Utilising Phosphogypsum and Biomass Fly Ash By-Products in Alkali-Activated Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Paste Composition and Design
2.3. Test Methods
3. Results
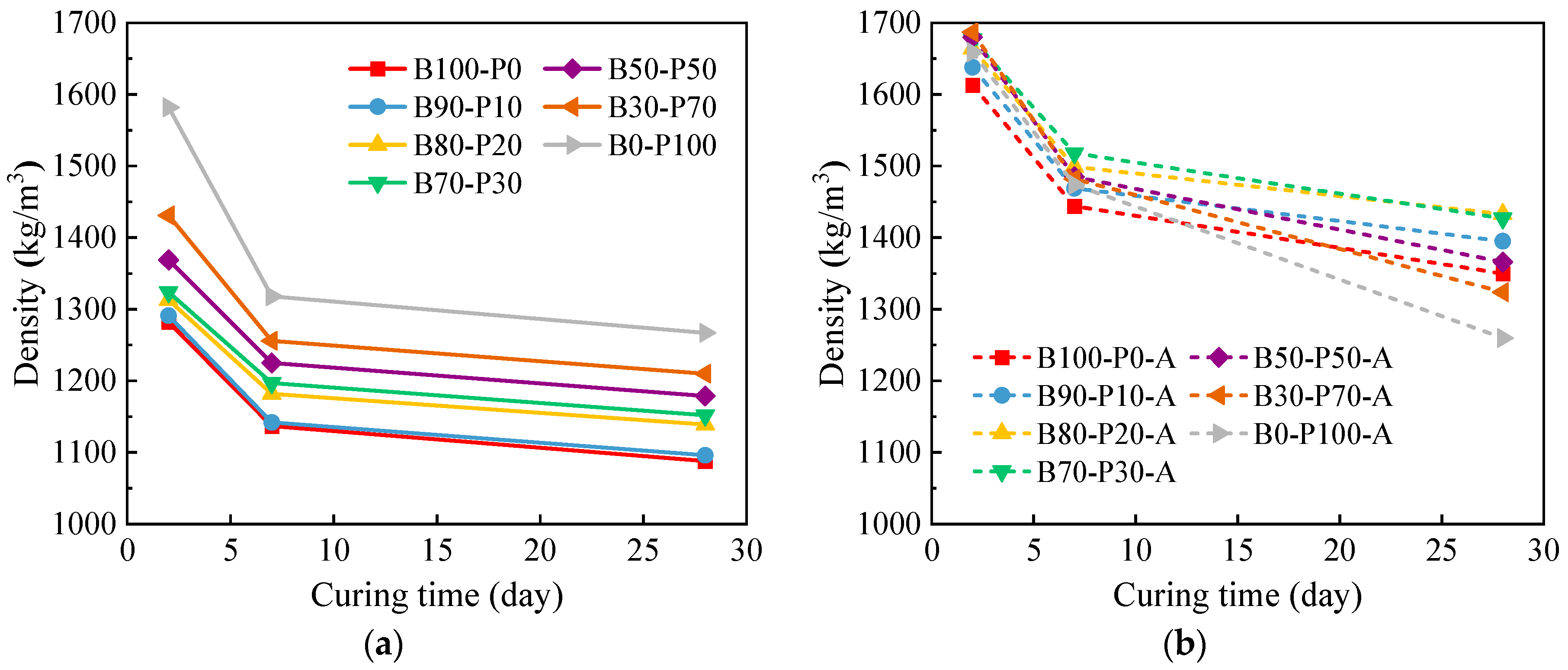
3.1. Density
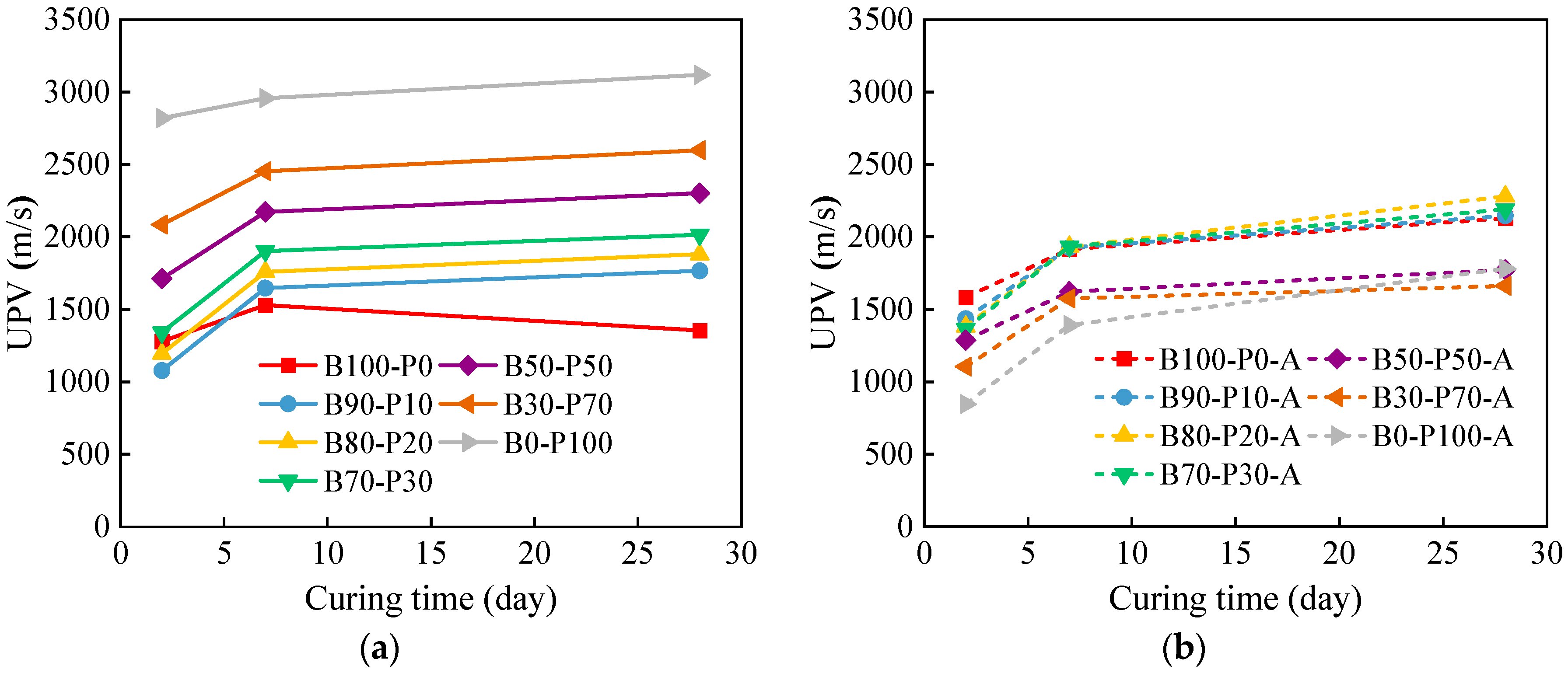
3.2. Ultrasonic Pulse Velocity
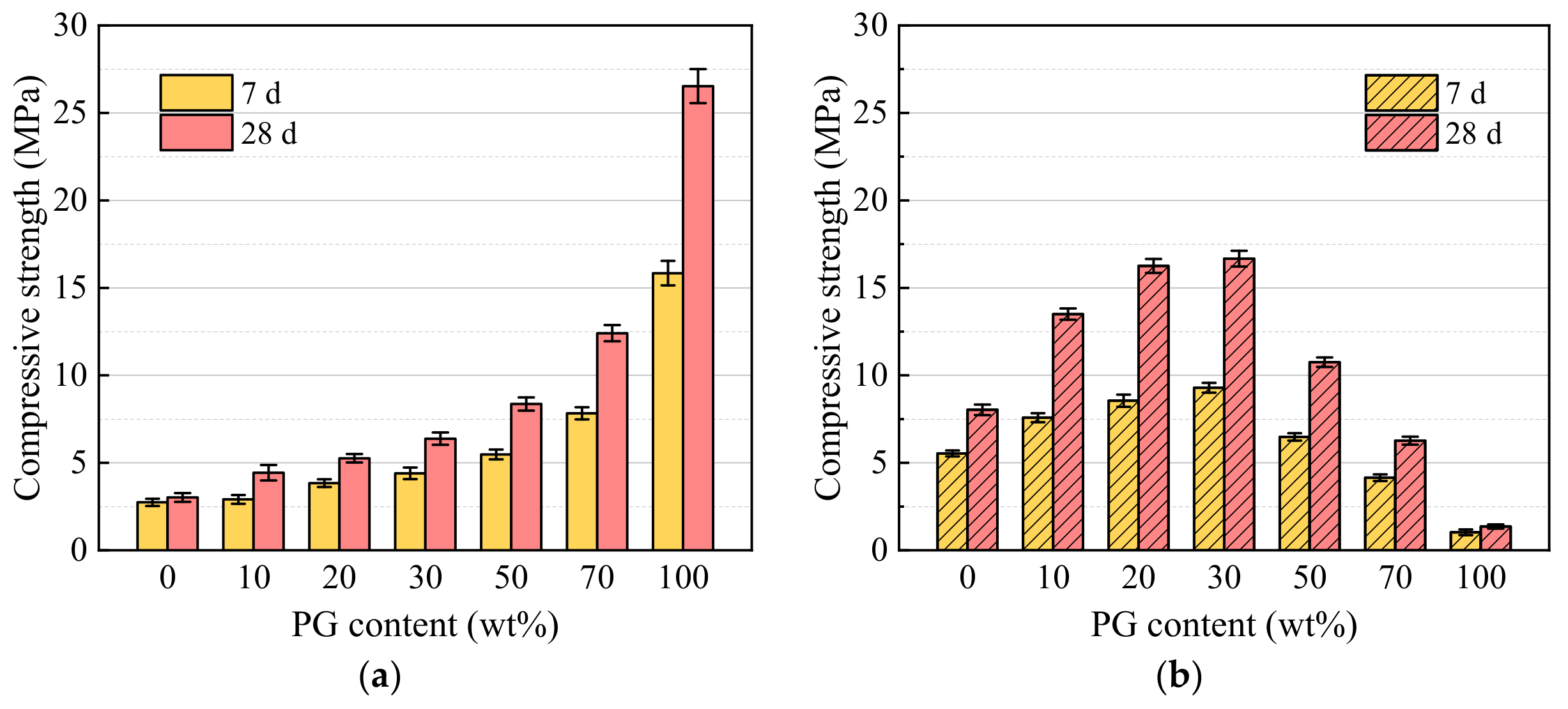
3.3. Compressive Strength
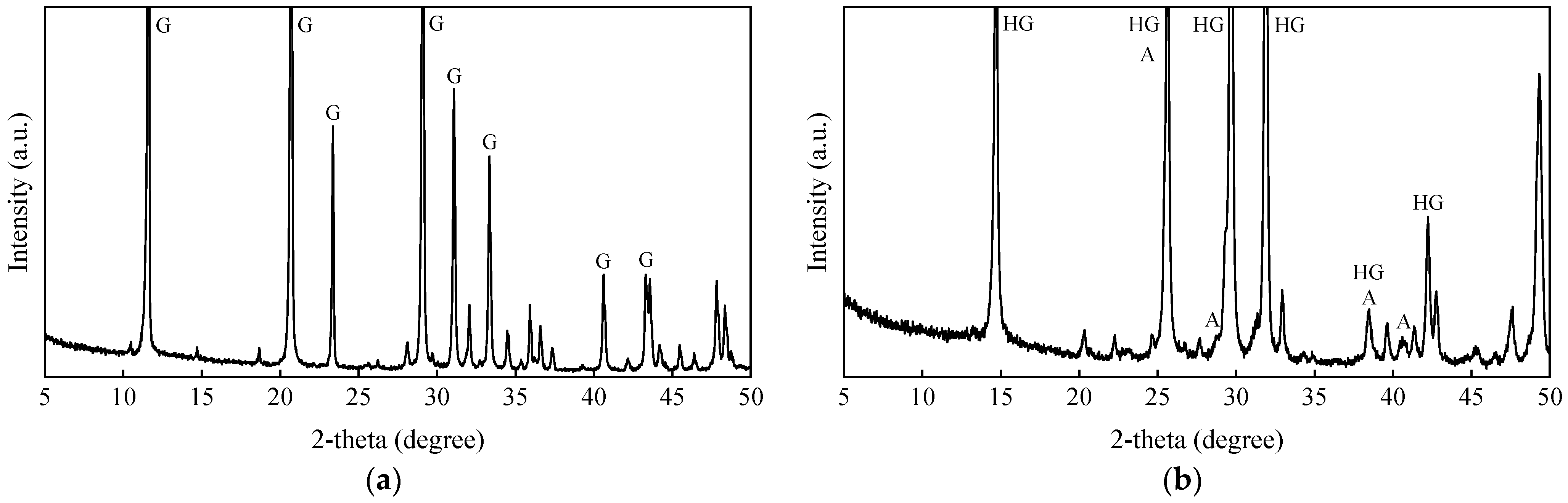
3.4. XRD Study
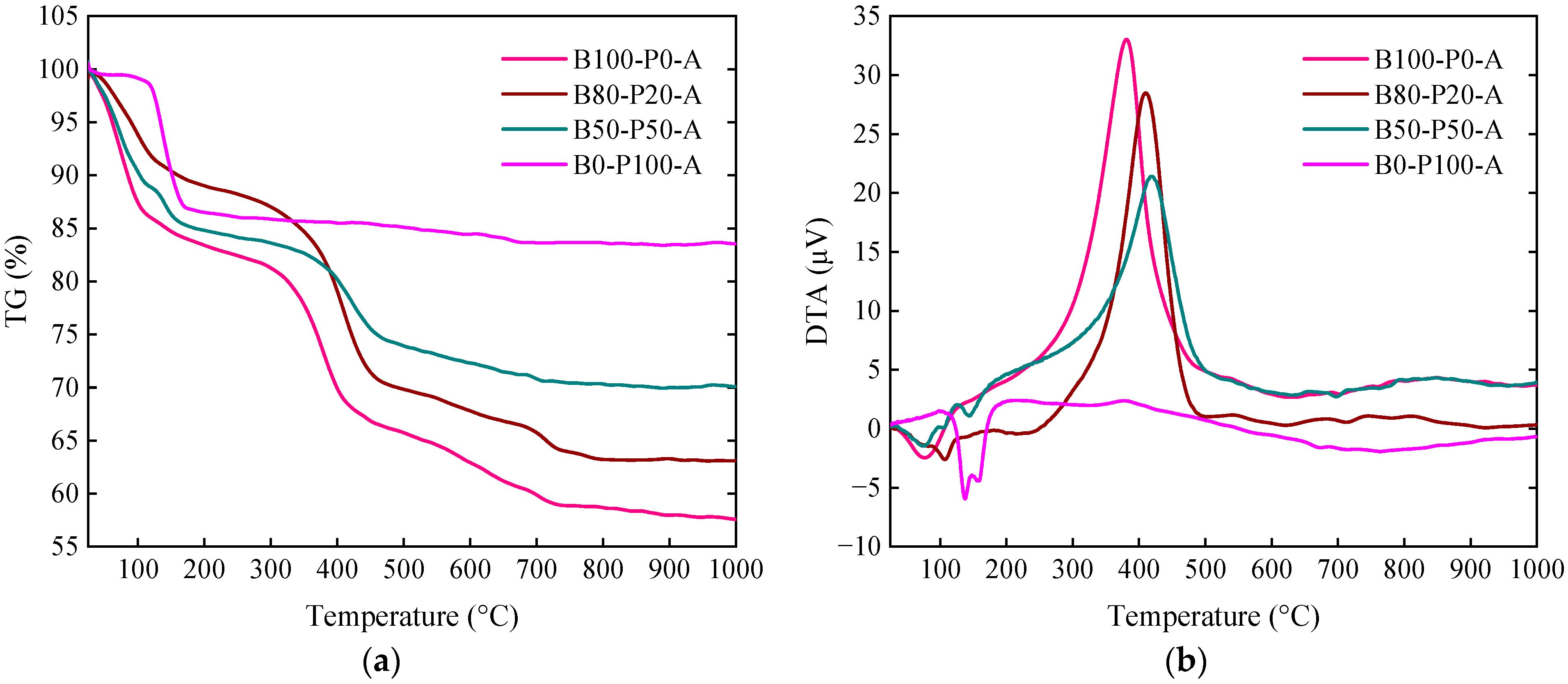
3.5. DTA/TG Study
3.6. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuenca, J.; Rodríguez, J.; Martín-Morales, M.; Sánchez-Roldán, Z.; Zamorano, M. Effects of Olive Residue Biomass Fly Ash as Filler in Self-Compacting Concrete. Constr. Build. Mater. 2013, 40, 702–709. [Google Scholar] [CrossRef]
- Demirbas, A. Potential Applications of Renewable Energy Sources, Biomass Combustion Problems in Boiler Power Systems and Combustion Related Environmental Issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Rosenberg, O.; Persson, T.; Högbom, L.; Jacobson, S. Effects of Wood-Ash Application on Potential Carbon and Nitrogen Mineralisation at Two Forest Sites with Different Tree Species, Climate and N Status. For. Ecol. Manag. 2010, 260, 511–518. [Google Scholar] [CrossRef]
- International Energy Agency. Key World Energy Statistics 2021; OECD: Paris, France, 2021. [Google Scholar]
- Scurlock, J.M.O.; Hall, D.O. The Contribution of Biomass to Global Energy Use (1987). Biomass 1990, 21, 75–81. [Google Scholar] [CrossRef]
- Hughes, E. Biomass Cofiring: Economics, Policy and Opportunities. Biomass Bioenergy 2000, 19, 457–465. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Jin, H.; Li, Z.; Liu, G.; Xing, F.; Tang, L. Exploring the Carbon Capture and Sequestration Performance of Biochar-Artificial Aggregate Using a New Method. Sci. Total Environ. 