Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture
Abstract
:1. Introduction
2. Review Motivation and Arrangement of Literature
3. Pesticide Persistence and Sustainable Agriculture
4. Factors Governing Pesticide Degradation and Behavior in Soils
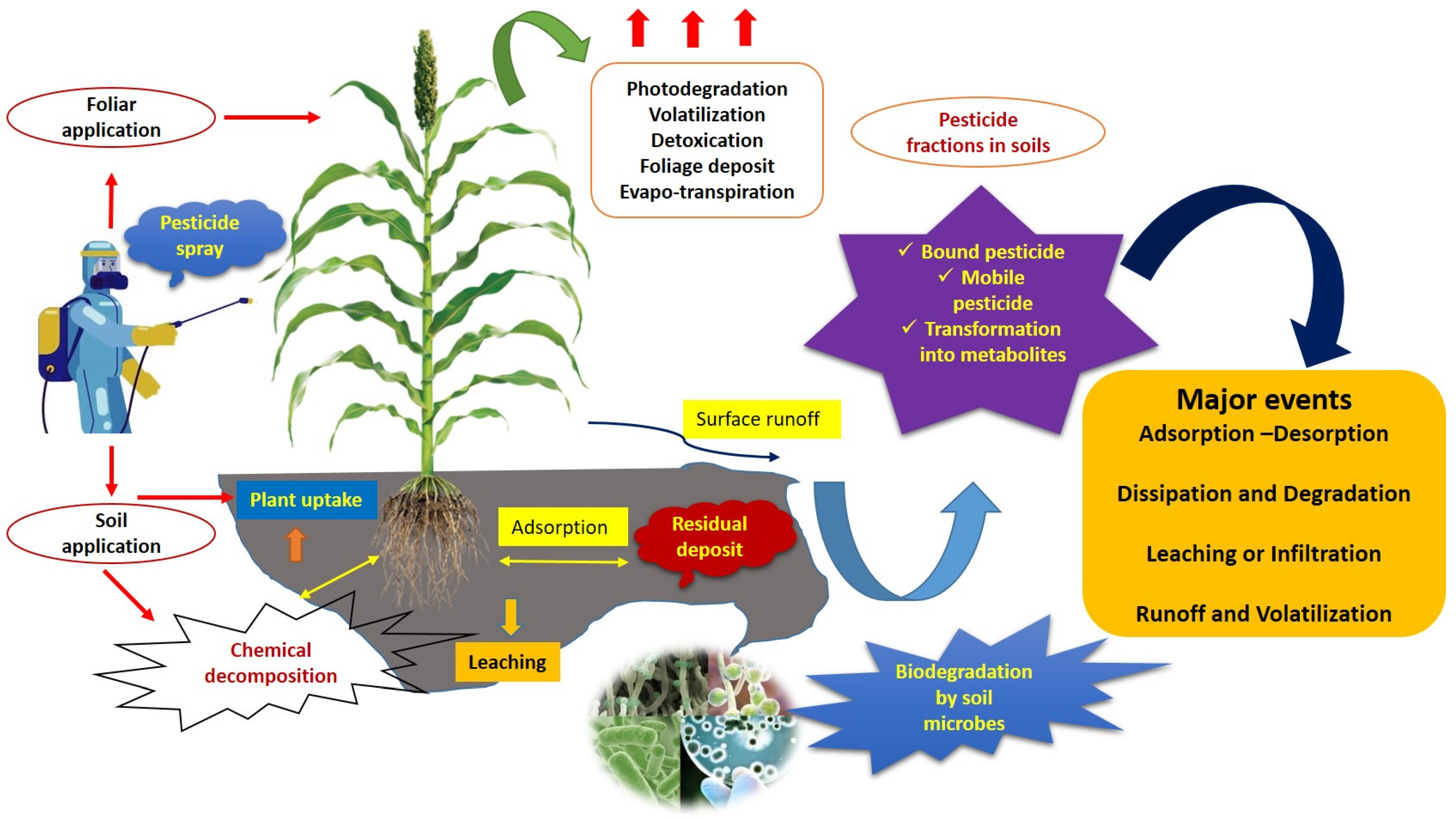
5. Pesticide Chemodynamics and Distribution
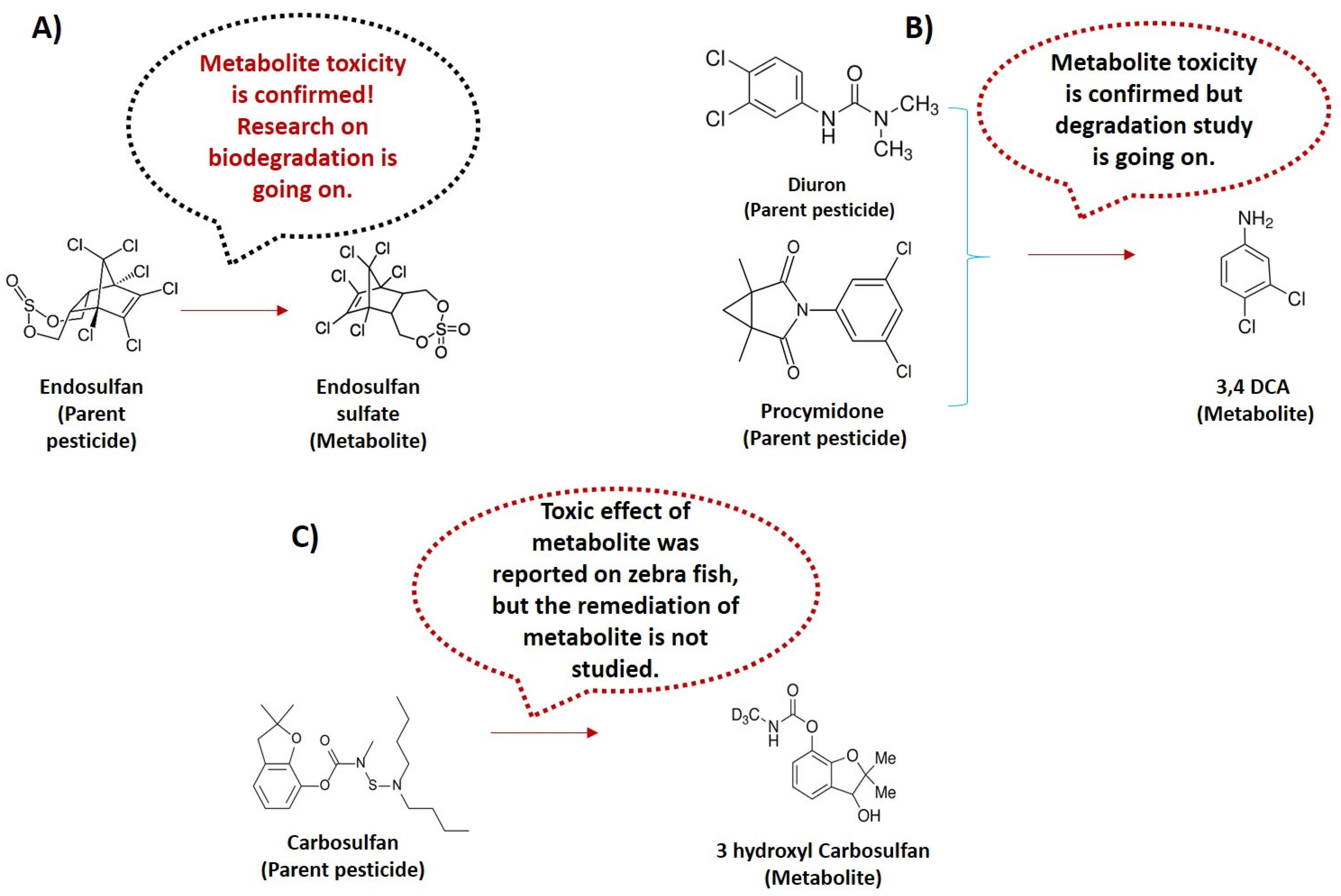
6. Transformation of Metabolites from Parent Pesticide
7. Pesticide Monitoring and Risk Assessment
8. Safety Guidelines for Pesticide and Good Agricultural Practice
9. Sustainable Management of Pesticides in Soils
- Revise the existing MRL and PHI and consider the plant back interval (PBI) (if any) to ensure the least impact of residual pesticides on the following crops on a regional basis. Pesticide behavior is varied due to regional factors and diversified climatic factors. Thus, micro-climatic pesticide monitoring is recommended to address this global concern. In addition, regular synchronization with global regulatory bodies is necessary to augment necessary amendments of safety guidelines regarding pesticide application to enable good agricultural practices (GAP) to be followed;
- Consider the transformation of parent pesticides into toxic metabolites and study the persistence of those toxic metabolites. The parent pesticide may be transformed into toxic metabolites. These toxic metabolites are considered hidden threats to global agriculture and off-target biota due to limited data and meticulous persistence monitoring. The global monitoring of pesticides should be revised to include transformed metabolites and their persistence until complete mineralization or transformation into non-toxic intermediates has occurred to ensure overall pesticide safety guidelines;
- In general, only a small fraction of the pesticide is required to control the target pests, whereas several-fold higher doses can be deposited at the application sites or off-target biota due to conventional applications. As a result, advanced technologies, including controlled released pesticide formulations, adopting integrated pest management (IPM) approach, and pesticide detox mechanisms, should be explored for sustainable management of pesticides in soils. The choice and maintenance of pesticide sprayer machines have a significant impact on spray drift and distribution at the application sites [107]. A technical check, regular calibration, types of spraying machine (e.g., aerial sprayer or machine-operated sprayer), and nozzle design all affect the quality of pesticide distribution. These crucial research gaps should be addressed to achieve sustainable management of pesticides in soils;
- Pesticide-contaminated soils can be managed through green and sustainable strategies, including biochar, biological enzymes, phytoremediations, and nanoremediation. These technologies are environmentally friendly and affordable for large-scale applications. A solitary application and/or combined technologies will be wondrous options for the sustainable management of pesticides. Thus, simultaneous studies can be employed for sustainable remediation of pesticides from contaminated sites using green and affordable approaches;
- A holistic synchronization of global reference laboratory-derived data with the regional analytical data should be a wondrous option for minimizing analytical error and certifying precise analysis of pesticide residues and their behavior at application sites. In recent years, statistical modeling data have become popular for predicting pesticide residue and environmental fate. However, there is a data gap between modeling-derived pesticide data and real-field incurred data. Thus, environmental modeling data should be corroborated with field-incurred data to establish reliable and reproducible analytical data. The overview of sustainable management of pesticide contamination is presented in Figure 9.
