Rapid Degradation of Carbamazepine in Wastewater Using Dielectric Barrier Discharge-Assisted Fe3⁺/Sodium Sulfite Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dielectric Barrier Discharge System
2.2. Experimental Reagents and Equipment
2.3. Setting of Initial Experimental Conditions
2.3.1. Degradation of CBZ Wastewater by DBD
2.3.2. Degradation of CBZ by DBD in Conjunction with Catalysts
2.3.3. Reactive Species and Degradation Mechanisms
2.4. Analytical Methods
3. Results and Discussion
3.1. Optimizing CBZ Degradation: Impact of Key Operational Parameters
3.2. Investigation of the Optimal System
3.3. Investigation of Factors Affecting Degradation Efficiency Under the Optimal System
3.4. Analysis of Response Surface Methodology
3.5. Exploration of the Optimal Degradation System for CBZ
3.6. Analysis of Degradation Pathways for CBZ
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strokal, M.; Bai, Z.; Franssen, W.; Hofstra, N.; Koelmans, A.A.; Ludwig, F.; Ma, L.; van Puijenbroek, P.; Spanier, J.E.; Vermeulen, L.C.; et al. Urbanization: An increasing source of multiple pollutants to rivers in the 21st century. Npj Urban Sustain. 2021, 1, 24. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (ppcps) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J. Water Process. Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, T.; Wang, D.; Zhao, Y.; Jin, Y.; Yang, G. The occurrence of typical psychotropic drugs in the aquatic environments and their potential toxicity to aquatic organisms—A review. Sci. Total. Environ. 2023, 900, 165732. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. A review of emerging pollutant treatment technologies applied in wastewater treatment plants. China Resour. Compre. 2021, 39, 96–98. [Google Scholar]
- Li, W.; Li, G.; Fan, L.; Liu, R. Research progress on PPCPs in the Yangtze River estuary and adjacent areas. Environ. Prot. 2022, 50, 44–50. [Google Scholar]
- Wang, X. Experimental Study on the Degradation of Carbamazepine by Potassium Ferrate Oxidation. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. [Google Scholar]
- Wang, W.; Zhang, S.; Jia, Y.; Xiao, G.; Zhang, J.; Xian, W.; Zhou, S. Catalytic removal of carbamazepine from water using nitrogen-doped sludge carbon and persulfate. J. Qinghai Univ. 2022, 40, 9–17. [Google Scholar]