2023, 859, 160423. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Miller, A.; Llamazos, E.; Fonseca, F.; Baxter, L. Biomass Fly Ash in Concrete: Mixture Proportioning and Mechanical Properties. Fuel 2008, 87, 365–371. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of Geopolymer Mortar System Containing High Calcium Biomass Wood Ash as a Partial Substitution to Fly Ash: An Early Age Evaluation. Compos. Part B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Forteza, R.; Far, M.; Seguí, C.; Cerdá, V. Characterization of Bottom Ash in Municipal Solid Waste Incinerators for Its Use in Road Base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ramakrishna, S.; Gupta, M.K. Towards Zero Waste Manufacturing: A Multidisciplinary Review. J. Clean. Prod. 2017, 168, 1230–1243. [Google Scholar] [CrossRef]
- Cheah, C.B.; Ramli, M. The Engineering Properties of High Performance Concrete with HCWA–DSF Supplementary Binder. Constr. Build. Mater. 2013, 40, 93–103. [Google Scholar] [CrossRef]
- Maschio, S.; Tonello, G.; Piani, L.; Furlani, E. Fly and Bottom Ashes from Biomass Combustion as Cement Replacing Components in Mortars Production: Rheological Behaviour of the Pastes and Materials Compression Strength. Chemosphere 2011, 85, 666–671. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of Wood Ash in Concrete Manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass Fly Ash Effect on Fresh and Hardened State Properties of Cement Based Materials. Compos. Part B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Kirkelund, G.M.; Jensen, P.E.; Geiker, M.R.; Ottosen, L.M. Impact of Production Parameters on Physiochemical Characteristics of Wood Ash for Possible Utilisation in Cement-Based Materials. Resour. Conserv. Recycl. 2019, 145, 230–240. [Google Scholar] [CrossRef]
