Impact of Conversion of the Caatinga Forest to Different Land Uses on Soil and Root Respiration Dynamics in the Brazilian Semiarid Region
Abstract
1. Introduction
2. Materials and Methods
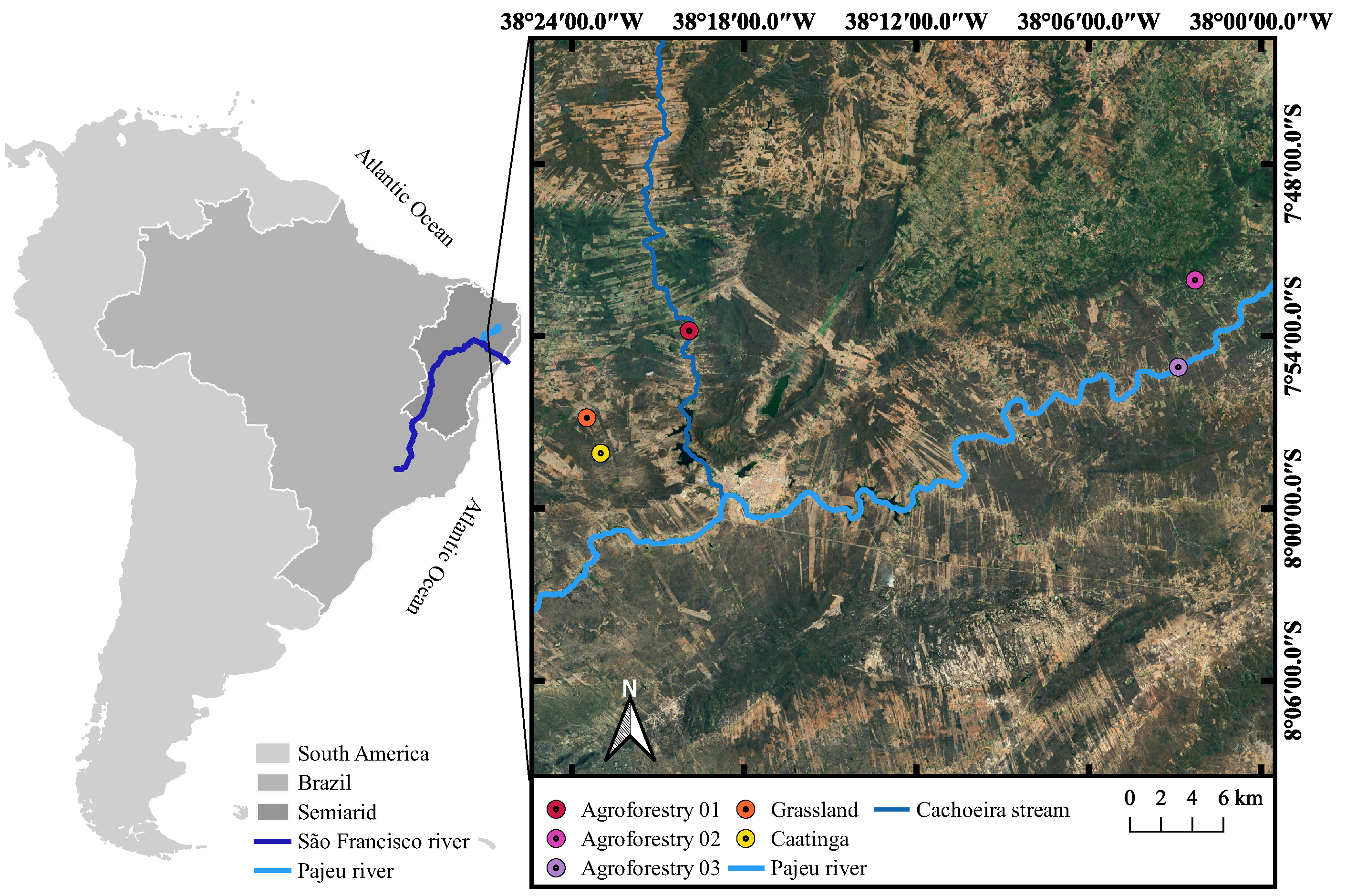
2.1. Study Sites
2.2. Experimental Procedures, Data Collection, and Analysis
2.2.1. Soil Respiration, Moisture, and Temperature Measurements
2.2.2. Data Analysis
2.3. Soil Carbon and Microbiological Attributes
2.4. Statistical Analysis
3. Results
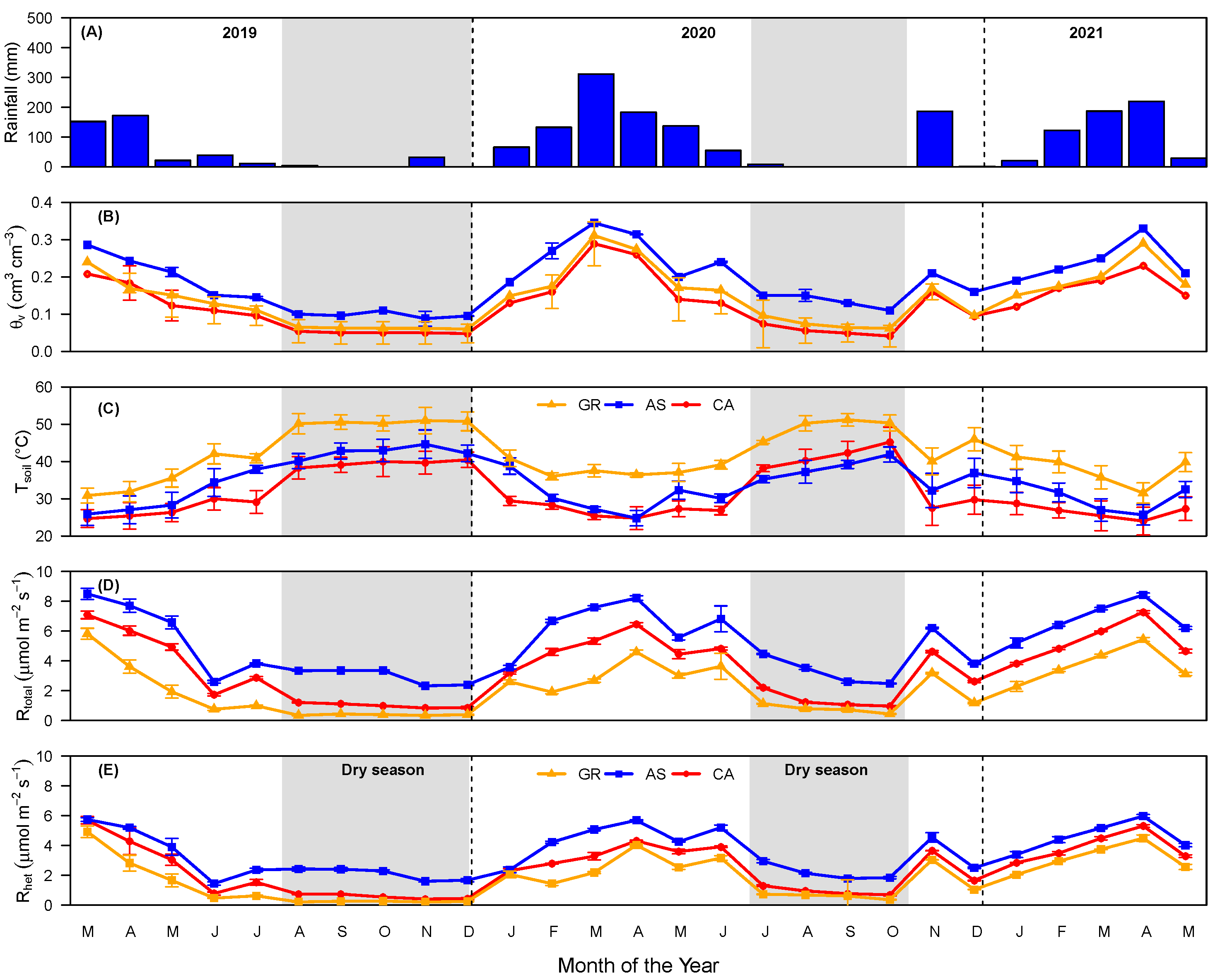
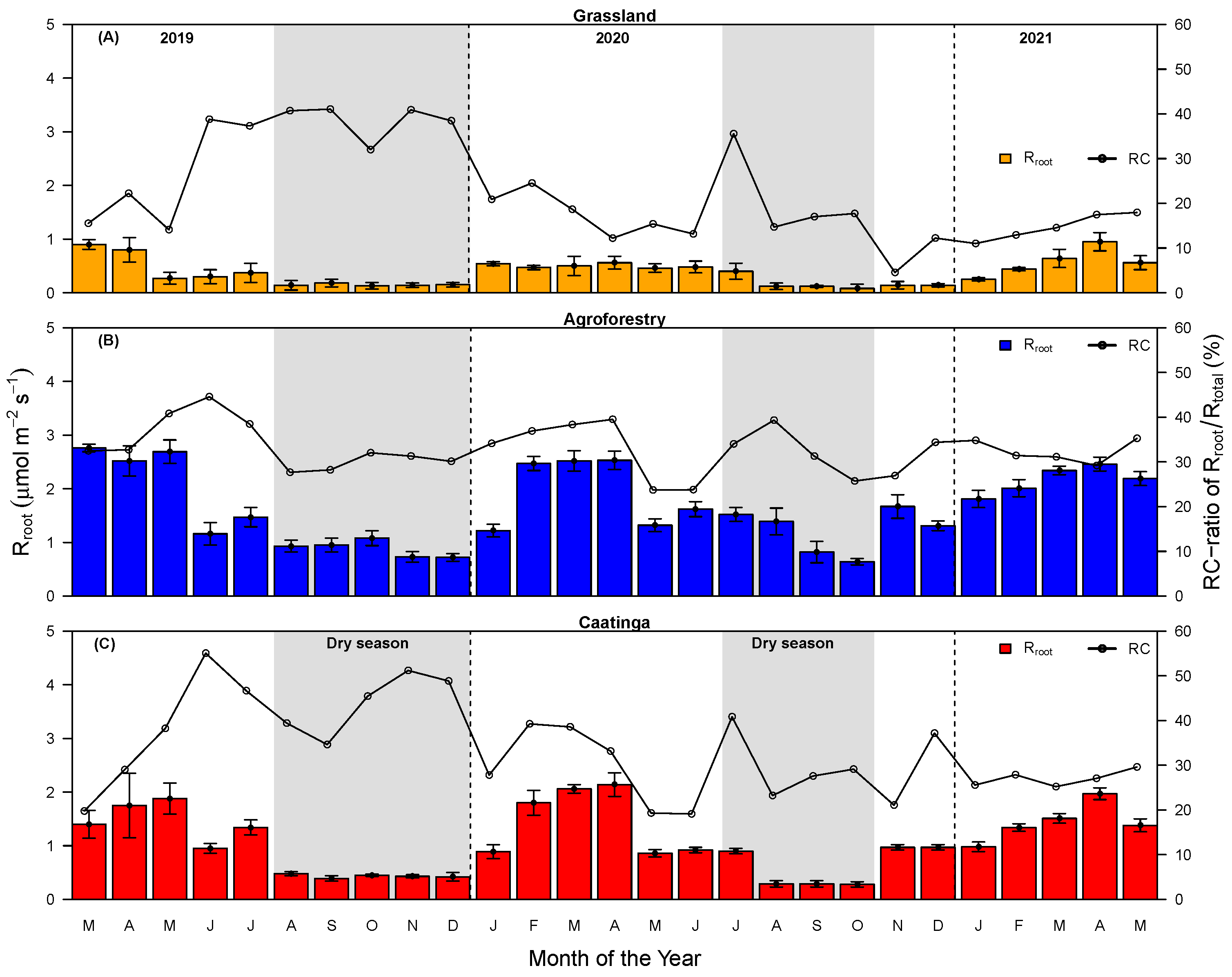
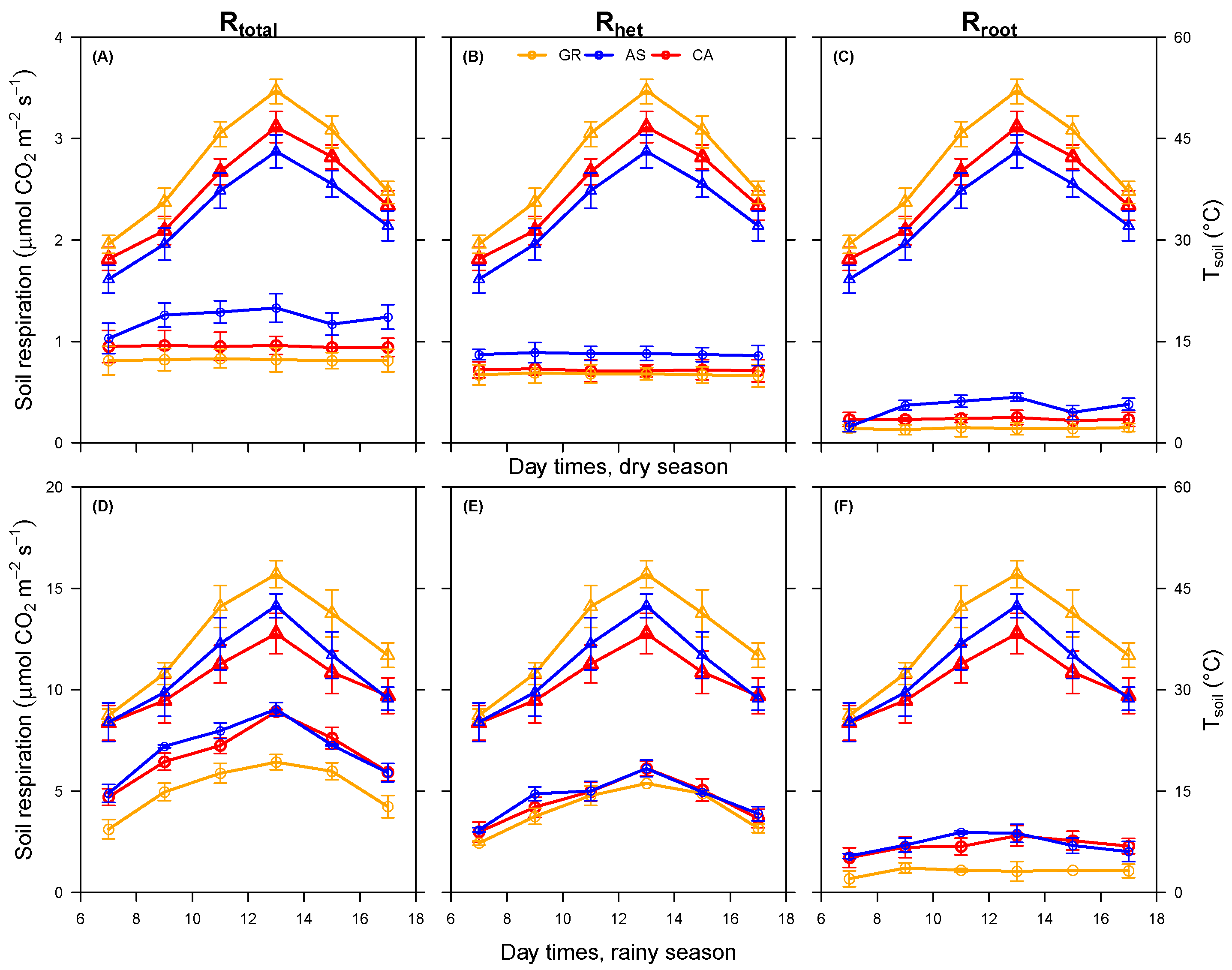
3.1. Soil Total, Heterotrophic, and Root Respiration Responses
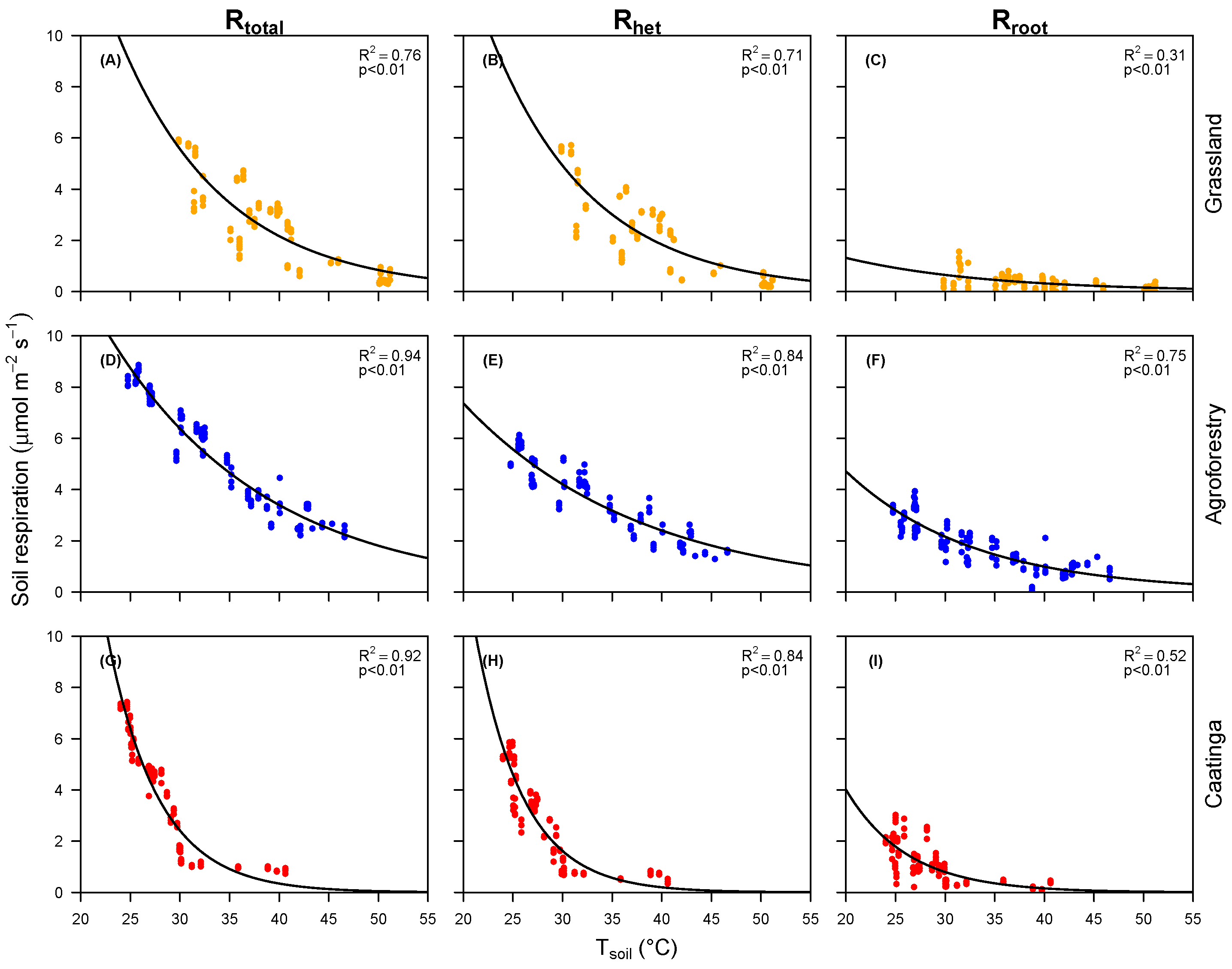
3.2. Soil Respiration and Moisture and Temperature Relationship
3.3. TOC and Microbiological Attributes
4. Discussion
4.1. Soil Respiration Response to Environmental Factors
4.2. TOC and Microbiological Attributes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, J.M.C.; Barbosa, L.C.F.; Leal, I.R.; Tabarelli, M. The Caatinga: Understanding the Challenges. In Caatinga: The Largest Tropical Dry Forest Region in South America; Silva, J.M.C., Barbosa, L.C.F., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–19. [Google Scholar]
- Araujo, H.F.P.; Garda, A.A.; Girão e Silva, W.A.; Nascimento, N.F.F.; Mariano, E.F.; Silva, J.M.C. The Caatinga region is a system and not an aggregate. J. Arid Environ. 2022, 203, 104778. [Google Scholar] [CrossRef]
- Barros, M.F.; Ribeiro, E.M.S.; Vanderlei, R.S.; de Paula, A.S.; Sila, A.B.; Wirth, R.; Cianciaruso, M.V.; Tabarelli, M. Resprouting drives successional pathways and the resilience of Caatinga dry forest in human-modified landscapes. For. Ecol. Manag. 2021, 481, 118881. [Google Scholar] [CrossRef]
