Biology and Ecology of the European Eel as Revealed by an Original Sampling Technique Performed in a Deep and Large Riverine Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Techniques
2.3. Data Processing
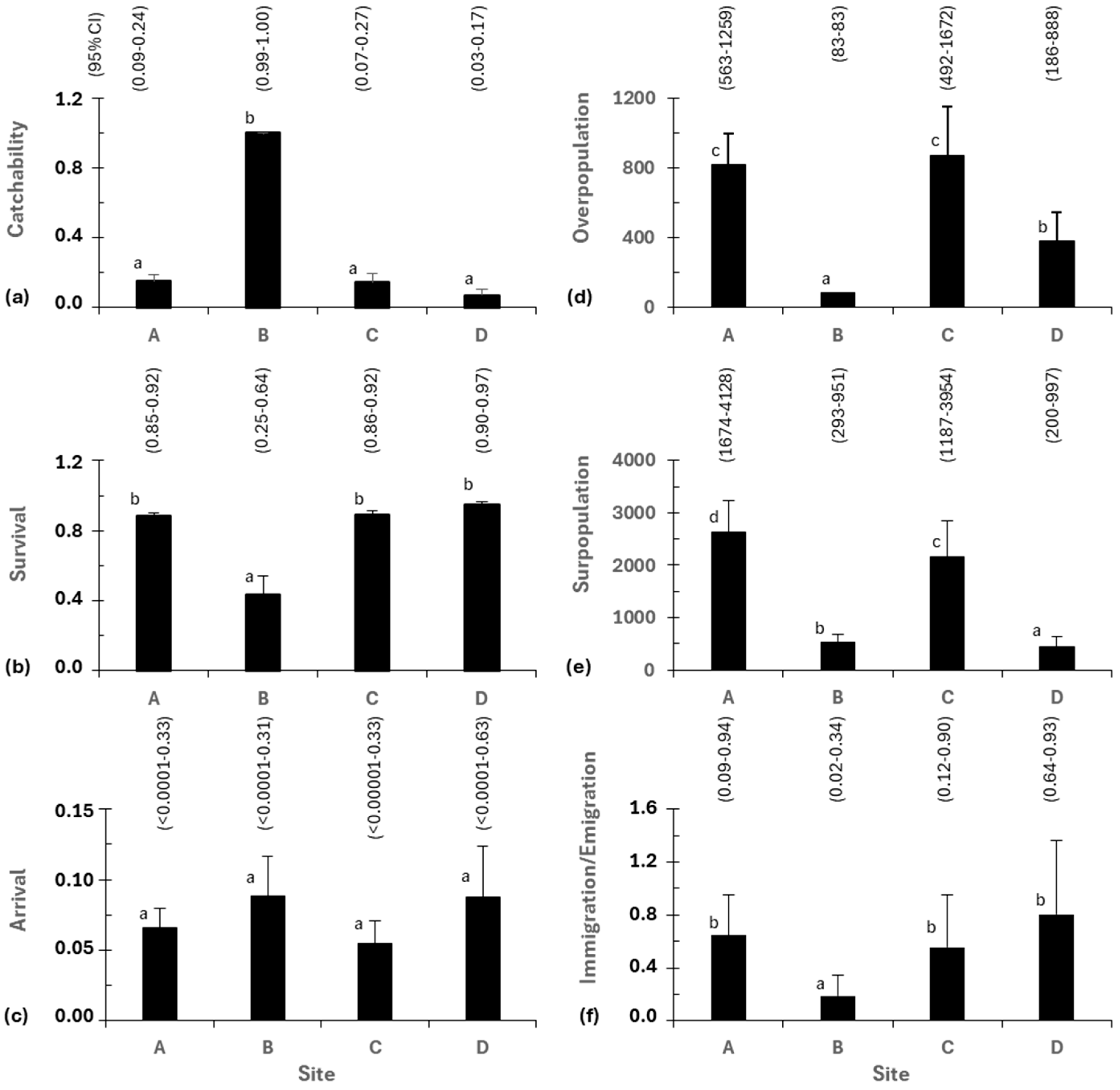
3. Results
3.1. Fauna Diversity
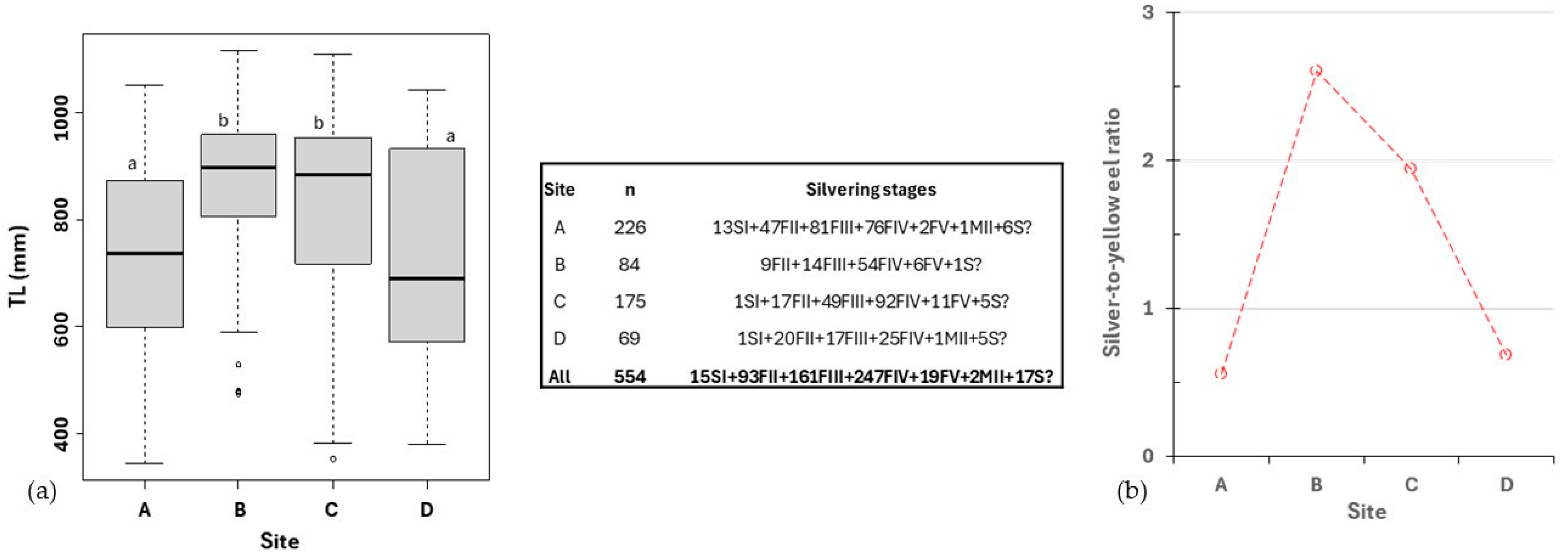
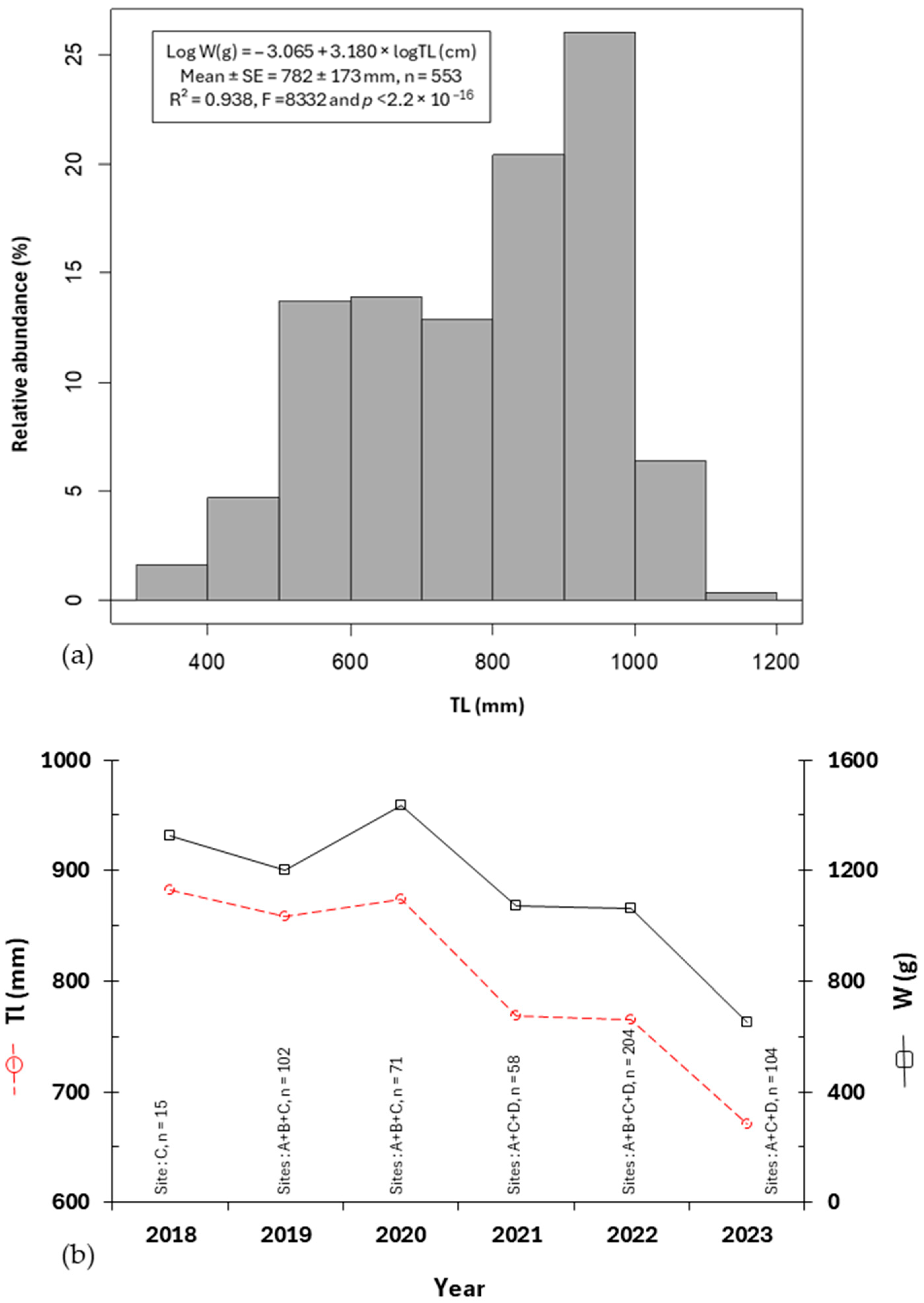
3.2. Eel Sampling Performance, Size, and Silvering Stage
3.3. Increment TL of Recaptured Eels
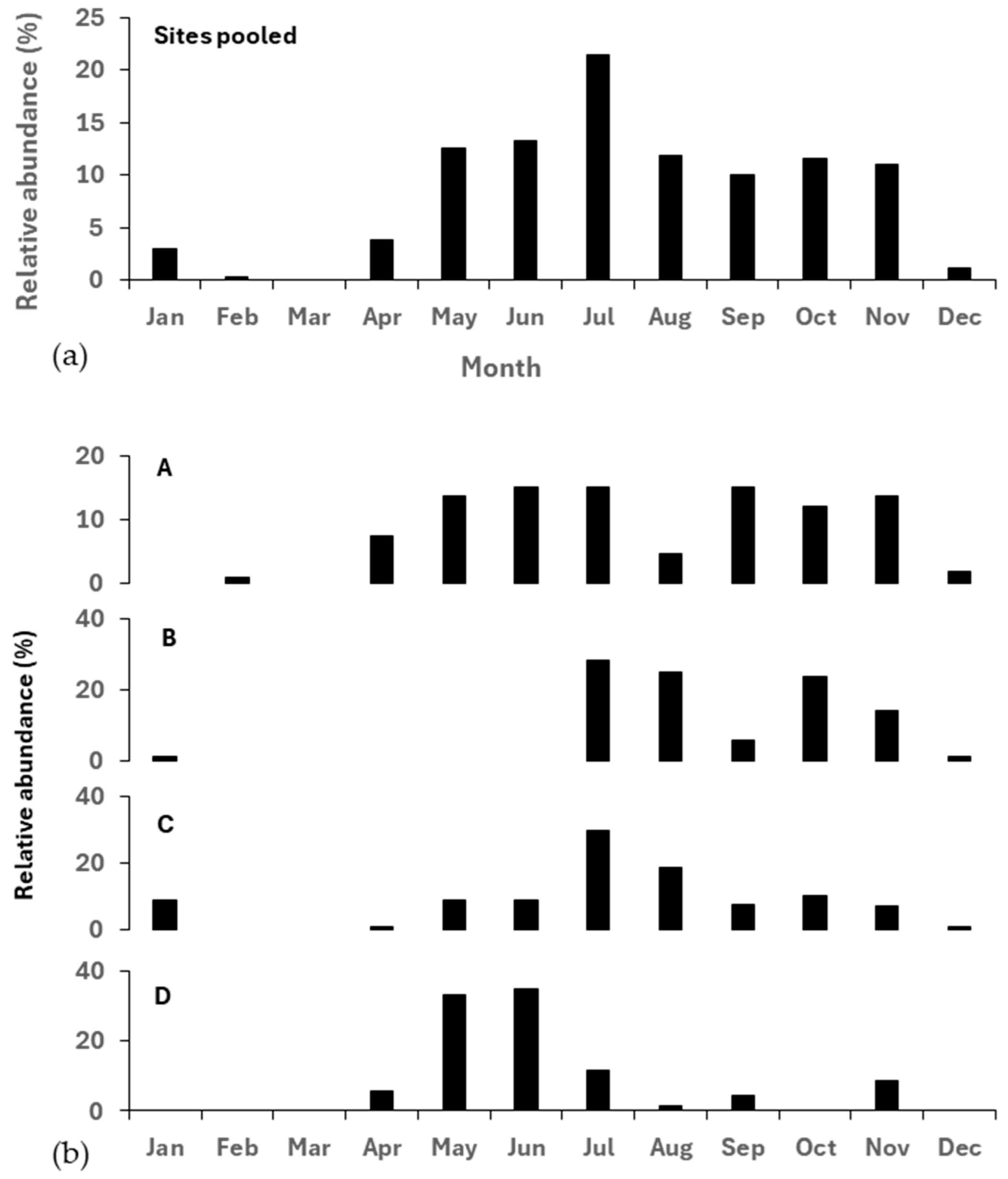
3.4. Monthly Pattern of Catching Eels
3.5. Demographic Parameters of Eels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacoby, D.M.P.; Casselman, J.M.; Crook, V.; DeLucia, M.-B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Ragauskas, A.; Butkauskas, D. The formation of the population genetic structure of the European eel Anguilla anguilla (L.): A short review. Ekologija 2013, 59, 143–154. [Google Scholar] [CrossRef]
- Jellyman, D.J. An enigma: How can freshwater eels (Anguilla spp.) be such a successful genus yet be universally threatened? Rev. Fish. Biol. Fisheries 2022, 32, 701–718. [Google Scholar] [CrossRef]
- Moriarty, C. The Yellow Eel. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 89–105. [Google Scholar]
- Tesch, F.W.; Thorpe, J.E. The Eel, 3rd ed.; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Daverat, F.; Limburg, K.; Thibault, I.; Shiao, J.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Wickström, H. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar. Ecol. Prog. Ser. 2006, 308, 231–241. [Google Scholar] [CrossRef]
- Harrod, C.; Grey, J.; McCarthy, T.K.; Morrissey, M. Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 2005, 144, 673–683. [Google Scholar] [CrossRef]
- Denis, J.; Rabhi, K.; Le Loc’h, F.; Lasram, F.B.R.; Boutin, K.; Kazour, M.; Diop, M.; Gruselle, M.-C.; Amara, R. Role of estuarine habitats for the feeding ecology of the European eel (Anguilla anguilla L.). PLoS ONE 2022, 17, e0270348. [Google Scholar] [CrossRef]