- Ayobami, A.B. Performance of Wood Bottom Ash in Cement-Based Applications and Comparison with Other Selected Ashes: Overview. Resour. Conserv. Recycl. 2021, 166, 105351. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Geiker, M.R.; Ottosen, L.M. Reaction Mechanisms of Wood Ash for Use as a Partial Cement Replacement. Constr. Build. Mater. 2021, 286, 122889. [Google Scholar] [CrossRef]
- Ban, C.C.; Nordin, N.S.A.; Ken, P.W.; Ramli, M.; Hoe, K.W. The High Volume Reuse of Hybrid Biomass Ash as a Primary Binder in Cementless Mortar Block. Am. J. Appl. Sci. 2014, 11, 1369–1378. [Google Scholar] [CrossRef]
- Cheah, C.B.; Ramli, M. The Implementation of Wood Waste Ash as a Partial Cement Replacement Material in the Production of Structural Grade Concrete and Mortar: An Overview. Resour. Conserv. Recycl. 2011, 55, 669–685. [Google Scholar] [CrossRef]
- Pereira, V.M.; Geraldo, R.H.; Cruz, T.A.M.; Camarini, G. Valorization of Industrial By-Product: Phosphogypsum Recycling as Green Binding Material. Clean. Eng. Technol. 2021, 5, 100310. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of Calcination Modification of Phosphogypsum and Its Effect on the Hydration Properties of Phosphogypsum-Based Supersulfated Cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a Construction Material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Campos, M.P.; Costa, L.J.P.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum Recycling in the Building Materials Industry: Assessment of the Radon Exhalation Rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of Phosphogypsum for Cemented Paste Backfill and Its Environmental Effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- López, F.A.; Gázquez, M.; Alguacil, F.J.; Bolívar, J.P.; García-Díaz, I.; López-Coto, I. Microencapsulation of Phosphogypsum into a Sulfur Polymer Matrix: Physico-Chemical and Radiological Characterization. J. Hazard. Mater. 