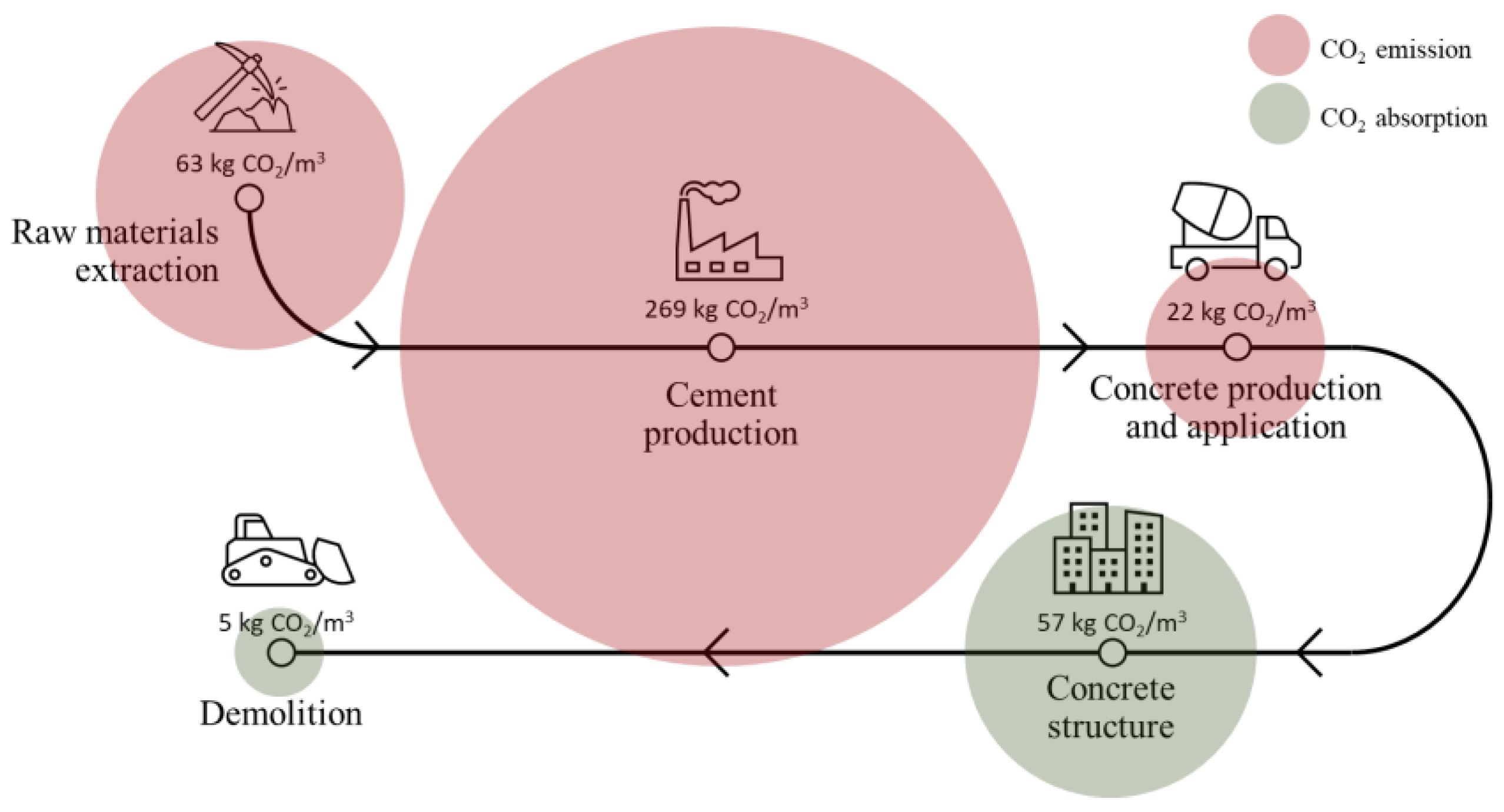
Strategies for OPC Paste Carbonation: Relationship between Microstructure, Performance and Net CO2 Balance
Abstract
:1. Introduction
2. Overview of Hydration and Carbonation of Cement-Based Materials
2.1. Hydration of Cement-Based Materials
2.2. Carbonation of Cement-Based Materials
3. Carbonation Curing of Cementitious Materials
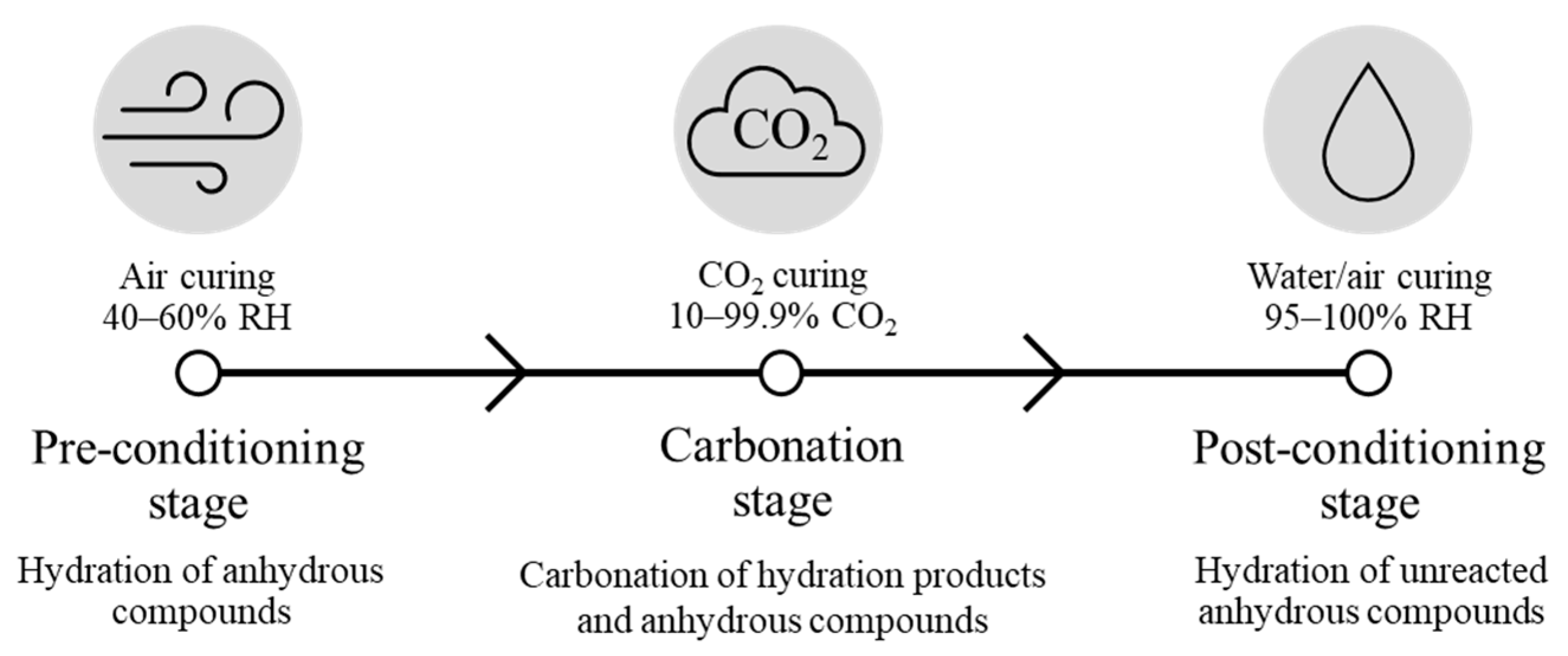
3.1. Carbonation Curing Process
3.2. Reactions and Phase Development
3.3. Carbonation Impact on Microstructure and Performance
3.4. Impact on Carbon Capture
4. Carbonation of Cementitious Materials during Mixing
4.1. Carbonation Mixing Process
4.2. Reactions and Phase Development
4.3. Carbonation Impact on Microstructure and Material Performance
4.4. Impact on Carbon Capture
5. Carbonation of Hydrated Cement Waste from Demolished Concrete
5.1. Carbonation of Hydrated Cement Waste
5.2. Reactions and Phase Development
5.3. Carbonation Impact on Microstructure and Performance
5.4. Impact on Carbon Capture
6. Overview
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency and World Business Council for Sustainable Development. Cement Technology Roadmap 2009: Carbon Emissions Reductions up to 2050; IEA: Paris, France, 2009.
- Filippo, J.D.; Karpman, J.; DeShazo, J.R. The impacts of policies to reduce CO2 emission within the concrete supply chain. Cem. Concr. Compos. 2019, 101, 67–82. [Google Scholar] [CrossRef]
- International Energy Agency and Cement Sustainability Initiative. Technology Roadmap: Low-Carbon Transition in the Cement Industry; IEA: Paris, France, 2018.
- United Nations Environment Programme; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Pan, S.-Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Metha, P.K. Reducing the environmental impact of concrete: Concrete can be durable and environmentally friendly. Concr. Int. 2001, 10, 61–66. [Google Scholar]