- ICES. Report of the Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL) and Country Reports 2017–2018; ICES Scientific Reports; ICES Advisory Committee: Gdańsk, Poland, 2018. [Google Scholar]
- Dekker, W. The history of commercial fisheries for European commenced only a century ago. Fish. Manag. Ecol. 2019, 26, 6–19. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels, and Country Reports 2018–2019; Walker, A., Ed.; ICES Scientific Reports; ICES: Copenhagen, Denmark, 2019. [Google Scholar]
- Ibbotson, A.; Smith, J.; Scarlett, P.; Aprahamian, M.W. Colonisation of freshwater habitats by the European eel Anguilla anguilla. Freshw. Biol. 2002, 47, 1696–1706. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Dynamics of upstream movements of the European eel Anguilla anguilla in an inland area of the river Meuse over the last 20 years. Environ. Biol. Fish 2016, 99, 223–235. [Google Scholar] [CrossRef][Green Version]
- Nzau Matondo, B.; Ovidio, M. Decreased stock entering the Belgian Meuse is associated with the loss of colonisation behaviour in yellow-phase European eels. Aquat. Living Resour. 2018, 31, 7. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL), and Country Reports 2019–2020; Pohlmann, J.-D., Ed.; ICES Scientific Reports; ICES: Copenhagen, Denmark, 2020. [Google Scholar]
- Imbert, H.; Labonne, J.; Rigaud, C.; Lambert, P. Resident and migratory tactics in freshwater European eels are size-dependent. Freshw. Biol. 2010, 55, 1483–1493. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Chassot, E.; Rivot, E. Fluctuations in European eel (Anguilla anguilla) recruitment resulting from environmental changes in the Sargasso Sea. Fish. Oceanogr. 2008, 17, 32–44. [Google Scholar] [CrossRef]
- Drouineau, H.D.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater eels: A symbol of the effects of global change. Fish. Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- Friedland, K.D.; Miller, M.J.; Knights, B. Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES J. Mar. Sci. 2007, 64, 519–530. [Google Scholar] [CrossRef]