10. Conclusions and Future Outlook
- Pesticide fate and degradation patterns are controlled by specific regional conditions and application periods (i.e., season). As a result, seasonal variations in pesticide dissipation may occur at the application field. Additionally, application methods and types of formulation may also govern the pesticide behavior and environmental fate. Thus, spatial and temporal variations in pesticide fate and transport should be considered during safety guideline assessment of pesticide application in the field;
- Poor monitoring of pesticides is another issue that neglects obsolete pesticides and their derivatives. For instance, DDT, endosulfan, and HCH and their derivatives may linger in the soils even decades after their last application. Therefore, regular monitoring is needed to detect traces of obsolete pesticides and their toxic derivatives in soils. To minimize the uncertainty of the persistence of pesticides in the soil, poor monitoring of pesticides must be improved and synchronized with global pesticide safety policy updates;
- Lack of public awareness and consciousness about the safe use of pesticides on farming land and ignorance of personal safety measures is another crucial concern with respect to developing countries. In general, farmers and end-users of pesticides neglect the safety measures during pesticide spraying and have limited access to the right information regarding MRL, recommended dose of pesticides, proper disposal of empty pesticide containers, and the use of personal protection equipment (PPE). In particular, the point source of pesticides from sprayers, disposed empty containers, and spills during handling are underestimated, as reported by the TOPPS–Life project [108]. Eventually, that negligence will emerge as the key factor for point source contamination of waters by plant protection products (PPP). Thus, increasing public awareness by providing proper information about safety guidelines for pesticides and the training of farmers should be included;
- Due to persistence and higher toxicity, several old formulations of pesticides have been banned or restricted for extensive use on farming lands. The rejection of such obsolete pesticide formulations was the initial step toward the sustainability of global agriculture and the introduction of new/novel formulations of pesticides, which have non-persistence and lower toxicity to mammals;
- During pesticide fate and transport studies, the major events, including environmental fate (e.g., adsorption, dissipation, degradation, plant uptake, and soil deposit), transport of pesticide (e.g., leaching, surface runoff, and sub-surface seepage), and loss of pesticide (e.g., photodegradation, volatilization, detoxification, and evapotranspiration), should be considered.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and Distribution of Pesticide Residues in Soil: Non-Dietary Human Health Risk Assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Nandi, R.; Kim, J.E.; Islam, T. Remediation of chemical pesticides from contaminated sites through potential microorganisms and their functional enzymes: Prospects and challenges. Environ. Technol. Innov. 2021, 23, 101777. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.; Adeyi, O.; Arnold, R.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; Breysse, P.N.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Shin, W.S.; Masud, M.A.A.; Nandi, R.; Islam, T. A critical review of sustainable pesticide remediation in contaminated sites: Research challenges and mechanistic insights. Environ. Pollut. 2024, 341, 122940. [Google Scholar] [CrossRef]
- Nandi, R.; Kwak, S.Y.; Lee, S.H.; Sarker, A.; Kim, H.J.; Lee, D.J.; Heo, Y.J.; Kyung, K.S.; Kim, J.E. Dissipation Characteristics of Spirotetramat and Its Metabolites in Two Phenotypically Different Korean Vegetables under Greenhouse Conditions. Food Addit. Contam. Part A 2022, 39, 964–976. [Google Scholar] [CrossRef]