- Araujo, H.F.P.; Canassa, N.F.; Machado, C.C.C.; Cianciaruso, M.V.; Tabarelli, M. Human disturbance is the major driver of vegetation changes in the Caatinga dry forest region. Sci. Rep. 2023, 13, 18440. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.d.S.; Pessoa, L.G.M.; Silva, E.M.d.; Freire, M.B.G.d.S.; de Souza, E.S.; Oresca, D.; Silva, J.O.N.d.; Júnior, G.B.; Bezerra, A.C.; Santos, E.S.d. Changes in Soil C, N, and P Concentrations and Stocks after Caatinga Natural Regeneration of Degraded Pasture Areas in the Brazilian Semiarid Region. Sustainability 2024, 16, 8737. [Google Scholar] [CrossRef]
- Lima, J.R.d.S.; Souza, R.M.S.; de Sá Barreto Sampaio, E.V.; Antonino, A.C.D.; de Souza, E.S.; de Medeiros, É.V.; Duda, G.P.; Ferreira, C.R.P.C.; Menezes, R.S.C.; Hammecker, C. Moisture, temperature and respiration of two soil classes under pasture and tropical dry forest in the semiarid brazilian region. J. Arid Environ. 2023, 214, 104981. [Google Scholar] [CrossRef]
- Nissan, A.; Alcolombri, U.; Peleg, N.; Galili, N.; Jimenez-Martinez, J.; Molnar, P.; Holzner, M. Global warming accelerates soil heterotrophic respiration. Nat. Commun. 2023, 14, 3452. [Google Scholar] [CrossRef]
- Hanson, P.; Edwards, N.; Garten, C.; Andrews, J. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Tiruvaimozhi, Y.V.; Sankaran, M. Soil respiration in a tropical montane grassland ecosystem is largely heterotroph-driven and increases under simulated warming. Agric. For. Meteorol. 2019, 276–277, 107619. [Google Scholar] [CrossRef]
- Yan, J.; Tong, M.; Liu, J.; Li, J.; Li, H. Temperature and moisture sensitivities of soil respiration vary along elevation gradients: An analysis from long-term field observations. Ecol. Evol. 2024, 912, 169150. [Google Scholar] [CrossRef]
- Chen, S.; Whang, J.; Zang, T.; Hou, Z. Climatic, soil, and vegetation controls of the temperature sensitivity (Q10) of soil respiration across terrestrial biomes. Glob. Ecol. Conserv. 2020, 22, e00955. [Google Scholar] [CrossRef]
- González-Ubierna, S.; Lai, R. Modelling the effects of climate factors on soil respiration across mediterranean ecosystems. J. Arid Environ. 2019, 165, 46–54. [Google Scholar] [CrossRef]