- Lee, Y.-Y.; Fan, C.; Haque, F. Hybrid combination of advanced oxidation and biological processes for the micropollutant removal of carbamazepine. Npj Clean Water 2022, 5, 60. [Google Scholar] [CrossRef]
- Xue, Y.; Kamali, M.; Kakavandi, B.; Costa, M.E.V.; Thompson, I.P.; Huang, W.; Appels, L.; Dewil, R. Activation of peracetic acid by a magnetic biochar-ferrospinel AFe2O4 (A = Cu, Co, or Mn) nanocomposite for the degradation of carbamazepine—A comparative and mechanistic study. Chem. Eng. J. 2024, 490, 151932. [Google Scholar] [CrossRef]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jafari, A.J.; Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in aop technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Shan, C.; Li, H.; Yin, Y.; Pan, B. toward selective oxidation of contaminants in aqueous systems. Environ. Sci. Technol. 2021, 55, 14494–14514. [Google Scholar] [CrossRef]
- McGachy, L.; Sedlak, D.L. From theory to practice: Leveraging chemical principles to improve the performance of peroxydisulfate-based in situ chemical oxidation of organic contaminants. Environ. Sci. Technol. 2023, 58, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, H.; Jin, W.; Chu, W.; Xu, Z. The performance and mechanism of iron-mediated chemical oxidation: Advances in hydrogen peroxide, persulfate and percarbonate oxidation. J. Environ. Sci. 2022, 128, 181–202. [Google Scholar] [CrossRef]
- Xu, L.; Wong, P.K.; Jiang, Z.; Yu, J.C. Iodide-mediated selective photocatalytic treatment of phenolic pollutants. Appl. Catal. B Environ. 2023, 338, 123080. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Shen, Y. Theoretical insights of the pharmaceutical compound fluoxetine in water: Role in direct photolysis and indirect photolysis by free radicals. J. Environ. Sci. 2023, 142, 269–278. [Google Scholar] [CrossRef]
- Bueno, I.; He, H.; Kinsley, A.C.; Ziemann, S.J.; Degn, L.R.; Nault, A.J.; Beaudoin, A.L.; Singer, R.S.; Wammer, K.H.; Arnold, W.A. Biodegradation, photolysis, and sorption of antibiotics in aquatic environments: A scoping review. Sci. Total. Environ. 2023, 897, 165301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, X.; Zheng, L.; Liu, Y.; Zhao, Y.; Huang, S.; Li, S. Synergistic catalysis degradation of amoxicillin by dbd plasma-catalyst system constructed by dbd plasma and Ce0.5Bi0.5VO4/HCP coating. Process. Saf. Environ. Prot. 2023, 181, 416–428. [Google Scholar] [CrossRef]
