New Wine in Old Bottles: The Sustainable Application of Slow Sand Filters for the Removal of Emerging Contaminants, a Critical Literature Review
Abstract
:1. Introduction
1.1. Contaminants of Emerging Concern
1.2. Legislative Approaches to Water Treatment
1.3. Water Stress
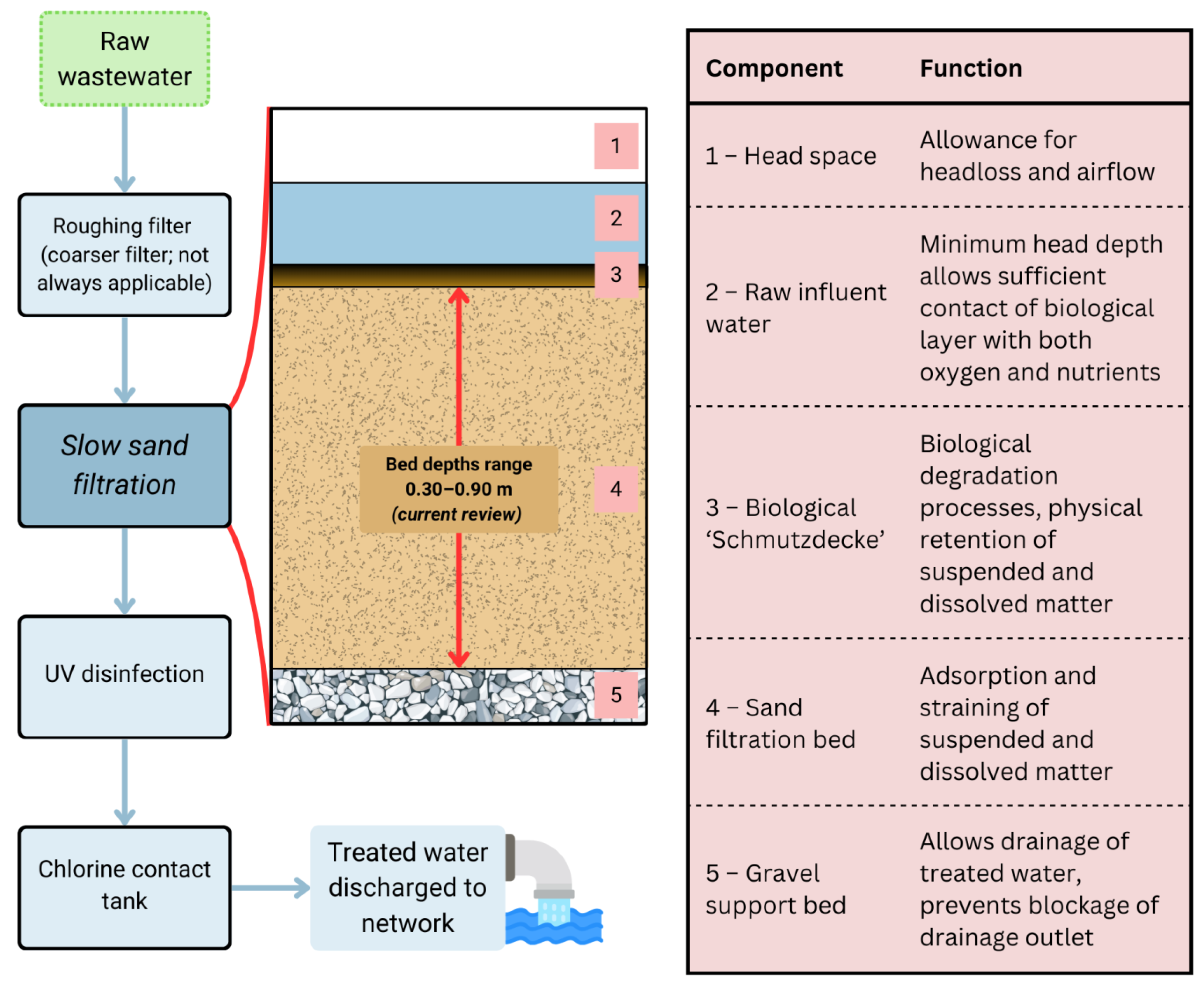
2. Slow Sand Filtration and Operation
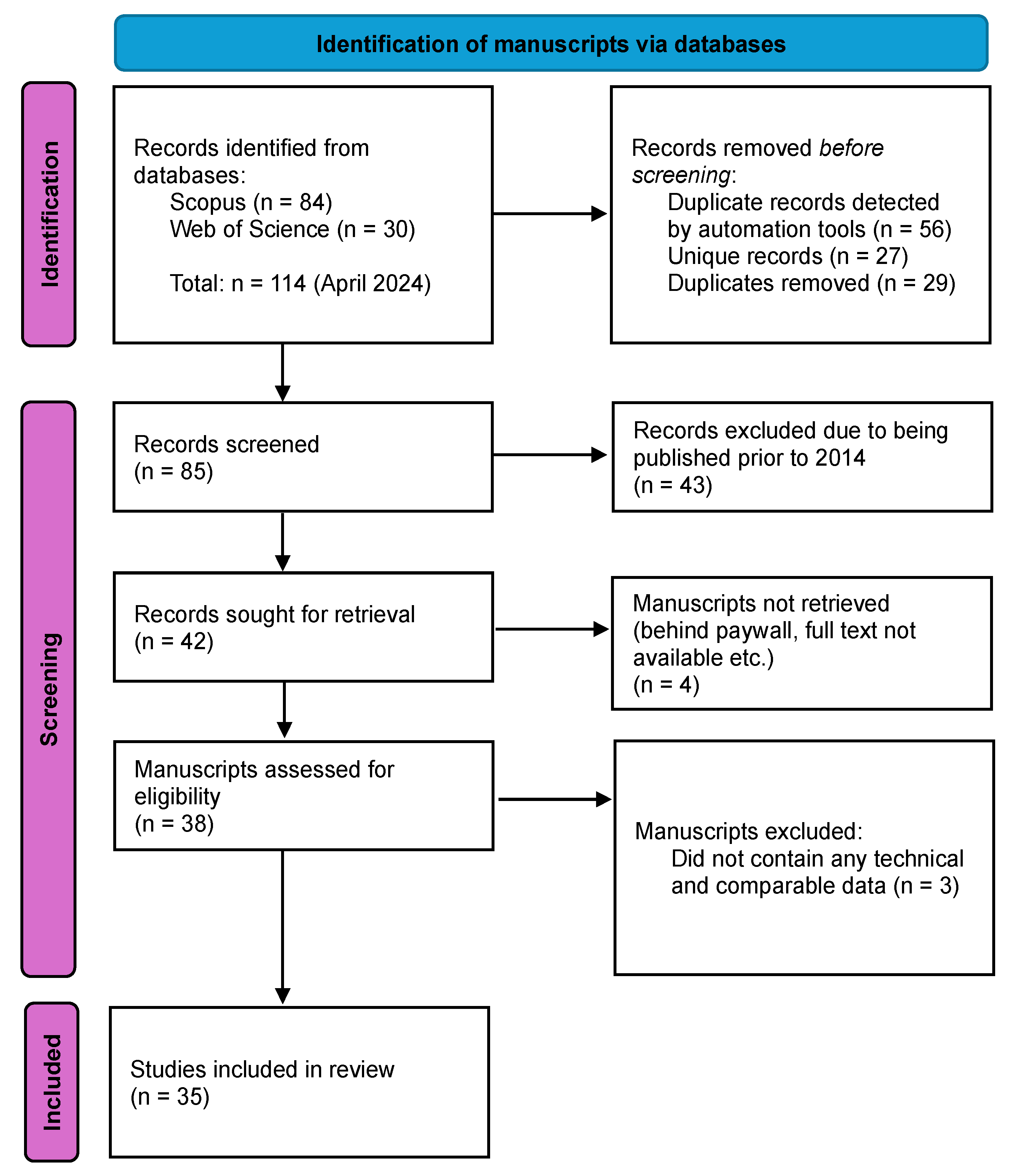
3. Methods Used for the Review of Literature
4. Key Design Parameters of SSFs
- Characteristics of the filter bed;
- The impact of location and climate on water demand and filter operation;
- Multimedia filters and the use of alternative materials.