- Feunteun, E. Management and restoration of European eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Robinet, T.; Feunteun, E. Sublethal effects of exposure to chemical compounds: A cause for the decline in Atlantic eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; Mladineo, I.; Konecny, R.; Yoshimizu, M.; Groman, D.; Munoz, P. Diseases of eels in an international perspective: Workshop on eel diseases at the 15th international conference on the diseases of fish and shellfish, Split, Croatia, 2011. Bull.Eur. Assoc. Fish Pathol. 2012, 32, 109–115. [Google Scholar]
- Muñoz, P.; Barcala, E.; Peñalver, J.; Romero, D. Can inorganic elements affect herpesvirus infections in European eels? Environ. Sci. Pollut. Res. 2019, 26, 35266–35269. [Google Scholar] [CrossRef]
- Delrez, N.; Zhang, H.; Lieffrig, F.; Mélard, C.; Farnir, F.; Boutier, M.; Donohoe, O.; Vanderplasschen, A. European eel restocking programs based on wild-caught glass eels: Feasibility of quarantine stage compatible with implementation of prophylactic measures prior to scheduled reintroduction to the wild. J. Nat. Conserv. 2021, 59, 125933. [Google Scholar] [CrossRef]
- Belpaire, C.G.J.; Goemans, G.; Geeraerts, C.; Quataert, P.; Parmentier, K.; Hagel, P.; De Boer, J. Decreasing eel stocks: Survival of the fattest? Ecol. Freshw. Fish 2009, 18, 197–214. [Google Scholar] [CrossRef]
- Pedersen, M.I.; Rasmussen, C.H. Yield per recruit from stocking two different sizes of eel (Anguilla anguilla) in the brackish Roskilde Fjord. ICES J. Mar. Sci. 2016, 73, 158–164. [Google Scholar] [CrossRef]
- Pike, C.; Crook, V.; Gollock, M. Anguilla anguilla. The IUCN Red List of Threatened Species 2020: E.T60344A152845178. 2020. Available online: https://www.fishsec.org/app/uploads/2022/11/200709-Assessment-10.2305_IUCN.UK_.2020-2.RLTS_.T60344A152845178.en_.pdf (accessed on 23 October 2024).