- Song, S.; Zhang, H.; Han, S.; Xiao, S.; Du, Y.; Hu, K.; Wang, H.; Wu, C. Activation of persulfate by a water falling film dbd process for the enhancement of enrofloxacin degradation. Chemosphere 2022, 301, 134667. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Lei, H.; Yao, X.; Fang, Y.; Cheng, X. Investigation of high-concentration toluene degradation by dbd plasma under different operating parameters. J. Phys. D Appl. Phys. 2023, 56, 315201. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Li, X.; Zhou, R.-W.; Huang, J.; Chen, W.; Wang, F.-P.; Lu, X.-Y.; Wen, Q. Degradation of high-concentration simulated organic wastewater by dbd plasma. Water Sci. Technol. 2019, 80, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guo, H.; Ji, Z.; Ou, X.; Chen, H.; Fan, X. Cellular foam-based trickle-bed dbd reactor for plasma-assisted degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2023, 311, 123317. [Google Scholar] [CrossRef]
- Cao, Y.; Qu, G.; Li, T.; Jiang, N.; Wang, T. Review on reactive species in water treatment using electrical discharge plasma: Formation, measurement, mechanisms and mass transfer. Plasma Sci. Technol. 2018, 20, 103001. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Guo, H.; Pan, S.; Puyang, C.; Su, Y.; Qiao, W.; Han, J. Insights into water film dbd plasma driven by pulse power for ibuprofen elimination in water: Performance, mechanism and degradation route. Sep. Purif. Technol. 2021, 277, 119415. [Google Scholar] [CrossRef]
- Tang, Q.; Lin, S.; Jiang, W.; Lim, T. Gas phase dielectric barrier discharge induced reactive species degradation of 2,4-dinitrophenol. Chem. Eng. J. 2009, 153, 94–100. [Google Scholar] [CrossRef]
- Sang, W.; Lu, W.; Mei, L.; Jia, D.; Cao, C.; Li, Q.; Wang, C.; Zhan, C.; Li, M. Research on different oxidants synergy with dielectric barrier discharge plasma in degradation of orange G: Efficiency and mechanism. Sep. Purif. Technol. 2021, 277, 119473. [Google Scholar] [CrossRef]