4.1. Characteristics of the Filter Bed
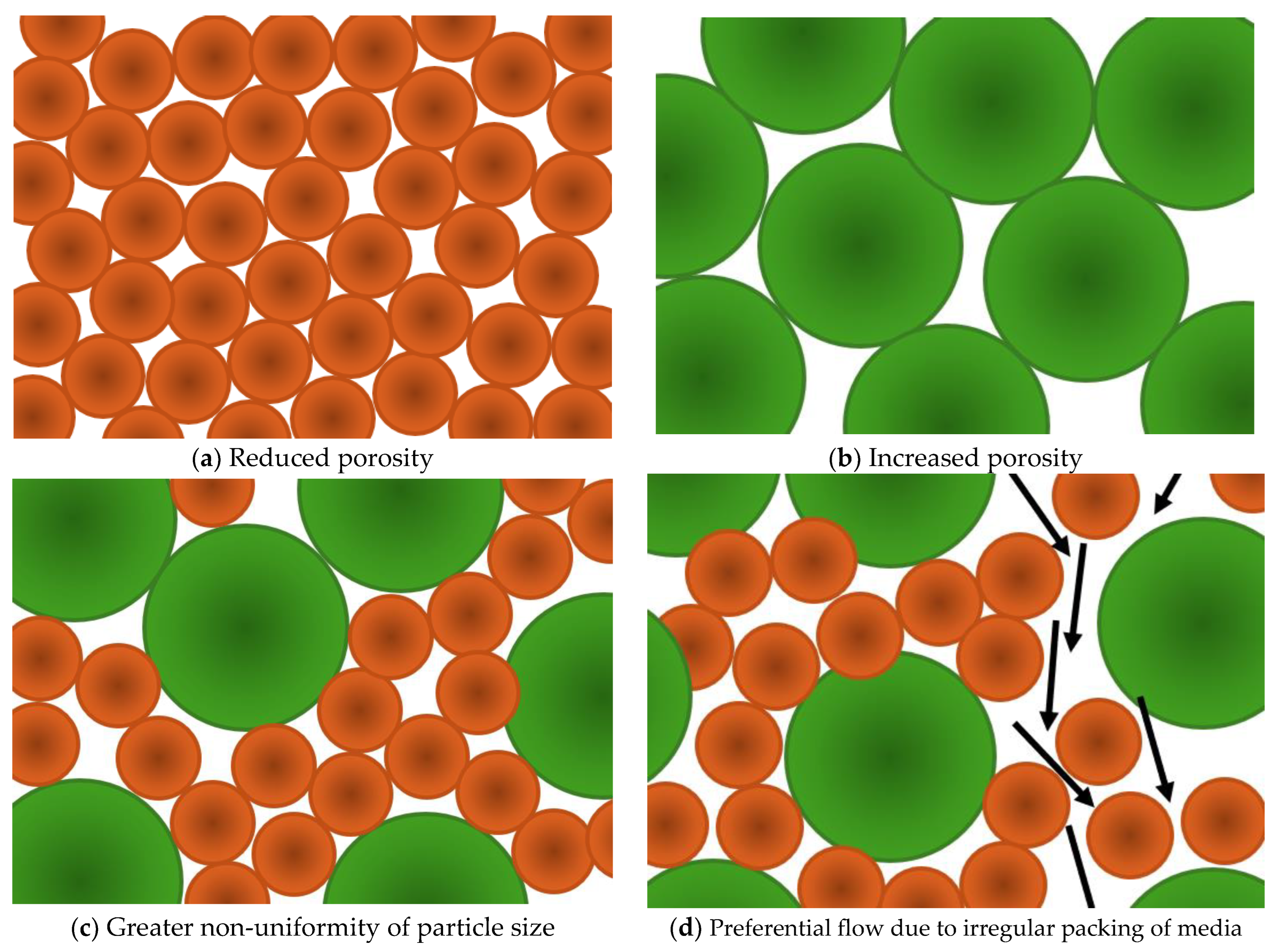
4.1.1. Particle Size Distribution
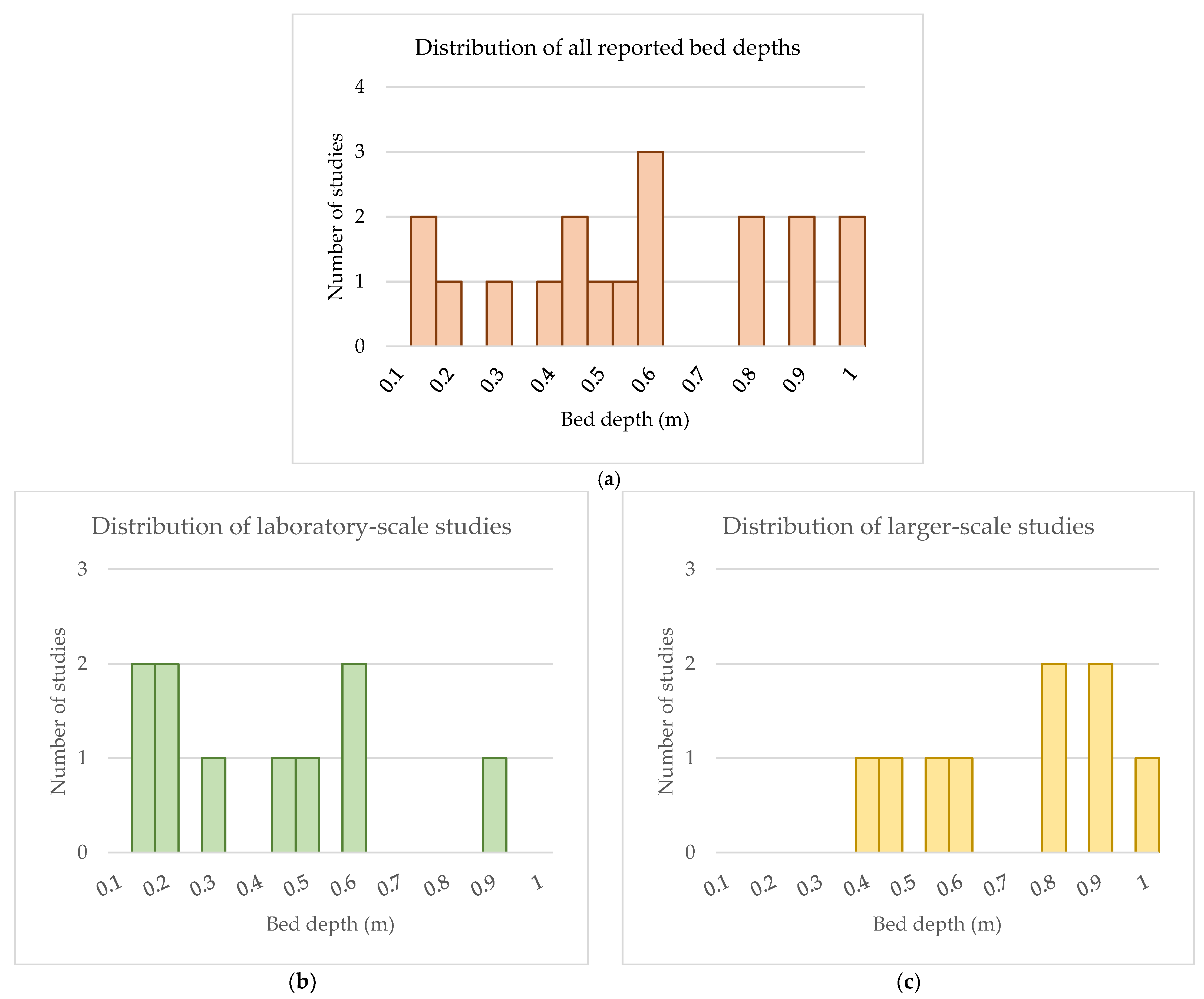
4.1.2. Depth of Media Material
4.2. The Impact of Location and Climate on Water Demand and Filter Operation
4.2.1. Operation Modes: Continuous Versus Intermittent
4.2.2. Maintenance Cycles
4.3. Multimedia Filters and the Use of Alternative Materials
4.3.1. Activated Carbon and Biochar
4.3.2. Alternative Sustainable Treatment Materials
| Sawdust Treatment | Contaminants or Indicators Measured | Removal Efficiency | Reference |
|---|---|---|---|
| Washed, dried, and ground. | Heavy metal ions such as Pb2+, Cd2+ and Ni2+ | 25.98–64.32% | [128] |
| Washed and dried. | Nitrogen species (NH3-N, NO3-N, and NO2-N) | Max. 98% removal | [129] |
| Washed, treated with solutions of citric acid and NaPO2H2, washed and dried. | Heavy metals Cr2+, Ni2+, Zn2+, and Cu2+ | Cr2+ removal 76% for all contact times. Remaining ion removal increased with contact time to max. 75% at 24 h. | [130] |
| Washed, treated with H2SO4 at 60 °C, dried, and ground. | Antibiotic meropenem (C17H25N3O5S) | Up to 98.6% removed by treated sawdust and up to 92.4% by untreated sawdust. | [60] |
| Treated sequentially with solutions of: H3PO4, H2SO4, KO, H and then distilled H2O. Washed and dried. | Antibiotic vancomycin (C66H75Cl2N9O24) | 63% removal by treated sawdust compared to 15% removal by untreated sawdust. | [123] |
| Treated with NaOH and H2SO4, washed, and dried. Then, chemically modified with FeH6O3 and Al2O3 | As5+ and F− | Removal efficiency was not reported | [125] |
4.4. Interactions Between PPCPs and the Biological Layer of the SSF
4.5. Summary of Engineering Observations
- A bed depth of >0.6 m
- A particle effective size (D10) between 0.15 and 0.40 mm
- A coefficient of uniformity ≤ 2
- The sand must have a high silica content.
- Lower bound for D60
- a.
- When D10 = 0.15 mm, and CU = 1, D60 = 0.15 mm.
- b.
- When D10 = 0.40 mm, and CU = 1, D60 = 0.40 mm.
- Upper bound for D60
- a.
- When D10 = 0.15 mm, and CU = 2.0, D60 = 0.30 mm.
- b.
- When D10 = 0.40 mm, and CU = 2.0, D60 = 0.80 mm.
5. Conclusions
- Optimal SSF design parameters for efficient contaminants removal include:
- a.
- a filter bed depth of at least 0.6 m;
- b.
- a filtration medium with recommended particle sizes in the range of 0.15–0.40 mm;
- c.
- a highly uniform filtration medium with a coefficient of uniformity < 2 is recommended.
- The selection of sand material (such as high silica (SiO2) content or quartz) is influential as biofilm adhesion, particle size, and friability may be impacted.
- A harmonious approach to reporting particle sizes, filtration rates, hydraulic retention/residence times, and performance indicators (i.e., removal rates) is not currently used in experimental work on SSF. A robust standard method of particle size characterization, highlighting effective size D10, is especially needed as well as the media coefficients of uniformity.
- The analysis of the studies using alternative filter media demonstrated the potential of lignocellulosic adsorbents for the removal of some common water contaminants as well as CECs such as antibiotics.
- The dimensions of the SSF are at the discretion of its application. Household continuous or intermittently run filters are more compact units; thus, supernatant head height is smaller to accommodate oxygen incorporation to promote biofilm growth during pause periods. Larger filters, typical of continuously run SSFs, have greater supernatant depths as the biological layer is putatively stimulated more and is thus more active.