- Qin, S.; Peng, Q.; Dong, Y.; Qi, Y.; Li, Z.; Guo, Y.; Liu, X.; Xiao, S.; Liu, X.; Jia, J.; et al. Role of ambient climate in the response of soil respiration to different grassland management measures. Agric. For. Meteorol. 2023, 334, 109439. [Google Scholar] [CrossRef]
- Barneze, A.S.; Whitaker, J.; McNamara, N.P.; Ostle, N.J. Interactive effects of climate warming and management on grassland soil respiration partitioning. Eur. J. Soil Sci. 2024, 75, e13491. [Google Scholar] [CrossRef]
- Lei, J.; Guo, X.; Zeng, Y.; Zhou, J.; Gao, Q.; Yang, Y. Temporal changes in global soil respiration since 1987. Nat. Commun. 2021, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Rodríguez-Caballero, E.; Sánchez-Cañete, E.P.; Domingo, F.; Cantón, Y. Temporal dynamics of dryland soil CO2 efflux using high-frequency measurements: Patterns and dominant drivers among biocrust types, vegetation and bare soil. Geoderma 2022, 405, 115404. [Google Scholar] [CrossRef]
- Azevedo, L.C.B.; Ferreira, A.S.; Rodovalho, N.S.; Ferreira, L.F.R.; Kumar, A. Microbial contribution to the carbon flux in the soil: A literature review. Rev. Bras. Ciênc. Solo 2024, 48, e0230065. [Google Scholar] [CrossRef]
- Yan, J.; Feng, Y.; Li, J.; Li, H.; Ding, G. Response of soil respiration and Q10 to temperature and moisture in naturally regenerated and bare lands based on an 11-year observation period. CATENA 2022, 208, 105711. [Google Scholar] [CrossRef]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, J.; Curiel Yuste, J.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef]
- Davidson, E.A.; Samanta, S.; Caramori, S.S.; Savage, K. The dual arrhenius and michaelis–menten kinetics model for decomposition of soil organic matter at hourly to seasonal time scales. Glob. Change Biol. 2012, 18, 371–384. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, J.; Jiang, J.; Gu, K.; Zhu, C.; Pan, C.; Yin, W. Effect of Soil Volumetric Water Content on the CO2 Diffusion Coefficient. Ecosystems 2023, 15, 12637. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Meng, H.; Zheng, Z.; Zhao, Y.; Zhang, T. Response of Soil Respiration and Its Components to Precipitation Exclusion in Vitex negundo Var. Heterophylla Shrubland of the Middle Taihang Mountain in North China. Front. Environ. Sci. 2021, 9, 712301. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, Y.; Fu, G.; Song, H.; Li, G.; Xiao, R. Asymmetric effect of increased and decreased precipitation in different periods on soil and heterotrophic respiration in a semiarid grassland. Agric. For. Meteorol. 2020, 291, 108039. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Rodler, A.S.; Kuffner, M.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol. Biochem. 2011, 43, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Frissell, M.; Hao, D.; Tang, X.; Berryman, E.; Bond-Lamberty, B. The global contribution of roots to total soil respiration. Glob. Ecol. Biogeogr. 2022, 31, 685–699. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bond-Lamberty, B.; Desai, A.R.; Lavoie, M.; Risk, D.; Tang, J.; Todd-Brown, K.; Vargas, R. The value of soil respiration measurements for interpreting and modeling terrestrial carbon cycling. Plant Soil 2017, 413, 1–25. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Fan, L.C.; Yang, M.Z.; Han, W. Soil respiration under different land uses in eastern china. PLoS ONE 2015, 10, e0124198. [Google Scholar] [CrossRef]
- Lima, J.R.d.S.; Souza, R.; da Silva, E.; Souza, E.; Emanuella, J.; Oliveira, S.; Medeiros, E.; Pessoa, L.G.; Antonino, A.C.D.; Hammecker, C. Impacts of land-use changes on soil respiration in the semi-arid region of brazil. Rev. Bras. Ciênc. Solo 2020, 44, e0200092. [Google Scholar] [CrossRef]
- Simões, V.J.L.; Souza, E.; Leite, M.; Souza, R.; Silva, J.; Torres Sales, A.; Tabosa, J.; Lima, J.; Antonino, A. Physical-hydric attributes and soil CO2 efflux in pastoral systems in a brazilian semi-arid environment. Agrofor. Syst. 2023, 97, 1421–1433. [Google Scholar] [CrossRef]