- Heshmati, A.; Nili-Ahmadabadi, A.; Rahimi, A.; Vahidinia, A.; Taheri, M. Dissipation Behavior and Risk Assessment of Fungicide and Insecticide Residues in Grape under Open-Field, Storage and Washing Conditions. J. Clean. Prod. 2020, 270, 122287. [Google Scholar] [CrossRef]
- Amekawa, Y.; Bumrungsri, S.; Wayo, K.; Gebre, G.G.; Hongsibsong, S. Pesticide Use under Public Good Agricultural Practices Standard: A Comparative Study in Thailand. Agriculture 2022, 12, 606. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kwak, S.Y.; Sarker, A.; Lee, S.H.; Choi, J.W.; Oh, J.E.; Abdulkareem, L.; Kim, J.E. Uptake and translocation of fungicide picarbutrazox in greenhouse cabbage: The significance of translocation factors and home processing. Environ. Sci. Pollut. Res. 2023, 30, 40919–40930. [Google Scholar] [CrossRef]
- Sarker, A.; Yoo, J.H.; Jeong, W.T. Environmental fate and metabolic transformation of two non-ionic pesticides in soil: Effect of biochar, moisture, and soil sterilization. Chemosphere 2023, 345, 140458. [Google Scholar] [CrossRef]
- Anagnostopoulou, K.; Nannou, C.; Evgenidou, E.; Lambropoulou, D. Overarching Issues on Relevant Pesticide Transformation Products in the Aquatic Environment: A Review. Sci. Total Environ. 2022, 815, 152863. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Lee, S.H.; Kwak, S.Y.; Nam, A.J.; Kim, H.J.; Kim, J.E. Residue Monitoring and Risk Assessment of Cyazofamid and Its Metabolite in Korean Cabbage Under Greenhouse Conditions. Bull. Environ. Contam. Toxicol. 2020, 105, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Sara Taha, A.; Sundas, R.Q.; Man-Qun, W. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Islam, T.; Rahman, S.; Nandi, R.; Kim, J.E. Uncertainty of pesticides in foodstuffs, associated environmental and health risks to humans—A critical case of Bangladesh with respect to global food policy. Environ. Sci. Pollut. Res. 2021, 28, 54448–54465. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Istriningsih; Dewi, Y.A.; Yulianti, A.; Hanifah, V.W.; Jamal, E.; Dadang; Sarwani, M.; Mardiharini, M.; Anugrah, I.S.; Darwis, V.; et al. Farmers’ Knowledge and Practice Regarding Good Agricultural Practices (GAP) on Safe Pesticide Usage in Indonesia. Heliyon 2022, 8, e08708. [Google Scholar] [CrossRef]
- Leong, W.H.; Teh, S.Y.; Hossain, M.M.; Nadarajaw, T.; Zabidi-Hussin, Z.; Chin, S.Y.; Lai, K.S.; Lim, S.H.E. Application, Monitoring and Adverse Effects in Pesticide Use: The Importance of Reinforcement of Good Agricultural Practices (GAPs). J. Environ. Manag. 2020, 260, 109987. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, D.; Kumari, A.; Sharma, G.; Rajput, S.; Arora, S.; Kaur, R. Pesticide Residues Degradation Strategies in Soil and Water: A Review. Int. J. Environ. Sci. Technol. 2023, 20, 3537–3560. [Google Scholar] [CrossRef]
- SANTE. Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-Approval Control and Monitoring Purposes. SANTE/2000/12830, Rev.1. 2021. Available online: https://food.ec.europa.eu/system/files/2021-03/pesticides_ppp_app-proc_guide_res_mrl-guidelines-2020-12830.pdf (accessed on 15 September 2024).
- Baran, N.; Rosenbom, A.E.; Kozel, R.; Lapworth, D. Pesticides and their metabolites in European groundwater: Comparing regulations and approaches to monitoring in France, Denmark, England and Switzerland. Sci. Total Environ. 2022, 842, 156696. [Google Scholar] [CrossRef]
- Crépet, A.; Luong, T.M.; Baines, J.; Boon, P.E.; Ennis, J.; Kennedy, M.; Massarelli, I.; Miller, D.; Nako, S.; Reuss, R.; et al. An International Probabilistic Risk Assessment of Acute Dietary Exposure to Pesticide Residues in Relation to Codex Maximum Residue Limits for Pesticides in Food. Food Control 2021, 121, 107563. [Google Scholar] [CrossRef]
- IRAC. The IRAC Mode of Action Classification. 2024. Available online: https://irac-online.org/modes-of-action/ (accessed on 20 August 2024).