- The impact of contaminants such as PPCPs on biofilm growth varied widely across studies, highlighting the need for further research under environmentally relevant conditions (i.e., realistic influent quality characteristics) to assess long-term effects and explore mitigation strategies.
- The adsorbent materials such as AC and biochar have previously demonstrated high PPCP removal rates from water. Published works investigating novel lignocellulosic adsorbents were analyzed in this review and, considering the lower carbon footprint in the manufacture of such materials, their capacity to replace AC is promising.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| AMR | Antimicrobial resistance |
| CAWST | Center for Affordable Water and Sanitation Technology |
| CEC | contaminant of emerging concerns |
| cm | centimeters |
| cm∙h−1 | Centimeters per hour |
| CSO | combines sewer overflow |
| CU | Co-efficient of uniformity (D60/D10) |
| D10 | Effective size (mm) |
| DEET | diethyltoluamide |
| EC | European Commission |
| EDC | endocrine-disrupting chemicals |
| EPA | Environmental Protection Agency |
| EPS | Exopolymeric substance |
| EQS | environmental quality standard |
| GAC | granular activated carbon |
| HLR | hydraulic loading rate |
| HRT | hydraulic retention time |
| L | Liters |
| m∙h−1 | Meters per hour |
| µg∙L−1 | Micrograms per liter |
| mL∙min−1 | Milliliters per minute |
| NSAID | non-steroidal anti-inflammatory drug |
| NTU | nephelometric turbidity units |
| PAC | powdered activated carbon |
| PBDE | polybrominated diphenyl ether |
| pKOW | partition coefficient (measure of the hydrophobicity of a substance) |
| PNEC | predicted no-effect concentration |
| POU | point-of-use |
| PPCP | pharmaceuticals and personal care products |
| RM | sand removal method |
| SA | surface agitation method |
| SD | standard deviation |
| SDG | sustainable development goal |
| SM | stirring method |
| SSF | slow sand filtration |
| TP | total phosphorus |
| UN | United Nations |
| uPBT | ubiquitous, persistent, bioaccumulative, toxic |
| V∙cm−1 | Volts per centimeter |
| WaSH | water supply, sanitation, and hygiene |
| WFD | Water Framework Directive |
References
- Nasser Fava, N.D.M.; Terin, U.C.; Freitas, B.L.S.; Sabogal-Paz, L.P.; Fernandez-Ibañez, P.; Anthony Byrne, J. Household Slow Sand Filters in Continuous and Intermittent Flows and their Efficiency in Microorganism’s removal from River Water. Environ. Technol. 2022, 43, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Welz, P.J. Biosand Reactors for Municipal and Industrial Wastewater Treatment: Status Quo, Challenges and Opportunities. Processes 2024, 12, 641. [Google Scholar] [CrossRef]
- Ellis, K.V. Slow sand filtration as a technique for the tertiary treatment of municipal sewages. Water Res. 1987, 21, 403–410. [Google Scholar] [CrossRef]
- Duckett, D.; Troldborg, M.; Hendry, S.; Cousin, H. Making waves: Promoting municipal water reuse without a prevailing scarcity driver. Water Res. 2024, 249, 120965. [Google Scholar] [CrossRef]
- Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse (Text with EEA Relevance). 2020. Available online: http://data.europa.eu/eli/reg/2020/741/oj (accessed on 4 June 2024).