- Kwon, E.; Ahn, J.; Cho, B.; Park, D. A study on development of recycled cement made from waste cementitious powder. Constr. Build. Mater. 2015, 83, 174–180. [Google Scholar] [CrossRef]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Re 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- The European Cement Association. Cementing the European Green Deal: Reaching Climate Neutrality along the Cement and Concrete Value Chain by 2050; CEMBUREAU: Brussels, Belgium, 2018. [Google Scholar]
- Global Cement and Concrete Association. Concrete Future: The GCCA 2050 Cement and Concrete Industry Roadmap for Net Zero Concrete; GCCA: London, UK, 2020. [Google Scholar]
- Low Emissions Intensity Lime & Cement—Leilac, Project—Leilac. Available online: https://cordis.europa.eu/project/id/654465 (accessed on 8 June 2021).
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 abatement strategies in cement industry: A review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef]
- Habert, G.; Billard, C.; Rossi, P.; Chen, C.; Roussel, N. Cement production technology improvement compared to factor 4 objectives. Cem. Concr. Res. 2010, 40, 820–826. [Google Scholar] [CrossRef]
- Jang, J.; Kim, G.; Kim, H.; Lee, H. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Schneider, M.; Hoenig, V.; Ruppert, J.; Rickert, J. The cement plant of tomorrow. Cem. Concr. Res. 2023, 173, 107290. [Google Scholar] [CrossRef]
- European Comission, Joint Research Centre, Publications Office. Decarbonisation Options for the Cement Industry. 2023. Available online: https://data.europa.eu/doi/10.2760/174037 (accessed on 18 August 2023).