2011, 192, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Cuadri, A.A.; Navarro, F.J.; García-Morales, M.; Bolívar, J.P. Valorization of Phosphogypsum Waste as Asphaltic Bitumen Modifier. J. Hazard. Mater. 2014, 279, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental Impact and Management of Phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Hermawan; Djayaprabha, H.S.; Nguyen, H.A. Utilizing Phosphogypsum Waste to Improve the Mechanical and Durability Performances of Cement-Free Structural Mortar Containing Ground Granulated Blast Furnace Slag and Calcium Oxide. J. Build. Eng. 2023, 72, 106557. [Google Scholar] [CrossRef]
- Leškevičienė, V.; Nizevičienė, D. Influence of the Setting Activators on the Physical Mechanical Properties of Phosphoanhydrite. Chem. Ind. Chem. Eng. Q 2014, 20, 233–240. [Google Scholar] [CrossRef]
- Bumanis, G.; Zorica, J.; Bajare, D.; Korjakins, A. Technological Properties of Phosphogypsum Binder Obtained from Fertilizer Production Waste. Energy Procedia 2018, 147, 301–308. [Google Scholar] [CrossRef]
- Vaičiukyniene, D.; Nizevičiene, D.; Kantautas, A.; Bocullo, V.; Kiele, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Peng, J.; Li, J.; Yin, S. Recycling of Phosphogypsum to Prepare Gypsum Plaster: Effect of Calcination Temperature. J. Build. Eng. 2022, 45, 103511. [Google Scholar] [CrossRef]