- González, O.; Bayarri, B.; Aceña, J.; Pérez, S.; Barceló, D. Treatment Technologies for Wastewater Reuse: Fate of Contaminants of Emerging Concern. In Advanced Treatment Technologies for Urban Wastewater Reuse; Fatta-Kassinos, D., Dionysiou, D.D., Kümmerer, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 5–37. [Google Scholar] [CrossRef]
- Jones, E.R.; van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237–245. Available online: https://api.semanticscholar.org/CorpusID:233882995 (accessed on 2 July 2024). [CrossRef]
- European Environment Agency. European Waters—Assessment of Status and Pressures 2018; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Afful, S.; Awudza, J.A.M.; Twumasi, S.K.; Osae, S. Determination of indicator polychlorinated biphenyls (PCBs) by gas chromatography–electron capture detector. Chemosphere 2013, 93, 1556–1560. [Google Scholar] [CrossRef]
- Wu, Z.; He, C.; Han, W.; Song, J.; Li, H.; Zhang, Y.; Jing, X.; Wu, W. Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: A review. Environ. Res. 2020, 187, 109531. [Google Scholar] [CrossRef] [PubMed]
- Budeli, P.; Ekwanzala, M.D.; Unuofin, J.O.; Momba, M.N.B. Endocrine disruptive estrogens in wastewater: Revisiting bacterial degradation and zymoremediation. Environ. Technol. Innov. 2021, 21, 101248. [Google Scholar] [CrossRef]
- Elskens, M.; Van Langenhove, K.; Carbonnel, V.; Brion, N.; Eisenreich, S.J. Dynamics of estrogenic activity in an urban river receiving wastewater effluents: Effect-based measurements with CALUX. Water Emerg. Contam. Nanoplastics 2023, 2, 9. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Samanipour, S.; Rapp-Wright, H.; Bieber, S.; Letzel, T.; O’brien, J.W.; Marczylo, T.; Gant, T.W.; Vineis, P.; Barron, L.P. Bridging knowledge gaps in human chemical exposure via drinking water with non-target screening. Crit. Rev. Environ. Sci. Technol. 2024, 1–25, ahead-of-print. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sahoo, S.; Yadav, A.K.; Patil, S.A. Green wall system coupled with slow sand filtration for efficient greywater management at households. NPJ Clean Water 2023, 6, 73. [Google Scholar] [CrossRef]
- Rapp-Wright, H.; Regan, F.; White, B.; Barron, L.P. A year-long study of the occurrence and risk of over 140 contaminants of emerging concern in wastewater influent, effluent and receiving waters in the Republic of Ireland. Sci. Total Environ. 2023, 860, 160379. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy (EU 2020/1161). 2020, pp. 32–35. Available online: http://data.europa.eu/eli/dec_impl/2020/1161/oj (accessed on 24 September 2024).
- Gomez Cortes, L.; Marinov, D.; Sanseverino, I.; Navarro, C.A.; Niegowska, C.M.; Porcel, R.E.; Stefanelli, F.; Lettieri, T. Selection of Substances for the 4th Watch List Under the Water Framework Directive; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Lettieri, T.; Napierska, D.; Loos, R.; Marinov, D.; Sanseverino, I. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- European Communities. Establishing a Framework for Community Action in the Field of Water Policy. 2000, pp. 1–72. Available online: http://data.europa.eu/eli/dir/2000/60/2014-11-20 (accessed on 22 October 2023).
- Jones, E.R.; Bierkens, M.F.P.; Wanders, N.; Sutanudjaja, E.H.; van Beek, L.P.H.; van Vliet, M.T.H. Current wastewater treatment targets are insufficient to protect surface water quality. Commun. Earth Environ. 2022, 3, 221. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.; Rui, X.; Wang, Y.; Zhu, N. Drivers of change behind the spatial distribution and fate of typical trace organic pollutants in fresh waste leachate across China. Water Res. 2024, 263, 122170. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste. 1999, pp. 1–19. Available online: http://data.europa.eu/eli/dir/1999/31/2024-08-04 (accessed on 9 November 2024).
- Gao, J.; Oloibiri, V.; Chys, M.; Audenaert, W.; Decostere, B.; He, Y.; Van Langenhove, H.; Demeestere, K.; Van Hulle, S.W.H. The present status of landfill leachate treatment and its development trend from a technological point of view. Rev. Environ. Sci. Bio/Technol. 2015, 14, 93–122. [Google Scholar] [CrossRef]
- Jamrah, A.; AL-Zghoul, T.M.; Al-Qodah, Z. An Extensive Analysis of Combined Processes for Landfill Leachate Treatment. Water 2024, 16, 1640. [Google Scholar] [CrossRef]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.M.D.S.; Navoni, J.A.; de Medeiros, G.F.; Mina, I.M.C.A.P. Ecotoxicological assessment of estuarine surface waters receiving treated and untreated sanitary wastewater. Environ. Monit. Assess. 2022, 194, 908. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Li, J.; Rothwell, J.; Hurley, R. Acute riverine microplastic contamination due to avoidable releases of untreated wastewater. Nat. Sustain. 2021, 4, 793–802. [Google Scholar] [CrossRef]
- Powley, H.R.; Dürr, H.H.; Lima, A.T.; Krom, M.D.; Van Cappellen, P. Direct Discharges of Domestic Wastewater are a Major Source of Phosphorus and Nitrogen to the Mediterranean Sea. Environ. Sci. Technol. 2016, 50, 8722–8730. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Off. J. 2000, 43, 1–83. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02000L0060-20141120#tocId101 (accessed on 5 December 2023).
- Cooper, R.J.; Hiscock, K.M. Two decades of the EU Water Framework Directive: Evidence of success and failure from a lowland arable catchment (River Wensum, UK). Sci. Total. Environ. 2023, 869, 161837. [Google Scholar] [CrossRef]
- Northern Ireland Environment Agency (NIEA); Department of Agriculture, Environment and Rural Affairs (DAERA). Water Framework Directive Statistics Report 2021 February 2023. Available online: https://www.daera-ni.gov.uk/articles/water-framework-directive-statistics (accessed on 15 January 2024).
- European Commission. Proposal for a Directive of the European Parliament and of the Council Amending Directive 2000/60/EC Establishing a Framework for Community Action in the Field of Water Policy, Directive 2006/118/EC on the Protection of Groundwater Against Pollution and Deterioration and Directive 2008/105/EC on Environmental Quality Standards in the Field of Water Policy; European Commission: Brussels, Belgium, 26 October 2022. [Google Scholar]
- Kaiser, R.A.; Taing, L.; Bhatia, H. Antimicrobial Resistance and Environmental Health: A Water Stewardship Framework for Global and National Action. Antibiotics 2022, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Naddaf, M. 40 million deaths by 2050: Toll of drug-resistant infections to rise by 70%. Nature 2024, 633, 747–748. [Google Scholar] [CrossRef]
- European Environment Agency. Water Resources Across Europe—Confronting Water Stress: An Updated Assessment; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs (UN DESA). The Sustainable Development Goals Report 2016; UN DESA: New York, NY, USA, July 2016; Available online: https://desapublications.un.org/publications/sustainable-development-goals-report-2016 (accessed on 8 December 2023).
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- Koncagül, E.; Tran, M.; Connor, R. The United Nations World Water Development Report 2021: Valuing Water; Facts and Figures; UNESCO: Paris, France, 2021; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000375751 (accessed on 9 March 2024).
