Vertical Variation in Temperature Sensitivity of Soil Organic Carbon Mineralization in Changbai Mountain, China: A Microcosm Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Soil Sampling
2.3. Sample Analysis
2.3.1. Organic Carbon Mineralization
2.3.2. Soil Properties
2.4. Statistical Analysis
3. Results
3.1. Vertical Variation Characteristics of Soil Organic Carbon Mineralization
3.2. Vertical Variation Characteristics of Organic Carbon Mineralization Temperature Sensitivity (Q10)
3.3. Kinetic Characteristics of Organic Carbon Mineralization
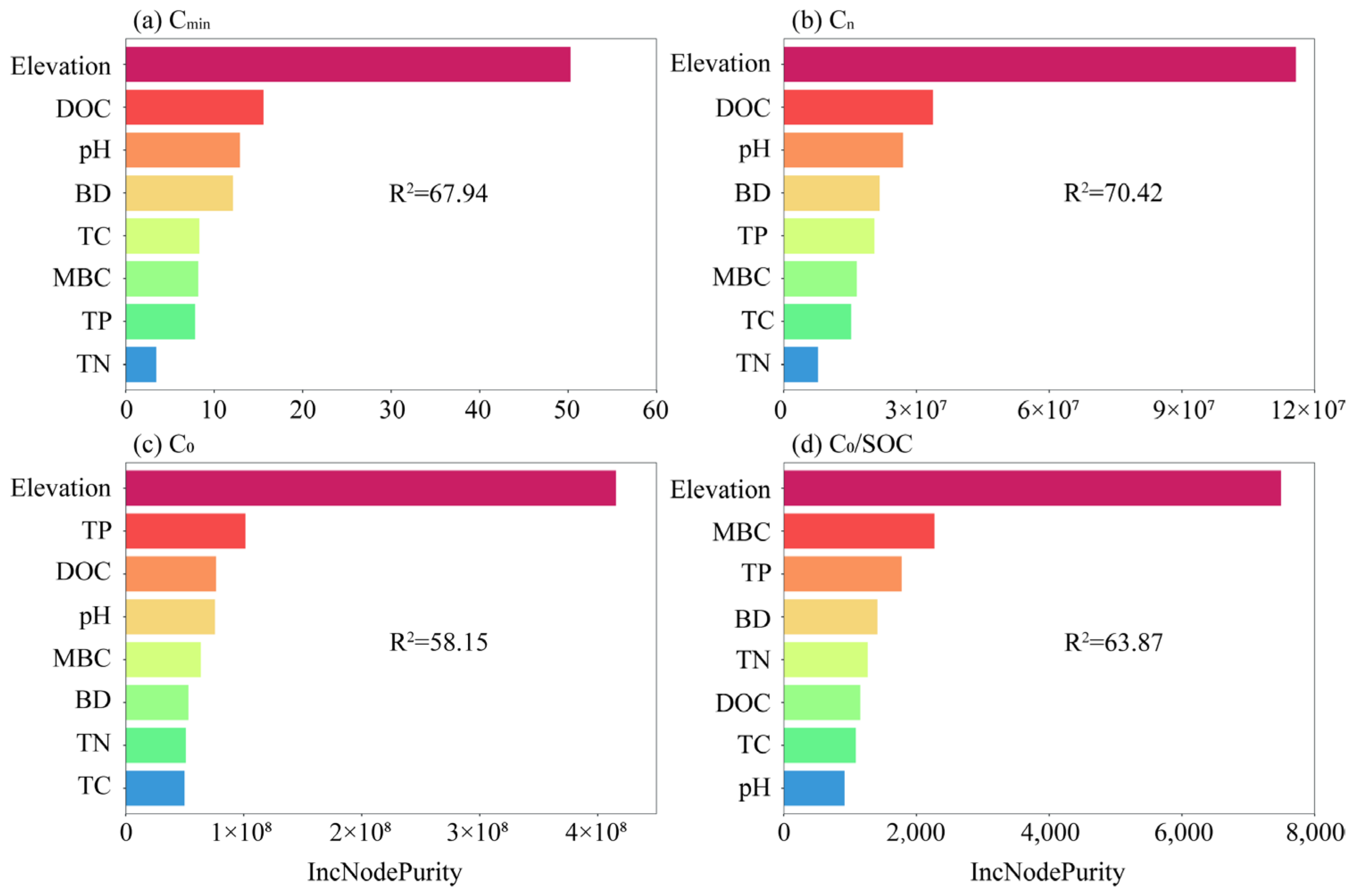
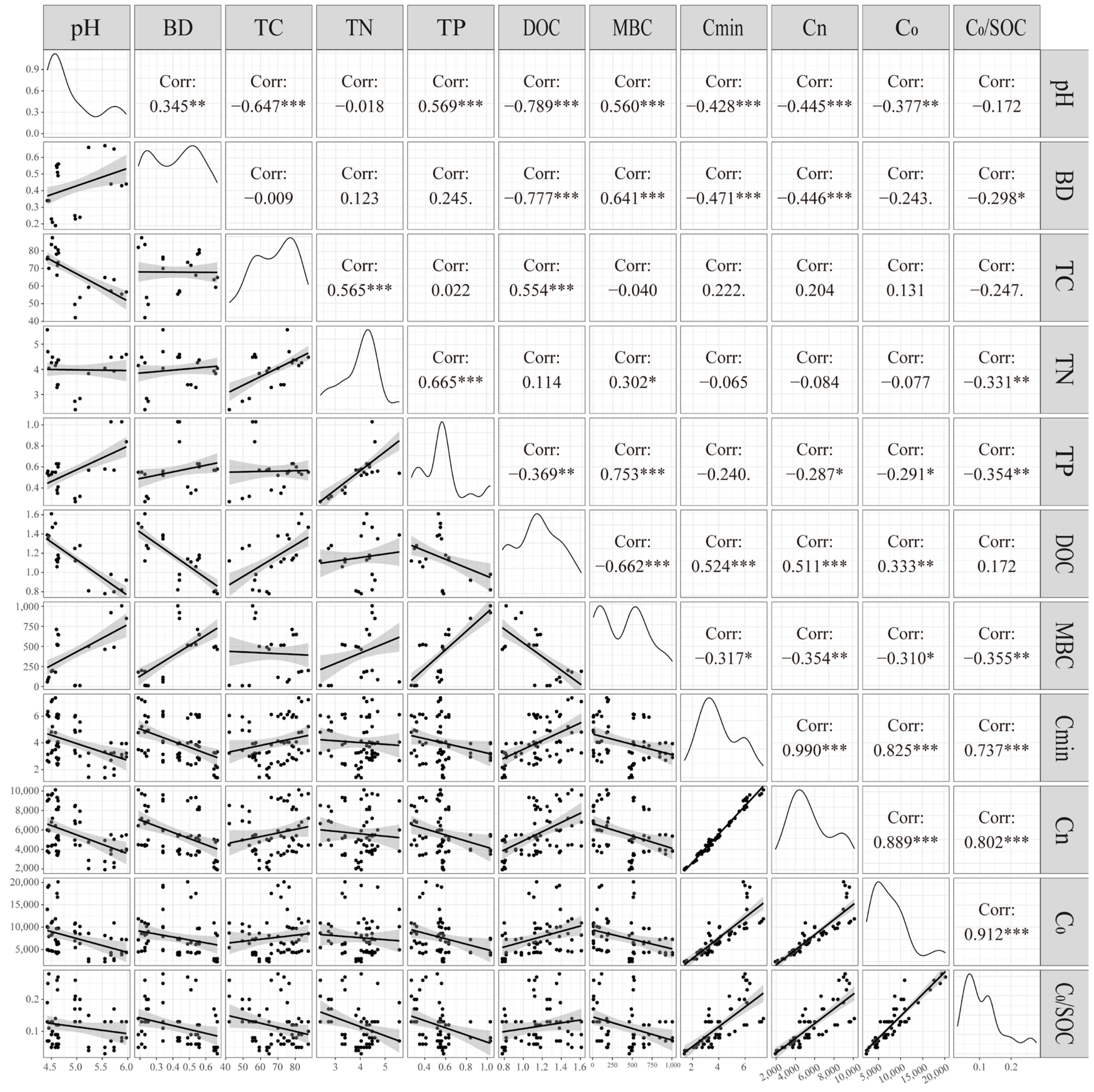
3.4. Influence Factors of Organic Carbon Mineralization
4. Discussion
4.1. Responses of Soil Organic Carbon Mineralization to Warming in Vertical Zones
4.2. Analysis of Driving Factors of Soil Organic Carbon Mineralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Synthesis Report: Climate Change 2023. In Proceedings of the Panel’s 58th Session, Interlaken, Switzerland, 13–19 March 2023. [Google Scholar]
- Hicks Pries, C.E.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, V.M.; Yao, F.; Ye, J.; Bu, R.C.; Ma, R.A.; Lin, J.J.; Kurganova, I.; Wang, X.G.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Zheng, Y.J.; Ma, R.Y.; Yu, K.; Han, Z.Q.; Xiao, S.Q.; Li, Z.F.; Wu, S.Q.; Wang, J.Y.; Luo, Y.Q.; et al. Increased soil release of greenhouse gases shrinks terrestrial carbon uptake enhancement under warming. Glob. Chang. Biol. 2020, 26, 4601–4613. [Google Scholar] [CrossRef] [PubMed]
- Haaf, D.; Six, J.; Doetterl, S. Global patterns of geo-ecological controls on the response of soil respiration to warming. Nat. Clim. Chang. 2021, 11, 623–627. [Google Scholar] [CrossRef]
- Wang, M.M.; Guo, X.W.; Zhang, S.; Xiao, L.J.; Mishra, U.; Yang, Y.H.; Zhu, B.; Wang, G.C.; Mao, T.; Jiang, T.; et al. Global soil profiles indicate depth-dependent soil carbon losses under a warmer climate. Nat. Commun. 2022, 13, 5514. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Tang, Y.H.; Son, Y. Why are East Asian ecosystems important for carbon cycle research? Sci. China Life Sci. 2010, 53, 753–756. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Wang, Q.K.; Liu, S.G.; Tian, P. Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Glob. Chang. Biol. 2018, 24, 2841–2849. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Ruan, H.H.; Luo, Y.Q. Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biol. Biochem. 2010, 42, 1811–1815. [Google Scholar] [CrossRef]