- CITES. Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III; Annual Report of the Secretariat; CITES: Geneva, Switzerland, 2024; 79p. [Google Scholar]
- ICES. Report of the 2012 Session of the Joint EIFAAC/ICES Working Group on Eels (WGEEL) and Country Reports 2011–2012; ICES Scientific Report; ICES: Copenhagen, Denmark, 2012. [Google Scholar]
- Panfili, J.; Darnaude, A.; Lin, Y.; Chevalley, M.; Iizuka, Y.; Tzeng, W.; Crivelli, A. Habitat residence during continental life of the European eel Anguilla anguilla investigated using linear discriminant analysis applied to otolith Sr:Ca ratios. Aquat. Biol. 2012, 15, 175–185. [Google Scholar] [CrossRef]
- Tahri, M.; Panfili, J. 13-year population survey of the critically endangered European eel in the southern Mediterranean region (Algeria). J. Fish Biol. 2023, 102, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Drouineau, H.; Lambert, P.; Boutron, O.; Nicolas, D. Spatio-temporal variations in glass eel recruitment at the entrance pathways of a Mediterranean delta. ICES J. Mar. Sci. 2022, 79, 1874–1887. [Google Scholar] [CrossRef]
- Baras, E.; Jeandrain, D.; Serouge, B.; Philippart, J.C. Seasonal variations in time and space utilization by radio-tagged yellow eels Anguilla anguilla (L.) in a small stream. Hydrobiologia 1998, 371/372, 187–198. [Google Scholar] [CrossRef]
- Glova, G.J.; Jellyman, D.J. Size-related differences in diel activity of two species of juvenile eel (Anguilla) in a laboratory stream. Ecol. Freshw. Fish 2000, 9, 210–218. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Philippart, J.C.; Ovidio, M. Assessment of the entering stock, migration dynamics and fish pass fidelity of European eel in the Belgian Meuse River. River Res. Appl. 2017, 33, 292–301. [Google Scholar] [CrossRef]
- Jacoby, D.M.P.; Piper, A.T. What acoustic telemetry can and cannot tell us about fish biology. J. Fish Biol. 2023, 1–25. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Backory, L.; Dupuy, G.; Amoussou, G.; Oumarou, A.A.; Gelder, J.; Renardy, S.; Benitez, J.-P.; Dierckx, A.; Dumonceau, F.; et al. Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study. Fishes 2023, 8, 137. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Fontaine, F.; Detrait, O.; Poncelet, C.; Vandresse, S.; Orban, P.; Gelder, J.; Renardy, S.; Benitez, J.P.; Dierckx, A.; et al. Glass Eel Restocking Experiments in Typologically Different Upland Rivers: How Much Have We Learned about the Importance of Recipient Habitats? Water 2023, 15, 3133. [Google Scholar] [CrossRef]
- Belpaire, C.; Breine, J.; Wichelen, J.V.; Nzau Matondo, B.; Ovidio, M.; Verhelst, P.; Teunen, L.; Bervoets, L.; Dumonceau, F.; Vlietinck, K. Report on the eel stock, fishery and other impacts in Belgium, 2020–2021. In Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL), and Country Reports 2020–2021; ICES Scientific Reports; ICES: Copenhagen, Denmark, 2021. [Google Scholar]
- Baras, E.; Philippart, J.C.; Salmon, B. Estimation of migrant yellow eels stock in large rivers through the survey of fish passes: A preliminary investigation in the River Meuse (Belgium). In Stock Assessment in Inland Fisheries; Cowx, I.G., Ed.; Oxford Fishing News Books (Blackwell): London, UK, 1996; pp. 314–325. [Google Scholar]
- Behrmann-Godel, J.; Eckmann, R. A preliminary telemetry study of the migration of silver European eel (Anguilla anguilla L.) in the RiverMosel, Germany. Ecol. Freshw. Fish 2003, 12, 196–202. [Google Scholar] [CrossRef]
- Schwarz, C.J.; Arnason, A.N. A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, 120–139. [Google Scholar] [CrossRef]
- Pledger, S.; Pollock, K.H.; Norris, J.L. Open Capture–Recapture Models with Heterogeneity: II. Jolly–Seber Model. Biometrics 2010, 66, 883–890. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Rollin, X.; Ovidio, M. An evaluation of restocking practice and demographic stock assessment methods for cryptic juvenile European eel in upland rivers. Sustainability 2020, 12, 1124. [Google Scholar] [CrossRef]
- Tesch, F.W. Der Aal; Paul Parey: Hamburg/Berlin, Germany, 1983; 340p. [Google Scholar]
- Tesch, F.W. Suivi des anguilles argentées dans les rivières Weser et Elbe. Fischö Kologie 1994, 7, 47–59. [Google Scholar]
- Jonsson, N. Influence du débit, de la température de l’eau et de la lumière sur la migration des poissons dans les rivières. Nord. J. Freshw. Res. 1991, 66, 20–35. [Google Scholar]
- Coutant, C.C.; Whitney, R.R. Comportement des poissons en relation avec le passage à travers les turbines hydroélectriques: Une revue. Trans. Am. Fish. Soc. 2000, 129, 351–380. [Google Scholar] [CrossRef]
- Benitez, J.-P.; Dierckx, A.; Rimbaud, G.; Nzau Matondo, B.; Renardy, S.; Rollin, X.; Gillet, A.; Dumonceau, F.; Poncin, P.; Philippart, J.-C.; et al. Assessment of Fish Abundance, Biodiversity and Movement Periodicity Changes in a Large River over a 20-Year Period. Environments 2022, 9, 22. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Durif, C.; Guibert, A.; Elie, P. Morphological discrimination of the silvering stages of the European eel. In Eels at the Edge Science, Status, and Conservation Concerns; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 103–111. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 12 September 2024).