- UNESCO World Water Assessment Programme. The United Nations World Water Development Report 2021—Valuing Water; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2021. [Google Scholar]
- UNICEF. Why We Must Act Now to Overcome the Global Water Crisis. Available online: https://www.unicef.org/wash/water-scarcity (accessed on 19 September 2024).
- Kuzma, S.; Bierkens, M.F.; Lakshman, S.; Luo, T.; Saccoccia, L.; Sutanudjaja, E.H.; Van Beek, R. Aqueduct 4.0: Updated Decision-Relevant Global Water Risk Indicators; Technical note; World Resources Institute: Washington, DC, USA. [CrossRef]
- Elmahdi, A.; Jeong, J. From Debt to Sustainability: Advancing Wastewater Projects in Developing Countries through Innovative Financing Mechanisms—The Role of Debt-for-Climate Swaps. Climate 2024, 12, 122. [Google Scholar] [CrossRef]
- Otoo, M.; Drechsel, P.; Hanjra, M.A. Section IV—Wastewater for Agriculture, Forestry and Aquaculture; Drechsel, P., Hanjra, M.A., Eds.; Taylor and Francis Ltd., London, UK. 2018. Available online: https://www.iwmi.cgiar.org/Publications/Books/PDF/resource_recovery_from_waste-section-IV.pdf (accessed on 26 May 2024).
- World Water Assessment Programme (United Nations). Wastewater: The Untapped Resource: The United Nations World Water Development Report.; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Maffettone, R.; Gawlik, B.M. Technical Guidance Water Reuse Risk Management for Agricultural Irrigation Schemes in Europe; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar] [CrossRef]
- United Nations—Water. The Sustainable Development Goal 6 Global Acceleration Framework. 2020. Available online: https://www.unwater.org/sites/default/files/app/uploads/2020/07/Global-Acceleration-Framework.pdf (accessed on 15 January 2024).
- Transforming Our World: The 2030 Agenda for Sustainable Development Transforming our world: The 2030 Agenda for Sustainable Development. 2015. Available online: https://digitallibrary.un.org/record/3923923?v=pdf (accessed on 18 September 2024).
- Brady, J.; Gray, N.F. Reform of the Irish water sector: Opportunities and challenges. Proc. Inst. Civ. Eng. Water Manag. 2017, 170, 165–174. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Waterbase—UWWTD: Urban Waste Water Treatment Directive—Reported Data. January 2023. Available online: https://www.eea.europa.eu/en/datahub/datahubitem-view/6244937d-1c2c-47f5-bdf1-33ca01ff1715 (accessed on 12 March 2024).
- Giakoumis, T.; Voulvoulis, N. Combined sewer overflows: Relating event duration monitoring data to wastewater systems’ capacity in England. Environ. Sci. Water Res. Technol. 2023, 9, 707–722. [Google Scholar] [CrossRef]
- Lamon, A.W.; Maciel, P.M.F.; Campos, J.R.; Corbi, J.J.; Dunlop, P.S.M.; Fernandez-Ibañez, P.; Byrne, J.A.; Sabogal-Paz, L.P. Household slow sand filter efficiency with schmutzdecke evaluation by microsensors. Environ. Technol. 2022, 43, 4042–4053. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Arkatkar, A.; Singh, S.; Rabbani, A.; Medina, J.D.S.; Ong, E.S.; Habashy, M.M.; Jadhav, D.A.; Rene, E.R.; Mungray, A.K. Physico-chemical and biological treatment strategies for converting municipal wastewater and its residue to resources. Chemosphere 2021, 282, 130881. [Google Scholar] [CrossRef] [PubMed]
- King-Nyamador, G.; Amoatey, P.K.; Amoah, S.; Adu-Ampong, B. Optimal bed thickness and effective size for improving wastewater quality for irrigation. Int. J. Energy Environ. Eng. 2021, 12, 175–190. [Google Scholar] [CrossRef]
- Letshwenyo, M.W.; Lebogang, L. Assessment of roughing and slow sand filter modified with slag and clinker ash for removal of microorganisms from secondary effluent. Environ. Technol. 2020, 41, 3004–3015. [Google Scholar] [CrossRef]
- Oki, L.R.; Bodaghi, S.; Lee, E.; Haver, D.; Pitton, B.; Nackley, L.; Mathews, D.M. Elimination of Tobacco mosaic virus from irrigation runoff using slow sand filtration. Sci. Hortic. 2017, 217, 107–113. [Google Scholar] [CrossRef]
- Suleiman, M.; Schröder, C.; Kuhn, M.; Simon, A.; Stahl, A.; Frerichs, H.; Antranikian, G. Microbial biofilm formation and degradation of octocrylene, a UV absorber found in sunscreen. Commun. Biol. 2019, 2, 430. [Google Scholar] [CrossRef]
- Zhang, L.; Carvalho, P.N.; Bollmann, U.E.; EI-taliawy, H.; Brix, H.; Bester, K. Enhanced removal of pharmaceuticals in a biofilter: Effects of manipulating co-degradation by carbon feeding. Chemosphere 2019, 236, 124303. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, E.R.; Harmsen, D.J.H.; Blankert, B.; Wessels, L.P.; van der Meer, W.G.J. Effect of minimal pre-treatment on reverse osmosis using surface water as a source. Desalination 2021, 509, 115056. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Juntunen, P.; Rajala, R.; Haume, E.; Niemelä, A. Costs of tertiary treatment of municipal wastewater by rapid sand filter with coagulants and UV. Water Sci. Technol.: Water Supply 2003, 3, 145–152. [Google Scholar] [CrossRef]
- Tretsiakova-McNally, S.; Solan, B.; Currie, R.; Akinsanmi, O.; Arnscheidt, J.; McDermott, R.; Coleman, H.M. A ligno-cellulosic bio-adsorbent derived from sawdust waste for the removal of meropenem antibiotic dissolved in water. In Proceedings of the European Waste Water Management Conference, Birmingham, UK, 29 January 2019; Available online: http://ewwmconference.com/ (accessed on 15 January 2024).
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Adin, A. Slow granular filtration for water reuse. Water Sci. Technol. Water Supply 2003, 3, 123–130. [Google Scholar] [CrossRef]
- Casas, M.E.; Bester, K. Can those organic micro-pollutants that are recalcitrant in activated sludge treatment be removed from wastewater by biofilm reactors (slow sand filters)? Sci. Total Environ. 2015, 506–507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.; McBratney, D.; Ní Eidhin, C.; Page, D.; Minihan, M.; Campbell, R.; Fitzgerald, J.; Doris, B.; MacDonald, B.; Gallagher, J.; et al. Water Treatment Manual: Filtration. December 2020. Available online: www.epa.ie (accessed on 16 February 2024).
- Page, M.; Moher, D.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.E.; Chou, R. Supplementary Material to: PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. 2021. Available online: https://www.bmj.com/content/372/bmj.n160 (accessed on 26 May 2024).