- Kaur, S.; Chowdhary, S.; Kumar, D.; Bhattacharyya, R.; Banerjee, D. Organophosphorus and Carbamate Pesticides: Molecular Toxicology and Laboratory Testing. Clin. Chim. Acta 2023, 551, 117584. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.R.; Shah, J.; Khawaja, M.A.; Gul, K. DDT Residue in Soil and Water in and around Abandoned DDT Manufacturing Factory. Environ. Monit. Assess. 2009, 155, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yun, X.; Ruan, Z.; Lu, C.; Shi, Y.; Qin, Q.; Men, Z.; Zou, D.; Du, X.; Xing, B.; et al. Review of Hexachlorocyclohexane (HCH) and Dichlorodiphenyltrichloroethane (DDT) Contamination in Chinese Soils. Sci. Total Environ. 2020, 749, 141212. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, Y.; Chen, W.; Chen, W.; Yuen, D.A.; Ding, Y.; Chen, Y.; Mao, Y.; Qi, S. Sources and Transformation Pathways for Dichlorodiphenyltrichloroethane (DDT) and Metabolites in Soils from Northwest Fujian, China. Environ. Pollut. 2018, 235, 560–570. [Google Scholar] [CrossRef]
- Tsiantas, P.; Tzanetou, E.N.; Karasali, H.; Kasiotis, K.M. A Dieldrin Case Study: Another Evidence of an Obsolete Substance in the European Soil Environment. Agriculture 2021, 11, 314. [Google Scholar] [CrossRef]
- Hensen, B.; Olsson, O.; Kümmerer, K. The Role of Irradiation Source Setups and Indirect Phototransformation: Kinetic Aspects and the Formation of Transformation Products of Weakly Sunlight-Absorbing Pesticides. Sci. Total Environ. 2019, 695, 133808. [Google Scholar] [CrossRef]
- Hintze, S.; Glauser, G.; Hunkeler, D. Influence of Surface Water—Groundwater Interactions on the Spatial Distribution of Pesticide Metabolites in Groundwater. Sci. Total Environ. 2020, 733, 139109. [Google Scholar] [CrossRef]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound Pesticide Residues in Soils: A Review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef]
- Boesten, J.J.T.I. Proposal for Field-Based Definition of Soil Bound Pesticide Residues. Sci. Total Environ. 2016, 544, 114–117. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade Impact of Maximum Residue Limits in Fresh Fruits and Vegetables. Food Policy 2022, 106, 102203. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; Alghamdi, A.G. Identification of Pesticide Residues and Prediction of Their Fate in Agricultural Soil. Water Air Soil Pollut. 2020, 231, 284. [Google Scholar] [CrossRef]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Brown, C.D. Prediction of the Adsorption of Lonizable Pesticides in Soils. J. Agric. Food Chem. 2007, 55, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Rice, P.J.; Arthur, E.L.; Barefoot, A.C. Advances in Pesticide Environmental Fate and Exposure Assessments. J. Agric. Food Chem. 2007, 55, 5367–5376. [Google Scholar] [CrossRef]
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef]
- Yue, L.; Ge, C.J.; Feng, D.; Yu, H.; Deng, H.; Fu, B. Adsorption–Desorption Behavior of Atrazine on Agricultural Soils in China. J. Environ. Sci. 2017, 57, 180–189. [Google Scholar] [CrossRef]
- Medina-Pastor, P.; Triacchini, G. The 2018 European Union Report on Pesticide Residues in Food. EFSA J. 2020, 18, e06057. [Google Scholar] [CrossRef]
- Lewis, S.E.; Silburn, D.M.; Kookana, R.S.; Shaw, M. Pesticide Behavior, Fate, and Effects in the Tropics: An Overview of the Current State of Knowledge. J. Agric. Food Chem. 2016, 64, 3917–3924. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhao, Q.; Wang, C.; Cui, Y.; Li, J.; Chen, A.; Liang, G.; Jiao, B. Occurrence, Temporal Variation, Quality and Safety Assessment of Pesticide Residues on Citrus Fruits in China. Chemosphere 2020, 258, 127381. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A Worldwide Review of Currently Used Pesticides’ Monitoring in Agricultural Soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Zhang, S.; Yang, H. Acceleration of the Herbicide Isoproturon Degradation in Wheat by Glycosyltransferases and Salicylic Acid. J. Hazard. Mater. 2015, 283, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate Pesticides an Emerging Environmental Contaminant: Pollution, Toxicity, Bioremediation Progress, and Remaining Challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Delgado-Moreno, L.; Rodríguez-Liébana, J.A. A Review of the Impact of Wastewater on the Fate of Pesticides in Soils: Effect of Some Soil and Solution Properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef]
- Park, B.K.; Joo, K.S.; Heo, M.J. Evaluation of Pesticide Residues in Vegetables and Risk Assessment from Incheon, Korea. Environ. Sci. Pollut. Res. 2023, 30, 43795–43803. [Google Scholar] [CrossRef]
- Liang, Z.; Mahmoud Abdelshafy, A.; Luo, Z.; Belwal, T.; Lin, X.; Xu, Y.; Wang, L.; Yang, M.; Qi, M.; Dong, Y.; et al. Occurrence, Detection, and Dissipation of Pesticide Residue in Plant-Derived Foodstuff: A State-of-the-Art Review. Food Chem. 2022, 384, 132494. [Google Scholar] [CrossRef]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of Glyphosate and Aminomethylphosphonic Acid in Loess Soil under Different Combinations of Temperature, Soil Moisture and Light/Darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef]
- Barbieri, M.V.; Peris, A.; Postigo, C.; Moya-Garcés, A.; Monllor-Alcaraz, L.S.; Rambla-Alegre, M.; Eljarrat, E.; López de Alda, M. Evaluation of the Occurrence and Fate of Pesticides in a Typical Mediterranean Delta Ecosystem (Ebro River Delta) and Risk Assessment for Aquatic Organisms. Environ. Pollut. 2021, 274, 115813. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Bilal, M.; Singh, A.K.; Iqbal, H.M.N.; Kim, T.H.; Boczkaj, G.; Athmaneh, K.; Ashraf, S.S. Bio-Mitigation of Organic Pollutants Using Horseradish Peroxidase as a Promising Biocatalytic Platform for Environmental Sustainability. Environ. Res. 2023, 239, 117192. [Google Scholar] [CrossRef]