- Sang, L.; Liu, X.; Sun, D.D.; Yang, Y.F.; Yang, J.S.; Wang, Z.K.; Li, Y.Z.; Zhou, D.; Ning, K.; Guan, B.; et al. Effect of tidal hydrology on soil anaerobic CO2 production of freshwater marsh in the Yellow River estuary wetland, China. Ecol. Indic. 2022, 145, 109747. [Google Scholar] [CrossRef]
- Okello, J.; Bauters, M.; Verbeeck, H. Temperature sensitivity of soil organic carbon respiration along a forested elevation gradient in the Rwenzori Mountains, Uganda. Biogeosciences 2023, 20, 719–735. [Google Scholar] [CrossRef]
- Reddy, R.; DeLaune, R. Biogeochemistry of Wetlands: Science and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Xu, X.; Luo, Y.; Zhou, J. Carbon quality and the temperature sensitivity of soil organic carbon decomposition in a tallgrass prairie. Soil Biol. Biochem. 2012, 50, 142–148. [Google Scholar] [CrossRef]
- Ding, J.Z.; Chen, L.Y.; Zhang, B.B.; Liu, L.; Yang, G.B.; Fang, K.; Chen, Y.L.; Li, F.; Kou, D.; Ji, C.J.; et al. Linking temperature sensitivity of soil CO2 release to substrate, environmental, and microbial properties across alpine ecosystems. Glob. Biogeochem. Cycles 2016, 30, 1310–1323. [Google Scholar] [CrossRef]
- Li, J.; Nie, M.; Pendall, E.; Reich, P.B.; Pei, J.M.; Noh, N.J.; Zhu, T.; Li, B.; Fang, C.M. Biogeographic variation in temperature sensitivity of decomposition in forest soils. Glob. Chang. Biol. 2020, 26, 1873–1885. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.S.; Ning, K.; Wang, A.D.; Wang, Q.X.; Wang, X.H.; Wang, S.W.; Lv, Z.B.; Zhao, Y.J.; Yu, J.B. Temperature sensitivity of anaerobic CO2 production in soils of Phragmites australis marshes with distinct hydrological characteristics in the Yellow River estuary. Ecol. Indic. 2021, 124, 107409. [Google Scholar] [CrossRef]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Piñeiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Zhang, L.; Li, H.Q.; He, H.D.; Wei, Y.X.; Luo, J.; Zhang, G.R.; Huang, Y.R.; Li, Y.N.; Zhou, H.K. Soil physicochemical properties and vegetation structure along an elevation gradient and implications for the response of alpine plant development to climate change on the northern slopes of the Qilian Mountains. J. Mt. Sci. 2018, 15, 1006–1019. [Google Scholar] [CrossRef]