- Poon, C.S.; Shen, P.; Jiang, Y.; Ma, Z.; Xuan, D. Total recycling of concrete waste using accelerated carbonation: A review. Cem. Concr. Res. 2023, 173, 107284. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, J.; Duan, Z. Strategies to accelerate CO2 sequestration of cement-based materials and their application prospects. Constr. Build. Mater. 2022, 314, 125646. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q.; Huang, T.; Peng, W. Mineralization and utilization of CO2 in construction and demolition wastes recycling for building materials: A systematic review of recycled concrete aggregate and recycled hardened cement powder. Sep. Purif. Technol. 2022, 298, 121512. [Google Scholar] [CrossRef]
- Li, L.; Wu, M. An overview of utilizing CO2 for accelerated carbonation treatment in the concrete industry. J. CO2 Util. 2022, 60, 102000. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Lee, C.-H.; Chen, W.-S. Mineral carbonation using alkaline waste and byproducts to reduce CO2 emissions in Taiwan. Environ. Chem. Lett. 2023, 21, 865–884. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Luan, C.; Zhang, G.; Fu, L.; Abomohra, A.E.-F. Accelerated carbonation technology for enhanced treatment of recycled concrete aggregates: A state-of-the-art review. Constr. Build. Mater. 2021, 282, 122671. [Google Scholar] [CrossRef]
- Tam, V.W.T.; Butera, A.; Le, K.N.; Li, W. Utilising CO2 technologies for recycled aggregate concrete: A critical review. Constr. Build. Mater. 2020, 250, 118903. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Carriço, A.; Bogas, J.A.; Guedes, M. Thermoactivated cementitious materials—A review. Constr. Build. Mater. 2020, 250, 118873. [Google Scholar] [CrossRef]
- Moir, G. Chapter 1—Cements. In Advance Concret Technology; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2003; Volume 3, pp. 3–45. [Google Scholar]
- Hewlett, P.C.; Liska, M. Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Prentice Hall: UK, 2012; p. 872. [Google Scholar]
- MacLaren, D.C.; White, M.A. Cement: Its chemistry and properties. J. Chem. Educ. 2003, 80, 623–635. [Google Scholar] [CrossRef]
- Van Oss, H.G.; Padovani, A.C. Cement Manufacture and the Environment Part I: Chemistry and Technology. J. Ind. Ecol. 2002, 6, 89–105. [Google Scholar] [CrossRef]
- Beaudoin, J.; Odler, I. Hydration, Setting and Hardening of Portland Cement. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 157–242. [Google Scholar]
- Zhang, M.; Bachu, S. Review of integrity of existing wells in realtion to CO2 geological storage: What do we know? Int. J. Greenh. Gas Control 2011, 5, 826–840. [Google Scholar] [CrossRef]
- Scrivener, K.; Juilland, P.; Monteiro, P. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Juilland, P.; Gallucci, E.; Flatt, R.; Scrivener, K. Dissolution theory applied to the induction period in alite hydration. Cem. Concr. Res. 2010, 40, 831–844. [Google Scholar] [CrossRef]
- Juilland, P.; Nicoleau, L.; Arvidson, R.S.; Gallucci, E. Advances in dissolution understanding and their implications for cement hydration. RILEM Tecnhical Lett. 2017, 2, 90–98. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Krautwurst, N.; Nicoleau, L.; Dietzsch, M.; Lieberwirth, I.; Labbez, C.; Fernandez-Martinez, A.; Driessche, A.V.; Barton, B.; Leukel, S.; Tremel, W. Two-Step Nucleation Process of Calcium Silicate Hydrate, the Nanobrick of Cement. Chem. Mater. 2018, 30, 2894–2904. [Google Scholar] [CrossRef]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Rao, N.V.; Meena, T. A review on carbonation study in concrete. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032011. [Google Scholar]
- Zhou, Y.; Gencturk, B.; ASCE, A.M.; William, K.; ASCE, F.; Attar, A. Carbonation-Induced and Chloride-Induced Corrosion in Reinforced Concrete Structures. Am. Soc. Civ. Eng. 2014, 27, 04014245. [Google Scholar] [CrossRef]
- Haselbach, L.; Thomle, J. An alternative mechanism for accelerated carbon sequestration in concrete. Sustain. Cities Soc. 2014, 12, 25–30. [Google Scholar] [CrossRef]
- Visser, J.H.M. Influence of the carbon dioxide concentration on the resistance to carbonation of concrete. Constr. Build. Mater. 2014, 67, 8–13. [Google Scholar] [CrossRef]
- Šavija, B.; Lukovic’, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Liu, B.; Qin, J.; Shi, J.; Jiang, J.; Wu, X.; He, Z. New perspectives on utilization of CO2 sequestration technologies in cement-based materials. Constr. Build. Mater. 2021, 272, 121660. [Google Scholar] [CrossRef]
- Zajac, M.; Irbe, L.; Bullerjahn, F.; Hilbig, H.; Haha, M.B. Mechanisms of carbonation hydration hardening in Portland cements. Cem. Concr. Res. 2022, 152, 106687. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Haha, M.B.; Deja, J. CO2 Mineralization Methods in Cement and Concrete Industry. Energies 2022, 15, 3597. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Ealy age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Winnefeld, F.; Leemann, A.; German, A.; Lothenbach, B. CO2 storage in cement and concrete by mineral carbonation. Curr. Opin. Green Sustain. Chem. 2022, 38, 100672. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y.; Ghouleh, Z. Effect of Initial Curing on Carbonation of Lightweight Concrete Masonry Units. ACI Mater. J. 2013, 110, 441–450. [Google Scholar]