- Abdiyev, K.; Azat, S.; Kuldeyev, E.; Ybyraiymkul, D.; Kabdrakhmanova, S.; Berndtsson, R.; Khalkhabai, B.; Kabdrakhmanova, A.; Sultakhan, S. Review of Slow Sand Filtration for Raw Water Treatment with Potential Application in Less-Developed Countries. Water 2023, 15, 2007. [Google Scholar] [CrossRef]
- Al-Herrawy, A.Z.; Gad, M.A. Assessment of two different drinking water treatment plants for the removal of free-living amoebae, Egypt. Iran. J. Parasitol. 2017, 12, 413–422. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85029773359&partnerID=40&md5=3f6269b0b8eb8ebc3c2d442e43756e17 (accessed on 16 May 2024). [PubMed]
- Haaken, D.; Schmalz, V.; Dittmar, T.; Worch, E.; Stolte, S. Modification of slow sand filtration of secondary effluents by electric fields: Electrokinetics and influence factors. AQUA Water Infrastruct. Ecosyst. Soc. 2022, 71, 697–708. [Google Scholar] [CrossRef]
- Jaeel, A.J.; Abdulkathum, S. Sustainable pollutants removal from wastewater using sand filter: A review. In Proceedings of the International Conference on Advances in Sustainable Engineering and Applications, ICASEA 2018—Proceedings, Wasit, Iraq, 14–15 March 2018; pp. 179–183. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, M.K.; Kumar, S. Biofiltration technique for removal of waterborne pathogens. In Waterborne Pathogens: Detection and Treatment; Butterworth-Heinemann: Hyderabad, India. 2020; pp. 123–141. [Google Scholar] [CrossRef]
- Fitriani, N.; Mohamed, R.M.S.R.; Affandi, M.; Nurdin, R.R.; Kurniawan, S.B. Performance of Intermittent Slow Sand Filter Processing Units in Treating Food Court Wastewater. J. Ecol. Eng. 2023, 24, 117–139. [Google Scholar] [CrossRef]
- Ni’matuzahroh, N.; Fitriani, N.; Nuswantara, E.N.; Affandi, M.; Prasongsuk, S.; Kurniawan, S.B. Isolation and Characterization of Schmutzdecke in Slow Sand Filter for Treating Domestic Wastewater. J. Ecol. Eng. 2022, 23, 76–88. [Google Scholar] [CrossRef]
- Fitriani, N.; Theresia, L.; O’Marga, T.T.N.; Kurniawan, S.B.; Supriyanto, A.; Abdullah, S.R.S.; Rietveld, L.C. Performance of a modified and intermittently operated slow sand filter with two different mediums in removing turbidity, ammonia, and phosphate with varying acclimatization periods. Heliyon 2023, 9, e22577. [Google Scholar] [CrossRef]
- Fitriani, N.; Ni’matuzahroh, N.; O’marga, T.T.N.; Mohamed, R.M.S.R.; Wahyudianto, F.E.; Imron, M.F.; Isnadina, D.R.M.; Soedjono, E.S. Optimization of Slow Sand Filtration for the Raw Municipal Wastewater Treatment by Using the Blood Cockle (Anadara granosa) Shell as an Alternative Filter Media through the Response Surface Methodology. J. Ecol. Eng. 2022, 23, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, N.; Kurniawan, S.B.; Imron, M.F.; Maulana, I.I.; Soedjono, E.S.; Mohamed, R.M.S.R.; Othman, N.B.; Ni’Matuzahroh, N.; Kusuma, M.N. System dynamic modelling to assess the influential factors affecting roughing filter and slow sand filter performance in treating culinary wastewater. J. Water Process Eng. 2023, 56, 104274. [Google Scholar] [CrossRef]
- Casas, M.E.; Larzabal, E.; Matamoros, V. Exploring the usage of artificial root exudates to enhance the removal of contaminants of emerging concern in slow sand filters: Synthetic vs. real wastewater conditions. Sci. Total Environ. 2022, 824, 153978. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Wastewater treatment by slow sand filters using uncoated and iron-coated fine sand: Impact of hydraulic loading rate and media depth. Environ. Sci. Pollut. Res. 2019, 26, 34148–34156. [Google Scholar] [CrossRef]
- Hojo, L.Y.C.P.; de P, R.V.; Lautenschlager, S.R.; Sabogal-Paz, L.P. Household slow sand filters operating in continuous and intermittent flows: Computational fluid dynamics simulation and validation by tracer experiments. Chem. Eng. Sci. 2022, 247, 117058. [Google Scholar] [CrossRef]
- Maiyo, J.K.; Dasika, S.; Jafvert, C.T. Slow Sand Filters for the 21st Century: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1019. [Google Scholar] [CrossRef]
- Malvern Instruments Limited. A Basic Guide to Particle Characterization 2. Better Understanding of Products, Ingredients and Processes. Worcestershire. 2015. Available online: https://www.cif.iastate.edu/files/inline-files/Particle%20Characterization%20Guide.pdf (accessed on 13 May 2024).
- Das, B.M.; Sobhan, K. Principles of Geotechnical Engineering, 8th ed.; Cengage Learning: Boston, MA, USA, 2012; Available online: https://docviewer.xdocs.net/view.php (accessed on 23 May 2024).
- Information Management Unit and Northern Ireland Water. Information Request NIW 2425071; Information Management Unit and Northern Ireland Water: Belfast, Ireland, 2024. [Google Scholar]
- Torres, A.; Simoni, M.U.; Keiding, J.K.; Müller, D.B.; zu Ermgassen, S.O.; Liu, J.; Jaeger, J.A.; Winter, M.; Lambin, E.F. Sustainability of the global sand system in the Anthropocene. One Earth 2021, 4, 639–650. [Google Scholar] [CrossRef]
- Krumenacher, M.J. Reality Check on a Purported Global Sand Shortage Sensationalism Extrapolated From Isolated Occurrences to Global Phenomena. Electron. Green J. 2022, 47, 1–11. [Google Scholar] [CrossRef]
- Bazargan, J.; Eskandari, R. Investigating the Influence of Filter Uniformity Coefficient and Effective Pore Size on Critical Hydraulic Gradient and Maximum Erosion of Dispersive and Non-Dispersive Samples.JWSR. 2013. Available online: www.SID.ir (accessed on 26 May 2024).
- Limited, H.I. A Guidebook to Particle Size Analysis. 2022. Available online: https://cmrf.research.uiowa.edu/sites/cmrf.research.uiowa.edu/files/HORIBA_A%20Guidebook%20to%20Particle%20Size%20Analysis%20-%202022.pdf (accessed on 30 June 2024).