- Du, B.; Kang, H.; Pumpanen, J.; Zhu, P.H.; Yin, S.; Wang, Z.; Kong, F.Q.; Liu, C.J. Soil organic carbon stock and chemical composition along an altitude gradient in the Lushan Mountain, subtropical China. Ecol. Res. 2014, 29, 433–439. [Google Scholar] [CrossRef]
- Zhang, B.P.; Yao, Y.H. Implications of mass elevation effect for the altitudinal patterns of global ecology. J. Geogr. Sci. 2016, 26, 871–877. [Google Scholar] [CrossRef]
- Wang, D.; He, N.P.; Wang, Q.; Lv, Y.L.; Wang, Q.F.; Xu, Z.W.; Zhu, J.X. Effects of temperature and moisture on soil organic matter decomposition along elevation gradients on the Changbai Mountains, Northeast China. Pedosphere 2016, 26, 399–407. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.J.; Wu, Z.; Du, H.; Zong, S.; Ma, S. Potential distribution shifts of plant species under climate change in Changbai Mountains. China. Forests 2019, 10, 498. [Google Scholar] [CrossRef]
- Garten, C.T.; Hanson, P.J. Measured forest soil C stocks and estimated turnover times along an elevation gradient. Geoderma 2006, 136, 342–352. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wu, H.T.; Liu, J.P.; Liu, D.D.; Lin, Y.L.; Kang, Y.J.; Guan, Q.; Zhang, Z.S. Vertical characteristics of soil carbon, nitrogen and phosphorus contents and ecological stoichiometry in the Changbai Mountains. Chin. J. Environ. Sci. 2023, 5, 7–15+81. [Google Scholar]
- Li, J.Y.; Chen, P.; Li, Z.G.; Li, L.Y.; Zhang, R.Q.; Hu, W.; Liu, Y. Soil aggregate-associated organic carbon mineralization and its driving factors in rhizosphere soil. Soil Biol. Biochem. 2023, 186, 109182. [Google Scholar] [CrossRef]
- Fierer, N.; Colman, B.P.; Schimel, J.P.; Jackson, R.B. Predicting the temperature dependence of microbial respiration in soil: A continental-scale analysis. Glob. Biogeochem. Cycles 2006, 20, GB3026. [Google Scholar] [CrossRef]
- Huang, J.X.; Xiong, D.C.; Liu, X.F.; Yang, Z.J.; Xie, J.S.; Yang, Y.S. Effects of warming on soil organic carbon mineralization: A review. Acta Ecol. Sin. 2017, 37, 12–24. [Google Scholar]