- Tonucci, R.G.; Vogado, R.F.; Silva, R.D.; Pompeu, R.C.F.F.; Oda-Souza, M.; Souza, H.A.d. Agroforestry system improves soil carbon and nitrogen stocks in depth after land-use changes in the brazilian semi-arid region. Rev. Bras. Ciênc. Solo 2023, 47, e0220124. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.; Feng, X.; Antonino, A.; Montenegro, S.; Souza, E.; Porporato, A. Vegetation response to rainfall seasonality and interannual variability in tropical dry forests. Hydrol. Process. 2016, 30, 3583–3595. [Google Scholar] [CrossRef]
- Baillie, I.C. Soil survey staff 1999, soil taxonomy. Soil Use Manag. 2001, 17, 57–60. [Google Scholar] [CrossRef]
- Kelting, D.L.; Burger, J.A.; Edwards, G.S. Estimating root respiration, microbial respiration in rhizosphere, and root-free soil respiration in forest soils. Soil Biol. Biochem. 1998, 30, 961–968. [Google Scholar] [CrossRef]
- Baah-Acheamfour, M.; Carlyle, C.; Bork, E.; Chang, S. Forest and perennial herbland cover reduce microbial respiration but increase root respiration in agroforestry systems. Agric. For. Meteorol. 2020, 280, 107790. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J. On the temperature dependence of soil respiration. Funct. Ecol. 2012, 83, 315–323. [Google Scholar] [CrossRef]
- Davidson, E.A.; Belk, E.L.; Boone, R.D. Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob. Change Biol. 1998, 4, 217–227. [Google Scholar] [CrossRef]
- Lai, L.; Zhao, X.; Jiang, L.; Wang, Y.; Luo, L.; Zheng, Y. Soil respiration in different agricultural and natural ecosystems in an arid region. PLoS ONE 2012, 10, e48011. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K. The metabolic quotient for co2 (qco2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Brookes, P.; Powlson, D.; Jenkinson, D. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Islam, K.; Weil, R. Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol. Fertil. Soils 1998, 27, 408–416. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Ross, D.S. Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Sparling, G. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Redwood City, CA, USA; Palo Alto: Santa Clara, CA, USA, 1960; pp. 278–292. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 6 November 2023).