- Zhang, D.; Cai, X.; Jaworska, B. Effect of pre-carbonation hydration on long-term hydration of carbonation-cured cement-based materials. Constr. Build. Mater. 2020, 231, 117122. [Google Scholar] [CrossRef]
- Qian, X.; Wang, J.; Fang, Y.; Wang, L. Carbon dioxide as an admixture for better performance of OPC-based concrete. J. CO2 Util. 2018, 25, 31–38. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime. Cem. Concr. Compos. 2019, 97, 78–88. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. A maturity approach to estimate compressive strength developmentof CO2-cured concrete blocks. Cem. Concr. Compos. 2018, 85, 153–160. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Xia, J.; Wu, R.-J.; Shen, X.-Y.; Chen, J.-J.; Zhao, Y.-X.; Jin, W.-L. An overview on the influence of various parameters on the fabrication and engineering properties of CO2-cured cement-based composites. J. Clean. Prod. 2022, 366, 132968. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Shao, Y. Flue gas carbonation curing of cement paste and concrete at ambient pressure. J. Clean. Prod. 2021, 313, 127943. [Google Scholar] [CrossRef]
- Shi, C.; Liu, M.; He, P.; Ou, Z. Factors affecting kinetics of CO2 curing of concrete. J. Sustain. Cem.-Based Mater. 2012, 1, 24–33. [Google Scholar]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zonghui, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influenceof porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. Carbon sequestration through accelerated carbonation of BOF slag: Influence of particle size characteristics. Chem. Eng. J. 2016, 298, 26–35. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Effect of accelerated carbonation curing on near surface properties of concrete. Eur. J. Environ. Civ. Eng. 2020, 1964–8189, 2116–7214. [Google Scholar] [CrossRef]
- Li, X.; Ling, T.-C. Instant CO2 curing for dry-mix pressed cement pastes: Consideration of CO2 concentrations coupled with further water curing. J. CO2 Util. 2020, 38, 348–354. [Google Scholar] [CrossRef]
- Liu, L.; Yilun, L.; Tian, X.; Chen, X. Superior CO2 uptake and enhanced compressive strength for carbonation curing of cement-based materials via flue gas. Constr. Build. Mater. 2022, 346, 128364. [Google Scholar] [CrossRef]
- Lu, B.; Drissi, S.; Liu, J.; Hu, X.; Song, B.; Shi, C. Effect of temperature on CO2 curing, compressive strength and microstructure of cement paste. Cem. Concr. Res. 2022, 157, 106827. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Shao, Y. Effect of Carbonation Mixing on CO2 Uptake and Strength Gain in Concrete. J. Mater. Civ. Eng. 2017, 29, 04017176. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Y.; Zhang, Y.; Chang, J. Changes in mineral composition, growth of calcite crystal, and promotion ofphysico-chemical properties induced by carbonation of β-C2S. J. CO2 Util. 2019, 34, 149–162. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; van Breugel, K. Understanding the adhesion mechanisms between C-S-H and fillers. Cem. Concr. Res. 2017, 100, 275–283. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- He, P.; Shi, C.; Tu, Z.; Poon, C.S.; Zhang, J. Effect of further water curing on compressive strength and microstructure of CO2-cured concrete. Cem. Concr. Compos. 2016, 72, 80–88. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Surface scaling of CO2-cured concrete exposed to freeze-thaw cycles. J. CO2 Util. 2018, 27, 137–144. [Google Scholar] [CrossRef]
- Almeida, A.; Tonoli, G.; Santos, S.; Savastano, H., Jr. Improved durability of vegetable fiber reinforced cement compositesubject to accelerated carbonation at early age. Cem. Concr. Compos. 2013, 42, 49–58. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetrationand weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Ouyang, X.; Wang, L.; Xu, S.; Ma, Y.; Ye, G. Surface characterization of carbonated recycled concrete fines and its effect on the rheology, hydration and strength development of cement paste. Cem. Concr. Compos. 2020, 114, 103809. [Google Scholar] [CrossRef]
- Ahmad, S. Accelerated carbon dioxide sequestration. In Carbon Dioxide Sequestration in Cementitious Construction Materials; Elsevier Ltd.: Saudi Arabia, 2018; pp. 81–101. [Google Scholar]
- Kwasny, J.; Basheer, P.M.; Russell, M. CO2 Sequestration in Cement-Based Materials During Mixing Process Using Carbonated Water and Gaseous CO2. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, West Lafayette, IN, USA, 24–26 July 2014. [Google Scholar]
- Silva, A.; Nogueira, R.; Bogas, J.A.; Rodrigues, M. Influence of carbon dioxide as a mixture component on the cement hydration. In Proceedings of the 4th International RILEM Conference: Microstructure Related Durability of Cementitious Composites, Delft, The Netherlands, 29 April–25 May 2021. [Google Scholar]
- Dodds, W.S.; Stutzman, L.F.; Sollami, B.J. Carbon dioxide solubility in water. Ind. Eng. Chem. 1956, 1, 92–95. [Google Scholar] [CrossRef]
- Nogueira, R.; Silva, A.; Bogas, J.A.; Gomes, M.G. Early-age hydration of Portland cement under forced carbonation. 2024; To be submited soon. [Google Scholar]
- Jorge, F.C.; Pereira, C.; Ferreira, J.M. Wood-cement composites: A review. Holz Roh Werkst. 2004, 62, 370–377. [Google Scholar] [CrossRef]
- CarbonCure. CarbonCure Technologies. 2021. Available online: https://www.carboncure.com/technology/ (accessed on 7 June 2021).