- Abdelhadi, M.; Abdelhadi, N.; El-Hasan, T.; Hadi, N.A. Optimization of Phosphogypsum By-Production Using Orthophosphoric Acid as Leaching Solvent with DifferentTemperatures and Leaching Time Periods. Earth Sci. Res. 2018, 7, 28. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Making of Anhydrite Cement from Waste Gypsum. Cem. Concr. Res. 2000, 30, 571–577. [Google Scholar] [CrossRef]
- Wu, M.; Shen, W.; Xiong, X.; Zhao, L.; Yu, Z.; Sun, H.; Xu, G.; Zhao, Q.; Wang, G.; Zhang, W. Effects of the Phosphogypsum on the Hydration and Microstructure of Alkali Activated Slag Pastes. Constr. Build. Mater. 2023, 368, 130391. [Google Scholar] [CrossRef]
- Yuan, J.F.; Liu, B.; Dong, X.J. Study on Early Hydration Properties of Phosphogypsum-Based Cement. Concrete 2014, 8, 92–94, 97. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kielė, A.; Janavičius, E.; Pupeikis, D. Effect of Phosphogypsum on the Stability upon Firing Treatment of Alkali-Activated Slag. Constr. Build. Mater. 2018, 184, 485–491. [Google Scholar] [CrossRef]
- Pinto, S.R.; Angulski da Luz, C.; Munhoz, G.S.; Medeiros-Junior, R.A. Durability of Phosphogypsum-Based Supersulfated Cement Mortar against External Attack by Sodium and Magnesium Sulfate. Cem. Concr. Res. 2020, 136, 106172. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Strength, Fracture and Durability Characteristics of Ambient Cured Alkali—Activated Mortars Incorporating High Calcium Industrial Wastes and Powdered Reagents. Crystals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Ates, F.; Park, K.T.; Kim, K.W.; Woo, B.-H.; Kim, H.G. Effects of Treated Biomass Wood Fly Ash as a Partial Substitute for Fly Ash in a Geopolymer Mortar System. Constr. Build. Mater. 2023, 376, 131063. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Beltrán, M.G.; López, M.; Agrela, F. Effects of Treatments on Biomass Bottom Ash Applied to the Manufacture of Cement Mortars. J. Clean. Prod. 2017, 154, 424–435. [Google Scholar] [CrossRef]