- Chai, L.K.; Wong, M.H.; Hansen, H.C.B. Degradation of Chlorpyrifos in Humid Tropical Soils. J. Environ. Manag. 2013, 125, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Zuo, H.G.; Ding, Y.J.; Miao, S.S.; Jiang, C.; Yang, H. Biotic and Abiotic Degradation of Pesticide Dufulin in Soils. Environ. Sci. Pollut. Res. 2014, 21, 4331–4342. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, A.M. A Review of Environmental and Health Effects of Organochlorine Pesticide Residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Yigit, N.; Velioglu, Y.S. Effects of Processing and Storage on Pesticide Residues in Foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 3622–3641. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Xie, D.; Chen, C.; Li, C.; Wang, Q. Influence of Cd on Atrazine Degradation and the Formation of Three Primary Metabolites in Water under the Combined Pollution. Environ. Sci. Pollut. Res. 2021, 28, 16081–16091. [Google Scholar] [CrossRef]
- Supreeth, M.; Raju, N. Biotransformation of Chlorpyrifos and Endosulfan by Bacteria and Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 5961–5971. [Google Scholar] [CrossRef]
- Sarker, A.; Lee, S.H.; Kwak, S.Y.; Nandi, R.; Kim, J.E. Comparative catalytic degradation of a metabolite 3,5-dichloroaniline derived from dicarboximide fungicide by laccase and MnO2 mediators. Ecotoxicol. Environ. Saf. 2020, 196, 110561. [Google Scholar] [CrossRef]
- Riise, G.; Lundekvam, H.; Wu, Q.L.; Haugen, L.E.; Mulder, J. Loss of Pesticides from Agricultural Fields in SE Norway—Runoff through Surface and Drainage Water. Environ. Geochem. Health 2004, 26, 269–276. [Google Scholar] [CrossRef]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Butkovskyi, A.; Jing, Y.; Bergheim, H.; Lazar, D.; Gulyaeva, K.; Odenmarck, S.R.; Norli, H.R.; Nowak, K.M.; Miltner, A.; Kästner, M.; et al. Retention and Distribution of Pesticides in Planted Filter Microcosms Designed for Treatment of Agricultural Surface Runoff. Sci. Total Environ. 2021, 778, 146114. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Lee, S.G.; Jang, J.R. Vegetative Filter Strip (Vfs) Applications for Runoff and Pollution Management in the Saemangeum Area of Korea. Irrig. Drain. 2016, 65, 168–174. [Google Scholar] [CrossRef]
- Cui, J.; Wang, F.; Gao, J.; Zhai, W.; Zhou, Z.; Liu, D.; Wang, P. Bioaccumulation and Metabolism of Carbosulfan in Zebrafish (Danio rerio) and the Toxic Effects of Its Metabolites. J. Agric. Food Chem. 2019, 67, 12348–12356. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Vela, N.; Navarro, G. Review. An Overview on the Environmental Behaviour of Pesticide Residues in Soils. Span. J. Agric. Res. 2007, 5, 357–375. [Google Scholar] [CrossRef]
- El-Aswad, A.F.; Fouad, M.R.; Badawy, M.E.I.; Aly, M.I. Effect of Calcium Carbonate Content on Potential Pesticide Adsorption and Desorption in Calcareous Soil. Commun. Soil Sci. Plant Anal. 2023, 54, 1379–1387. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A Review of the Global Pesticide Legislation and the Scale of Challenge in Reaching the Global Harmonization of Food Safety Standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mohan, K.; Ganesan, A.R.; Govarthanan, M.; Yusoff, A.R.M.; Gu, F.L. Persistence, toxicological effect and ecological issues of endosulfan—A review. J. Hazard. Mater. 2021, 416, 125779. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, S.Y.; Sarker, A.; Moon, J.K.; Kim, J.E. Optimization of a Multi-Residue Analytical Method during Determination of Pesticides in Meat Products by GC-MS/MS. Foods 2022, 11, 2930. [Google Scholar] [CrossRef]
- Afshari, M.; Karimi-Shahanjarini, A.; Khoshravesh, S.; Besharati, F. Effectiveness of Interventions to Promote Pesticide Safety and Reduce Pesticide Exposure in Agricultural Health Studies: A Systematic Review. PLoS ONE 2021, 16, e0245766. [Google Scholar] [CrossRef]
- Barchanska, H.; Sajdak, M.; Szczypka, K.; Swientek, A.; Tworek, M.; Kurek, M. Atrazine, Triketone Herbicides, and Their Degradation Products in Sediment, Soil and Surface Water Samples in Poland. Environ. Sci. Pollut. Res. 2017, 24, 644–658. [Google Scholar] [CrossRef]
- Pan, L.; Feng, X.; Cao, M.; Zhang, S.; Huang, Y.; Xu, T.; Jing, J.; Zhang, H. Determination and Distribution of Pesticides and Antibiotics in Agricultural Soils from Northern China. RSC Adv. 2019, 9, 15686–15693. [Google Scholar] [CrossRef]
- Han, Y.; Mo, R.; Yuan, X.; Zhong, D.; Tang, F.; Ye, C.; Liu, Y. Pesticide Residues in Nut-Planted Soils of China and Their Relationship between Nut/Soil. Chemosphere 2017, 180, 42–47. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, K.G.; Choi, E.A.; Kang, G.R.; Kim, T.S.; Yang, Y.S.; Moon, S.J.; Ha, D.R.; Kim, E.S.; Cho, B.S. Pesticide Residues in Leafy Vegetables, Stalk and Stem Vegetables from South Korea: A Long-Term Study on Safety and Health Risk Assessment. Food Addit. Contam. Part A 2015, 33, 105–118. [Google Scholar] [CrossRef]
- Park, B.K.; Kwon, S.H.; Yeom, M.S.; Joo, K.S.; Heo, M.J. Detection of Pesticide Residues and Risk Assessment from the Local Fruits and Vegetables in Incheon, Korea. Sci. Rep. 2022, 12, 9613. [Google Scholar] [CrossRef]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, Pesticides, Personal Care Products and Microplastics Contamination Assessment of Al-Hassa Irrigation Network (Saudi Arabia) and Its Shallow Lakes. Sci. Total Environ. 2020, 701, 135021. [Google Scholar] [CrossRef]
- Murugan, A.V.; Swarnam, T.P.; Gnanasambandan, S. Status and Effect of Pesticide Residues in Soils under Different Land Uses of Andaman Islands, India. Environ. Monit. Assess. 2013, 185, 8135–8145. [Google Scholar] [CrossRef]
- Siddique, Z.; Malik, A.U.; Asi, M.R.; Inam-ur-Raheem, M.; Iqbal, M.; Abdullah, M. Impact of Sonolytic Ozonation (O3/US) on Degradation of Pesticide Residues in Fresh Vegetables and Fruits: Case Study of Faisalabad, Pakistan. Ultrason. Sonochem. 2021, 79, 105799. [Google Scholar] [CrossRef]
- Rahman, M.; Hoque, M.S.; Bhowmik, S.; Ferdousi, S.; Kabiraz, M.P.; van Brakel, M.L. Monitoring of Pesticide Residues from Fish Feed, Fish and Vegetables in Bangladesh by GC-MS Using the QuEChERS Method. Heliyon 2021, 7, e06390. [Google Scholar] [CrossRef]