- Monkman, S.; Kenward, P.A.; Dipple, G.; MacDonald, M.; Raudsepp, M. Activation of cement hydration with carbon dioxide. J. Sustain. Cem.-Based Mater. 2018, 7, 160–181. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Y.; Gao, F.; Zhang, L.; Zhang, Z.; Liu, X. Study on high-efficiency CO2 absorption by fresh cement paste. Constr. Build. Mater. 2021, 270, 121364. [Google Scholar] [CrossRef]
- Monkman, S.; Lee, B.E.J.; Grandfield, K.; MacDonald, M.; Raki, L. The impacts of in-situ carbonate seeding on the early hydration of tricalcium silicate. Cem. Concr. Res. 2020, 136, 106179. [Google Scholar] [CrossRef]
- Monkman, S.; MacDonald, M.; Hooton, R.D.; Sandberg, P. Properties and durability of concrete produced usign CO2 as an accelerating admixture. Cem. Concr. Compos. 2016, 74, 218–224. [Google Scholar] [CrossRef]
- Monkman, S. Sustainable Ready Mixed Concrete Production Using Waste CO2: A Case Study. In Proceedings of the Conference: Recent Advances in Concrete Technology and Sustainability Issues: Fourteenth International Conference, Beijing, China, 30 October–2 November 2018. [Google Scholar]
- Lippiatt, N.; Ling, T.-C. Rapid hydration mechanism of carbonic acid and cement. J. Build. Eng. 2020, 31, 101357. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.-C.; Eggermont, S. Combining hydration and carbonation of cement using super-saturated aqueous CO2 solution. Constr. Build. Mater. 2019, 229, 116825. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of carbonated water to improve the mechanical properties and reduce the carbon footprint of cement-based materials with recycled aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of hydration of calcium silicates by carbon dioxide treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Tao, Y.; Rahul, A.V.; Lesage, K.; Yuan, Y.; Tittelboom, K.V.; Schutter, G.D. Stiffening control of cement-based materials using accelerators in inline mixing processes: Possibilities and challenges. Cem. Concr. Compos. 2021, 119, 103972. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Burgos-Cara, A.; Di Lorenzo, F.; Ruiz-Agudo, E.; Elert, K. Nonclassical Crystallization of Calcium Hydroxide via Amorphous Precursors and the Role of Additives. Cryst. Growth Des. 2020, 20, 4418–4432. [Google Scholar] [CrossRef]
- Nonat, A.; Lecoq, X.; Gauffinet, S. Calcium hydroxide concentration in solution: Parameter determining the kinetics of the early hydration of tricalcium silicate and the characteristics of the products. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997. [Google Scholar]
- Cuesta, A.; Zea-Garcia, J.D.; Londono-Zuluaga, D.; Torre, A.G.D.L.T.; Santacruz, I.; Vallcorba, O.; Dapiaggi, M.; Sanfélix, S.G.; Aranda, M.A.G. Multiscale understanding of tricalcium silicate hydration reactions. Sci. Rep. 2018, 8, 8544. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Kudlacz, K.; Cizer, O.; Ruiz-Agudo, E. Formation of amorphous calcium carbonate and its transformation into mesostructured calcite. CrystEngComm 2015, 17, 58–72. [Google Scholar] [CrossRef]
- Chen, J.J.; Sorelli, L.; Vandamme, M.; Ulm, F.-J.; Chanvillard, G. A Coupled Nanoindentation/SEM-EDS Study on Low Water/Cement RatioPortland Cement Paste: Evidence for C–S–H/Ca(OH)2 Nanocomposites. J. Am. Ceram. Soc. 2010, 93, 1484–1493. [Google Scholar] [CrossRef]
- Cembureau, Cement, Concrete & the Circular Economy. Cembureau, European Cement Association, Brussels, Belgium. 2016. Available online: https://circulareconomy.europa.eu/platform/sites/default/files/cement_concrete_the_circular_economy.pdf (accessed on 8 June 2021).