- Chen, C.; Ma, C.; Yang, Y.; Yang, X.; Demeestere, K.; Nikiforov, A.; Van Hulle, S. Degradation of micropollutants in secondary wastewater effluent using nonthermal plasma-based Aops: The roles of free radicals and molecular oxidants. Water Res. 2023, 235, 119881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Hao, M.; Yu, F.; Houda, C. Persistent degradation of 2,4-dichlorophenol in groundwater by persulfate synergize with Fe(iii)/CaSO3 system: Role of Fe(iv) and 1O2 oxidation. Sep. Purif. Technol. 2023, 334, 125979. [Google Scholar] [CrossRef]
- Li, L.; Zheng, D.; Gu, X.; Sun, C.; Liu, Y.; Dong, W.; Wu, Y. Mechanism of the improved Fe(iii)/persulfate reaction by gallic acid for ibuprofen degradation. Environ. Pollut. 2022, 314, 120318. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tong, Y.; Xue, Y.; Liu, Z.; Tang, M.; Huang, L.-Z.; Shao, S.; Fang, Z. Degradation of the β-blocker propranolol by sulfite activation using FeS. Chem. Eng. J. 2019, 385, 123884. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, C.; Chen, L.; Duan, J.; Li, F.; Liu, W. Different reaction mechanisms of SO4•− and •OH with organic compound interpreted at molecular orbital level in Co(ii)/peroxymonosulfate catalytic activation system. Water Res. 2022, 229, 119392. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, D.; Xia, D.; Li, Q. Orange peels biochar doping with Fe-Cu bimetal for PMS activation on the degradation of bisphenol A: A synergy of SO4•−, •OH, 1O2 and electron transfer. Chem. Eng. J. 2023, 471, 144832. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, J.; Guo, H. Sulfite activation by non-thermal plasma coupled with Fe2+ for ibuprofen degradation: In-depth insight into activation energy barrier and mechanism. Sep. Purif. Technol. 2024, 351, 128042. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Li, Z.; Han, J.; Guo, H. Sulfite activation by water film dielectric barrier discharge plasma for ibuprofen degradation: Efficiency, comparison of persulfate, mechanism, active substances dominant to pathway, and toxicity evaluation. Sep. Purif. Technol. 2023, 330, 125531. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Xu, D.; Liu, Y. Research progress on the pollution status and removal methods of PPCPs in China. Technol. Innov. Appl. 2021, 11, 130–133 + 136. [Google Scholar]
- Zhang, Q. Experimental Research on Low-Temperature Plasma Wastewater Treatment Technology and Application. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2018. [Google Scholar]