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman and CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous, Version 4.1-1; Cran: Washington, DC, USA, 2018; Available online: http://CRAN.Rproject.org/package=Hmisc (accessed on 12 September 2024).
- Russon, I.J.; Kemp, P.S.; Calles, O. Response of downstream migrating adult European eels (Anguilla anguilla) to bar racks under experimental conditions. Ecol. Freshw. Fish 2010, 19, 197–205. [Google Scholar] [CrossRef]
- Russon, I.J.; Kemp, P.S. Advancing multi-species fish passage: Behaviour of adult European eel (Anguilla anguilla) and brown trout (Salmo trutta) in response to accelerating flow. Ecol. Eng. 2011, 37, 2018–2024. [Google Scholar] [CrossRef]
- Piper, A.T.; Manes, C.; Siniscalchi, F.; Marion, A.; Wright, R.M.; Kemp, P.S. Response of seaward-migrating European eel (Anguilla anguilla) to manipulated flow fields. Proc. R. Soc. B 2015, 282, 1098. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.I. Long-term survival and growth of stocked eel, Anguilla anguilla (L.), in a small eutrophic lake. Dana 2000, 12, 71–76. [Google Scholar]
- Mazel, V.; Charrier, F.; Legault, A.; Laffaille, P. Long-term effects of passive integrated transponder tagging (PIT tags) on the growth of the yellow eel (Anguilla anguilla (Linnaeus, 1758)). J. Appl. Ichthyol. 2013, 29, 906–908. [Google Scholar] [CrossRef]
- Sonny, D. La dévalaison des poissons dans la Meuse moyenne belge. Cah. Ethol. 2009, 22, 267. [Google Scholar]
- Deelder, C.L. Factors affecting the migration of the silver eel in Dutch inland waters. J. Cons. Int. Explor. Mer. 1954, 20, 177–185. [Google Scholar] [CrossRef]
- Vøllestad, L.A.; Jonsson, B.; Hvidsten, N.A.; Naesje, T.F. Experimental test of environmental factors influencing the seaward migration of European silver eels. J. Fish Biol. 1994, 45, 641–651. [Google Scholar] [CrossRef]
- Vøllestad, L.A.; Jonsson, B.; Hvidsten, N.A.; Naesje, T.F.; Haraldstad, Ø.; Ruud-Hansen, J. Environmental factors regulating the seaward migration of European silver eels (Anguilla anguilla). Can. J. Fish. Aquat. Sci. 1986, 43, 1909–1916. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Delrez, N.; Bardonnet, A.; Vanderplasschen, A.; Joaquim-Justo, C.; Rives, J.; Benitez, J.P.; Dierckx, A.; Séleck, E.; Rollin, X.; et al. A complete check-up of European eel after eight years of restocking in an upland river: Trends in growth, lipid content, sex ratio and health status. Sci. Total Environ. 2022, 807, 151020. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Partial migration: Niche shift versus sexual maturation in fishes. Rev. Fish Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Naismith, I.A.; Knights, B. Migrations of elvers and juvenile European eels, Anguilla anguilla L., in the River Thames. J. Fish Biol. 1988, 33, 161–175. [Google Scholar] [CrossRef]
- White, E.M.; Knights, B. Environmental factors affecting migration of the European eel in the Rivers Severn and Avon, England. J. Fish Biol. 1997, 50, 1104–1116. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Monteiro, R.; Castro, N.; Quintella, B.R.; Almeida, P.R.; Domingos, I. Can a restocking event with European (glass) eels cause early changes in local biological communities and its ecological status? Glob. Ecol. Conserv. 2020, 21, e00884. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Quintella, B.R.; Almeida, P.R.; Monteiro, R.; Santos, J.; Portela, T.; Domingos, I. Early settlement and growth of stocked European glass eels in a fragmented watercourse. Fish. Manag. Ecol. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Bonnineau, C.; Scaion, D.; Lemaire, B.; Belpaire, C.; Thomé, J.-P.; Thonon, M.; Leermaker, M.; Gao, Y.; Debier, C.; Silvestre, F.; et al. Accumulation of neurotoxic organochlorines and trace elements in brain of female European eel (Anguilla anguilla). Environ. Toxicol. Pharmacol. 2016, 45, 346–355. [Google Scholar] [CrossRef]