- Emdadi, Z.; Asim, N.; Amin, M.H.; Yarmo, M.A.; Maleki, A.; Azizi, M.; Sopian, K. Development of Green Geopolymer Using Agricultural and Industrial Waste Materials with High Water Absorbency. Appl. Sci. 2017, 7, 514. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of Combined Activator of Ca(OH)2 and Na2CO3 on Workability and Compressive Strength of Alkali-Activated Ferronickel Slag System. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Zhu, C.; Pundienė, I.; Pranckevičienė, J.; Kligys, M. Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes. Materials 2022, 15, 8354. [Google Scholar] [CrossRef]
- Beltrán, M.G.; Barbudo, A.; Agrela, F.; Jiménez, J.R.; De Brito, J. Mechanical Performance of Bedding Mortars Made with Olive Biomass Bottom Ash. Constr. Build. Mater. 2016, 112, 699–707. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of Curing Conditions on Properties of High Calcium Fly Ash Geopolymer Containing Portland Cement as Additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Megat Johari, M.A.; Ahmad, Z.A.; Maslehuddin, M. Impacts of Silica Modulus on the Early Strength of Alkaline Activated Ground Slag/Ultrafine Palm Oil Fuel Ash Based Concrete. Mater. Struct. Constr. 2015, 48, 733–741. [Google Scholar] [CrossRef]
- Tong, S.; Yuqi, Z.; Qiang, W. Recent Advances in Chemical Admixtures for Improving the Workability of Alkali-Activated Slag-Based Material Systems. Constr. Build. Mater. 2021, 272, 121647. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of Sodium Carbonate/Sodium Silicate Activator on the Rheology, Geopolymerization and Strength of Fly Ash/Slag Geopolymer Pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement–Part 1: Determination of Strength. iTeh, Inc.: Newark, DE, USA, 2016.