- Obana, H. Determination of Neonicotinoid Pesticide Residues in Chromatography Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 2501–2505. [Google Scholar] [CrossRef]
- Del Prado-Lu, J.L. Insecticide Residues in Soil, Water, and Eggplant Fruits and Farmers’ Health Effects Due to Exposure to Pesticides. Environ. Health Prev. Med. 2015, 20, 53–62. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and Recently Used Pesticides in Central European Arable Soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide Residues in European Agricultural Soils—A Hidden Reality Unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Lehotay, S.J.; Geis-Asteggiante, L.; Kwon, H.; Mol, H.G.J.; van der Kamp, H.; Mateus, N.; Domingues, V.F.; Delerue-Matos, C. Analysis of Pesticide Residues in Strawberries and Soils by GC-MS/MS, LC-MS/MS and Two-Dimensional GC-Time-of-Flight MS Comparing Organic and Integrated Pest Management Farming. Food Addit. Contam. Part A 2014, 31, 262–270. [Google Scholar] [CrossRef]
- Karanasios, E.; Karasali, H.; Marousopoulou, A.; Akrivou, A.; Markellou, E. Monitoring of Glyphosate and AMPA in Soil Samples from Two Olive Cultivation Areas in Greece: Aspects Related to Spray Operators Activities. Environ. Monit. Assess. 2018, 190, 361. [Google Scholar] [CrossRef]
- Aznar, R.; Moreno-Ramón, H.; Albero, B.; Sánchez-Brunete, C.; Tadeo, J.L. Spatio-Temporal Distribution of Pyrethroids in Soil in Mediterranean Paddy Fields. J. Soils Sediments 2017, 17, 1503–1513. [Google Scholar] [CrossRef]
- Villanneau, E.J.; Saby, N.P.A.; Marchant, B.P.; Jolivet, C.C.; Boulonne, L.; Caria, G.; Barriuso, E.; Bispo, A.; Briand, O.; Arrouays, D. Which Persistent Organic Pollutants Can We Map in Soil Using a Large Spacing Systematic Soil Monitoring Design? A Case Study in Northern France. Sci. Total Environ. 2011, 409, 3719–3731. [Google Scholar] [CrossRef]
- Chiaia-Hernandez, A.C.; Keller, A.; Wächter, D.; Steinlin, C.; Camenzuli, L.; Hollender, J.; Krauss, M. Long-Term Persistence of Pesticides and TPs in Archived Agricultural Soil Samples and Comparison with Pesticide Application. Environ. Sci. Technol. 2017, 51, 10642–10651. [Google Scholar] [CrossRef]
- Muendo, B.M.; Lalah, J.O.; Getenga, Z.M. Behavior of Pesticide Residues in Agricultural Soil and Adjacent River Kuywa Sediment and Water Samples from Nzoia Sugarcane Belt in Kenya. Environmentalist 2012, 32, 433–444. [Google Scholar] [CrossRef]
- Omeje, J.S.; Asegbeloyin, J.N.; Ihedioha, J.N.; Ekere, N.R.; Ochonogor, A.E.; Abugu, H.O.; Alum, O.L. Monitoring of Pesticide Residues in Fresh Fruits and Vegetables Available in Nigerian Markets and Assessment of Their Associated Health Risks. Environ. Monit. Assess. 2022, 194, 516. [Google Scholar] [CrossRef]
- Dankyi, E.; Gordon, C.; Carboo, D.; Fomsgaard, I.S. Quantification of Neonicotinoid Insecticide Residues in Soils from Cocoa Plantations Using a QuEChERS Extraction Procedure and LC-MS/MS. Sci. Total Environ. 2014, 499, 276–283. [Google Scholar] [CrossRef]
- Ngabirano, H.; Birungi, G. Pesticide Residues in Vegetables Produced in Rural South-Western Uganda. Food Chem. 2022, 370, 130972. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Shalaby, S.E.M. Screening and Assessing of Pesticide Residues and Their Health Risks in Vegetable Field Soils from the Eastern Nile Delta, Egypt. Toxicol. Rep. 2022, 9, 1281–1290. [Google Scholar] [CrossRef]
- Mutengwe, M.T.; Chidamba, L.; Korsten, L. Monitoring Pesticide Residues in Fruits and Vegetables at Two of the Biggest Fresh Produce Markets in Africa. J. Food Prot. 2016, 79, 1938–1945. [Google Scholar] [CrossRef]
- Loha, K.M.; Lamoree, M.; de Boer, J. Pesticide Residue Levels in Vegetables and Surface Waters at the Central Rift Valley (CRV) of Ethiopia. Environ. Monit. Assess. 2020, 192, 546. [Google Scholar] [CrossRef]
- Sousa, E.S.; Pinto, L.; de Araujo, M.C.U. A Chemometric Cleanup Using Multivariate Curve Resolution in Liquid Chromatography: Quantification of Pesticide Residues in Vegetables. Microchem. J. 2017, 134, 131–139. [Google Scholar] [CrossRef]
- Mac Loughlin, T.M.; Peluso, M.L.; Etchegoyen, M.A.; Alonso, L.L.; de Castro, M.C.; Percudani, M.C.; Marino, D.J.G. Pesticide Residues in Fruits and Vegetables of the Argentine Domestic Market: Occurrence and Quality. Food Control 2018, 93, 129–138. [Google Scholar] [CrossRef]
- Calderon, R.; García-Hernández, J.; Palma, P.; Leyva-Morales, J.B.; Zambrano-Soria, M.; Bastidas-Bastidas, P.J.; Godoy, M. Assessment of Pesticide Residues in Vegetables Commonly Consumed in Chile and Mexico: Potential Impacts for Public Health. J. Food Compos. Anal. 2022, 108, 104420. [Google Scholar] [CrossRef]
- deNux, C.; Hou, A.; Fultz, L. Evaluation of Organic and Synthetic Herbicide Applications on Weed Suppression in a Conventional Cropping System in Louisiana. Sustainability 2024, 16, 3019. [Google Scholar] [CrossRef]
- Conrad, S.R.; White, S.A.; Santos, I.R.; Sanders, C.J. Assessing Pesticide, Trace Metal, and Arsenic Contamination in Soils and Dam Sediments in a Rapidly Expanding Horticultural Area in Australia. Environ. Geochem. Health 2021, 43, 3189–3211. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Moura, A.C.M.; Lago, I.N.; Cardoso, C.F.; dos Reis Nascimento, A.; Pereira, I.; Vaz, B.G. Rapid Monitoring of Pesticides in Tomatoes (Solanum lycopersicum L.) during Pre-Harvest Intervals by Paper Spray Ionization Mass Spectrometry. Food Chem. 2020, 310, 125938. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.H.; Kim, G.S. Impact of the Positive List System (PLS) on the Banana Market in Korea. J. Asia Pac. Econ. 2020, 25, 718–732. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Z.; Fantke, P. Improved Plant Bioconcentration Modeling of Pesticides: The Role of Periderm Dynamics. Pest Manag. Sci. 2021, 77, 5096–5108. [Google Scholar] [CrossRef] [PubMed]
- Directive 2009/128/EC of the European Parliament and of the Council Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; Official Journal of the European Union L 309; European Union: Brussels, Belgium, 24 November 2009; pp. 71–86.