- Belpaire, C.; Geeraerts, C.; Evans, D.; Ciccotti, E.; Poole, R. The European eel quality database: Towards a pan-European monitoring of eel quality. Environ. Monit. Assess. 2011, 183, 273–284. [Google Scholar] [CrossRef]
- Marohn, L.; Jakob, E.; Hanel, R. Implications of facultative catadromy in Anguilla anguilla. Does individual migratory behaviour influence eel spawner quality? J. Sea Res. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Verhelst, P.; Buysse, D.; Reubens, J.; Pauwels, I.; Aelterman, B.; Van Hoey, S.; Goethals, P.; Coeck, J.; Tom Moens, T.; Mouton, A. Downstream migration of European eel (Anguilla anguilla L.) in an anthropogenically regulated freshwater system: Implications for management. Fish. Res. 2018, 199, 252–262. [Google Scholar] [CrossRef]
- Calles, O.; Elghagen, J.; Nyqvist, D.; Harbicht, A.; Nilsson, A. Efficient and timely downstream passage solutions for European silver eels at hydropower dams. Ecol. Eng. 2021, 170, 106350. [Google Scholar] [CrossRef]
- Podda, C.; Palmas, F.; Pusceddu, A.; Sabatini, A. When the Eel Meets Dams: Larger Dams’ Long-Term Impacts on Anguilla anguilla (L., 1758). Front. Environ. Sci. 2022, 10, 876369. [Google Scholar] [CrossRef]
- Rodeles, A.A.; Galicia, D.; Miranda, R. A Simple Method to Assess the Fragmentation of Freshwater Fish Meta-Populations: Implications for River Management and Conservation. Ecol. Indic. 2021, 125, 107557. [Google Scholar] [CrossRef]
- Milt, A.W.; Diebel, M.W.; Doran, P.J.; Ferris, M.C.; Herbert, M.; Khoury, M.L.; Moody, A.T.; Neeson, T.M.; Ross, J.; Treska, T.; et al. Minimizing Opportunity Costs to Aquatic Connectivity Restoration while Controlling an Invasive Species. Conserv. Biol. 2018, 32, 894–904. [Google Scholar] [CrossRef]
- Rahel, F.J. Intentional Fragmentation as a Management Strategy in Aquatic Systems. Bioscience 2013, 63, 362–372. [Google Scholar] [CrossRef]
- Larinier, M.; Travade, F. Downstream migration: Problems and facilities. Bull. Fr. Pêche Piscic. 1999, 364, 181–207. [Google Scholar] [CrossRef]
- Schmutz, S.; Mielach, C. Review of Existing Research on Fish Passage through Large Dams and Its Applicability to Mekong Mainstream Dams; MRC Technical Paper, No. 48; Mekong River Commission: Phnom Penh, Cambodia, 2015; 155p. [Google Scholar]
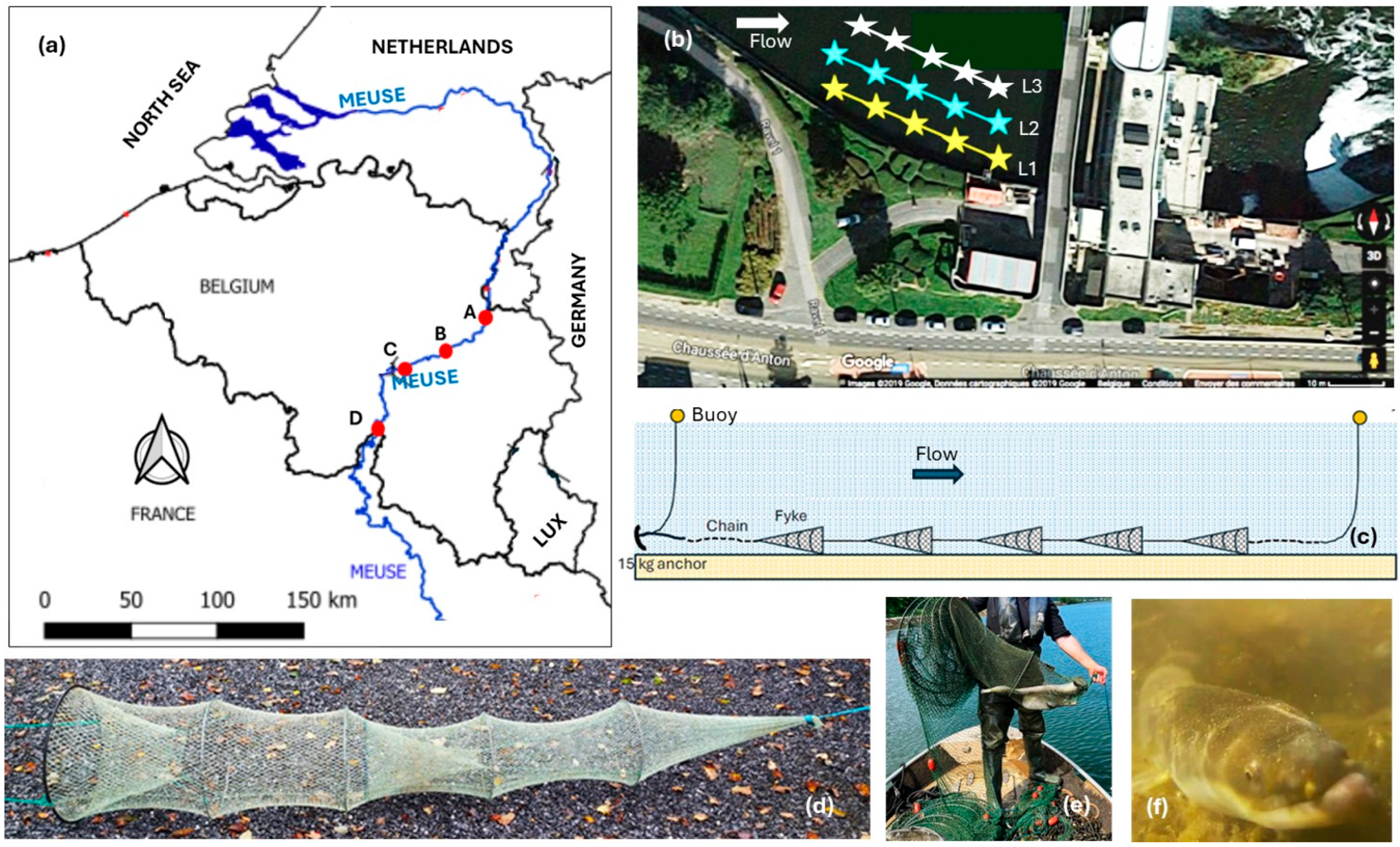


| Distance (km) from | Type of Current Construction | Dam Number from Downstream | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site Name | Code | Mouth | Source | Wide (m) | Deep (m) | Temperature (°C) | Flow Rate (m3·s−1) | ||
| Lixhe | A | 323.0 | 612.8 | 230.0 | 6.0–8.0 | 14.7 (±6.2) | 219.3 (±221.0) | Dam | 1 |
| Ampsin | B | 368.3 | 567.5 | 150.0 | 6.0 | 15.6 (±6.3) | 208.9 (±190.3) | Dam and ship lock | 4 |
| Andenne | C | 386.8 | 549.0 | 125.0 | 6.0 | 13.4 (±6.1) | - | Dam and ship lock | 5 |
| Hastière | D | 448.8 | 487.0 | 125.0 | 4.0 | - | 156.6 (±145.0) | Dam and ship lock | 15 |
| Model Description | AICc | Delta AICc | AICc Weight | Model Likelihood | n Parameters | Deviance | −2log(L) |
|---|---|---|---|---|---|---|---|
| Site A | |||||||
| {p(.), ϕ(.), pent(t), N(.)} * | 337.49 | 0.00 | 1.00 | 1.00 | 16 | −663.30 | 302.70 |
| {p(.), ϕ(t), pent(t), N(.)} | 356.33 | 18.84 | 0.00 | 0.00 | 24 | −745.29 | 220.71 |
| {p(t), ϕ(.), pent(t), N(.)} | 388.84 | 51.35 | 0.00 | 0.00 | 29 | −739.60 | 226.40 |
| {p(t), ϕ(t), pent(t), N(.)} | 399.85 | 62.36 | 0.00 | 0.00 | 47 | −761.04 | 204.97 |
| Site B | |||||||
| {p(.), ϕ(t), pent(t), N(.)} | 70.94 | 0.00 | 0.66 | 1.00 | 14 | −335.19 | 36.86 |