- Sáez, P.V.; Osmani, M. A diagnosis of construction and demolition waste generation and recovery practice in the European Union. J. Clean. Prod. 2019, 241, 188400. [Google Scholar]
- Oikonomou, N.D. Recycled concrete aggregates. Cem. Concr. Compos. 2005, 27, 315–318. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.-L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and demolition waste best management practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, Z.; Wu, H.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C.; Mo, K.H. Valorization of waste powders from cement-concrete life cycle: A pathway to circular future. J. Clean. Prod. 2020, 268, 122358. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; He, X.; Zhao, R.; Yang, J.; Su, Y. Nano particles prepared from hardened cement paste by wet grinding and its utilization as an accelerator in Portland cement. J. Clean. Prod. 2021, 283, 124632. [Google Scholar] [CrossRef]
- Tereza, P.; Koci, V.; Sefflova, M. Study Replacement of Cement with Recycled Cement Powder and the and the Environmental Assessment. Solid State Phenom. 2016, 249, 136–141. [Google Scholar]
- Bouarroudj, M.E.; Rémond, S.; Bulteel, D.; Potier, G.; Michel, F.; Zhao, Z.; Courard, L. Use of grinded hardened cement pastes as mineral addition for mortars. J. Build. Eng. 2021, 34, 101863. [Google Scholar] [CrossRef]
- Bordy, A.; Younsi, A.; Aggoun, S.; Fiorio, B. Cement substitution by a recycled cement paste fine: Role of the residual anhydrous clinker. Constr. Build. Mater. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, Y.W. Utilization of waste concrete powder as a substitution material for cement. Constr. Build. Mater. 2012, 30, 500–504. [Google Scholar] [CrossRef]
- Cong, L.; Jialin, L.; Jing, C.; Hailun, W.; Dong, L. Study on the Application of Recycled Fine Powder in Ready-Mixed Concrete. MATEC Web Conf. 2019, 278, 01010. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Ling, T.-C.; Cheng, X.; Mo, K.H. Effect of particle size and CO2 treatment of waste cement powder on properties of cement paste. Can. J. Civ. Eng. 2020. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Yamasaki, A.; Iizuka, A.; Fujii, M.; Kumagai, K.; Yanagisawa, Y. Development of a process for producing high-purity calcium carbonate (CaCO3) from waste cement using pressurized CO2. Environ. Prog. 2005, 24, 162–170. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Microstructure changes of waste hydrated cement paste induced by accelerated carbonation. Constr. Build. Mater. 2015, 76, 360–365. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Chen, W.; Jie, Z.; Zhuanzhuan, K.; Pu, S.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Tenza-Abril, A.J. Microstructure of thermoactivated recycled cement pastes. Cem. Concr. Res. 2020, 138, 106226. [Google Scholar] [CrossRef]
- Carriço, A.; Real, S.; Bogas, J.A.; Pereira, M.F.C. Mortars with thermo activated recycled cement: Fresh and mechanicalcharacterisation. Constr. Build. Mater. 2020, 256, 119502. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Pereira, M. Mechanical characterization of thermal activated low-carbon recycledcement mortars. J. Clean. Prod. 2019, 218, 377–389. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Ling, T.-C.; Cheng, X.; Pan, S.-Y.; Mo, K.H. CO2 treatment of hydrated cement powder: Characterization and application consideration. J. Mater. Civ. Eng. 2021, 33, 04021041. [Google Scholar] [CrossRef]
- Wu, H.; Liang, C.; Xiao, J.; Ma, Z. Properties and CO2-curing enhancement of cement-based materials containing various sources of waste hardened cement paste powder. J. Build. Eng. 2021, 44, 102677. [Google Scholar] [CrossRef]
- Silva, A.; Nogueira, R.; Bogas, A.; Abrantes, J.; Wawrzynczak, D.; Sciubidlo, A.; Majchrzak-Kuceba, I. Valorisation of recycled cement paste: Feasibility of a short duration carbonation process. Materials 2022, 15, 6001. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Nogueira, R.; Bogas, A. Recycled cement paste carbonation: The industrial advance of the short carbonation process. In Proceedings of the 13th Central European Congress on Concrete Engineering, CCC 2022, Zakopane, Polony, 13–14 September 2022. [Google Scholar]