- Directive 2009/127/EC of the European Parliament and of the Council of 21 October 2009 Amending Directive 2006/42/EC with Regard to Machinery for Pesticide Application; European Union: Brussels, Belgium, 2009.
- TOPPS Life Project. Available online: https://www.topps-life.org/topps-life-project.html (accessed on 24 November 2024).







| Region | Country | Study Site | Studied Pesticides | Analytical Method | Salient Findings | Reference |
|---|---|---|---|---|---|---|
| Asia | China | Vegetable fields and orchards near a river basin in North China | Atrazine, chlorpyrifos, tebuconazole, thiamethoxam, pymetrozine, and difenoconazole | Extraction by QuEChERS and analysis by HPLC-MS/MS | Among 47 studied pesticides, 6 pesticides showed higher concentrations | [73] |
| China | Three soil samples of a nut-growing region in China | Six organochlorines, one organophosphate, and six pyrethroid group pesticides | A simple methanol extraction, followed by GC-ECD analysis | Among 29 pesticides, organochlorine was detected in 78.9% of soils and pyrethroid in 65.8% | [74] | |
| Korea | Minor and leafy vegetables from the Gwangju and Jeonnam regions of Korea | A multi-residue pesticide analysis strategy for 230 pesticides was performed | Modified QuEChERS extraction, followed by GC-MS and LC-MS/MS analysis | Among the samples, 1.4% of vegetables exceeded the Korean MRL and posed a dietary risk to Korean consumers | [75] | |
| Korea | Twenty types of fruit and vegetables were collected from the local market in Incheon (Korea) | Multi-residue pesticide analysis comprising the currently used pesticides (CUPs) in Korea | HPLC, GC-ECD, GC-MS/MS, LC-MS/MS analysis for multi-pesticide analysis | Chlorfenapyr, procymidone, etofenprox, pendimethalin, fluopyram, and azoxystrobin were found to be the most detected pesticides | [76] | |
| Nepal | Agricultural farms practicing traditional farming and IPM | Seven organophosphate and eight organochlorine pesticides | QuEChERS extraction, followed by LC-MS/MS analysis | IPM-derived soil showed negligible dietary health risks as compared to conventional farming soil | [1] | |
| Saudi Arabia | Drainage channel, lagoon wetland in the Arabian Gulf and eastern province of Saudi Arabia | Multi-residue pesticide analysis strategy | SPE extraction, methanol washing through an ultrasonic bath, followed by UHPLC-MS/MS analysis | Among the pesticides, chlorpyrifos, diazinon, and bifenthrin showed a risk to the studied biota | [77] | |
| India | Soil samples from different land uses on Andaman Island, India | Multi-pesticide residue analysis protocol from the soil was used | A robust QuEChERS extraction, followed by GC-MS analysis | Detection of endosulfan and DDT (41.7%), followed by Aldrin (16.7%), in the Andaman Island soil samples | [78] | |
| Pakistan | Local market-derived vegetables and fruits in Faisalabad, Pakistan | Thiamethoxam, imidacloprid, acetamiprid, thiacloprid, and carbendazim | HPLC-UVD and LC-MS/MS analysis for simultaneous pesticide residues | Local market vegetables and fruits were contaminated with pesticides and exceeded the Codex MRL | [79] | |
| Bangladesh | Vegetables, fish, and fish feed collected from a local market in Bangladesh | 17 organochlorine, 5 pyrethroid, and 3 organophosphate pesticides | QuEChERS extraction and GC-MS analysis for simultaneous pesticide detection | Among the studied samples, the majority of vegetables and a few fish samples showed pesticides exceeding MRL | [80] | |
| Japan | A total of 12 vegetable and fruit samples collected in Osaka, Japan | Five neonicotinoids (Imidacloprid, thiacloprid, acetamiprid, thiamethoxam, and nitenpyram) | Methanol extraction, SPE clean-up, followed by LC-MS analysis | Five pesticides showed acceptable recovery (70–95%) during the 0.1 and 1.0 mg/kg spiking test | [81] | |
| Philippines | Topsoil, water, and eggplant fruit samples collected from the eggplant farm in Pangasinan | Malathion, cypermetrhin, chlorpyrifos, profenfos, and triazophos | Acetonitrile-based SPE extraction, followed by GC-ECD or GC-NPD analysis | Among the studied samples, eggplant fruit showed the highest concentration of pesticides, followed by plant and water samples | [82] | |
| Europe | Czech Republic | Seventy-five arable soil samples collected from central Europe (Czech Republic) | A total of 53 pesticides and 15 transformation products (metabolites) | QuEChERS extraction, followed by LC-MS/MS analysis | Triazines and conazoles were the most detected pesticides, followed by simazine and their transformation products | [83] |
| 10 EU countries | Various crop samples such as cereal, root crops, vegetables, fruits, and beans from 10 EU countries | Multi-pesticides, including organochloride, organophosphate, pyrethroids, and triazole pesticides | QuEChERS extractions and LC-MS/MS, GC-HRMS analysis | Twenty percent of total eggplant samples tested positive for pesticides and posed a risk to human health due to continuous exposure to pesticide-contaminated fruit of eggplant | [84] | |
| Portugal | Strawberries are grown under organic and IPM farming technology | A total of 170 targeted pesticides through a multi-pesticide analysis strategy | QuEChERS, followed by GC-MS/MS and LC-MS/MS analysis | Strawberries from organic farming had no detectable pesticides, while nine pesticides were detected in IPM samples | [85] | |
| Greece | Soil samples from the olive farm located in Southern Greece | Glyphosate and its primary metabolite AMPA | QuEChERS extraction, followed by LC-MS/MS | Longer persistence of primary metabolite AMPA was evident over the parent glyphosate | [86] | |