| {p(.), ϕ(.), pent(t), N(.)} * | 72.31 | 1.36 | 0.34 | 0.51 | 12 | −328.13 | 42.91 |
| {p(t), ϕ(.), pent(t), N(.)} | 111.49 | 40.54 | 0.00 | 0.00 | 24 | −328.90 | 43.15 |
| {p(t, ϕ(t), pent(t), N(.)} | 137.01 | 60.07 | 0.00 | 0.00 | 31 | −335.19 | 36.86 |
| Site C | |||||||
| {p(.), ϕ(t), pent(t), N(.)} | 171.97 | 0.00 | 0.97 | 1.00 | 22 | −693.00 | 120.90 |
| {p(.), ϕ(.), pent(t), N(.)} * | 178.59 | 6.62 | 0.04 | 0.04 | 14 | −666.09 | 147.81 |
| {p(t), ϕ(.), pent(t), N(.)} | 214.94 | 42.97 | 0.00 | 0.00 | 35 | −688.34 | 125.56 |
| {p(t, ϕ(t), pent(t), N(.)} | 220.34 | 48.37 | 0.00 | 0.00 | 39 | −696.32 | 117.58 |
| Site D | |||||||
| {p(.), ϕ(.), pent(t), N(.)} * | 114.03 | 0.00 | 1.00 | 1.00 | 9 | −169.61 | 92.75 |
| {p(.), ϕ(t), pent(t), N(.)} | 133.06 | 19.03 | 0.00 | 0.00 | 19 | −184.20 | 78.17 |
| {p(t), ϕ(.), pent(t), N(.)} | 140.23 | 26.20 | 0.00 | 0.00 | 23 | −195.06 | 67.30 |
| {p(t, ϕ(t), pent(t), N(.)} | 140.98 | 26.96 | 0.00 | 0.00 | 24 | −199.38 | 62.98 |
| Family | Species | Common Name | Ecological Group | n (%) | |||
|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||
| Fish | |||||||
| Anguillidae | |||||||
| European eel | Anguilla anguilla | Eurytopic | 226 (10.32) | 84 (14.89) | 175 (11.21) | 69 (13.50) | |
| Cyprinidae | |||||||
| Roach | Rutilus rutilus | Eurytopic | 103 (4.70) | 15 (2.66) | 14 (0.90) | - | |
| Common bream | Abramis brama | Eurytopic | 6 (0.27) | - | 1 (0.06) | - | |
| Common bleak | Alburnus alburnus | Eurytopic | 2 (0.09) | 1 (0.18) | 2 (0.13) | - | |
| Silver bream | Blicca bjoerkna | Eurytopic | 1 (0.05) | - | - | - | |
| Tench | Tinca tinca | Limnophilic | 18 (0.82) | - | - | - | |
| Crucian carp | Carassius carassius | Limnophilic | 1 (0.05) | - | - | - | |
| Barbel | Barbus barbus | Rheophilic | - | - | - | 6 (1.17) | |
| Chub | Leuciscus cephalus | Rheophilic | - | - | 1 (0.06) | - | |
| Esocidae | |||||||
| Pike | Esox lucius | Limnophilic | - | 1 (0.18) | - | - | |
| Gobiidae | |||||||
| Round goby | Neogobius melanostomus # | Limnophilic | 1567 (71.52) | 364 (64.54) | 1264 (80.97) | 111 (21.72) | |
| Percidae | |||||||
| Perch | Perca fluviatilis | Limnophilic | 236 (10.77) | 68 (12.06) | 84 (5.38) | 6 (1.17) | |
| Zander | Sander lucioperca | Limnophilic | 9 (0.41) | - | 6 (0.38) | 4 (0.78) | |
| Ruffe | Gymnocephalus cernua | Limnophilic | - | - | - | 2 (0.39) | |
| Salmonidae | |||||||
| Trout | Salmo trutta | Rheophilic | - | - | 1 (0.06) | - | |
| Silurae | |||||||
| Catfish | Silurus glanis # | Eurytopic | 22 (1.00) | 31 (5.50) | 13 (0.83) | 313 (61.25) | |
| TOTAL | 2191 (100.00) | 564 (100.00) | 1561 (100.00) | 511 (100.00) | |||
| TL (mm) | W (g) | Day Ratio: | ||||||
|---|---|---|---|---|---|---|---|---|
| Trapping Period | Site | Total Number | Mean ± SE | Range | Mean ± SE | Range | n Control Days | with Eel/Total Days |
| 2018 | ||||||||
| 5 September 2018–18 December 2018 | C | 15 | 883 ± 123 | 520–986 | 1324 ± 481 | 291–2205 | 6 | 1.00 |
| 2019 | ||||||||
| 17 July 2019–15 January 2020 | A | 28 | 841 ± 128 | 570–1007 | 1239 ± 625 | 270–2525 | 22 | 0.64 |
| 10 July 2019–23 January 2020 | B | 30 | 891 ± 111 | 588–1035 | 1271 ± 437 | 340–2063 | 37 | 0.81 |
| 26 July 2019–23 January 2020 | C | 44 | 847 ± 141 | 575–1076 | 1127 ± 644 | 238–2715 | 26 | 0.62 |
| 2020 | ||||||||
| 2 July 2020–17 December 2020 | A | 21 | 861 ± 135 | 554–1051 | 1425 ± 593 | 337–2243 | 30 | 0.70 |
| 2 July 2020–17 December 2020 | B | 24 | 871 ± 137 | 477–1042 | 1452 ± 627 | 172–2422 | 29 | 0.86 |
| 2 July 2020–17 December 2020 | C | 26 | 886 ± 115 | 623–1092 | 1432 ± 518 | 438–2673 | 33 | 0.79 |
| 2021 | ||||||||
| 7 September 2021–23 December 2021 | A | 38 | 731 ± 171 | 344–963 | 904 ± 545 | 83–1980 | 11 | 1.00 |
