroz, K.D.A. Microencapsulation of essential oil of molle (Schinus molle) against the aphid Macrosiphum euphorbiae. J. Pharm. Negat. Results 2022, 13, 1019–1023. [Google Scholar] [CrossRef]
- Torres, F.C.; Lucas, A.M.; Lucia, V.; Ribeiro, S.; Martins, J.R.; Von Poser, G.; Guala, M.S.; Elder, H.V.; Cassel, E. Influence of essential oil fractionation by vacuum distillation on acaricidal activity against the cattle tick. Arch. Biol. Technol. 2012, 55, 613–621. [Google Scholar] [CrossRef]
- Rey-Valeirón, C.; Guzmán, L.; Saa, L.R.; López-Vargas, J.; Valarezo, E. Acaricidal activity of essential oils of Bursera graveolens (Kunth) Triana & Planch and Schinus molle L. on unengorged larvae of cattle tick Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). J. Essent. Oil Res. 2017, 29, 344–350. [Google Scholar] [CrossRef]
- Mulderij-Jansen, V.; Pundir, P.; Grillet, M.E.; Lakiang, T.; Gerstenbluth, I.; Duits, A.; Tami, A.; Bailey, A. Effectiveness of Aedes-borne infectious disease control in Latin America and the Caribbean region: A scoping review. PLoS ONE 2022, 17, e0277038. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Lutomiah, J.; Chepkorir, E.; Tchouassi, D.P. Evolving dynamics of Aedes-borne diseases in Africa: A cause for concern. Curr. Opin. Insect Sci. 2022, 53, 100958. [Google Scholar] [CrossRef] [PubMed]
- Unger, H.W.; Acharya, S.; Arnold, L.; Wu, C.; van Eijk, A.M.; Gore-Langton, G.R.; ter Kuile, F.O.; Lufele, E.; Chico, R.M.; Price, R.N.; et al. The effect and control of malaria in pregnancy and lactating women in the Asia-Pacific region. Lancet Glob. Health 2023, 11, e1805–e1818. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.S.; Rogerson, S.J. Pathogenicity and virulence of malaria: Sticky problems and tricky solutions. Virulence 2023, 14, 2150456. [Google Scholar] [CrossRef] [PubMed]
- George, A.M.; Ansumana, R.; de Souza, D.K.; Niyas, V.K.M.; Zumla, A.; Bockarie, M.J. Climate change and the rising incidence of vector-borne diseases globally. Int. J. Infect. Dis. 2024, 139, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Kaseya, J.; Dereje, N.; Tajudeen, R.; Ngongo, A.N.; Ndembi, N.; Fallah, M.P. Climate change and malaria, dengue and cholera outbreaks in Africa: A call for concerted actions. BMJ Glob. Health 2024, 9, e015370. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: Climate change and the spread of vector-borne diseases, including dengue, malaria, lyme disease, and west nile virus infection. Med. Sci. Monit. 2024, 30, e943546. [Google Scholar] [CrossRef] [PubMed]
- Paz, S. Climate change: A driver of increasing vectorborne disease transmission in non-endemic areas. PLoS Med. 2024, 21, e1004382. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Rocklöv, J.; Ebi, K.L. Climate change and cascading risks from infectious disease. Infect. Dis. Ther. 2022, 11, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Pontifes, P.A.; Jourdain, F.; Diagne, C.; Leroy, B.; Vaissière, A.C.; Tolsá-García, M.J.; Salles, J.M.; Simard, F.; Courchamp, F. The rising global economic costs of invasive Aedes mosquitoes and Aedes-borne diseases. Sci. Total Environ. 2024, 933, 173054. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.A.S.; Souza-Santos, L.P. Environmental risk assessment (ERA) of pyriproxyfen in non-target aquatic organisms. Aquat. Toxicol. 2020, 222, 105448. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Miao, J.; Zhang, W.; Wang, Q.; Sun, C.; Wang, L.; Pan, L. Occurrence, distribution, and health risk assessment of pyrethroid and neonicotinoid insecticides in aquatic products of China. Sci. Total Environ. 2024, 919, 170880. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Sedaghat, M.M.; Sanei-Dehkordi, A.; Amani, A. Plant-derived essential oils; their larvicidal properties and potential application for control of mosquito-borne diseases. Galen Med. J. 2019, 8, 1532. [Google Scholar] [CrossRef] [PubMed]