- Wang, G.B.; Zhou, Y.; Xu, X.; Ruan, H.H.; Wang, J.S. Temperature Sensitivity of Soil Organic Carbon Mineralization along an Elevation Gradient in the Wuyi Mountains, China. PLoS ONE 2013, 8, e53914. [Google Scholar] [CrossRef][Green Version]
- Pold, G.; Stuart Grandy, A.; Melillo, J.M.; DeAngelis, K.M. Changes in substrate availability drive carbon cycle response to chronic warming. Soil Biol. Biochem. 2017, 110, 68–78. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Takriti, M.; Wild, B.; Schnecker, J.; Mooshammer, M.; Knoltsch, A.; Lashchinskiy, N.; Alves, R.J.E.; Gentsch, N.; Gittel, A.; Mikutta, R.; et al. Soil organic matter quality exerts a stronger control than stoichiometry on microbial substrate use efficiency along a latitudinal transect. Soil Biol. Biochem. 2018, 121, 212–220. [Google Scholar] [CrossRef]
- Wei, L.; Razavi, B.S.; Wang, W.Q.; Zhu, Z.K.; Liu, S.L.; Wu, J.S.; Kuzyakov, Y.; Ge, T. Labile carbon matters more than temperature for enzyme activity in paddy. soil. Soil Biol. Biochem. 2019, 135, 134–143. [Google Scholar] [CrossRef]
- Kang, Y.J.; Wu, H.T.; Zhang, Y.F.; Wu, Q.; Guan, Q.; Lu, K.L.; Lin, Y.L. Differential distribution patterns and assembly processes of soil microbial communities under contrasting vegetation types at distinctive altitudes in the Changbai Mountain. Front. Microbiol. 2023, 14, 1152818. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.D.; Newkirk, K.M.; Rullo, G.M. Carbon dioxide and methane fluxes by a forest soil under laboratory-controlled moisture and temperature conditions. Soil Biol. Biochem. 1998, 30, 1591–1597. [Google Scholar] [CrossRef]
- Bradford, M.A.; Davies, C.A.; Frey, S.D.; Maddox, T.R.; Melillo, J.M.; Mohan, J.E.; Reynolds, J.F.; Treseder, K.K.; Wallenstein, M.D. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 2008, 11, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Watts, B.W.; Davies, C.A. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob. Chang. Biol. 2010, 16, 1576–1588. [Google Scholar] [CrossRef]
- Zheng, J.J.; Huang, S.Y.; Jia, X.; Tian, Y.; Mu, Y.; Liu, P.; Zha, T.S. Spatial variation and controlling factors of temperature sensitivity of soil respiration in forest ecosystems across China. Chin. J. Plant Ecol. 2020, 44, 687–698. [Google Scholar] [CrossRef]