| 7 September 2021–9 November 2021 | C | 17 | 825 ± 173 | 352–988 | 1341 ± 727 | 70–2896 | 9 | 0.89 |
| 7 September 2021–8 November 2021 | D | 3 | 935 ± 40 | 906–963 | 1651 ± 198 | 1433–1820 | 6 | 0.50 |
| 2022 | ||||||||
| 29 March 2022–11 October 2022 | A | 83 | 704 ± 165 | 358–1040 | 792 ± 529 | 76–2120 | 30 | 0.83 |
| 7 July 2022–11 October 2022 | B | 30 | 824 ± 161 | 474–1115 | 1303 ± 698 | 150–2962 | 20 | 0.80 |
| 29 March 2022–11 October 2022 | C | 51 | 823 ± 190 | 414–1110 | 1345 ± 780 | 105–2889 | 35 | 0.63 |
| 29 March 2022–20 September 2022 | D | 40 | 775 ± 184 | 448–1030 | 1075 ± 794 | 142–2665 | 24 | 0.63 |
| 2023 | ||||||||
| 21 February 2023–18 July 2023 | A | 56 | 666 ± 137 | 385–943 | 648 ± 433 | 80–1880 | 11 | 1.00 |
| 21 February 2023–29 August 2023 | C | 22 | 709 ± 170 | 381–999 | 686 ± 491 | 93–1810 | 11 | 0.82 |
| 21 February 2023–29 August 2023 | D | 26 | 650 ± 174 | 286–1042 | 637 ± 598 | 131–2076 | 15 | 0.87 |
| TL at Tagging (mm) | TL Increment (mm.day−1) | ||||||
|---|---|---|---|---|---|---|---|
| Time Class Between Tagging and Recapture (Month) | Silvering Index at Tagging | Site (-Recapture Site Change) | n | Mean ± SE | Range | Mean ± SE | Range |
| <1 | FII | A | 2 | 560 ± 2 | 558–561 | 0.421 ± 0.191 | 0.286–0.556 |
| C | 1 | 720 | - | 0 | - | ||
| D | 1 | 539 | - | 0 | - | ||
| FIII | A | 1 | 660 | - | 0.222 | - | |
| C | 1 | 824 | - | 0.133 | - | ||
| FIV | B | 1 | 994 | - | 0.214 | - | |
| C | 2 | 932 ± 26 | 914–950 | 0.107 ± 0.051 | 0.071–0.143 | ||
| D | 1 | 1009 | - | 0 | - | ||
| 1–6 | FII | A | 2 | 570 ± 38 | 543–597 | 0.302 ± 0.427 | 0–0.604 |
| D | 1 | 560 | - | 0.286 | - | ||
| FIII | A | 2 | 615 ± 29 | 594–635 | 0.197 ± 0.050 | 0.162–0.232 | |
| C | 1 | 893 | - | 0.064 | - | ||
| D-A | 1 | 600 | - | 0.614 | - | ||
| FIV | A | 1 | 870 | - | 0.260 | - | |
| C | 1 | 1016 | - | 0.086 | |||
| 7–12 | FI | A | 2 | 505 ± 0 | 505-505 | 0.551 | 0.543–0.558 |
| FII | A | 1 | 549 | - | 0.064 | - | |
| FIII | A | 1 | 666 | - | 0.337 | - | |
| A-C | 1 | 742 | - | 0.121 | - | ||
| C | 1 | 575 | - | 0.485 | - | ||
| C-D | 1 | 687 | - | 0.019 | - | ||
| FIV | A | 1 | 796 | - | 0.326 | - | |
| D | 2 | 950 ± 18 | 937–963 | 0.009 ± 0.004 | 0.007–0.012 | ||
| 13–18 | FII | A | 3 | 561 ± 60 | 526–630 | 0.419 ± 0.017 | 0.399–0.429 |
| D | 1 | 716 | - | 0.258 | - | ||
| FIII | D-A | 1 | 600 | - | 0.138 | - | |
| 19–24 | FIII | C | 2 | 717 ± 170 | 597–837 | 0.271 ± 0.149 | 0.166–0.377 |
| FIV | C | 1 | 912 | - | 0.030 | - | |
| >24 | FII | C | 1 | 674 | - | 0.197 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzau Matondo, B.; Ovidio, M.; Lerquet, M.; Colson, D.; Sonny, D. Biology and Ecology of the European Eel as Revealed by an Original Sampling Technique Performed in a Deep and Large Riverine Ecosystem. Sustainability 2024, 16, 10607. https://doi.org/10.3390/su162310607
Nzau Matondo B, Ovidio M, Lerquet M, Colson D, Sonny D. Biology and Ecology of the European Eel as Revealed by an Original Sampling Technique Performed in a Deep and Large Riverine Ecosystem. Sustainability. 2024; 16(23):10607. https://doi.org/10.3390/su162310607
Chicago/Turabian StyleNzau Matondo, Billy, Michaël Ovidio, Marc Lerquet, Dylan Colson, and Damien Sonny. 2024. "Biology and Ecology of the European Eel as Revealed by an Original Sampling Technique Performed in a Deep and Large Riverine Ecosystem" Sustainability 16, no. 23: 10607. https://doi.org/10.3390/su162310607
APA StyleNzau Matondo, B., Ovidio, M., Lerquet, M., Colson, D., & Sonny, D. (2024). Biology and Ecology of the European Eel as Revealed by an Original Sampling Technique Performed in a Deep and Large Riverine Ecosystem. Sustainability, 16(23), 10607. https://doi.org/10.3390/su162310607