- Costa, A.R.D.; Matos, S.R.C.; Camarini, G.; Gonçalves, J.P. Hydration of Sustainable Ternary Cements Containing Phosphogypsum. Sustain. Mater. Technol. 2021, 28, e00280. [Google Scholar] [CrossRef]
- Amziane, S.; Sonebi, M. Overview on Biobased Building Material Made with Plant Aggregate. RILEM Tech. Lett. 2016, 1, 31–38. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Evaluation of Slag Characteristics on the Reaction Kinetics and Mechanical Properties of Na2CO3 Activated Slag. Constr. Build. Mater. 2017, 131, 334–346. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-Activated Binders Based on Ground Granulated Blast Furnace Slag and Phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Tamošaitis, G.; Kantautas, A.; Nizevičienė, D.; Pupeikis, D. Porous Alkali-Activated Materials Based on Municipal Solid Waste Incineration Ash with Addition of Phosphogypsum Powder. Constr. Build. Mater. 2021, 301, 123962. [Google Scholar] [CrossRef]
- Hamdi, N.; Ben Messaoud, I.; Srasra, E. Production of Geopolymer Binders Using Clay Minerals and Industrial Wastes. Comptes Rendus Chim. 2019, 22, 220–226. [Google Scholar] [CrossRef]
- de Oliveira, L.B.; de Azevedo, A.R.G.; Marvila, M.T.; Pereira, E.C.; Fediuk, R.; Vieira, C.M.F. Durability of Geopolymers with Industrial Waste. Case Stud. Constr. Mater. 2022, 16, e00839. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Chen, L.; Dick, W.A. Alkali-Activated Complex Binders from Class C Fly Ash and Ca-Containing Admixtures. J. Hazard. Mater. 2010, 173, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ozbakkaloglu, T. Behavior of Low-Calcium Fly and Bottom Ash-Based Geopolymer Concrete Cured at Ambient Temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Analcime Mineral Data. Available online: https://www.webmineral.com/data/Analcime.shtml (accessed on 15 January 2024).
- Xu, M.; Jing, Z.; Van Orman, J.A.; Yu, T.; Wang, Y. Density of NaAlSi2O6 Melt at High Pressure and Temperature Measured by In-Situ X-Ray Microtomography. Minerals 2020, 10, 161. [Google Scholar] [CrossRef]
- Asadi Ardebili, A.; Villoria Sáez, P.; González Cortina, M.; Tasán Cruz, D.M.; Rodríguez Sáiz, Á.; Atanes-Sánchez, E. Mechanical Characterization of Gypsum Mortars with Waste from the Automotive Sector. Constr. Build. Mater. 2023, 370, 130675. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Balevičius, G.; Vaičiukynas, V.; Kantautas, A.; Jakevičius, L. Synergic Effect between Two Pozzolans: Clinoptilolite and Silica Gel by-Product in a Ternary Blend of a Portland Cement System. Constr. Build. Mater. 2022, 344, 128155. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, Z.; Zhang, B.; Xie, J.; Chen, C.; Tan, J.; Zhao, C. Effects of Phosphogypsum Substitution on the Performance of Ground Granulated Blast Furnace Slag/Fly Ash-Based Alkali-Activated Binders. J. Build. Eng. 2023, 70, 106387. [Google Scholar] [CrossRef]
- McAdie, H.G. The Effect of Water Vapor Upon the Dehydration of CaSO4·2H2O. Can. J. Chem. 2011, 42, 792–801. [Google Scholar] [CrossRef]