- Papp, L.A.; Cardinali-Rezende, J.; de Souza Júdice, W.A.; Sanchez, M.B.; Araújo, W.L. Low biological phosphorus removal from effluents treated by slow sand filters. Appl. Microbiol. Biotechnol. 2022, 106, 5797–5809. [Google Scholar] [CrossRef] [PubMed]
- El-kamah, H.M.; Doma, H.S.; El-Shafai, S.A. Non-Conventional Municipal Wastewater Treatment System Suitable for Egyptian Villages and Small Communities. Res. J. Pharm. Biol Chem. Sci. 2017, 8, 2024–2031. [Google Scholar]
- Chibueze, C.V.; Nnaji, C.C.; Mama, C.N.; Nwankwo, E.J. Integrated treatment of stormwater using multistage filtration (MSF) for domestic application (reuse). Water SA 2024, 50, 154–165. [Google Scholar] [CrossRef]
- Platias, S.; Vatalis, K.I.; Charalampides, G. Suitability of Quartz Sands for Different Industrial Applications. Procedia Econ. Financ. 2014, 14, 491–498. [Google Scholar] [CrossRef]
- Cohen, B.; Shipley, D.S.; Tong, A.R.; Casaroli, S.J.G.; Petrie, J.G. Precipitation of iron from concentrated chloride solutions: Literature observations, challenges and preliminary experimental results. Miner. Eng. 2005, 18, 1344–1347. [Google Scholar] [CrossRef]
- Mittal, Y.; Srivastava, P.; Pandey, S.; Yadav, A.K. Development of nature-based sustainable passive technologies for treating and disinfecting municipal wastewater: Experiences from constructed wetlands and slow sand filter. Sci. Total Environ. 2023, 900, 165320. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.Y.; He, X.J.; Chen, Q.; Zhang, H.; Liu, X.Y. Improved prediction of water retention characteristic based on soil gradation and clay fraction. Geoderma 2021, 404, 115293. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products. Sci. Total Environ. 2018, 635, 1182–1190. [Google Scholar] [CrossRef]
- Bun, S.; Chawaloesphonsiya, N.; Ermukdakul, T.; Pungrasmi, W.; Suwannasilp, B.B.; Painmanakul, P. Suspended solid and nitrate removal from aquaculture system wastewater by different approaches. Desalination Water Treat. 2017, 81, 87–94. [Google Scholar] [CrossRef]
- Maharjan, N.; Kuroda, K.; Dehama, K.; Hatamoto, M.; Yamaguchi, T. Development of slow sponge sand filter (SpSF) AS a post-treatment of UASB-DHS reactor effluent treating municipal wastewater. Water Sci. Technol. 2016, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Ehrampoush, M.H.; Eslami, H.; Ghaneian, M.T.; Fallahzadeh, H.; Talebi, P.; Fard, R.F.; Ebrahimi, A.A. Removal of linear alkylbenzene sulfonate and turbidity from greywater by a hybrid multi-layer slow sand filter microfiltration ultrafiltration system. J. Clean. Prod. 2019, 211, 922–931. [Google Scholar] [CrossRef]
- Karakurt-Fischer, S.; Rien, C.; Sanz-Prat, A.; Szewzyk, R.; Hübner, U.; Drewes, J.E.; Selinka, H.-C. Fate and Transport of Viruses within a High-Rate Plug-Flow Biofilter Designed for Non-Membrane-Based Indirect Potable Reuse Applications. ACS ES&T Water 2021, 1, 1229–1239. [Google Scholar] [CrossRef]
- Li, J. Removal of Selected Pharmaceuticals and Personal Care Products using Greater Duckweed Constructed Wetland Followed by GAC Sandwich Slow Sand Filter. Ph.D. Thesis, University College London, London, UK, 2019. [Google Scholar]
- Biosand Filter Construction Manual. August 2012. Available online: www.cawst.org (accessed on 27 May 2024).
- Vu, C.T.; Wu, T. Enhanced Slow Sand Filtration for the Removal of Micropollutants from Groundwater. Sci. Total Environ. 2022, 809, 152161. [Google Scholar] [CrossRef] [PubMed]
- Guchi, E. Review on Slow Sand Filtration in Removing Microbial Contamination and Particles from Drinking Water. Am. J. Food Nutr. 2015, 3, 47–55. [Google Scholar] [CrossRef]
- Lubarsky, H.; Fava, N.d.M.N.; Freitas, B.L.S.; Terin, U.C.; Oliveira, M.; Lamon, A.W.; Pichel, N.; Byrne, J.A.; Sabogal-Paz, L.P.; Fernandez-Ibañez, P. Biological Layer in Household Slow Sand Filters: Characterization and Evaluation of the Impact on Systems Efficiency. Water 2022, 14, 1078. [Google Scholar] [CrossRef]
- Terin, U.C.; Sabogal-Paz, L.P. Microcystis aeruginosa and microcystin-LR removal by household slow sand filters operating in continuous and intermittent flows. Water Res. 2019, 150, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.; Skinner, B.; Cantwell, R.E. Impact of surface maintenance on BioSand filter performance and flow. J. Water Health 2017, 15, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Hunt, J.; Lingos, A.; Platje, J.; Vieira, L.W.; Will, M.; Gavriletea, M.D. The Unsustainable Use of Sand: Reporting on a Global Problem. Sustainability 2021, 13, 3356. [Google Scholar] [CrossRef]
- Greene, T. Lough Neagh: Major Review into Sand Dredging Will Include Long-Awaited Survey of the Lake Bed. The Detail. Available online: https://www.thedetail.tv/articles/lough-neagh-survey (accessed on 22 July 2024).
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Ajala, O.J.; Ogunniyi, S.; Muritala, K.B. Thermochemical co-conversion of biomass-plastic waste to biochar: A review. Green Chem. Eng. 2024, 5, 31–49. [Google Scholar] [CrossRef]
- Silori, R.; Zang, J.; Raval, N.P.; Giri, B.S.; Mahlknecht, J.; Mora, A.; Dueñas-Moreno, J.; Tauseef, S.M.; Kumar, M. Adsorptive removal of ciprofloxacin and sulfamethoxazole from aqueous matrices using sawdust and plastic waste-derived biochar: A sustainable fight against antibiotic resistance. Bioresour. Technol. 2023, 387, 129537. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into chemical regeneration of activated carbon for water treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Emslie, D.; Siddiqua, S.; Crawford, B.; Teece, W. Biofilm Formation and Effectiveness of Biosand Filtration Systems with Typical and Innovative Filter Media. Geotech. Geol. Eng. 2022, 40, 1251–1266. [Google Scholar] [CrossRef]
- Bonné, P.A.C.; Hofman, J.A.M.H.; der Hoek, J.P. Long term capacity of biological activated carbon filtration for organics removal. Water Sci. Technol. Water Supply 2002, 2, 139–146. [Google Scholar] [CrossRef]
- Vedenyapina, M.D.; Kurmysheva, A.Y.; Kulaishin, S.A. Regeneration of Activated Carbons after Adsorption of Biologically Active Organic Compounds on Them (A Review). Solid Fuel Chem. 2024, 58, 24–39. [Google Scholar] [CrossRef]
- Olaniyan, P.O.; Nadim, M.M.; Subir, M. Detection and binding interactions of pharmaceutical contaminants using quartz crystal microbalance—Role of adsorbate structure and surface functional group on adsorption. Chemosphere 2023, 311, 137075. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Meng, N.; Liao, Y. Photothermal Conversion Porous Organic Polymers: Design, Synthesis, and Applications. Small Methods 2024, 8, 2301554. [Google Scholar] [CrossRef] [PubMed]
- Wolska, J.; Frankowski, M.; Jenczyk, J.; Wolski, L. Highly sulfonated hyper-cross-linked polymers as promising adsorbents for efficient and selective removal of ciprofloxacin from water. Sep. Purif. Technol. 2024, 343, 127147. [Google Scholar] [CrossRef]
- Dastyar, F.; Dinari, M. Preparation of pyridine based porous organic polymers as new adsorbents to remove Congo red dye from aqueous media. J. Polym. Res. 2024, 31, 315. [Google Scholar] [CrossRef]
- Olupot, P.W.; Menya, E.; Jjagwe, J.; Wakatuntu, J.; Román, F.; Hensel, O. Optimization of roughing filtration unit for a handwashing wastewater recirculation point-of-use system. Water Sci. Technol. 2022, 86, 2528–2544. [Google Scholar] [CrossRef]
- Sain, U.; Bishnoi, N.R. Assessment of removal efficiency of total suspended solids (TSS) from secondary treated effluent using a multigrade multimedia filtration technique. Ann. Agri Bio Res. 2018, 23, 74–77. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85063578213&partnerID=40&md5=65550e59fc61f4ca9eec8b9b0fe125da (accessed on 21 February 2024).