- Budiman, H.; Stang, E.; Daud, A.; Amiruddin, R. Essential oil as a new tool for larvicidal Aedes aegypti: A systematic review. Gac. Sanit. 2021, 35, S459–S462. [Google Scholar] [CrossRef] [PubMed]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; Amaral, F.M.M.do; Coutinho, D.F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Trop. 2020, 212, 105705. [Google Scholar] [CrossRef]
- Massebo, F.; Mohammed, M.T.; Tadesse, M.; Bekele, T.; Balkew, M.; Gebre-Michael, T. Evaluation on larvicidal effects of essential oils of some local plants against Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Diptera, Culicidae) in Ethiopia. Afr. J. Biotechnol. 2009, 8, 4183–4188. [Google Scholar]
- Abdelgaleil, S.A.M.; El-Sabrout, A.M. Composition, toxicity and developmental potential of three essential oils on the West Nile virus mosquito, Culex pipiens L. Int. J. Pest Manag. 2020, 69, 175–183. [Google Scholar] [CrossRef]
- De Oliveira Barbosa Bitencourt, R.; de Souza Faria, F.; Marchesini, P.; Reis dos Santos-Mallet, J.; Guedes Camargo, M.; Rita Elias Pinheiro Bittencourt, V.; Guedes Pontes, E.; Baptista Pereira, D.; Siqueira de Almeida Chaves, D.; da Costa Angelo, I. Entomopathogenic fungi and Schinus molle essential oil: The combination of two eco-friendly agents against Aedes aegypti larvae. J. Invertebr. Pathol. 2022, 194, 107827. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzúa, A. Insecticidal activity of essential oils from native medicinal plants of Central Argentina against the house fly, Musca domestica (L.). Parasitol. Res. 2009, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.E.D.M.; Abou-Taleb, H.K.; Abdelgaleil, S.A.M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J. Asia-Pac. Entomol. 2017, 20, 133–139. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chavez, H.; Enciso-Roca, E.C.; Común-Ventura, P.W.; Hañari-Quispe, R.D.; Figueroa-Salvador, L.; Loyola-Gonzales, E.L.; Pari-Olarte, J.B.; Aljarba, N.H.; Alkahtani, S.; et al. GC-MS profile, antioxidant activity, and in silico study of the essential oil from Schinus molle L. leaves in the presence of mosquito juvenile hormone-binding protein (mJHBP) from Aedes aegypti. BioMed Res. Int. 2022, 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deveci, O.; Sukan, A.; Tuzun, N.; Kocabas, E. Chemical composition, repellent and antimicrobial activity of Schinus molle L. Artic. J. Med. Plants Res. 2010, 4, 2211–2216. [Google Scholar]
- Almeida, A.R.; Oliveira, N.D.; Pinheiro, F.A.S.D.; de Morais, W.A.; Ferreira, L.D.S. Challenges encountered by natural repellents: Since obtaining until the final product. Pestic. Biochem. Physiol. 2023, 195, 105538. [Google Scholar] [CrossRef]
- AnnaDurai, K.S.; Chandrasekaran, N.; Velraja, S.; Hikku, G.S.; Parvathi, V.D. Essential oil nanoemulsion: An emerging eco-friendly strategy towards mosquito control. Acta Trop. 2024, 257, 107290. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Hussain, N.; Touqeer, S.I.; Khalil-Ur-Rahman; Shamshad, H.; Abbas, N. Formulation of mentha piperita-based nanobiopesticides and assessment of the pesticidal and antimicrobial potential. Life 2024, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Velho, M.C.; Cossetin, L.F.; de Godoi, S.N.; Santos, R.C.V.; Gündel, A.; Monteiro, S.G.; Ourique, A.F. Nanobiopesticides: Development and inseticidal activity of nanoemulsions containing lemongrass or eucalyptus oils. Nat. Prod. Res. 2020, 35, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology Contains Nonbinding Recommendations; FDA: Chicago, IL, USA, 2014.
- European Commission. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System. EUGreenDeal. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 14 September 2024).