- Fu, S.L.; Liu, M.Q.; Zhang, W.X.; Shao, Y.H. A review of recent advances in the study of geographical distribution and ecological functions of soil fauna diversity. Biodivers. Sci. 2022, 30, 22435. [Google Scholar] [CrossRef]
- Liu, D.D.; Liu, D.; Yu, H.X.; Wu, H.T. Strong variations and shifting mechanisms of altitudal diversity and abundance patterns in soil oribatid mites (Acari: Oribatida) on the Changbai Mountain, China. Appl. Soil Ecol. 2023, 186, 104808. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Fierer, N.; Craine, J.M.; McLauchlan, K. Litter quality and the temperature sensitivity of decomposition. Ecology 2005, 86, 320–326. [Google Scholar] [CrossRef]
- Li, Q.; Leroy, F.; Zocatelli, R. Abiotic and biotic drivers of microbial respiration in peat and its sensitivity to temperature change. Soil Biol. Biochem. 2021, 153, 108077. [Google Scholar] [CrossRef]
- Haddix, M.L.; Plante, A.F.; Conant, R.T.; Six, J.; Steinweg, M.; Magrini-Bair, K.; Drijber, R.A.; Morris, S.J.; Paul, E.A. The Role of Soil Characteristics on Temperature Sensitivity of Soil Organic Matter. Soil Sci. Soc. Am. J. 2007, 75, 56. [Google Scholar] [CrossRef]
- Craine, J.M.; Fierer, N.; Mclauchlan, K.K. Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat. Geosci. 2010, 3, 854–857. [Google Scholar] [CrossRef]
- Bosatta, E.; Ågren, G.I. Soil organic matter quality interpreted thermodynamically. Soil Biol. Biochem. 1999, 31, 1889–1891. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, N.P.; Zhu, J.X.; Xu, L.; Yu, G.R.; Niu, S.L.; Sun, X.M.; Wen, X.F. Regional variation in the temperature sensitivity of soil organic matter decomposition in China’s forests and grasslands. Glob. Chang. Biol. 2017, 23, 3393–3402. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Zhang, T.L.; Chen, B.Y. Dynamics of soluble organic carbon and its relation to mineralization of soil organic carbon. Acta Pedol. Sin. 2004, 41, 544–552. [Google Scholar]