- Silva, A.; Nogueira, R.; Bogas, A.; Wawrzynczak, D.; Sciubidlo, A.; Majchrzak-Kuceba, I. Parametric study towards optimization of a short duration carbonation process of recycled cement paste. Materials 2022, 15, 6513. [Google Scholar] [CrossRef] [PubMed]
- Carriço, A.; Bogas, J.A.; Hu, S.; Real, S.; Costa Pereira, M.F. Novel separation process for obtaining recycled cement and high-quality recycled sand from waste hardened concrete. J. Clean. Prod. 2021, 309, 127375. [Google Scholar] [CrossRef]
- Skocek, J.; Zajac, M.; Haha, M.B. Carbon Capture and Utilization by mineralization of cement pastes derived from recycled concrete. NatureResearch 2020, 10, 5614. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, A.; Fujii, M.; Yamasaki, A.; Yanagisawa, Y. Development of a New CO2 Sequestration Process Utilizing the Carbonation of Waste Cement. Ind. Eng. Chem. Res. 2004, 43, 7880–7887. [Google Scholar] [CrossRef]
- Holmes, N.; Kelliher, D.; Tyrer, M. Simulating cement hydration using HYDCEM. Constr. Build. Mater. 2020, 239, 117811. [Google Scholar] [CrossRef]
- Stepkowska, E.; Aviles, M.; Blanes, J.; Perez-Rodriguez, J. Gradual transformation of Ca(OH)2 into CaCO3 on cement hydration: XRD study. J. Therm. Anal. Calorim. 2007, 87, 189–198. [Google Scholar] [CrossRef]
- Bier, T.A.; Kropp, J.; Hilsdorf, H. Formation of Silica gel During Carbonation of Cementitious Systems Containing Slag Cements. ACI Symp. Pap. 1989, 114, 1413–1428. [Google Scholar]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. 2009, 42, 515–525. [Google Scholar] [CrossRef]
- Black, L.; Garbev, K.; Gee, I. Surface carbonation of synthetic C-S-H samples: A comparison between fresh and aged C-S-H using X-ray photoelectron spectroscopy. Cem. Concr. Res. 2008, 38, 745–750. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Huang, J.; Yu, K.; Zhong, G.; Chen, F.; Chen, X.; Yang, W.; Wang, Y. Effects of temperature, humidity and CO2 concentration on carbonation of cement-based materials: A review. Constr. Build. Mater. 2022, 346, 128399. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; He, P.; Liu, J.; Shi, C. A review on the modelling of carbonation of hardened and fresh cement-based materials. Cem. Concr. Compos. 2022, 125, 104315. [Google Scholar] [CrossRef]
- van der Zee, S.; Zeman, F. Production of carbon negative precipitated calcium carbonate from waste concrete. Can. J. Chem. Eng. 2016, 94, 2153–2159. [Google Scholar] [CrossRef]
- Ravikumar, D.; Zhang, D.; Keoleian, G.; Miller, S.; Sick, V.; Li, V. Carbon dioxide utilization in concrete curing or mixing might not produce a net climate benefit. Nat. Commun. 2021, 12, 855–868. [Google Scholar] [CrossRef]




| Mixture | Process | Compressive Strength Variation | CO2 Uptake (%wt of Clinker) | Refs. | ||
|---|---|---|---|---|---|---|
| Wet-mix concrete (w/b = 0.45) | PC 1: 20 h/26 °C/50% | +26% at 3 days | 14.1% | [69] | ||
| CS 2: 6 h/99%/0.69 atm after vacuum | +5% at 28 days | |||||
| 1 day | 7 days | 28 days | ||||
| Wet-mix concrete (w/b = 0.4) | PC 1: 0 h/25 °C/50% | −17% | 44% | 41% | 7.5% | [56] |
| PC 1: 4 h/25 °C/50% | −46% | −13% | −8% | 21.3% | ||
| PC 1: 18 h/25 °C/50% | −13% | 15% | −1% | 24.2% | ||
| CS 2: 4 h/100%/1 atm | ||||||
| Dry-mix paste (w/b = 0.15) | 3 days | [70] | ||||
| CS 2: 2 h/1%/1 atm | +1.2% | 9.6% | ||||
| CS 2: 2 h/3%/1 atm | +3.8% | 13.2% | ||||
| CS 2: 2 h/10%/1 atm | +3.7% | 16.6% | ||||
| CS 2: 2 h/20%/1 atm | +10.7% | 19.5% | ||||
| Dry-mix mortar (w/b = 0.15) | 28 days | [57] | ||||
| PC 1: 0 h | −3.2% | 9.8% | ||||
| PC 1: 5 h | −3.2% | 9.0% | ||||
| PC 1: 11 h | +13.5% | 7.9% | ||||
| PC 1: 23 h | +12.8% | 6.8% | ||||
| PC 1: 71 h | +37.8% | 6.1% | ||||
| CS 2: 1 h/99%/1 atm | ||||||
| Dry-mix mortar (w/b = 0.3) | PC 1: 2 h/60%/20 °C | 3 days | 7 days | 28 days | [71] | |
| CS 2: 1 h/100%/1 atm | +9.0% | +4.5% | +0.0% | 19% | ||
| CS 2: 5 h/100%/1 atm | +10.5% | +4.5% | −10.7% | 21% | ||
| CS 2: 12 h/100%/1 atm | +15.0% | +9.1% | −10.7% | 23% | ||
| Dry-mix paste (w/b = 0.18) | PC 1: 2 h | +212.5% | 7.9% | [72] | ||
| CS 2: 2 h/20%/1.5 atm | +1.4% | 17.6% | ||||