- Marques, T.; Mendes, K.; Mutti, P.; Medeiros, S.; Silva, L.; Perez-Marin, A.; Campos, S.; Lucio, P.; Lima, K.; Reis, J.; et al. Environmental and biophysical controls of evapotranspiration from seasonally dry tropical forests (caatinga) in the brazilian semiarid. Agric. For. Meteorol. 2020, 287, 1–15. [Google Scholar] [CrossRef]
- Macedo, R.S.; Letícia, M.; Lambais, E.O.; Lambais, G.R.; Bakker, A.P. Effects of degradation on soil attributes under Caatinga in the Brazilian semiarid. Rev. Árvore 2023, 47, e4702. [Google Scholar] [CrossRef]
- de Oliveira, M.L.; dos Santos, C.A.C.; Perez-Marin, A.M.; Santos, C.A.G. Effects of human-induced land degradation on water and carbon fluxes in two different Brazilian dryland soil covers. Sci. Total Environ. 2021, 792, 148458. [Google Scholar] [CrossRef]
- Lopes, V.S.; Cardoso, I.M.; Fernandes, O.R.; Rocha, G.C.; Simas, F.N.B.; de Melo Moura, W.; Santana, F.C.; Veloso, G.V.; da Luz, J.M.R. The establishment of a secondary forest in a degraded pasture to improve hydraulic properties of the soil. Soil Tillage Res. 2020, 198, 104538. [Google Scholar] [CrossRef]
- Das, S.; Deb, S.; Sahoo, S.S.; Sahoo, U.K. Soil microbial biomass carbon stock and its relation with climatic and other environmental factors in forest ecosystems: A review. Sci. Rep. 2023, 43, 933–945. [Google Scholar] [CrossRef]
- Jiang, Z.; Bian, H.; Xu, L.; Li, M.; He, N. Pulse effect of precipitation: Spatial patterns and mechanisms of soil carbon emissions. Front. Ecol. Evol. 2021, 9, 673310. [Google Scholar] [CrossRef]
- Ito, A. Constraining size-dependence of vegetation respiration rates. Sci. Rep. 2020, 10, 4304. [Google Scholar] [CrossRef] [PubMed]
- Martínková, J.; Hájek, T.; Adamec, L.; Klimešová, J. Growth, root respiration and photosynthesis of a root-sprouting short-lived herb after severe biomass removal. Tree Physiol. 2021, 284, 151915. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, S.; Luo, Y. Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob. Change Biol. 2007, 13, 761–775. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Song, Y.; Zhu, B. Patterns of soil respiration and its temperature sensitivity in grassland ecosystems across china. Biogeosciences 2018, 15, 5329–5341. [Google Scholar] [CrossRef]
- Bian, H.; Li, C.; Zhu, J.; Li, M.; Zheng, S.; He, N. Soil Moisture Affects the Rapid Response of Microbes to Labile Organic C Addition. Front. Ecol. Evol. 2022, 10, 857185. [Google Scholar] [CrossRef]
- Darrouzet-Nardi, A.; Reed, S.; Grote, E.; Belnap, J. Patterns of longer-term climate change effects on CO2 efflux from biocrusted soils differ from those observed in the short term. Biogeosciences 2018, 15, 4561–4573. [Google Scholar] [CrossRef]
- Miao, Y.; Han, H.; Du, Y.; Zhang, Q.; Jiang, L.; Hui, D.; Wan, S. Nonlinear responses of soil respiration to precipitation changes in a semiarid temperate steppe. Sci. Rep. 2017, 7, 45782. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, C.; Chen, X.; Yao, J.; Jiang, L.; Liu, M. Home-field advantage in soil respiration and its resilience to drying and rewetting cycles. J. Geophys. Res. Biogeosci. 2021, 750, 141736. [Google Scholar] [CrossRef]
- Meena, A.; Hanief, M.; Dinakaran, J.; Rao, K. Soil moisture controls the spatio-temporal pattern of soil respiration under different land use systems in a semi-arid ecosystem of delhi, india. Ecol. Process. 2020, 9, 15. [Google Scholar] [CrossRef]
- Wei, D.; Yue, L.; Pei, H.; Jiaqi, Z.; Haichai, J.; Chen, N.; Yinghui, L. Nitrogen Addition Decreases Soil Respiration without Changing the Temperature Sensitivity in a Semiarid Grassland. Agric. For. Meteorol. 2020, 11, 129–139. [Google Scholar] [CrossRef][Green Version]
- Ferreira, C.; Antonino, A.; Sampaio, E.V.D.S.; Correia, K.; Lima, J.; Soares, W.; Menezes, R. Soil CO2 efflux measurements by alkali absorption and infrared gas analyzer in the brazilian semiarid region. Rev. Bras. Ciênc. Solo 2018, 42, e0160563. [Google Scholar] [CrossRef]
- Niu, F.; Chen, J.; Xiong, P.; Wang, Z.; Zhang, H.; Xu, B. Responses of soil respiration to rainfall pulses in a natural grassland community on the semi-arid loess plateau of china. Nat. Commun. 2019, 178, 199–208. [Google Scholar] [CrossRef]
- Kurganova, I.; Lopes de Gerenyu, V.; Khoroshaev, D.; Myakshina, T.; Sapronov, D.; Zhmurin, V. Temperature Sensitivity of Soil Respiration in Two Temperate Forest Ecosystems: The Synthesis of a 24-Year Continuous Observation. Forest 2022, 13, 1374. [Google Scholar] [CrossRef]
- Wang, X.; Piao, S.; Ciais, P.; Janssens, I.A.; Reichstein, M.; Peng, S.; Wang, T. Are ecological gradients in seasonal q10 of soil respiration explained by climate or by vegetation seasonality? Soil Biol. Biochem. 2010, 42, 1728–1734. [Google Scholar] [CrossRef]