| Spain | A total of 33 rice field sites during the rice production period | A total of 10 pyrethroid insecticides | Ultrasonic extraction followed by GC-MS analysis | The irrigation from the wastewater treatment plant was determined to be the pesticide contamination source in the paddy field | [87] | |
| Poland | Sediment, soil, and surface water from agricultural and forest field | Atrazine and triketone herbicides, including their metabolites | Acetonitrile extraction and HPLC-DAD analysis | Atrazine was not detected in the soil samples, but the transformation product was still detected (41%) in the soil | [72] | |
| France | Forest, agricultural soils, and grassland | Persistent organic pollutants (POPs) such as DDT, DDD, lindane | Acetone-assisted pressure liquid extraction and HPLC-MS | The wide transport of POPs was documented in the study regions | [88] | |
| Switzerland | Archived farming soils, orchards, vineyards | A total of 80 polar pesticides and 90+ transformation products | Pressurized liquid extraction and LC-HRMS analysis | A decade-long study noticed the transformation of 50% of parent pesticides into transformation products (TP) | [89] | |
| Africa | Kenya | Nzoia sugarcane belt sub-catchment water samples and soil samples adjacent to a river | Organochlorine pesticides and herbicides | Soxhlet extraction, followed by GC-MS and LC-MS analysis | The concentrations of some detected pesticides crossed the limit of EU-MRL | [90] |
| Nigeria | Vegetables and fruits available at the Nigerian market | Organochlorine and organophosphate group pesticides | DCM extraction and florisil clean-up, followed by GC-FPD analysis | Among the 38 tested pesticides, the levels of six pesticides were found to be over the MRL | [91] | |
| Ghana | Soil samples collected from cocoa plantation farm | Neonicotinoid pesticides | QuEChERS extraction, followed by LC-MS/MS | Multi-residue analysis was optimized for neonicotinoid determination | [92] | |
| Uganda | Fresh vegetables from the rural region in southwest Kabale district | Cypermethrin, dimethoate, malathion, metalaxyl, profenofos, dichlorvos, and mancozeb | AOAC suggested QuEChERS, followed by LC-MS/MS and GC-MS/MS analysis | The terminal residue was found to be over the MRL in sprayed and market-derived vegetable samples | [93] | |
| Egypt | Arable soils from vegetable fields near the Eastern Nile Delta region | Multi-pesticide analysis targeting 33 compounds through simultaneous analysis | QuEChERS extraction and LC-MS/MS and GC-MS/MS analysis | Chlorpyrifos and propamocarb were found to be the most detected residual pesticides | [94] | |
| South Africa | Fresh fruits and vegetables collected from the biggest South African market | A total of 74 pesticides are commonly used in the vegetables of African agriculture. | Modified QuEChERS extraction and analysis by GC-ECD | Boscalid, endosulfan, profenofos, and procymidone exceeded the national MRL | [95] | |
| Ethiopia | Vegetables and surface water near the Rift Valley in Ethiopia | DDT, α-cyhalothrin, profenofos, metalaxyl, β-endosulfan | QuEChERS extraction and analysis by GC-MS/MS | Approximately 2–12% of samples exceeded the EU MRL, and regular monitoring was suggested. | [96] | |
| USA | Brazil | Fresh vegetables and fruits from respective regions in Brazil | Carbaryl, carbofuran, carbendazim, flutriafol, fuberidazole, and thiabendazole | AOAC QuEChERS and HPLC-DAD analysis as a new method | Seven studied pesticides showed good performance during a simultaneous analysis | [97] |
| Argentina | Domestic market of Argentina (fresh fruits and vegetables) | Multi-residue pesticides, including 35 compounds | QuEChERS extraction and GC-MS analysis | Approximately 60% of samples were positive for pesticides but were within the MRL limit | [98] | |
| Chile, Mexico | Commonly consumed vegetables in Mexico and Chile | A total of 22 pesticides is analyzed for this study | QuEChERS multi-residue analysis by GC-MS/MS | Among studied pesticides, 10 residue pesticides were found in vegetables, and a greater number were in Mexican (7) samples than in Chilian (3) | [99] | |
| Louisiana | Two different soils from the traditional cropping system in Louisiana, USA | Synthetic and organic herbicides | Performance evaluation of herbicide for weed suppression | Synthetic herbicides were more efficient during weed control than organic herbicides | [100] | |
| Australia | New South Wales | Sediments from the fast-growing farming land in Australia | Targeted for 97 pesticides from the sediments | Methanol extraction of sediments followed by LC-MS/MS | The detected pesticides numbered 10 out of 97 target compounds | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, A.; Kim, D.; Jeong, W.-T. Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture. Sustainability 2024, 16, 10741. https://doi.org/10.3390/su162310741
Sarker A, Kim D, Jeong W-T. Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture. Sustainability. 2024; 16(23):10741. https://doi.org/10.3390/su162310741
Chicago/Turabian StyleSarker, Aniruddha, Do Kim, and Won-Tae Jeong. 2024. "Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture" Sustainability 16, no. 23: 10741. https://doi.org/10.3390/su162310741
APA StyleSarker, A., Kim, D., & Jeong, W.-T. (2024). Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture. Sustainability, 16(23), 10741. https://doi.org/10.3390/su162310741