- López, A.; Castro, S.; Andina, M.J.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Ruffinengo, S.; Daniel Maggi, M.; Negri, P.; Brasesco, C. Bioactivity of microencapsulated essentials oils and perspectives of their use in the control of Varroa destructor. Bull. Insectol. 2014, 67, 81–86. [Google Scholar]
- Cui, G.Z.; Zhu, J.J. Pheromone-based pest management in china: Past, present, and future prospects. J. Chem. Ecol. 2016, 42, 557–570. [Google Scholar] [CrossRef]
- Infante, F. Pest management strategies against the coffee berry borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food Chem. 2018, 66, 5275–5280. [Google Scholar] [CrossRef]
- Kabir, M.H.; Nur-E-Alam, S.M.; Datta, A.; Tan, M.L.; Rahman, M.S. Understanding vegetable farmers’ adoption, dis-adoption, and non-adoption decisions of pest management by pheromone trapping. PLoS ONE 2023, 18, e0292254. [Google Scholar] [CrossRef]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Lang, J.; Chidawanyika, F.; Khan, Z.R.; Schuman, M.C. Ecological chemistry of pest control in push-pull intercropping systems: What we know, and where to go? Chimia 2022, 76, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Zhang, A.; Angeli, S.; Abubeker, S.; Michel, C.; Feng, Y.; Rodriguez-Saona, C. Behavioral and antennal responses of Drosophila suzukii (diptera: Drosophilidae) to volatiles from fruit extracts. Environ. Entomol. 2015, 44, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Badji, C.A.; Dorland, J.; Kheloul, L.; Bréard, D.; Richomme, P.; Kellouche, A.; De Souza, C.R.A.; Bezerra, A.L.; Anton, S. Behavioral and antennal responses of Tribolium confusum to Varronia globosa essential oil and its main constituents: Perspective for their use as repellent. Molecules 2021, 26, 4393. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Domínguez, A.; Guerrero, Á.; Quero, C. Inhibitory effect of thymol on pheromone-mediated attraction in two pest moth species. Sci. Rep. 2021, 11, 1223. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Curkovic, T.; Ceballos, R. Behavioral and antennal responses of Lobesia botrana (Lepidoptera: Tortricidae) to volatiles from the non-host plant Schinus molle L. (Anacardiaceae). Chil. J. Agric. Res. 2019, 79, 165–171. [Google Scholar] [CrossRef]
- Bedini, S.; Djebbi, T.; Ascrizzi, R.; Farina, P.; Pieracci, Y.; Echeverría, M.C.; Flamini, G.; Trusendi, F.; Ortega, S.; Chiliquinga, A.; et al. Repellence and attractiveness: The hormetic effect of aromatic plant essential oils on insect behavior. Ind. Crops Prod. 2024, 210, 118–122. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Kasmi, A.; Hammami, M.; Raoelison, E.G.; Abderrabba, M.; Bouajila, J.; Ducamp, C. Chemical composition and behavioral effects of five plant essential oils on the green pea aphid Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Chem. Biodivers. 2017, 14, e1600464. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef] [PubMed]
- Kesraoui, S.; Andrés, M.F.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and indirect effects of essential oils for sustainable crop protection. Plants 2022, 11, 2144. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The application of essential oils as a next-generation of pesticides: Recent developments and future perspectives. Zeitschrift Fur Naturforschung. Sect. C J. Biosci. 2020, 75, 183–204. [Google Scholar] [CrossRef]
- Arnouts, S.; Brown, S.; De Ar43, M.L.; Donabedian, M.; Charlier, J. Technology Readiness Levels for vaccine and drug development in animal health: From discovery to life cycle management. Front Vet. Sci. 2022, 21, 1016959. [Google Scholar] [CrossRef] [PubMed]