- Delmond, B.; Tretsiakova-McNally, S.; Solan, B.; McDermott, R.; Audoin, A. A Sustainable Method to Reduce Vancomycin Concentrations in Water Using Timber Waste. Water Air Soil Pollut. 2023, 234, 81. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Sahu, O. Arsenate and fluoride removal from groundwater by sawdust impregnated ferric hydroxide and activated alumina (SFAA). Groundw. Sustain. Dev. 2021, 12, 100490. [Google Scholar] [CrossRef]
- Akinsanmi, O.; Kaya; Tretsiakova-McNally, S.; Arnscheidt, J.; Altayan, M.; Coleman, H. Tackling Antimicrobial Resistance: Adsorption of meropenem and ciprofloxacin on ligno-cellulosic substrate from sawdust. In Proceedings of the European Waste Water Management Conference, Birmingham, UK, 16–17 July 2019. [Google Scholar]
- Sanchez, J.L.; Hodge, D.B.; Davis, M.F.; Paës, G.; Zoghlami, A. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.Y.; Le Lv, M.; Kong, Y.; Yu, Y.W.; Xu, X.Y. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Yasemin, B.; Zeki, T. Removal of heavy metals from aqueous solution by sawdust adsorption. J. Environ. Sci. 2007, 19, 160–166. [Google Scholar]
- Harmayani, K.D.; Anwar, A.H.M.F. Adsorption kinetics and equilibrium study of nitrogen species onto radiata pine (Pinus radiata) sawdust. Water Sci. Technol. 2016, 74, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, H.; Leiviskä, T.; Heiderscheidt, E.; Postila, H.; Tanskanen, J. Removal of metals from industrial wastewater and urban runoff by mineral and bio-based sorbents. J. Environ. Manag. 2018, 209, 316–327. [Google Scholar] [CrossRef]
- Li, J.; Han, X.; Brandt, B.W.; Zhou, Q.; Ciric, L.; Campos, L.C. Physico-chemical and biological aspects of a serially connected lab-scale constructed wetland-stabilization tank-GAC slow sand filtration system during removal of selected PPCPs. Chem. Eng. J. 2019, 369, 1109–1118. [Google Scholar] [CrossRef]
- Onesios, K.M.; Bouwer, E.J. Biological removal of pharmaceuticals and personal care products during laboratory soil aquifer treatment simulation with different primary substrate concentrations. Water Res. 2012, 46, 2365–2375. [Google Scholar] [CrossRef]
- Pompei, C.M.E.; Ciric, L.; Canales, M.; Karu, K.; Vieira, E.M.; Campos, L.C. Influence of PPCPs on the performance of intermittently operated slow sand filters for household water purification. Sci. Total Environ. 2017, 581–582, 174–185. [Google Scholar] [CrossRef]
- Pompei, C.M.E.; Campos, L.C.; Vieira, E.M.; Tucci, A. The impact of micropollutants on native algae and cyanobacteria communities in ecological filters during drinking water treatment. Sci. Total Environ. 2022, 822, 153401. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Zhang, X.; Huang, X.; Wang, F.; Zhu, S.; Li, X.; Guan, Y. Multi-omics analysis on seasonal variations of the biofilm microbial community in a full-scale pre-denitrification biofilter. Environ. Sci. Pollut. Res. 2023, 30, 24284–24298. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, Y.; Huang, L.; He, G. Biofilm growth on cohesive sediment deposits: Laboratory experiment and model validation. Hydrobiologia 2017, 799, 261–274. [Google Scholar] [CrossRef]
- D’Haese, A.; Le-Clech, P.; Van Nevel, S.; Verbeken, K.; Cornelissen, E.R.; Khan, S.J.; Verliefde, A.R. Trace organic solutes in closed-loop forward osmosis applications: Influence of membrane fouling and modeling of solute build-up. Water Res. 2013, 47, 5232–5244. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ngwenya, B.T.; Butler, I.B.; Kurlanda, H.; Elphick, S.C. Coupled interactions between metals and bacterial biofilms in porous media: Implications for biofilm stability, fluid flow and metal transport. Chem. Geol. 2013, 337–338, 20–29. [Google Scholar] [CrossRef]
- Alnahhal, S.Y.; Elfari, A.A.; Afifi, S.A.; Aljubb, A.E.R. Using slow sand filter for organic matter and suspended solids removal as post-treatment unit for wastewater effluent. Environ. Sci. Water Res. Technol. 2023, 10, 490–497. [Google Scholar] [CrossRef]
- Livingston, P.; Slack, D. Management of the schmutzdecke layer in a slow sand filter to reuse drainage water from a greenhouse. Adv. Mater. Res. 2014, 931, 834–838. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Oruganti, R.K.; Katam, K.; Show P., L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A comprehensive review on the use of algal-bacterial systems for wastewater treatment with emphasis on nutrient and micropollutant removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]

| Name of Substance/Group of Substances | Typical Application of the Substance [17] | Max. Predicted Environmental Concentration (PEC) (µg/L) | Predicted No-Effect Concentration (PNEC) (µg/L) | |
|---|---|---|---|---|
| Metaflumizone | Insecticide | 0.30 | 0.0654 | |
| Amoxicillin | Antibiotic | 1.28 a | 0.078 | |
| Ciprofloxacin | Antibiotic | 7.00 | 0.089 | |
| Sulfamethoxazole | Antibiotic | <10 | 16 | |
| Trimethoprim | Antibiotic | <10 | 120 | |
| Venlafaxine (and O-desmethylvenlafaxine) | Antidepressant (antidepressant metabolite) | 0.20 | 0.038 * [18] | |
| Azole compounds: | Clotrimazole | Antifungal pharmaceuticals or food protection products | 0.016 * | 1 |
| Fluconazole | 0.06 * | 9.46 | ||
| Imazalil | 0.43 | 0.8 | ||
| Ipconazole | 0.2719 | 0.27 | ||
| Metconazole | 1.2 | 0.0582 [18] | ||
| Miconazole | No data | 0. 4 | ||
| Penconazole | 3.3 | 6 | ||
| Prochloraz | 3 | 10 | ||
| Tebuconazole | No data | 1 | ||
| Tetraconazole | 3 | 1.9 | ||
| Dimoxystrobin | Fungicide | 16.42 | 0.0316 | |
| Famoxadone | Fungicide | 1.80 | 0.14 | |
| Study | Location | Temperature (°C) | Frequency of Cleanings | Influent | Reference |
|---|---|---|---|---|---|
| Coal slag SSF | Botswana | 14–17 | Twice over 17 weeks | Real wastewater treatment plant discharge | [54] |
| EPA SSF recommendations | Ireland | >6 * | Every 1–2 weeks to months | Real wastewater | [64] |
| SSF | São Paulo state, Brazil | 25 | Every 30 days | River water | [51] |
| SSF | São Paulo state, Brazil | 20–25 | Every 1–3 weeks | Real wastewater treatment plant discharge | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbett, H.; Solan, B.; Tretsiakova-McNally, S.; Fernandez-Ibañez, P.; McDermott, R. New Wine in Old Bottles: The Sustainable Application of Slow Sand Filters for the Removal of Emerging Contaminants, a Critical Literature Review. Sustainability 2024, 16, 10595. https://doi.org/10.3390/su162310595
Corbett H, Solan B, Tretsiakova-McNally S, Fernandez-Ibañez P, McDermott R. New Wine in Old Bottles: The Sustainable Application of Slow Sand Filters for the Removal of Emerging Contaminants, a Critical Literature Review. Sustainability. 2024; 16(23):10595. https://doi.org/10.3390/su162310595
Chicago/Turabian StyleCorbett, Hayley, Brian Solan, Svetlana Tretsiakova-McNally, Pilar Fernandez-Ibañez, and Rodney McDermott. 2024. "New Wine in Old Bottles: The Sustainable Application of Slow Sand Filters for the Removal of Emerging Contaminants, a Critical Literature Review" Sustainability 16, no. 23: 10595. https://doi.org/10.3390/su162310595
APA StyleCorbett, H., Solan, B., Tretsiakova-McNally, S., Fernandez-Ibañez, P., & McDermott, R. (2024). New Wine in Old Bottles: The Sustainable Application of Slow Sand Filters for the Removal of Emerging Contaminants, a Critical Literature Review. Sustainability, 16(23), 10595. https://doi.org/10.3390/su162310595