- Xin, L. Study on the Simultaneous Removal of Triclosan from Water by Dielectric Barrier Discharge Plasma and Activated Carbon Fiber. Master’s Thesis, Nanjing University, Nanjing, China, 2016. [Google Scholar]
- Doll, T.E.; Frimmel, F.H. Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195–202. [Google Scholar] [CrossRef]
- Sharma, V.K. Potassium ferrate(VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Miao, X.-S.; Yang, J.-J.; Metcalfe, C.D. Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wang, Q.; Li, Q.; Shang, B.; Rahaman, H.; Liang, J.; Ji, J.; Liu, W.; Zhai, J.; Wang, Q.; et al. Degradation mechanisms of carbamazepine by delta-MnO2: Role of protonation of degradation intermediates. Sci. Total. Environ. 2018, 640, 981–988. [Google Scholar] [CrossRef]
- Rao, Y.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef]



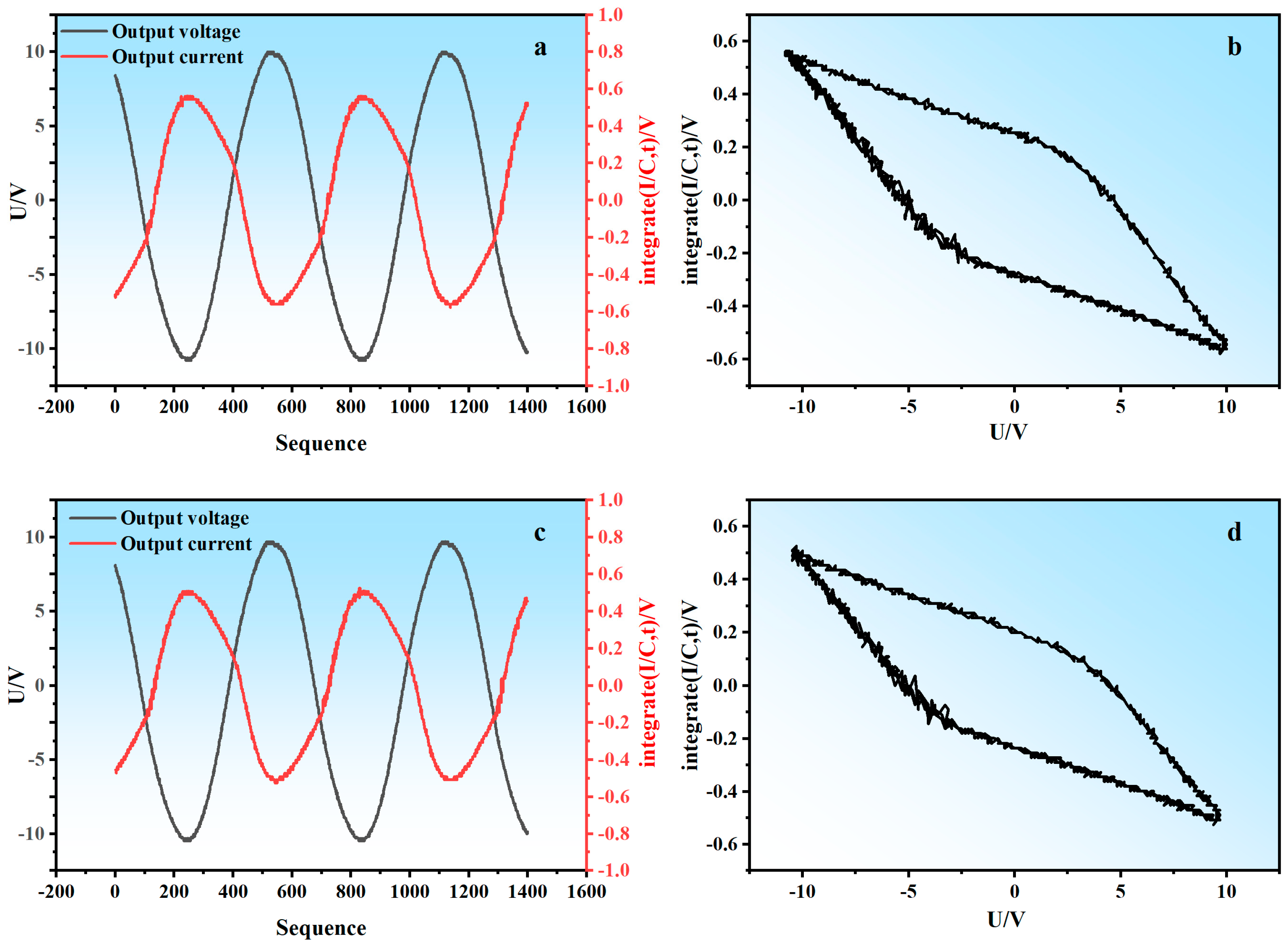


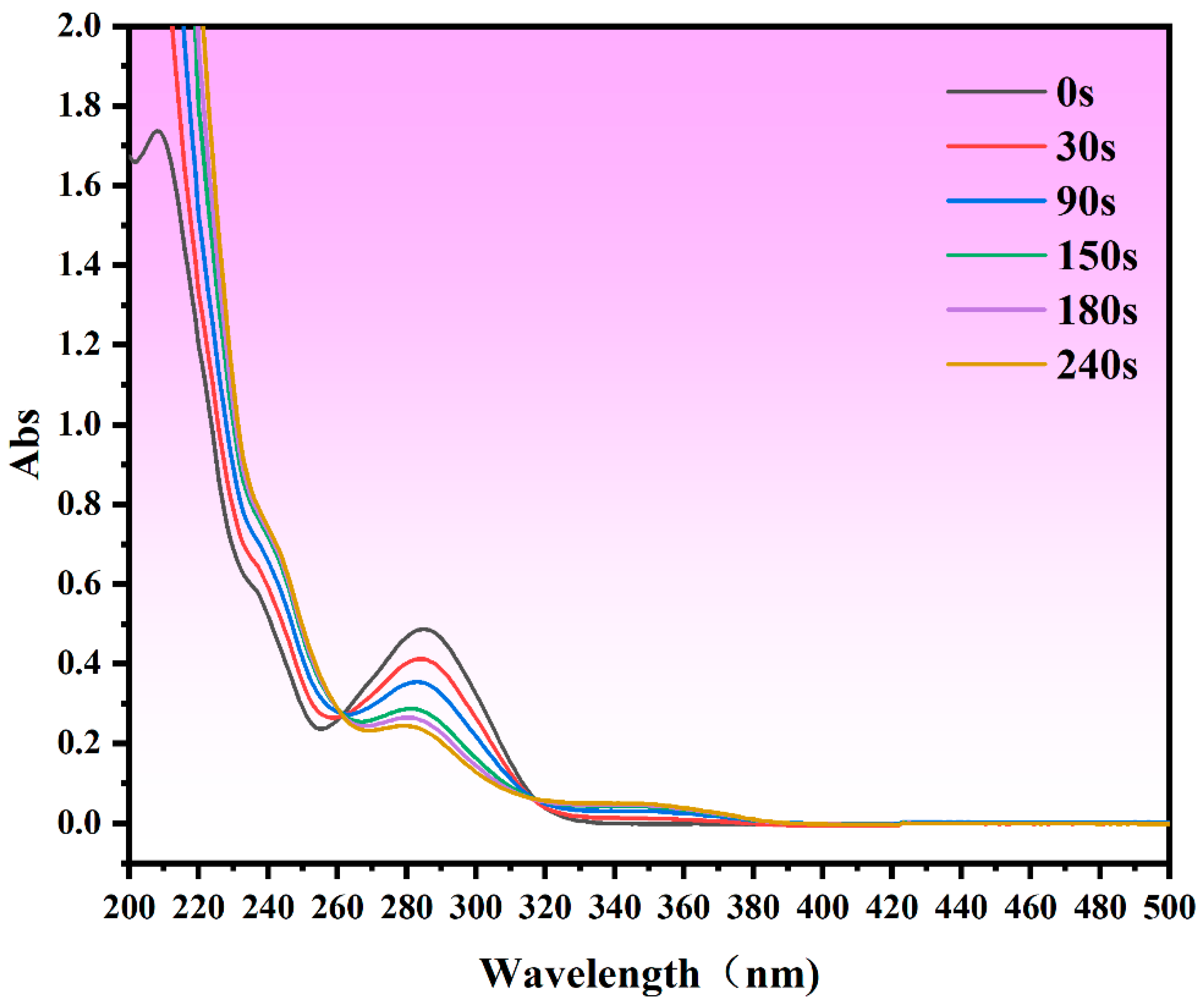

| Variable | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A-Sodium metabisulfite concentrationm (M) | 0.1 | 0.55 | 1 |
| B-pH | 3 | 5 | 7 |
| C-Fe3+ concentration(mg/L) | 0.5 | 1 | 1.5 |
| Sample | A | B | C | CBZ Degradation Efficiency | CBZ Degradation Efficiency |
|---|---|---|---|---|---|
| Sodium Metabisulfite Concentration (M) | pH | Fe3+ Concentration (mg/L) | Experimental Value (%) | Predicted Value (%) | |
| 1 | 0.1 | 3 | 1 | 96.000 | 99.940 |
| 2 | 1 | 3 | 1 | 70.836 | 70.610 |
| 3 | 0.1 | 7 | 1 | 93.600 | 93.830 |
| 4 | 1 | 7 | 1 | 60.211 | 56.270 |
| 5 | 0.1 | 5 | 0.5 | 83.334 | 81.260 |
| 6 | 1 | 5 | 0.5 | 30.911 | 33.010 |
| 7 | 0.1 | 5 | 1.5 | 92.869 | 90.770 |
| 8 | 1 | 5 | 1.5 | 70.057 | 72.130 |
| 9 | 0.55 | 3 | 0.5 | 68.327 | 66.460 |
| 10 | 0.55 | 7 | 0.5 | 55.384 | 57.230 |
| 11 | 0.55 | 3 | 1.5 | 93.609 | 91.770 |
| 12 | 0.55 | 7 | 1.5 | 78.687 | 80.550 |
| 13 | 0.55 | 5 | 1 | 66.000 | 62.800 |
| 14 | 0.55 | 5 | 1 | 60.000 | 62.800 |
| 15 | 0.55 | 5 | 1 | 62.000 | 62.800 |
| 16 | 0.55 | 5 | 1 | 63.000 | 62.800 |
| 17 | 0.55 | 5 | 1 | 63.000 | 62.800 |
| Atom | Natural Charge | Atomic Bond | Bond Length Å |
|---|---|---|---|
| N27 | 0.687 | N27-C26 | 1.374 |
| O30 | −0.283 | O30-C26 | 1.231 |
| N25 | −0.212 | N25-C26 | 1.395 |