- Héder, M. From NASA to EU: The evolution of the TRL scale in Public Sector Innovation. Innov. J. Public Sect. Innov. J. 2017, 22, 3. [Google Scholar]



| Country | Part | Extraction | Main Compound | Application | Pest | Biopesticide Activity | Ref |
|---|---|---|---|---|---|---|---|
| Argentina | L | HD | limonene (15.7%); α-phellandrene (13.8%); elmol (9.0%). | Contact (contaminated surface), Fumigation, Topical, Repellency assay | Rhizopertha dominica (Col.: Bostrichidae) | Adulticide (contact: LC50 = 41.2 mg/cm2; fumigation: LC50 = 0.6 mg/cm2; topical: LC50 = 0.88 mg/cm2). Repellency rate (at 1 h 67% at 120.0 μg/cm2). | [41] |
| F | limonene (40.3%); α-phellandrene (24.5%); β-myrcene (16.3%). | Adulticide (contact: LC50 = 236.4 mg/cm2; fumigation: LC50 = 0.8 mg/cm2; topical: LC50 = 8.88 mg/cm2). Repellency rate (at 1 h 67% at 120.0 μg/cm2). | |||||
| Argentina | F | SD | limonene (40.3%); α-phellandrene (24.5%); β-myrcene (16.4%). | Repellency/Attractivity assay, and antifeeding activity, Fumigant activity | Sitophilus oryzae (Col.: Curculionidae) | Strong feeding deterrent action (62%). | [42] |
| L | SD | limonene (15.7%); α-phellandrene (13.8%); elmol (9.0%). | Repellent effects (0.04 and 0.4% w/w). Fumigant activity (not toxic). | ||||
| Brazil | L | SD | α-pinene (60.0%); limonene (11.3%); β-pinene (9.2%). | Ingestion | Anticarsia gemmatalis (Lep.: Erebidae) | Larvicide (at 24 h 30.0% at 2.0% v/v). | [43] |
| Brazil | n.r. | HD | limonene (25.6%); bycyclogermacrene (22.9%); sabinene (19.7%). | Contact | Drosophila suzukii (Dip.: Drosophilidae) | Adulticide (at 24 h LC50 = 19.3 µL/mL and LC95 = 518.5 µL/mL). | [44] |
| Chile | F | SD | β-pinene (15.4%); α-phellandrene (15.0%); p-cymene (10.9%). | Contact, Repellency assay | Sitophilus zeamais (Col.: Curculionidae) | Adulticide (LC50 = 38.2 mL/kg and LC90 = 91.0 mL/kg). Repellency rate (70% at 4% v/m). | [20] |
| Ecuador | L | SD | Bicycloelemene (n.r); trans-caryophyllene (n.r); bicyclogermacrene (n.r). | Immersion | Premnotrypes vorax (Col.: Curculionidae) | Egg eclosion inhibition (at 24 h 47.5% at 5% m/v). Larvicide (at 48 h 25% at 10% m/v). Adulticide (at 24 h 25% at 10% m/v). | [45] |
| Egypt | L | HD | α-phellandrene (29.9%); β-phellandrene (21.1%); elemol (13.0%). | Fumigation | Tribolium confusum (Col.: Tenebrionidae) | Egg toxicity (at 7 days LC50 = 2.22 µL/L). Larvicide (at 7 days 55.1 µL/L). Adulticide (at 24 h LC50 = 46.9 µL/L). | [46] |
| Egypt | L | HD | α-phellandrene (29.8%); β-phellandrene (21.1%). | Ingestion | Aphis nerii (Hem.: Aphididae) | Adulticide (at 24 h LC50 = 0.12 mg/L and LC95 = 3.28 mg/L). | [47] |
| Mexico | L | HD | o-cymene (29.0%); α-pinene (15.5%); camphene (14.0%). | Ingestion | Bactericera cockerelli (Hem.: Triozidae) | Nymphicide (at 24 h 4th-instar LC50 = 329.4 ppm, LC90 = 662.3 ppm and 5th-instar LC50 523.8 ppm, LC90 = 1029.9 ppm). | [48] |
| Sitophilus zeamais (Col.: Curculionidae) | Adulticide (at 5th day LC50 = 781.5 ppm and LC90 = 1641.3 ppm). | ||||||
| Morocco | F | HD | limonene (23.2%); spathulenol (14.3%); β-ocimene (13.3%). | Contact, Fumigation, Repellency assay | Sitophilus oryzae (Col.: Curculionidae) | Adulticide (contact: at 24 h LC50 = 50.0 μL/cm2; fumigation: at 8 days 50% mortality at 5 μL/L). Repellency rate (30% at 150 µL/cm2). | [21] |
| L | HD | limonene (18.5%); longifolene (8.5%); γ-terpinene (8.2%). | Adulticide (contact: at 24 h LC50 = 100.0 μL/cm2; fumigation: at 8 days 75% mortality at 5 μL/L). Repellency rate (30% at 150 µL/cm2). | ||||