- Maes, S.L.; Dietrich, J.; Midolo, G.; Schwieger, S.; Kummu, M.; Vandvik, V.; Aerts, R.; Althuizen, I.H.J.; Biasi, C.; Björk, R.G.; et al. Environmental drivers of increased ecosystem respiration in a warming tundra. Nature 2024, 629, 105–113. [Google Scholar] [CrossRef]
- Tang, Z.; Sun, X.; Luo, Z.; He, N.; Sun, O. Effects of temperature, soil substrate, and microbial community on carbon mineralization across three climatically contrasting forest sites. Ecol. Evol. 2018, 8, 879–891. [Google Scholar] [CrossRef]
- Hou, T.; Wang, Y.; Guo, F.; Jia, Q.; Wu, X.; Wang, E.; Hong, J. Soil respiration characteristics and influencing factors for apple orchards in different regions on the loess plateau of shaanxi province. Sustainability 2021, 13, 4780. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Y.; Shi, P.; She, J.; Zhou, P. Temporal-spatial variation and controls of soil respiration in different primary succession stages on glacier forehead in gongga mountain, china. PLoS ONE 2012, 7, e42354. [Google Scholar] [CrossRef]
- Schulz, K.; Voigt, K.; Beusch, C.; Almeida-Cortez, J.S.; Kowarik, I.; Walz, A.; Cierjacks, A. Grazing deteriorates the soil carbon stocks of caatinga forest ecosystems in Brazil. For. Ecol. Manag. 2016, 367, 62–70. [Google Scholar] [CrossRef]
- Visscher, A.M.; Visscher, A.M.; Meli, P.; Fonte, S.J.; Zerbe, S.; Wellstein, C. Agroforestry enhances biological activity, diversity and soil-based ecosystem functions in mountain agroecosystems of Latin America: A meta-analysis. Glob. Change Biol. 2023, 30, e17036. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.D.; Harrison, R.B. The case for digging deeper: Soil organic carbon storage, dynamics, and controls in our changing world. Soil Syst. 2019, 28, 28. [Google Scholar] [CrossRef]
- Morais, D.H.O.; Silva, C.A.; Rosset, J.S.; Silva Souza, C.B.; Ozório, J.M.B.; Pierri Castilho, S.C.; Marra, L.M. Stock and indices of carbon management under different soil use systems. Braz. J. Environ. Sci. 2021, 56, 286–295. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, P.; Fu, B.; Wu, D.; Zhao, Z. Labile Fraction of Organic Carbon in Soils from Natural and Plantation Forests of Tropical China. Sustainability 2024, 16, 7836. [Google Scholar] [CrossRef]
- Costa, D.P.; Pereira, A.S.A.; Mendes, L.W.; França, R.F.; Silva, T.G.E.; Oliveira, J.B.; Duda, G.P.; Menezes, R.S.C.; Medeiros, E.V. Forest-to-pasture conversion modifies the soil bacterial community in Brazilian dry forest Caatinga. Sci. Total Environ. 2022, 810, 151943. [Google Scholar] [CrossRef]
- Parron, L.M.; Peixoto, R.T.d.G.; da Silva, K.; Brown, G.G. Traditional Yerba Mate Agroforestry Systems in Araucaria Forest in Southern Brazil Improve the Provisioning of Soil Ecosystem Services. Conservation 2024, 4, 115–138. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Barreto-Garcia, A.B.; Monroe, P.H.M.; Barros, W.T.; Nunes, M.R. Carbon in soil macroaggregates under coffee agroforestry systems: Modeling the effect of edaphic fauna and residue input. Appl. Soil Ecol. 2024, 202, 105604. [Google Scholar] [CrossRef]
- Nunes, J.; Araujo, A.; Nunes, L.; Lima, L.; Carneiro, R.; Salviano, A.; Tsai, S. Impact of land degradation on soil microbial biomass and activity in northeast brazil. Pedosphere 2012, 22, 88–95. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Rolando, J.; Kalu, S.; Karhu, K. Microbial soil quality indicators depending on land use and soil type in a semi-arid dryland in Kenya. Eur. J. Soil Biol. 2024, 121, 103626. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. in: PAUL, E. A. and LADD, J. N. (ed.). Soil Biochem. 1981, 5, 415–471. [Google Scholar] [CrossRef]
- Lepcha, N.T.; Devi, N.B. Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecol. Process. 2020, 9, 65. [Google Scholar] [CrossRef]
- Pezarico, C.R.; Vitorino, A.C.; Mercante, F.M.; Daniel, O. Indicadores de qualidade do solo em sistemas agroflorestais. Rev. Ciênc. Agrár. 2013, 56, 40–47. [Google Scholar] [CrossRef]
- Huang, R.; Crowther, T.W.; Sui, Y.; Sun, B.; Liang, Y. High stability and metabolic capacity of bacterial community promote the rapid reduction of easily decomposing carbon in soil. Commun. Biol. 2021, 4, 1376. [Google Scholar] [CrossRef] [PubMed]
- Somenahally, A.C.; McLawrence, J.; Chaganti, V.N.; Ganjegunte, G.K.; Obayomi, O.; Brady, J.A. Response of soil microbial Communities, inorganic and organic soil carbon pools in arid saline soils to alternative land use practices. Ecol. Indic. 2023, 150, 110227. [Google Scholar] [CrossRef]
- Cao, R.; Yang, W.; Chang, C.; Wang, Z.; Wang, Q.; Jiang, Y.; Li, H.; Tan, B. Soil microbial biomass carbon and freeze-thaw cycles drive seasonal changes in soil microbial quotient along a steep altitudinal gradient. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006325. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]