- Leifeld, J.; Fuhrer, J. The temperature response of CO2 production from bulk soils and soil fractions is related to soil organic matter quality. Biogeochemistry 2005, 75, 433–453. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Gren, G.I. Temperature and soil organic matter decomposition rates-synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Chen, Z.X.; Zhang, C.; Li, Q.; Song, X.Z.; Shi, M. Mechanism underlying temperature sensitivity of soil organic carbon decomposition: A review. Chin. J. Appl. Ecol. 2023, 34, 2575–2584. [Google Scholar]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, Y.; Feng, S.Z. Soil organic carbon mineralization with fresh organic substrate and inorganic carbon additions in a red soil is controlled by fungal diversity along a pH gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Novara, A.; Armstrong, A.; Gristina, L.; Semple, K.T.; Quinton, J.N. Effects of soil compaction, rain exposure and their interaction on soil carbon dioxide emission. Earth Surf. Process. Landf. 2012, 37, 994–999. [Google Scholar] [CrossRef]
- Chen, X.W.; Yang, J.M.; Liang, A.Z.; Zhang, X.P. Effects of soil compaction and tillage practices on carbon dioxide efflux in northeast China: Evidence from an incubation study. Pol. J. Environ. Stud. 2016, 25, 1769–1776. [Google Scholar] [CrossRef]
- Rong, H.; Fang, H.; Jiang, Y.Q.; Zhao, X.; Peng, X.H.; Sun, B.; Zhou, H. Effects of loose soil samples, repacked soil columns and compactness on soil organic carbon mineralization. Acta Pedol. Sin. 2022, 59, 1551–1560. [Google Scholar]
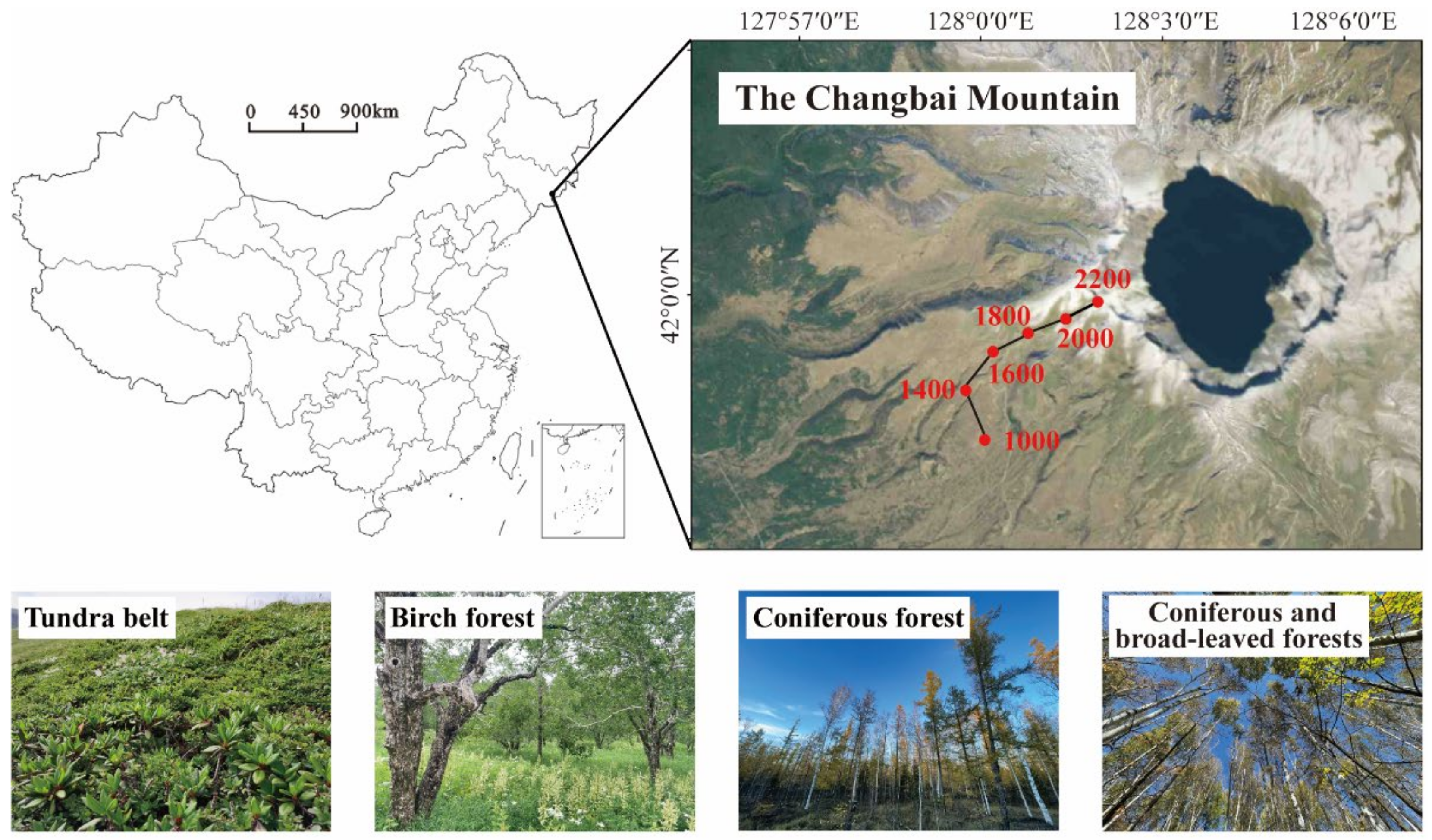




| Elevation (m) | Latitude–Longitude | Vegetation Zone | Vegetation Type (Main Composition) |
|---|---|---|---|
| 1000 | 128.15 E, 42.24 N | Coniferous and broad-leaved forests | Korean pine, White birch, Quercus mongolica, Acer mono, Tilia amurensis, Ulmus propinoua, and Fraxinus mandshurica |
| 1400 | 128.13 E, 42.14 N | Coniferous forest | Picea jezoensis, Abies nephrolepis, Pinus koraiensis, Korean pine, and Betula costata |
| 1600 | 128.07 E, 42.09 N | ||
| 1800 | 128.06 E, 42.06 N | Birch forest | Betula ermanii, and Rhododendron aureum |
| 2000 | 128.07 E, 42.06 N | ||
| 2200 | 128.07 E, 42.04 N | Tundra | Rhododendron aureum, Dryas octopetala, Vaccinum uliginosum, and Rhododendron redovoskianum |
| Index | Temperature | Elevation | Temperature × Elevation | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Cmin | 15.967 | <0.001 | 658.177 | <0.001 | 172.309 | <0.001 |