| C26 | 0.714 | N25-C7 | 1.433 |
| C1 | −0.087 | N25-C12 | 1.432 |
| C3 | −0.111 | C1-C3 | 1.399 |
| C5 | −0.081 | C3-C5 | 1.394 |
| C7 | 0.113 | C5-C7 | 1.399 |
| C8 | 0.161 | C7-C8 | 1.413 |
| C9 | −0.158 | C8-C9 | 1.409 |
| C11 | 0.144 | C8-C21 | 1.462 |
| C12 | 0.178 | C21-C23 | 1.553 |
| C13 | −0.077 | C23-C11 | 1.463 |
| C15 | −0.119 | C11-C12 | 1.411 |
| C17 | −0.090 | C12-C13 | 1.393 |
| C19 | −0.161 | C13-C15 | 1.392 |
| C21 | −0.347 | C15-C17 | 1.399 |
| C23 | −0.342 | C17-C19 | 1.390 |
| C19-C11 | 1.410 |
| Serial Number | Molecular Formula | Molecular Weight | Structural Formula |
|---|---|---|---|
| P-237 | C15H12N2O | 237 |  |
| P1-267 | C15H10N2O3 | 267 |  |
| P2-253 | C15H12N2O2 | 253 |  |
| P3-284 | C15H12N2O4 | 284 |  |
| P4-208 | C14H9NO | 208 | 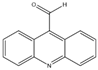 |
| P5-196 | C13H9NO | 196 |  |
| P6-224 | C14H9NO2 | 224 |  |
| P7-180 | C13H9N | 180 | 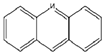 |
| P8-271 | C15H14N2O3 | 271 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Zhu, Y.; Zhou, Z.; Dong, Y.; Ni, Z.; Jiang, Z.; Liu, Z.; Chen, Z.; Wu, X.; Zheng, Q.; et al. Rapid Degradation of Carbamazepine in Wastewater Using Dielectric Barrier Discharge-Assisted Fe3⁺/Sodium Sulfite Oxidation. Sustainability 2024, 16, 10544. https://doi.org/10.3390/su162310544
Wei W, Zhu Y, Zhou Z, Dong Y, Ni Z, Jiang Z, Liu Z, Chen Z, Wu X, Zheng Q, et al. Rapid Degradation of Carbamazepine in Wastewater Using Dielectric Barrier Discharge-Assisted Fe3⁺/Sodium Sulfite Oxidation. Sustainability. 2024; 16(23):10544. https://doi.org/10.3390/su162310544
Chicago/Turabian StyleWei, Wei, Yulong Zhu, Zhenghan Zhou, Yuxiang Dong, Ziyan Ni, Zhongqi Jiang, Zhiquan Liu, Zhiyan Chen, Xiachun Wu, Qiyuan Zheng, and et al. 2024. "Rapid Degradation of Carbamazepine in Wastewater Using Dielectric Barrier Discharge-Assisted Fe3⁺/Sodium Sulfite Oxidation" Sustainability 16, no. 23: 10544. https://doi.org/10.3390/su162310544
APA StyleWei, W., Zhu, Y., Zhou, Z., Dong, Y., Ni, Z., Jiang, Z., Liu, Z., Chen, Z., Wu, X., Zheng, Q., & Zhu, S. (2024). Rapid Degradation of Carbamazepine in Wastewater Using Dielectric Barrier Discharge-Assisted Fe3⁺/Sodium Sulfite Oxidation. Sustainability, 16(23), 10544. https://doi.org/10.3390/su162310544