| Grassland | Agroforestry | Caatinga | |
|---|---|---|---|
| Physical attributes | |||
| Sand (g ) | 65.32 | 59.04 | 69.30 |
| Silt (g ) | 18.40 | 31.60 | 18.40 |
| Clay (g ) | 16.28 | 9.36 | 12.30 |
| Bulk Density (g ) | 1.61 | 1.32 | 1.45 |
| Particle Density (g ) | 2.61 | 2.47 | 2.57 |
| Total Porosity (%) | 38.3 | 46.6 | 43.6 |
| Textural Class | Sandy Loam | Sandy Loam | Sandy Loam |
| Chemical attributes | |||
| pH (O) | 7.30 | 6.81 | 7.11 |
| P (mg ) | 2.10 | 3.87 | 3.95 |
| ( ) | 0.60 | 1.30 | 1.40 |
| ( ) | 0.09 | 0.16 | 0.15 |
| ( ) | 0.18 | 0.76 | 0.09 |
| ( ) | 0.32 | 0.76 | 0.78 |
| + ( ) | 1.00 | 1.00 | 1.00 |
| SB ( ) | 1.19 | 2.28 | 2.42 |
| CEC ( ) | 2.19 | 3.29 | 3.42 |
| V (%) | 54.3 | 69.6 | 70.7 |
| m (%) | 0.0 | 0.0 | 0.0 |
| Organic Matter (%) | 1.40 | 3.56 | 3.47 |
| Respiration Types | Grassland | Agroforestry | Caatinga | |||
|---|---|---|---|---|---|---|
| (mol ) | v ( ) | (°C) | v ( ) | (°C) | v ( ) | (°C) |
| 0.87 *** | −0.87 *** | 0.93 *** | −0.95 *** | 0.91 *** | −0.91 *** | |
| 0.86 *** | −0.85 *** | 0.91 *** | −0.91 *** | 0.86 *** | −0.87 *** | |
| 0.79 *** | −0.87 *** | 0.89 *** | −0.93 *** | 0.89 *** | −0.86 *** | |
| Areas | Exponential Models (Equation (2)) | (Equation (3)) | |
|---|---|---|---|
| Grassland | = 94.2 × | 0.76 *** | 0.39 |
| = 93.7 × | 0.71 *** | 0.37 | |
| = 5.4 × | 0.31 ** | 0.49 | |
| Agroforestry | = 42.22 × | 0.94 *** | 0.53 |
| = 22.6 × | 0.84 *** | 0.57 | |
| = 22.4 × | 0.75 *** | 0.46 | |
| Caatinga | = 854.9 × | 0.92 *** | 0.14 |
| = 851.7 × | 0.86 ** | 0.12 | |
| = 101.86 × | 0.52 ** | 0.20 |
| Attributes | Layer (cm) | Grassland | Agroforestry | Caatinga |
|---|---|---|---|---|
| Dry Season | ||||
| 00–05 | 8.5 bB | 21.4 aB | 20.9 aB | |
| TOC (g C ) | 05–10 | 7.8 bB | 18.6 aB | 17.7 aB |
| 10–20 | 6.8 bA | 14.7 aB | 15.5 aB | |
| 00–05 | 0.21 cB | 0.52 aB | 0.42 bB | |
| SBR (mg C- ) | 05–10 | 0.18 cB | 0.44 aB | 0.37 bB |
| 10–20 | 0.14 cA | 0.38 aB | 0.32 bB | |
| 00–05 | 135 cB | 525 aB | 437 bB | |
| MBC (mg C ) | 05–10 | 126 cB | 498 aB | 407 bB |
| 10–20 | 113 cA | 476 aB | 388 bB | |
| 00–05 | 1.58 cA | 2.45 aA | 2.12 bB | |
| qMic (%) | 05–10 | 1.61 cB | 2.68 aA | 2.30 bB |
| 10–20 | 1.59 cB | 3.03 aA | 2.51 bB | |
| 00–05 | 1.55 aB | 0.99 bB | 0.96 bB | |
| (mg C- C ) | 05–10 | 1.35 aB | 0.88 bB | 0.90 bB |
| 10–20 | 1.24 aB | 0.79 bB | 0.82 bB | |
| Rainy Season | ||||
| 00–05 | 9.2 bA | 24.1 aA | 23.9 aA | |
| TOC (g C ) | 05–10 | 8.9 bA | 20.4 aA | 19.8 aA |
| 10–20 | 6.3 bA | 16.9 aA | 17.9 aA | |
| 00–05 | 0.29 cA | 0.67 aA | 0.61 bA | |
| SBR (mg C- ) | 05–10 | 0.21 cA | 0.55 aA | 0.48 bA |
| 10–20 | 0.16 bA | 0.46 aA | 0.45 aA | |
| 00–05 | 165 cA | 598 aA | 540 bA | |
| MBC (mg C ) | 05–10 | 145 cA | 547 aA | 512 bA |
| 10–20 | 115 cA | 521 aA | 479 bA | |
| 00–05 | 1.58 cA | 1.79 bB | 2.48 aA | |
| qMic (%) | 05–10 | 1.86 bA | 2.68 aA | 2.58 aA |
| 10–20 | 1.83 cA | 3.08 aA | 2.67 bA | |
| 00–05 | 1.76 aA | 1.12 bA | 1.13 bA | |
| (mg C- C ) | 05–10 | 1.45 aA | 1.01 bA | 0.94 bA |
| 10–20 | 1.40 aA | 0.88 bA | 0.94 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oresca, D.; Souza, E.S.d.; Souza, R.M.S.; Silva, J.R.I.; Moura, D.P.d.; Sampaio, E.V.d.S.B.; Hammecker, C.; Lima, J.R.d.S.; Menezes, R.S.C.; Pessoa, L.G.M.; et al. Impact of Conversion of the Caatinga Forest to Different Land Uses on Soil and Root Respiration Dynamics in the Brazilian Semiarid Region. Sustainability 2024, 16, 10652. https://doi.org/10.3390/su162310652
Oresca D, Souza ESd, Souza RMS, Silva JRI, Moura DPd, Sampaio EVdSB, Hammecker C, Lima JRdS, Menezes RSC, Pessoa LGM, et al. Impact of Conversion of the Caatinga Forest to Different Land Uses on Soil and Root Respiration Dynamics in the Brazilian Semiarid Region. Sustainability. 2024; 16(23):10652. https://doi.org/10.3390/su162310652
Chicago/Turabian StyleOresca, Denizard, Eduardo Soares de Souza, Rodolfo Marcondes Silva Souza, José Raliuson Inácio Silva, Débora Purcina de Moura, Everardo Valadares de Sá Barreto Sampaio, Claude Hammecker, José Romualdo de Sousa Lima, Rômulo Simões Cezar Menezes, Luiz Guilherme Medeiros Pessoa, and et al. 2024. "Impact of Conversion of the Caatinga Forest to Different Land Uses on Soil and Root Respiration Dynamics in the Brazilian Semiarid Region" Sustainability 16, no. 23: 10652. https://doi.org/10.3390/su162310652
APA StyleOresca, D., Souza, E. S. d., Souza, R. M. S., Silva, J. R. I., Moura, D. P. d., Sampaio, E. V. d. S. B., Hammecker, C., Lima, J. R. d. S., Menezes, R. S. C., Pessoa, L. G. M., Ferrão, N. G. d. M., & Antonino, A. C. D. (2024). Impact of Conversion of the Caatinga Forest to Different Land Uses on Soil and Root Respiration Dynamics in the Brazilian Semiarid Region. Sustainability, 16(23), 10652. https://doi.org/10.3390/su162310652