| CS 2: 672 h/20%/1.5 atm | at the end of CS | |||||
| Dry-mix paste (w/b = 0.15) | 3 days | 28 days | [73] | |||
| CS 2: 2 h/99.5%/1 atm | +17% | +8% | 7.3% | |||
| Mixture | Process | Compressive Strength Variation | CO2 Uptake | Refs. | ||
|---|---|---|---|---|---|---|
| Cement paste (w/b = 0.15) | Flow-through system Flow rate 5 L/min MD 1 240 s cCO2 2 99.5% | fc 3 variation −84% 3 days −83% 28 days | CO2 uptake 3.4%wt | [73] | ||
| Cement paste (w/b = 0.44) | Enclosed system cCO2 2 85 ± 5%v/v MD 1 45 min MD 1 90 min | fc 3 variation at 28 days −12% −13% | CO2 uptake 0.93%wt 1.12%wt | [86] | ||
| Ready-mix concrete (308 kg of cement and 77 kg of slag) | Enclosed system with injection of CO2 | No impact on durability fc 3 variation 3 days 7 days 28 days | CO2 uptake | [92] | ||
| 0.05%wt | +10% | +1.0% | +3.0% | 0.04%wt | ||
| 0.15%wt | −6.0% | −4.0% | −4.0% | 0.12%wt | ||
| 0.30%wt | −9.0% | −9.0% | −6.0% | 0.24%wt | ||
| (CO2/cement) | ||||||
| Ready-mix concrete (147.7 kg of cement and 73.9 kg of slag and of fly ash) | Enclosed system with injection of CO2 0.11%wt (CO2/cement) | Same fc 3 as a reference concrete with 4.3% more binder | Reduction in CO2 emissions 10.0 kg/m3 (CO2/concrete) | [93] | ||
| Ready-mix concrete (25% fly ash) | Enclosed system with injection of CO2 0.25%wt (CO2/cement) | fc 3 variation +24% 1 day +29% 2 days +23% 7 days +19% 28 days | N/A | [89] | ||
| Mortar (w/b = 0.5) | Enclosed system cCO2 2 99.5% | fc 3 variation 3 days 7 days 28 days | CO2 uptake | [90] | ||
| −1.9% | −5.7% | −3.3% | 0.44%wt | |||
| −9.6% | −7.1% | +5.6% | 1.32%wt | |||
| −7.7% | −5.7% | +8.9% | 2.20%wt | |||
| Mixture | Compressive Strength Variation | CO2 Uptake | Refs. |
|---|---|---|---|
| Cement paste (w/b = 0.5) | fc 1 at 28 days 6% reduction | N/A | [84] |
| Cement paste (w/b = 0.4) | fc 1 at 28 days 20 % reduction | N/A | [94] |
| Cement mortar (w/b = 0.4) | Reduction in fc 1 Porosity doubled and pores around 80 nm increased | N/A | [94] |
| Cement paste (12% limestone filler) (w/b = 0.4) | fc 1 variation at 1 day +18% | 0.61%wt | [95] |
| Mortar a/c = 0.4 | fc 1 variation 1 day 3 days 7 days −9% +18% +13% | 1.03%wt | [96] |
| HCW Origin | HCW Conditions 1 | Carbonation Process 2 | CO2 Uptake (%wt of HCW) | Mixture | Compressive Strength Variation | Refs. | |||
|---|---|---|---|---|---|---|---|---|---|
| Lab-made paste (w/b = 0.4) | <75 μm | N/A cCO2 99% 1 atm 20 °C RH 60% | 20.9 | 1 day 28 days | [111] | ||||
| 10% 3 | +16% | +4% | |||||||
| 20% 3 | +24% | +12% | |||||||
| 30% 3 | 4% | −5% | |||||||
| Cement paste (w/b = 0.4) | |||||||||
| Lab-made paste (w/b = 0.3) | 0–75 μm 75–150 μm | 28 days cCO2 20% 1 atm 20 °C RH 65% | 21.1 | at 28 days | [119] | ||||
| 10% 3 | +5% | 0% | |||||||
| 20% 3 | +8% | 0% | |||||||
| 30% 3 | 0% | −3% | |||||||
| Cement paste (w/b = 0.3) | 75 μm | 150 μm | |||||||
| Lab-made paste (w/b = 0.3) | <200 μm | 12 days cCO2 20% 1 atm 20 °C RH 70% | 19.3 | 30% 3 Cement paste (w/b = 0.4) | N/A | [81] | |||
| Lab-made paste (w/b = 0.2) (w/b = 0.3) (w/b = 0.4) | <75 μm | 28 days cCO2 20% 1 atm 20 °C RH 65% | 18.3 for 0.2 19.7 for 0.3 21.0 for 0.4 | at 28 days | [127] | ||||
| 5% 3 | +4% | −1% | 0% | ||||||
| 10% 3 | +7% | +7% | +5% | ||||||
| 15% 3 | +8% | +10% | +6% | ||||||
| 20% 3 | −6% | −2% | +9% | ||||||
| Cement paste (w/b = 0.3) | 0.2 | 0.3 | 0.4 | ||||||
| Lab-made paste (w/b = 0.45) | <250 μm WC 17% | 2 h cCO2 80% 1 atm 20 °C RH 70% | 19 | N/A | N/A | [129] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Nogueira, R.; Bogas, J.A. Strategies for OPC Paste Carbonation: Relationship between Microstructure, Performance and Net CO2 Balance. Sustainability 2024, 16, 361. https://doi.org/10.3390/su16010361
Silva A, Nogueira R, Bogas JA. Strategies for OPC Paste Carbonation: Relationship between Microstructure, Performance and Net CO2 Balance. Sustainability. 2024; 16(1):361. https://doi.org/10.3390/su16010361
Chicago/Turabian StyleSilva, André, Rita Nogueira, and José Alexandre Bogas. 2024. "Strategies for OPC Paste Carbonation: Relationship between Microstructure, Performance and Net CO2 Balance" Sustainability 16, no. 1: 361. https://doi.org/10.3390/su16010361