| Cn | 13.267 | <0.001 | 750.448 | <0.001 | 219.932 | <0.001 |
| C0 | 7.450 | 0.002 | 119.077 | <0.001 | 44.472 | <0.001 |
| C0/SOC | 3.634 | 0.003 | 72.294 | <0.001 | 46.275 | <0.001 |
| Q10 | — | — | 60.321 | <0.001 | — | — |
| Temperature (°C) | Elevation (m) | Ct = C0(1−e−kt) | R2 |
|---|---|---|---|
| 5 | 1000 | Ct = 2378.52(1−e−0.03t) | 0.996 |
| 1400 | Ct = 4277.07(1−e−0.02t) | 0.996 | |
| 1600 | Ct = 4349.18(1−e−0.03t) | 0.998 | |
| 1800 | Ct = 4895.36(1−e−0.02t) | 0.998 | |
| 2000 | Ct = 4605.27(1−e−0.03t) | 0.998 | |
| 2200 | Ct = 6232.53(1−e−0.02t) | 0.999 | |
| 15 | 1000 | Ct = 2919.59(1−e−0.03t) | 0.997 |
| 1400 | Ct = 6300.08(1−e−0.02t) | 0.997 | |
| 1600 | Ct = 6537.09(1−e−0.02t) | 0.994 | |
| 1800 | Ct = 9314.89(1−e−0.02t) | 0.997 | |
| 2000 | Ct = 7978.83(1−e−0.03t) | 0.980 | |
| 2200 | Ct = 8730.68(1−e−0.02t) | 0.993 | |
| 25 | 1000 | Ct = 7314.00(1−e−0.01t) | 0.998 |
| 1400 | Ct = 18,121.29(1−e−0.03t) | 0.997 | |
| 1600 | Ct = 9707.66(1−e−0.02t) | 0.997 | |
| 1800 | Ct = 17,412.72(1−e−0.01t) | 0.999 | |
| 2000 | Ct = 11,357.59(1−e−0.03t) | 0.997 | |
| 2200 | Ct = 10,583.67(1−e−0.02t) | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, Y.; Wu, H.; Liu, D.; Zhang, Z. Vertical Variation in Temperature Sensitivity of Soil Organic Carbon Mineralization in Changbai Mountain, China: A Microcosm Study. Sustainability 2024, 16, 1350. https://doi.org/10.3390/su16031350
Liu X, Zhang Y, Wu H, Liu D, Zhang Z. Vertical Variation in Temperature Sensitivity of Soil Organic Carbon Mineralization in Changbai Mountain, China: A Microcosm Study. Sustainability. 2024; 16(3):1350. https://doi.org/10.3390/su16031350
Chicago/Turabian StyleLiu, Xue, Yifan Zhang, Haitao Wu, Dandan Liu, and Zhongsheng Zhang. 2024. "Vertical Variation in Temperature Sensitivity of Soil Organic Carbon Mineralization in Changbai Mountain, China: A Microcosm Study" Sustainability 16, no. 3: 1350. https://doi.org/10.3390/su16031350
APA StyleLiu, X., Zhang, Y., Wu, H., Liu, D., & Zhang, Z. (2024). Vertical Variation in Temperature Sensitivity of Soil Organic Carbon Mineralization in Changbai Mountain, China: A Microcosm Study. Sustainability, 16(3), 1350. https://doi.org/10.3390/su16031350