| Saudi Arabia | F | HD | p-cymene (32.8%); β-pinene (19.0%); α-terpinene (18.3%). | Topical, Repellency assay | Tribolium castaneum (Col.: Tenebrionidae) | Adulticide (at 2 days 53.3% at 750.0 μL/mL). Repellency rate (73.6% at 750 μL/mL). | [28] |
| Topical | Trogoderma granarium (Col.: Dermestidae) | Adulticide (at 2 days 50% at 750.0 μL/mL). | |||||
| L | HD | p-cymene (69.4%); carvotanancetone (2.5%); calamenene (2.3%). | Topical | Tribolium castaneum (Col.: Tenebrionidae) | Adulticide (at 2 days 50.0% at 750.0 μL/mL). | ||
| Topical, Repellency assay | Trogoderma granarium (Col.: Dermestidae) | Adulticide (at 2 days 46.7% at 750.0 μL/mL). Repellency rate (72.3% at 750 μL/mL). | |||||
| Tunisia | F | HD | α-phellandrene (20.4%); limonene (17.7%); t-muurolol (11.0%). | Fumigation | Ectomyelois ceratoniae (Lep.: Pyralidae) | Adulticide (at 24 h LC50 = 7.9 μL/L). | [49] |
| Ephestia kuehniella (Lep.: Pyralidae) | Adulticide (at 24 h LC50 = 170.7 μL/L). Egg hactchability (74.6% at 459.5 μL/L). | ||||||
| L | α-phellandrene (17.1%); elemol (13.3%); t-muurolol (13.2%). | Fumigation | Ectomyelois ceratoniae (Lep.: Pyralidae) | Adulticide (at 24 h LC50 = 176.5 μL/L). | |||
| Turkey | AP | HD | limonene + β-phellandrene (13.7%); elemol (11.6%); p-cymene (9.6%). | Fumigation | Tetranychus urticae (Trom.: Tetranychidae) | Adulticide (at 96 h LC50 = 12.3 µL/L and LC90 = 36.8 µL/L). Larvicide (at 96 h LC50 = 8.4% µL/L and LC90 = 16.1 µL/L). | [50] |
| USA | n.r. | SD | n.r. | Incubation | Rhynchophorus ferrugineus (Col.: Curculionidae) | Cell mortality (at 24 h LC50 = 483.1 ppm). | [51] |
| Country | Part | Extraction | Main Compound | Application | Pest | Biological Activity | Ref |
|---|---|---|---|---|---|---|---|
| Argentina | n.r. | SD | β-phellandrene (28.3%); α-phellandrene (11.5%); camphene (7.9%). | Contact (contaminated surface) | Varroa destructor (Mes.: Varroidae) | Adulticide (at 24 h LD50 = 2.7 µL/mg) | [53] |
| Argentina | F, L, B | SD | sabinene (51.0%); β-pinene (11.2%); α-pinene (9.5%). | Contact and Ingestion | Varroa destructor (Mes.: Varroidae) | Efficiency: 11.3% mortality | [54] |
| Argentina | F, L, B | SD | sabinene (34.3%); α-pinene (8.4%); terpinen-4-ol (8.2%). | Immersion | Rhipicephalus microplus (Ixo.: Ixodidae) | Larvicide (LC50 = 4.4 µL/mL) | [55] |
| Ecuador | F | HD | p-cymene (40.0%); limonene (19.5%); myrcene (7.7%). | Contact | Rhipicephalus microplus (Ixo.: Ixodidae) | Larvicide (at 24 h LC90 = 1.3% v/v) | [56] |
| Ecuador | F | HD | p-cymene (40.0%); limonene (19.5%); myrcene (7.7%). | Contact | Rhipicephalus sanguineus (Ixo.: Ixodidae) | Larvicide (at 24 h LC50 = 0.2% v/v and LC90 = 0.8% v/v) | [24] |
| Country | Part | Extraction | Main Compound | Application | Pest | Activity | Ref |
|---|---|---|---|---|---|---|---|
| Argentina | L | HD | n.r | Fumigation | Musca domestica (Dipt.: Muscidae) | Adulticide (LC50 = 46.9 mg/dm3). | [76] |
| Brazil | L | HD | sabinene and bicyclogermacrene | Insecticidal activity in silico and in vitro Contact | Aedes aegypti (Dipt.: Culicidae) | Larvicide (S50 (median survival time) = 1 day at 0.01% v/v). | [75] |
| Brazil | L | SD | sylvestrene (27.1%), myrcene (26.4%), and sabinene (20.8%) | Contact | Culex quinquefasciatus (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 60.1 µg/mL). | [2] |
| Egypt | L | HD | α-phellandrene (29.8%), β-phellandrene (21.1%), and elemol (13.0%) | Immersion | Culex pipiens (Dipt.: Culicidae) | Comparative toxicity against all stages (LC50 = 12.8 mg/L). | [74] |