- Beaugnon, F.; Preturlan, J.G.D.; Fusseis, F.; Gouillart, E.; Quiligotti, S.; Wallez, G. From Atom Level to Macroscopic Scale: Structural Mechanism of Gypsum Dehydration. Solid State Sci. 2022, 126, 106845. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, J.; Liu, C.; Chen, X.; Zhao, Y. Dehydration Pathways of Gypsum and the Rehydration Mechanism of Soluble Anhydrite γ-CaSO4. ACS Omega 2019, 4, 7636–7642. [Google Scholar] [CrossRef] [PubMed]
- Košnář, Z.; Mercl, F.; Perná, I.; Tlustoš, P. Investigation of Polycyclic Aromatic Hydrocarbon Content in Fly Ash and Bottom Ash of Biomass Incineration Plants in Relation to the Operating Temperature and Unburned Carbon Content. Sci. Total Environ. 2016, 563–564, 53–61. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The Evolution of Strength and Crystalline Phases for Alkali-Activated Ground Blast Furnace Slag and Fly Ash-Based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Calcium Sulphate Hemihydrate Hydration Leading to Gypsum Crystallization. Prog. Cryst. Growth Charact. Mater. 2007, 53, 57–77. [Google Scholar] [CrossRef]
- Marinkovic, S.; Kostic-Pulek, A.; Popov, S.; Djinovic, J.; Trifunovic, P. The Possibility of Obtaining Beta-Anhydrite from Waste Nitrogypsum. J. Min. Metall. Sect. B Metall. 2004, 40, 89–100. [Google Scholar] [CrossRef]







| Compound Formula | BFA (wt%) | PG (wt%) | Compound Formula | BFA (wt%) | PG (wt%) |
|---|---|---|---|---|---|
| SiO2 | 22.9 | 2.13 | Y2O3 | 0.02 | 0.03 |
| Al2O3 | 2.63 | 0.11 | SrO | 0.09 | 0.26 |
| CaO | 31.5 | 39.5 | Na2O | 0.26 | – |
| MgO | 3.57 | 0.11 | Cr2O3 | 0.02 | – |
| SO3 | 0.88 | 48.4 | NiO | 0.01 | – |
| P2O5 | 2.71 | 1.09 | Rb2O | 0.02 | – |
| K2O | 4.00 | 0.05 | BaO | 0.17 | – |
| ZnO | 0.10 | 0.01 | CeO2 | – | 0.19 |
| TiO2 | 0.21 | 0.03 | Nd2O3 | – | 0.07 |
| MnO | 2.07 | 0.01 | Nb2O5 | – | 0.002 |
| Fe2O3 | 2.32 | 0.01 | F | – | 1.41 |
| CuO | 0.02 | 0.01 | LOI * | 26.5 | 6.578 |
| Sample | B100-P0 | B90-P10 | B80-P20 | B70-P30 | B50-P50 | B30-P70 | B0-P100 |
|---|---|---|---|---|---|---|---|
| BFA | 100 | 90 | 80 | 70 | 50 | 30 | 0 |
| PG | 0 | 10 | 20 | 30 | 50 | 70 | 100 |
| Sample | Binder Content | Na2CO3 Solution Concentration | Na2CO3/Na2SiO3 Solute Ratio | |
|---|---|---|---|---|
| BFA (wt%) | PG (wt%) | (wt%) | ||
| B100-P0-A | 100 | 0 | 15 | 0.90 |
| B90-P10-A | 90 | 10 | 15 | 0.90 |
| B80-P20-A | 80 | 20 | 15 | 0.90 |
| B70-P30-A | 70 | 30 | 15 | 0.90 |
| B50-P50-A | 50 | 50 | 15 | 0.90 |
| B30-P70-A | 30 | 70 | 15 | 0.90 |
| B0-P100-A | 0 | 100 | 15 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Pranckevičienė, J.; Pundienė, I.; Kizinievič, O. Utilising Phosphogypsum and Biomass Fly Ash By-Products in Alkali-Activated Materials. Sustainability 2024, 16, 1084. https://doi.org/10.3390/su16031084
Zhu C, Pranckevičienė J, Pundienė I, Kizinievič O. Utilising Phosphogypsum and Biomass Fly Ash By-Products in Alkali-Activated Materials. Sustainability. 2024; 16(3):1084. https://doi.org/10.3390/su16031084
Chicago/Turabian StyleZhu, Chengjie, Jolanta Pranckevičienė, Ina Pundienė, and Olga Kizinievič. 2024. "Utilising Phosphogypsum and Biomass Fly Ash By-Products in Alkali-Activated Materials" Sustainability 16, no. 3: 1084. https://doi.org/10.3390/su16031084