| Egypt | n.r. | HD | n.r. | Contact, Fumigation | Culex pipiens (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 141.0 mg/L). Adulticide (at 24 h LC50 = 6.92 mg/L). | [77] |
| Ethiopia | L | HD | n.r. | Contact | Aedes aegypti (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 9.6 ppm; LC90 = 15.0 ppm). | [73] |
| Anopheles arabiensis (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 21.0 ppm; LC90 = 37.3 ppm). | ||||||
| S | Contact | Aedes aegypti (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 14.5 ppm; LC90 = 28.5 ppm). | ||||
| Anopheles arabiensis (Dipt.: Culicidae) | Larvicide (at 24 h LC50 = 26.5 ppm; LC90 = 45.4 ppm). | ||||||
| Peru | L | HD | α-phellandrene (32.7%), D-limonene (12.6%), and β-phellandrene (12.2%) | Insecticidal activity in silico | Juvenile hormone-binding protein (mJHBP) from Aedes aegypti (Dipt.: Culicidae) | α-muurolene and γ-cadinene had the best biding energy on mJHBP (ΔG = −9:7 kcal/mol), followed by β-cadinene (ΔG = −9:5 kcal/mol). | [78] |
| Turkey | L | HD | δ-cadinene (11.3%), α-cadinol (10.8%), and α-phellandrene (6.9%) | Repellency assay | Blatta orientalis (Blatt.: Blattidae) | Repellency (93.3% at 35.4 µg/cm2). | [79] |
| Country | Part | Extraction | Main Compound | Formula/Technology | Dimension | Application | Pest | Activity | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Peru | n.r. | n.r. | D-limonene and α-phellandrene | EO and chitosan (1:2) microencapsulation with the spray-drier (1% w/w) | 78.4 μm | Contact (contaminated surface) | Macrosiphum euphorbiae (Hem.: Aphididae) | Adulticide (LC50 = 1.4 mg/cm2) | [54] |
| Argentinea | AP, F | HD | β-phellandrene (28.3%) and α-phellandrene (11.5%) | EO and Arabic rubber microencapsulation with atomizer (10% w/w) | 200.0 μm | Contact (contaminated surface), Evaporation, and Repellency assay | Varroa destructor (Mes.: Varroidae) | Adulticide (at 24 h, 0.25 g, contact: 30%; evaporation: 20%); Attractant effect | [87] |
| Argentina | L, B | SD | n.r. | EO and gum Arabic/maltodextrin (1:4) microencapsulation with the spray-drier (1% w/w) | 10–40 μm | Contact | Haematobia irritans (Dipt.: Muscidae) | Adulticide (at 2 h 32% at 100 mg/mL) | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalvenzi, L.; Durofil, A.; Cáceres Claros, C.; Pérez Martínez, A.; Guardado Yordi, E.; Manfredini, S.; Baldini, E.; Vertuani, S.; Radice, M. Unleashing Nature’s Pesticide: A Systematic Review of Schinus molle Essential Oil’s Biopesticidal Potential. Sustainability 2024, 16, 10444. https://doi.org/10.3390/su162310444
Scalvenzi L, Durofil A, Cáceres Claros C, Pérez Martínez A, Guardado Yordi E, Manfredini S, Baldini E, Vertuani S, Radice M. Unleashing Nature’s Pesticide: A Systematic Review of Schinus molle Essential Oil’s Biopesticidal Potential. Sustainability. 2024; 16(23):10444. https://doi.org/10.3390/su162310444
Chicago/Turabian StyleScalvenzi, Laura, Andrea Durofil, Carlos Cáceres Claros, Amaury Pérez Martínez, Estela Guardado Yordi, Stefano Manfredini, Erika Baldini, Silvia Vertuani, and Matteo Radice. 2024. "Unleashing Nature’s Pesticide: A Systematic Review of Schinus molle Essential Oil’s Biopesticidal Potential" Sustainability 16, no. 23: 10444. https://doi.org/10.3390/su162310444
APA StyleScalvenzi, L., Durofil, A., Cáceres Claros, C., Pérez Martínez, A., Guardado Yordi, E., Manfredini, S., Baldini, E., Vertuani, S., & Radice, M. (2024). Unleashing Nature’s Pesticide: A Systematic Review of Schinus molle Essential Oil’s Biopesticidal Potential. Sustainability, 16(23), 10444. https://doi.org/10.3390/su162310444









